Abstract
Objective:
To provide a multidimensional analysis of the learning curve in major laparoscopic colonic and rectal surgery and compare outcomes between right-sided versus left-sided resections.
Summary Background Data:
The laparoscopic learning curve is known to vary between surgeons, may be influenced by the patient selection and operative complexity, and requires appropriate case-mix adjustment.
Methods:
This is a descriptive single-center study using routinely collected clinical data from 900 patients undergoing laparoscopic surgery between November 1991 and April 2003. Outcome measures included operation time, conversion rate (CR), and readmission and postoperative complication rates. Multifactorial logistic regression analysis was used to identify patient-, surgeon-, and procedure-related factors associated with conversion of laparoscopic to open surgery. A risk-adjusted Cumulative Sum (CUSUM) model was used for evaluating the learning curve for right and left-sided resections.
Results:
The conversion rate for right-sided colonic resections was 8.1% (n = 457) compared with 15.3% for left-sided colorectal resections (n = 443). Independent predictors of conversion of laparoscopic to open surgery were the body mass index (BMI) (odds ratio [OR] = 1.07 per unit increase), ASA grade (OR = 1.63 per unit increase), type of resection (left colorectal versus right colonic procedures, OR = 1.5), presence of intra-abdominal abscess (OR = 5.0) or enteric fistula (OR = 4.6), and surgeon's experience (OR 0.9 per 10 additional cases performed). Having adjusted for case-mix, the CUSUM analysis demonstrated a learning curve of 55 cases for right-sided colonic resections versus 62 cases for left-sided resections. Median operative time declined with operative experience (P<0.001). Readmission rates and postoperative complications remained unchanged throughout the series and were not dependent on operative experience.
Conclusions:
Conversion rates for laparoscopic colectomy are dependent on a multitude of factors that require appropriate adjustment including the learning curve (operative experience) for individual surgeons. The laparoscopic model described can be used as the basis for performance monitoring between or within institutions.
The learning curve in laparoscopic colorectal surgery was evaluated using a case-mix adjusted Cumulative Sum (CUSUM) model analysis. A learning curve of 55 cases was evident for right-sided colonic resections versus 62 cases for left-sided resections. Readmission rates and postoperative complications remained unchanged throughout the series and were not dependent on operative experience.
Laparoscopic colorectal surgery has been used for inflammatory, benign and malignant disease entities and has been shown to reduce postoperative pain and length of hospital stay, provide faster recovery, and shown to be cost-effective in comparison to open surgery.1 As the demand for laparoscopic colorectal surgery increases, patient selection, case-mix, and laparoscopic outcomes such as conversion rates are expected to vary between surgeons and institutions.
Laparoscopic surgery requires a high degree of special resolution, dexterity, and technical skills. An initial training period is usually required for the majority of surgeons to become proficient in these complex procedures by continuous repetition of these tasks. As a result, one would anticipate that to become technically proficient at laparoscopic colorectal resections may require a longer training period than simpler procedures such as cholecystectomy.2 This initial training period or learning curve often consists of a steep gradient, which varies between surgeons and procedures, representing the rapid change in the ability to complete the task until “failure” is eliminated or reduced to a minimum constant rate. Ramsay et al3,4 reviewed 272 articles, which formally assessed the learning curve in minimal access (51%), other surgical (41%), and diagnostic (8%) procedures. The majority of studies used simple graphs (44%), arbitrarily splitting of the data into chronologic groups and performing univariate statistics with (9%) and without (60%) tests for trend. Advanced multivariate techniques were used sparingly (4%) and the Cumulative Sum (CUSUM) technique was used in only 2% of all studies without any formal adjustment for the patient risk factors or case mix.
The aim of the study is to provide a risk-adjusted multidimentional analysis of the learning curve in laparoscopic colorectal surgery and compare surgeon-specific and patient-specific outcomes between right- and left-sided resections. Particular emphasis is given to the adjustment of potential confounding factors (patient case-mix and procedural risk factors) that may affect the learning curve in colonic and rectal surgery.
PATIENTS AND METHODS
Consecutive patients undergoing elective or emergency colorectal surgery between November 1991 and April 2003 by the laparoscopic approach at the Cleveland Clinic Foundation were identified from the institutional review board approved database.5 The database provided a comprehensive dataset comprising of patient demographic characteristics, preoperative assessment, surgical treatment, postoperative course, pathology section, intraoperative and postoperative complications, readmission within 30 days, and length of stay in hospital. The study population was divided into 2 groups according to the type of operation performed: 1) right-sided colonic resections included ileocolic resection, right-hemicolectomy, and extended right-hemicolectomy; and 2) left-sided colonic resections included left hemicolectomy, sigmoid colectomy, and rectosigmoidectomy.
Study End Point and Risk Factors
The primary end-point was conversion of laparoscopic to open surgery defined an “early or unplanned” need for a midline laparotomy or an abdominal incision greater than 10 cm, for either completion of the operative procedure or extraction of the specimen. Secondary end-points were patient readmission to hospital within 30 days followings discharge, postoperative complications within 30 days from surgery, and operative time calculated from the first skin incision to the application of dressings in theater. Patient-specific, procedure-specific, and surgeon-specific factors were considered, comprising age, gender, BMI, diagnosis, American Society of Anesthesiology classification, operative procedure, operative time, cancer staging according to the preoperative and perioperative clinical findings, and histologic TNM classification for colorectal cancer. Intraoperative factors included the presence of intra-abdominal phlegmon, abscess, enteric-related fistulae, and adhesive tenacity (classed as no adhesions, loose filmy adhesions that can be separated by blunt dissection, adhesions requiring <50% or >50% of sharp dissection for separation, serosal injury, full thickness injury). Operative experience was represented as each individual surgeon's case sequence number. The operative sequence was categorized into 8 levels of increasing operative experience, each level representing 25 cases, except the highest group, which denoted experience beyond 175 cases.
Statistical Analysis
Continuous variables such as age and BMI were categorized into quartiles representing groups of increasing risk of conversion to open surgery. To reduce the influence of outlying values such as operative time, these were transformed logarithmically, thus assuming a near-normal distribution. Information on intraoperative risk factors was not recorded before January 2001 and to maximize the information extracted from the predictor variables the technique of median imputation was used to substitute for incomplete data.6 True adjustment for intraoperarive factors was feasible for the last 2 years of the study. Risk factors with a univariate P value of <0.25 were included in the multivariate logistic regression model. Each risk factor was manually entered into the model starting from the most relevant; smallest P value, and adding each factor in-turn. By observing the odds ratios, the 95% confidence intervals for each new factor, we were able to ascertain whether each variable should remain in the model or not. The final variable selection was based on clinical relevance and statistical significance. Operative experience is a surgeon-dependent variable; and although it was included in the final multifactorial model, this is merely done for illustrative purposes. It was not used in the estimation of the probability of conversion to open surgery and in the calculation of the risk-adjusted CUSUM chart.
Risk-Adjusted Cumulative Sum (RA-CUSUM) Chart
This is an extension of the original CUSUM method, which plots the difference between the cumulative expected laparoscopic conversions (calculated by the logistic regression model in this study) and the actual conversions to open surgery. The RA-CUSUM plot gives a visual representation on how far a surgeon's or group of surgeons' cumulative laparoscopic conversions are above or below the predicted cumulative conversions, taking into account the expected risk associated with a particular caseload.7,8 Every case in the series is plotted from left to right on the horizontal axis, and the line moves up for every case completed laparoscopically and down for every case that is converted to open surgery. For each patient, the probability of conversion to open surgery is determined by the logistic regression model, which in turn determines the magnitude by which the graph ascends or descends. For every nonconverted case, the graph ascends by an amount equal to the estimated probability of conversion and for every case that is converted the graph descends by an amount equal to the estimated probability of nonconversion. Therefore, if a laparoscopic case is converted in a high-risk patient, the surgeon's performance chart is not unduly penalized. The converse is true if conversion occurs in a low-risk patient. In the present study, the RA-CUSUM graph evaluated the learning curve for right-sided and left-sided resections for a number of surgeons; therefore, each case number represented the aggregated observed minus expected conversions for all staff during their first laparoscopic case, second case, and so on.
Software
The following statistical software package was used: “Statistical Package for the Social Sciences” version 11 for Windows (SPSS, Chicago, IL) and MLwiN Version 1.2 (University of London) for multilevel modeling.
RESULTS
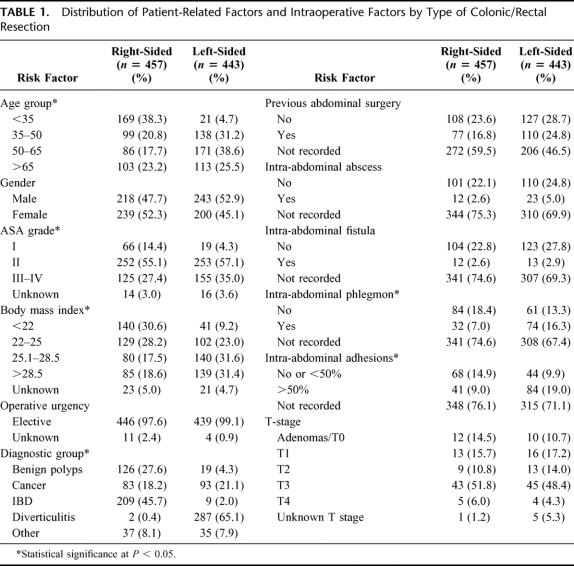
A total of 900 patients (461 men and 439 women) underwent laparoscopic colorectal surgery during the study period. The average age in the series was 50.3 ± 18.7 years (range, 10.1–92.4 years), and the average BMI was 25.8 ± 5.1 kg/m2 (range, 14.1–49.2 kg/m2). Surgery was performed for malignant disease in 176 cases (19.6%), diverticulitis in 289 cases (32.1%), inflammatory bowel disease in 218 cases (24.2%), and other pathologies in 72 cases (8.0%). The patients' preoperative characteristics, diagnosis, staging, and intraoperative factors for right-sided and left-sided resections are shown in Table 1. The patients' age, ASA grade, BMI, diagnosis, and presence of intraoperative phlegmon and adhesions differed significantly between right-sided and left-sided procedures (P < 0.05). Right-sided resections included 155 (17.2%) ileocolic resections, 151 (16.8%) right hemicolectomies, 137 (15.2) extended right hemicolectomies, whereas left-sided resections included 419 (46.5%) rectosigmoidectomies and 38 (4.2%) left hemicolectomies; 889 of 900 (98.7%) procedures were performed by one of 4 staff. The 2 senior staff performed laparoscopic surgery from1991 to 1998 and 1999 to 2003 and did 310 and 348 cases, respectively. The 2 junior staff were trained at the Department of Colorectal Surgery, Cleveland Clinic Foundation between 1997 and 1999 and 2000 and 2003 and performed 136 and 95 cases, respectively. With regards to the patient case selection, during the late part of the study left-sided resections were more frequently performed (54.9% versus 40.5%, P = 0.050) on patients with higher comorbidity (P = 0.034) with complex diverticular disease or cancer (P = 0.003).
TABLE 1. Distribution of Patient-Related Factors and Intraoperative Factors by Type of Colonic/Rectal Resection
Conversion to Open Surgery
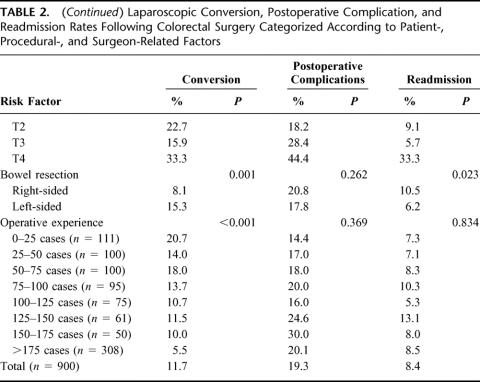
The conversion rates to open surgery, 30-day readmission rates, and postoperative complication rates for various risk factors are shown in Table 2. The rate of conversion to open surgery for right-sided resections was 8.1% (95% CI, 5.8%–11.0%) and for left sided resections 15.3% (95% CI, 12.1%–19.0%) (χ2 test = 11.485,1 df, P = 0.001). Reasons for conversion included: tumor fixity or inadequate distal clearance, n = 11 (9.6%); adhesions and inflammation, n = 38 (36.1%); abscess/fistula, n = 15 (14.2%); bleeding, n = 11 (10.4%); obesity, n = 11 (10.5%); poor views, n = 7 (6.7%); and other, n = 12 (11.4%).
TABLE 2. Laparoscopic Conversion, Postoperative Complication, and Readmission Rates Following Colorectal Surgery Categorized According to Patient-, Procedural-, and Surgeon-Related Factors
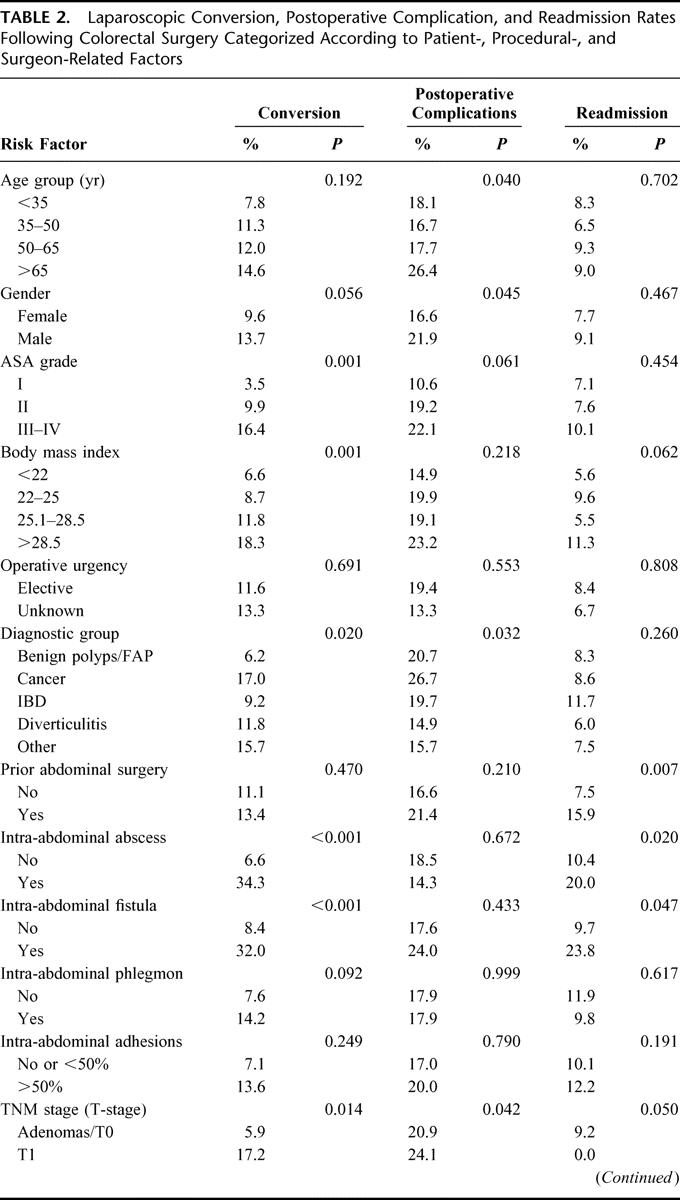
TABLE 2. (Continued) Laparoscopic Conversion, Postoperative Complication, and Readmission Rates Following Colorectal Surgery Categorized According to Patient-, Procedural-, and Surgeon-Related Factors
The relationship between BMI and conversion rate for right-sided and left sided resections is shown in Figure 1. Increasing BMI was shown to be associated with a higher conversion rate; adjusted odds ratio of 2.117 per 10 units increase in BMI, CI, 1.43–3.133. Having adjusted for the BMI, left sided resections were 1.701 times more likely to undergo conversion than the right-sided resections 95% CI, 1.084–2.67.
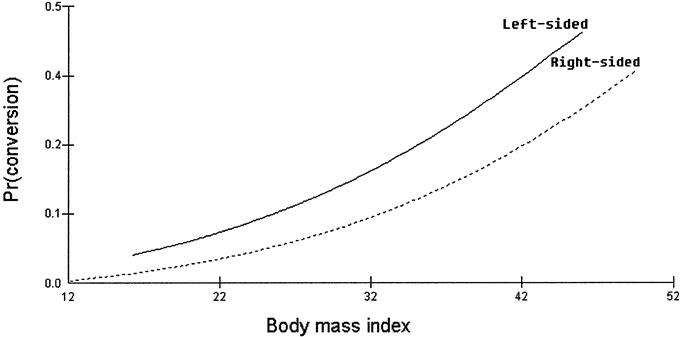
FIGURE 1. Effect of body mass index and type of resection (right-sided versus left-sided) on the conversion rate in laparosopic colorectal surgery.
On multivariate analysis, 6 factors were found to be independent predictors of conversion of laparoscopic to open surgery: the ASA grade, BMI, type of surgery, intra-abdominal abscess or fistula, and the operative experience. These factors are shown in Table 3 together with the β-coefficients, the odds ratios, and the 95% confidence intervals. First-order interactions between these independent risk factors were also considered, but none of these exceeded the significance threshold for inclusion into the model. Having adjusted for confounding variables, there was a 5.1-fold and 3.8-fold increase in the likelihood of conversion to open surgery during the first 25 cases and 25 to 50 cases, respectively, in comparison with the conversion rate following operative experiences beyond 175 cases. The model fitted the data well, as evidenced by the calibration Hosmer-Lemeshow ĉ statistic (9.692, 8 df, P = 0.287), representing the ability of the model to assign the correct probability of outcome to individual patients. The model discrimination (the ability to assign higher probability of conversion to patients who are actually converted) was satisfactory as shown by the area under the receiver operating characteristic curve of 0.759 (95% CI, 0.710–0.807).
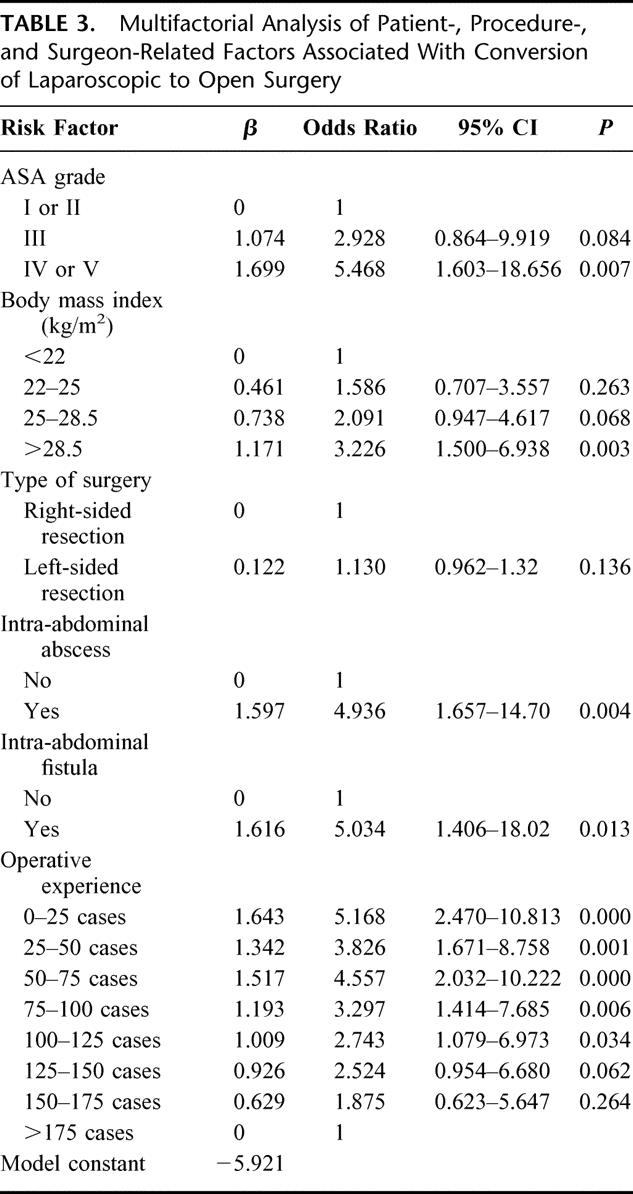
TABLE 3. Multifactorial Analysis of Patient-, Procedure-, and Surgeon-Related Factors Associated With Conversion of Laparoscopic to Open Surgery
The learning curve for right-sided laparoscopic procedures for all staff (n = 7) is shown in Figure 2. The expected conversion rate for each patient was calculated by excluding the operative experience for each surgeon from the multivariate model. A visual inspection of the aggregated risk-adjusted CUSUM plot showed that the conversion to open surgery was more frequent (ie, more negative values) at the beginning of the series and improved after the 55th consecutive case. The learning curve for left-sided laparoscopic bowel resections is shown in Figure 3. Following an initial training period of 40 cases, the conversion rate remained stable upto the 62nd case followed by an improvement in the conversion rate.
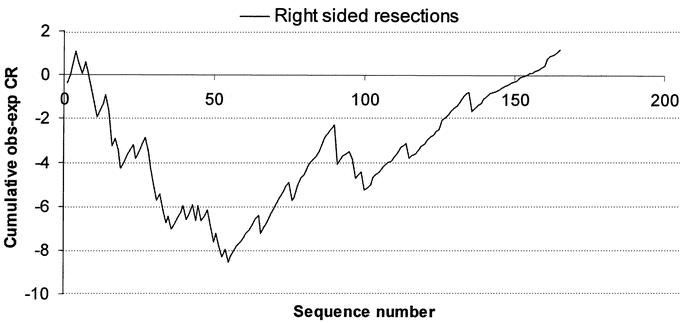
FIGURE 2. Learning curve for laparoscopic surgery for right-sided colonic resections for all staff at CCF. A risk-adjusted CUSUM chart is displayed for a series of 457 consecutive patients. The predicted conversion rate to open surgery was calculated based on a multivariate model based on the patient BMI, comorbidity, type of resection, presence of intra-abdominal fistulae, and abscesses.
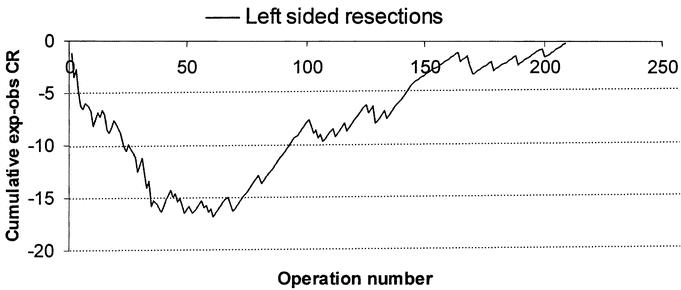
FIGURE 3. Learning curve for laparoscopic left-sided colonic resections for all staff at CCF.
Readmission and Complication Rates
Readmission rate for right-sided resections was 10.5% (n = 47) and 6.2% (n = 27) for left sided resections (χ2 test = 5.137,1 df, P = 0.023). Postoperative complication rates were similar between the 2 groups of patients (20.8% versus 17.8% for right and left-sided resections respectively). The type of adverse events included anastomotic leaks 2.6% (n = 24), bleeding/hematoma 1.6% (n = 15), superficial and deep abdominal sepsis 2.8% (n = 26), postoperative ileus 3.7% (n = 34), bowel obstruction 2.3% (n = 21), and other medical complications 6% (n = 54). The study did not demonstrate a significant learning curve based on the 30-day readmission rate or the postoperative complication rate. On multifactorial logistic regression analysis (not shown), ASA grade (OR per unit increase for ASA = 1.359; 95% CI, 1.021–1.810) and conversion to open surgery (OR = 1.742; 95% CI, 1.076–2.820) were the only 2 independent predictors of postoperative morbidity. Significant predictors for readmission following laparoscopic colorectal surgery were left-sided resections (OR = 0.467; 95% CI, 0.235–0.93), prior abdominal surgery (OR = 2.221; 95% CI, 1.143–4.31), and presence of intra-abdominal fistula (OR = 3.943; 95% CI, 1.251–12.42). Having adjusted for confounding variables, the readmission rate and complication rate remained stable chronologically throughout the study period.
Operative Time
The median operating time for the first 25 procedures was 180 minutes (range, 60–430 minutes) and was longer in comparison with all other groups of operative experience (Fig. 4). Median operating time at the end of the series (>175 cases) was 115 minutes (range, 35–490 minutes). On multivariate analysis of covariance, having adjusted for the risk factors that affected operating time (patient age: F1,823 = 8.823, P = 0013; BMI: F1,823 = 6.405, P = 0.012; and gender: F1,823 = 7.362, P = 0.007), a statistically significant difference in operating time (ie, reduction) was demonstrated with increasing operative experience (F7,823 = 10.030, P < 001). There was no significant difference in the operating time between right-sided and left sided resections (P = 0.115).
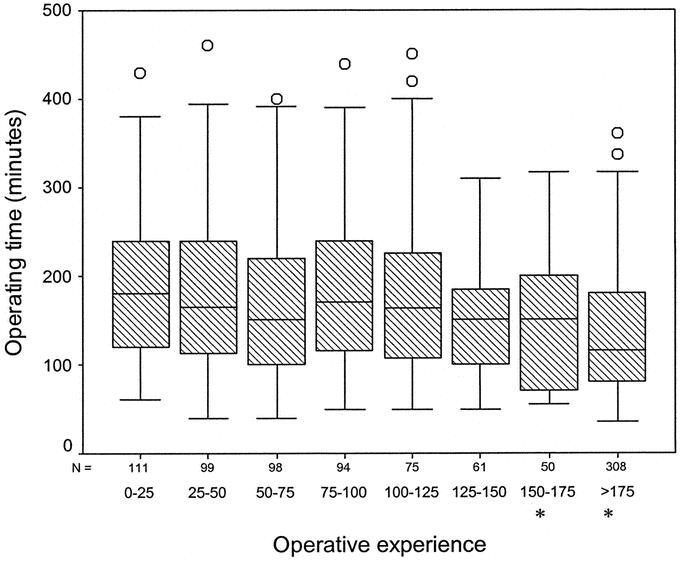
FIGURE 4. Box plots of median operative time groupedaccording to the level of operative experience. ANOVA = F7,823 = 10.030; P < 001. Asterisks denote statistical significance at a P value of <0.05.
DISCUSSION
The first step in the evaluation of the learning curve of any surgical intervention is the selection of an appropriate outcome measure that indirectly acts as a proxy in the measurement of the ability of a surgeon to perform that particular task on a temporal basis. Such outcome variables can be classified into 2 distinct categories: 1) measures of clinical process and task efficiency and 2) measures of patient outcome and quality assurance. The present study provided a multidimensional evaluation of the learning curve in left- and right-sided colorectal resections by addressing multiple indicators of surgical performance. These included operative time, conversion to open surgery, postoperative complications, and readmission rates, the first 2 addressing the process of care and the latter 2 representing patient outcomes as a surrogate markers of clinical effectiveness.
Previous publications have suggested that the learning curve in laparoscopic colorectal surgery ranges from 30 to 70 cases with the exception of Simons et al9 who reported a learning curve of 11 to 15 cases in a series of 144 patients. Schlachta et al,2 in a series of 461 patients, reported a learning curve of 30 cases, Bennet et al10 reported a learning curve of 40 cases among 1194 patients, and Dincler et al11 suggested that 70 to 80 cases may represent the learning curve for sigmoid colectomy. In the present study, the learning curve for right-sided colectomies was calculated as 55 cases and 62 cases for left-sided resections. Although these estimates may seem high for most trainee surgeons, these should only be used as guidance as the series began in the early 1990s at the time when technology and operative efficiency was inferior by today's standards, and these limiting factors may not be applicable in the modern era of laparoscopic surgery. In a recent multi-institutional randomized controlled trial of open versus laparoscopic colorectal surgery,12 the participating surgeons had performed at least 20 laparoscopically assisted colectomies with a conversion rate of 21%. The study did not demonstrate any disadvantage in the oncologic clearance of the cancer and similar recurrence rates between the 2 groups. The study investigators concluded that “any decrease in conversion rates would need to result from refining the process of patient selection, rather than from altering oncologic indications for conversion.”
One other possibility for the progressive improvement in outcomes could be a result of patient section as suggested by Agachan et al13 who stopped performing laparoscopic subtotal colectomies early in their series. In the present study, we adopted a less stringent policy for laparoscopic case selection and indeed the proportion of more challenging and high-risk cases rose with increasing experience. During the late part of the study, left-sided resections were more frequently performed on patients with higher comorbidity with complex diverticular disease. There is a natural tendency for experienced surgeons to be more liberal in their process of patient selection with the passage of time,14 and appropriate adjustment should be made to accommodate this change in case-mix. The present study provided a unique example of how predictive models of surgical outcomes can be used to evaluate surgical proficiency and characterize the learning profile of laparoscopic colorectal surgery by incorporating case-mix adjustment. A novel model is described based on a large patient cohort with sufficient power to make appropriate adjustment for patient-related factors, such as comorbidity and BMI, as well as procedure-related factors, which included the type of surgery, intraoperative abscess, and fistula.
Laparoscopic conversion to open surgery is known to vary among studies: 7% to 25% in large series and 2% to 41% in smaller series.15,16 Although conversion itself is not a complication, it is associated with a greater postoperative morbidity,16 as reflected in the results of our study. Risk factors associated with conversion to open surgery have been well described in the literature such as BMI,17 excessive tumor bulk,18 adhesions,19 diverticular phlegmon, Crohn's mass,20 and operative experience.2,21 Such studies have mainly focused on procedural or disease subsets, thus restricting their sample size, with conflicting information on the interrelation of these risk factors with outcome.22
Various studies have also shown that the number of intraoperative laparoscopic-related complications, conversion rate, and morbidity and mortality rates decreased with increasing experience.21,23 Operative experience showed a distinct influence on operating time in the present study, which is well documented by other investigators.21,24,25
However, we did not demonstrate a reduction in the readmission rate and complication rate with increasing experience despite a significant reduction in the operating time and conversion rate. The possible explanation for this paradox is the significant shift toward more complex and high-risk cases in the later part of the series, thus resulting in an overall stable complication and readmission rate. Similar findings were reported by Marusch et al21 in a multicenter study of 1658 patients, which showed that surgeons with experience of more than 100 laparoscopic colorectal operations were more likely to embark on more difficult cases with a conversion rate of 4.3% versus 6.9% for surgeons with experience of less than 100 procedures yet identical postoperative mortality and morbidity between the 2 groups.
CONCLUSION
The current study suggests that that it is possible to perform more difficult laparoscopic procedures with increasing experience without increasing postoperative morbidity. Because of the apparent shift in the case-mix and the multitude of factors that affect outcomes in laparoscopic colorectal surgery, appropriate case-mix adjustment should be made before any meaningful comparisons of task efficiency are made between or within centers. The reduction in the steepness of the learning curve poses a challenge to both trainees and trainers. Possible strategies include formal training courses in laparoscopic colorectal surgery, close intraoperative supervision by expert practitioners, and assistance from other well-trained staff.26
Footnotes
Presented at the Association of Coloproctology of Great Britain and Ireland Scientific Meeting, Birmingham, 2004 [Abstract: Colorectal Dis. 2004;6(suppl 1):4].
Reprints: Anthony J Senagore, MD, MS, MBA, Department of Surgery, Medical University of Ohio, Dowling Hall, 3065 Arlington Ave, Toledo, OH 43614. E-mail: asenagore@mco.edu.
REFERENCES
- 1.Senagore AJ, Duepree HJ, Delaney CP, et al. Cost structure of laparoscopic and open sigmoid colectomy for diverticular disease: similarities and differences. Dis Colon Rectum. 2002;45:485–490. [DOI] [PubMed] [Google Scholar]
- 2.Schlachta CM, Mamazza J, Seshadri PA, et al. Defining a learning curve for laparoscopic colorectal resections. Dis Colon Rectum. 2001;44:217–222. [DOI] [PubMed] [Google Scholar]
- 3.Ramsay CR, Wallace SA, Garthwaite PH, et al. Assessing the learning curve effect in health technologies: lessons from the nonclinical literature. Int J Technol Assess Health Care. 2002;18:1–10. [PubMed] [Google Scholar]
- 4.Ramsay CR, Grant AM, Wallace SA, et al. Statistical assessment of the learning curves of health technologies. Health Technol Assess. 2001;5:1–79. [DOI] [PubMed] [Google Scholar]
- 5.Delaney CP, Kiran RP, Senagore AJ, et al. Case-matched comparison of clinical and financial outcome after laparoscopic or open colorectal surgery. Ann Surg. 2003;238:67–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Statist Med. 1996;15:361–387. [DOI] [PubMed] [Google Scholar]
- 7.Poloniecki J, Valencia O, Littlejohns P. Cumulative risk adjusted mortality chart for detecting changes in death rate: observational study of heart surgery. BMJ. 1998;316:1697–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lovegrove J, Valencia O, Treasure T, et al. Monitoring the results of cardiac surgery by variable life-adjusted display. Lancet. 1997;350:1128–1130. [DOI] [PubMed] [Google Scholar]
- 9.Simons AJ, Anthone GJ, Ortega AE, et al. Laparoscopic-assisted colectomy learning curve. Dis Colon Rectum. 1995;38:600–603. [DOI] [PubMed] [Google Scholar]
- 10.Bennett CL, Stryker SJ, Ferreira MR, et al. The learning curve for laparoscopic colorectal surgery: preliminary results from a prospective analysis of 1194 laparoscopic-assisted colectomies. Arch Surg. 1997;132:41–44. [DOI] [PubMed] [Google Scholar]
- 11.Dincler S, Koller MT, Steurer J, et al. Multidimensional analysis of learning curves in laparoscopic sigmoid resection: eight-year results. Dis Colon Rectum. 2003;46:1371–1378. [DOI] [PubMed] [Google Scholar]
- 12.Clinical Outcomes of Surgical Therapy Study Group. A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med. 2004;350:2050–2059. [DOI] [PubMed] [Google Scholar]
- 13.Agachan F, Joo JS, Weiss EG, et al. Intraoperative laparoscopic complications: are we getting better? Dis Colon Rectum. 1996;39(suppl):14–19. [DOI] [PubMed] [Google Scholar]
- 14.Voitk AJ, Tsao SG, Ignatius S. The tail of the learning curve for laparoscopic cholecystectomy. Am J Surg. 2001;182:250–253. [DOI] [PubMed] [Google Scholar]
- 15.Gervaz P, Pikarsky A, Utech M, et al. Converted laparoscopic colorectal surgery. Surg Endosc. 2001;15:827–832. [DOI] [PubMed] [Google Scholar]
- 16.Marusch F, Gastinger I, Schneider C, et al. Importance of conversion for results obtained with laparoscopic colorectal surgery. Dis Colon Rectum. 2001;44:207–214. [DOI] [PubMed] [Google Scholar]
- 17.Pikarsky AJ, Saida Y, Yamaguchi T, et al. Is obesity a high-risk factor for laparoscopic colorectal surgery? Surg Endosc. 2002;16:855–858. [DOI] [PubMed] [Google Scholar]
- 18.Pandya S, Murray JJ, Coller JA, et al. Laparoscopic colectomy: indications for conversion to laparotomy. Arch Surg. 1999;134:471–475. [DOI] [PubMed] [Google Scholar]
- 19.Yong L, Deane M, Monson JR, et al. Systematic review of laparoscopic surgery for colorectal malignancy. Surg Endosc. 2001;15:1431–1439. [DOI] [PubMed] [Google Scholar]
- 20.Moorthy K, Shaul T, Foley RJ. Factors that predict conversion in patients undergoing laparoscopic surgery for Crohn's disease. Am JSurg. 2004;187:47–51. [DOI] [PubMed] [Google Scholar]
- 21.Marusch F, Gastinger I, Schneider C, et al. Experience as a factor influencing the indications for laparoscopic colorectal surgery and the results. Surg Endosc. 2001;15:116–120. [DOI] [PubMed] [Google Scholar]
- 22.Schlachta CM, Mamazza J, Seshadri PA, et al. Predicting conversion to open surgery in laparoscopic colorectal resections: a simple clinical model. Surg Endosc. 2000;14:1114–1117. [DOI] [PubMed] [Google Scholar]
- 23.Larach SW, Patankar SK, Ferrara A, et al. Complications of laparoscopic colorectal surgery: analysis and comparison of early vs. later experience. Dis Colon Rectum. 1997;40:592–596. [DOI] [PubMed] [Google Scholar]
- 24.Bruch HP, Schiedeck TH, Schwandner O. Laparoscopic colorectal surgery: a five-year experience. Dig Surg. 1999;16:45–54. [DOI] [PubMed] [Google Scholar]
- 25.Agachan F, Joo JS, Sher M, et al. Laparoscopic colorectal surgery: do we get faster? Surg Endosc. 1997;11:331–335. [DOI] [PubMed] [Google Scholar]
- 26.Hasan A, Pozzi M, Hamilton JR. New surgical procedures: can we minimise the learning curve? BMJ. 2000;320:171–173. [DOI] [PMC free article] [PubMed] [Google Scholar]