Abstract
Objective:
The purpose of this study was to evaluate whether ampullectomy can substitute for pancreatoduodenectomy (PD) in early ampullary cancer by clinicopathologic study.
Summary Background Data:
Although ampullectomy has been attempted in early ampullary cancer (pTis, pT1), the indication and extent of resection have not been established.
Methods:
Of 201 patients who had undergone PD for ampullary cancer between 1986 and 2002, 67 patients with a histologic diagnosis of pTis (n = 5) or pT1 (n = 62) cancer were analyzed retrospectively. Pathologic PD specimens were reviewed to analyze the cancer spread pattern, and medical records were reviewed for clinical outcomes.
Results:
The 5-year survival rate of the 66 patients with early ampullary cancer (excluding one mortality) was 83.7%. Recurrence was confirmed in 12 patients (18.2%) and all died because of the recurrence. Pathologic review showed that 22 patients (32.8%) had at least one risk factor for failure after ampullectomy: lymph node metastasis (n = 6, 9.0%), perineural invasion (n = 1), or mucosal tumor infiltration along the CBD or P-duct (n = 15, 22.4%). Mean lengths of invasion into the CBD or the P-duct beyond the sphincter of Oddi were 7.7 mm (range, 1–25 mm) or 6.3 mm (range, 2–18 mm), respectively. Moreover, these risk factors were not correlated with tumor size, histologic grade, or the gross morphology of the primary tumor, although pTis cancer or pT1 cancer sized 1.0 cm or less was found to be least associated with risk factors.
Conclusions:
Ampullectomy for early ampullary cancer should not be considered an alternative operation to PD because of the high possibility of recurrence. PD should be preferably performed for adequate radical resection, even in early ampullary cancer, and ampullectomy should be reserved for those who have pTis or pT1 cancer sized 1.0 cm or less with high operative risk.
The clinicopathologic features of early ampullary cancer (pTis, pT1 cancer) were investigated to determine the indications for ampullectomy. Despite a fairly good prognosis, a substantial number of early ampullary cancers had risk factors for failure after ampullectomy (lymph node metastasis, perineural invasion, common bile duct or pancreatic duct mucosal involvement). Pancreatoduodenectomy should be preferably performed for adequate radical resection even in early ampullary cancer, and ampullectomy should be reserved for pTis cancer or pT1 cancer sized 1.0 cm or less with a high operative risk.
Ampulla of Vater cancer has been reported to have a more favorable prognosis than pancreatic or bile duct cancer.1–3 A number of recent reports have documented a 5-year survival rate ranging from 34% to 67.7%.3–10 However, pancreatoduodenectomy (PD), the standard surgical strategy for ampullary cancer, is still associated with a high operative morbidity rate, despite a recent reduction in the operative mortality to less than 5%.11–13 For this reason, local resection, in other words, ampullectomy has been attempted not only for benign lesions but also for early cancer as an alternative to PD. Since the first ampullary resection by Halsted14 in 1899, increasing numbers of local resections for ampullary cancer have been reported. Most surgeons generally accept the role of local resection for small benign tumors, but controversy remains about expanding the indications for local resection to early ampullary cancer because of its high recurrence rate.15–19 Furthermore, the indication and extent of ampullectomy with curative intent have not been well defined because the number of ampullectomy cases for ampullary cancer is limited.
For ampullectomy to be a curative local resection for early ampullary cancer (defined as pTis and pT1 ampullary cancer in this study), an accurate comprehension of the tumor spread pattern is necessary for determining the indication and extent of local resection. However, few studies have offered adequate pathologic details of early ampullary cancer from this viewpoint. In this study, we evaluated the overall prognosis of ampullary cancer and investigated the pathologic features of early ampullary cancer by reviewing PD specimens to determine the feasibility of ampullectomy.
MATERIALS AND METHODS
Total Patients
Between 1986 and 2002, 201 patients underwent PD for ampulla of Vater cancer at the Seoul National University Hospital. Ampullary tumor was differentiated from other periampullary tumors by gross or microscopic evaluation of the tumor center. When a tumor was centered on the ampulla or extensively involved it, the tumor was regarded to be ampullary origin. Conversely, a tumor with peripheral extension into the ampulla was regarded to have originated from the duodenum, distal common bile duct (CBD), or the head of the pancreas. Mean patient age was 56.5 years (range, 29–80 years) and patients were composed of 103 men and 98 women. A conventional Whipple's procedure was performed in 98 patients, pylorus-preserving pancreatoduodenectomy (PPPD) in 100 (PPPD was first introduced in 1991), and pancreas head resection with segmental duodenectomy in 3. Microscopically curative resection (R0) was possible in 192 patients (95.5%).
Survival and Prognostic Factors
The following data were reviewed to identify prognostic factors: 1) preoperative clinical factors; age, sex, and presence of jaundice, 2) operation-related factors; type of resection (Whipple operation or PPPD), the presence of intraoperative transfusion, and 3) pathologic factors; size of tumor, gross morphology, histologic grade, the presence of a noninvasive adenomatous component (NAC), perineural invasion, lymphovascular invasion, depth of invasion, and lymph node metastasis.
Medical records were reviewed for preoperative clinical factors and operation-related factors, and pathologic slides were reexamined for pathologic factors. Gross morphology was not available in 9 cases because they had not been well described. Follow-up data were obtained by reviewing medical records or by direct patient contact. The median follow-up period was 58.9 ± 3.4 months (mean ± standard error of the mean) months (range, 2–203 months).
Predictability of Lymph Node Metastasis
For a successful local resection of ampullary cancer, it is essential to select an ampullary cancer without lymph node metastasis. Therefore, to identify factors capable of predicting the absence of lymph node metastasis at the preoperative stage, we examined other preoperatively assessable clinicopathologic factors (eg, depth of tumor, tumor size, gross morphology, and histologic grade) by reviewing pathologic slides after PD, and their relations with lymph node metastasis were evaluated.
Patients With Early Ampullary Cancer
Of the 201 patients, 67 patients were finally diagnosed as having early ampullary cancer; 5 patients had pTis and 62 pT1 cancer. The mean age of these 67 patients was 56.7 years (range, 37–77 years), and there were 39 men and 28 women. Whipple's procedure was performed in 29 patients, PPPD in 35, and pancreas head resection with segmental duodenectomy in 3. All patients with early ampullary cancer underwent curative operation.
Pathologic Evaluation of Early Ampullary Cancer
We defined early ampullary cancer as pTis or pT1 cancer (confined to mucosa or the sphincter of Oddi [SoO]) that had been confirmed by pathologic assessment after PD. To evaluate the locoregional spread pattern of early ampullary cancer, one pathologist specializing in the gastrointestinal tract reevaluated all pathologic PD specimens. The following factors were reviewed: tumor size, gross morphology, histologic grade, NAC, lymph node metastasis, perineural invasion, and lymphovascular invasion. In addition, we investigated whether tumors had spread proximally along the mucosal layer of the CBD and the pancreatic duct (P-duct). We measured the length from the proximal end of the SoO to the upper limit of the tumor, as shown in Figure 1. Tumor stage was based on the 6th edition of AJCC cancer staging.20
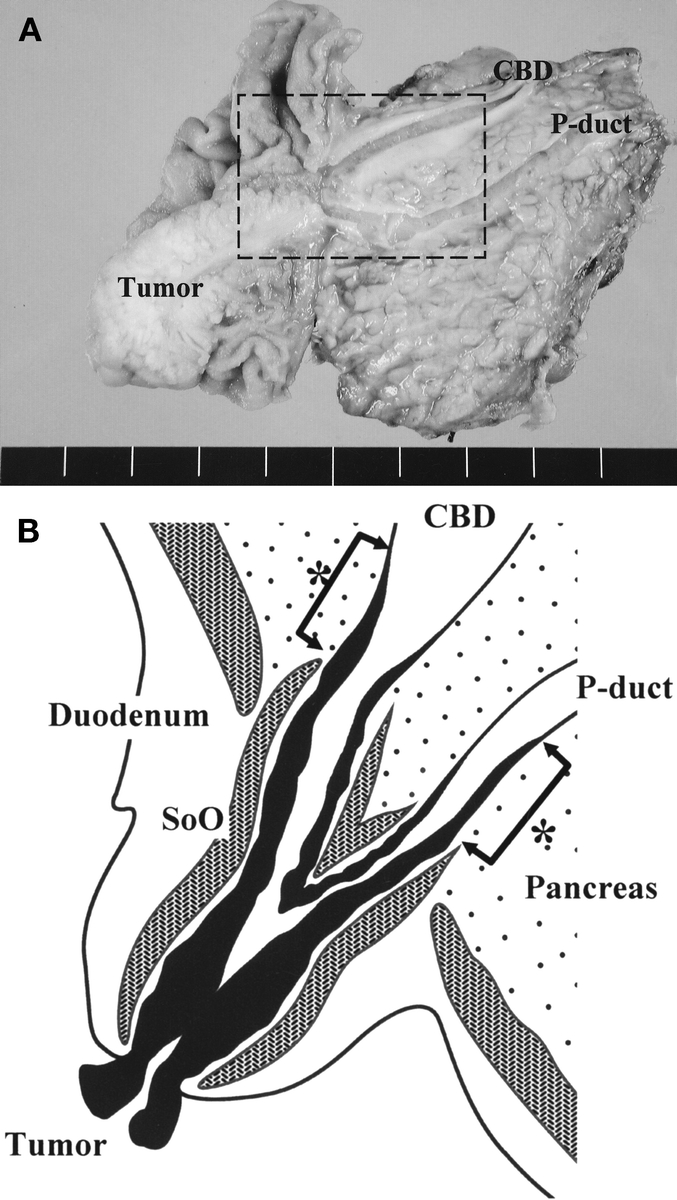
FIGURE 1. A, Surgical specimen after PD, which was resected for CBD and P-duct visualization at the same cross section. B, Schematic drawing of the rectangular area in A, showing mucosal tumor infiltration into the CBD and the P-duct of early ampullary cancer. One expert pathologist measured the length from the proximal end of the sphincter of Oddi to the upper limit of the mucosal tumor (*arrows).
We defined lymph node metastasis,2–4,6,8,21–23 perineural invasion,10,24 lymphovascular invasion,4 and CBD or P-duct mucosal involvement as risk factors for failure after ampullectomy. To identify factors capable of predicting these risk factors for ampullectomy at the preoperative stage, factors that can be assessed preoperatively and that have been proposed as indications for ampullectomy (eg, tumor size, histologic differentiation, and gross morphology) were examined by pathologic review; we designated these factors as indication factors for ampullectomy in this study. Thereafter, we investigated the correlation between these indication factors and the risk factors for ampullectomy.
Statistical Analysis
Survival was calculated by the Kaplan-Meier method, and to analyze differences, the log-rank test was used. Only variables that were statistically significant by univariate analysis were included in the multivariate analysis, which was performed using Cox proportional hazards regression. To identify correlations between the risk factors for failure after ampullectomy and the indication factors for ampullectomy, the χ2 test was used. Results were presented as mean ± the standard error of the mean. A P value of less than 0.05 was considered statistically significant.
RESULTS
Operative Mortality and Morbidity
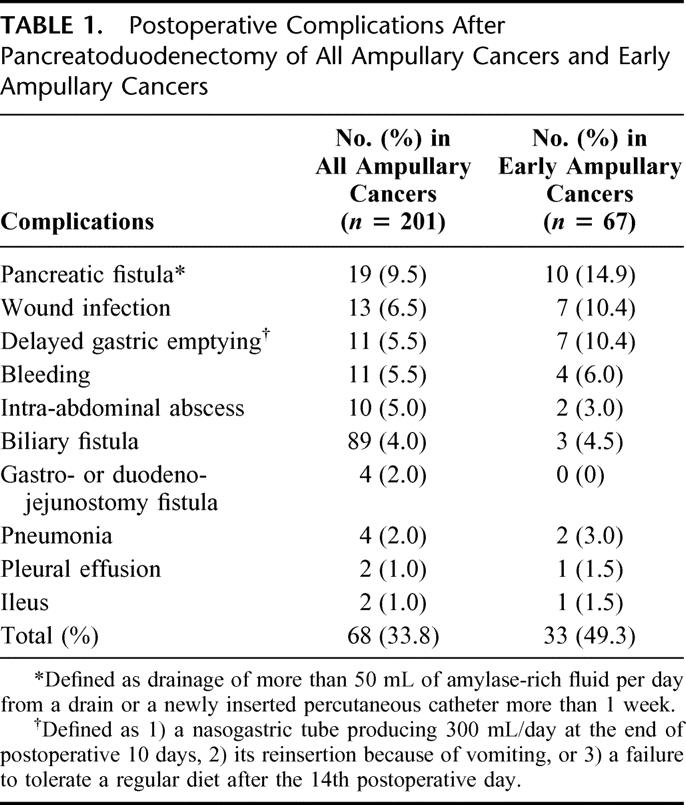
Operative mortality occurred in 2 (1.0%) of 201 patients because of postoperative bleeding and sepsis from pneumonia, respectively. One or more complications were present in 68 patients (33.8%). Table 1 details the postoperative complications observed. The most common complication was pancreatic fistula, which occurred in 19 patients (9.5%). In cases of early ampullary cancer, 1 (1.5%) operative mortality occurred due to sepsis from pneumonia, and operative morbidity occurred in 33 patients (49.3%), as shown in Table 1.
TABLE 1. Postoperative Complications After Pancreatoduodenectomy of All Ampullary Cancers and Early Ampullary Cancers
Survival Analysis of Total Cases
After excluding the 2 operative mortality cases, the overall 3-, 5-, and 10-year survival rates of patients with 199 resected ampullary cancers were 72.1%, 59.8%, and 49.2% (Fig. 2). Of these 199, 79 patients (39.7%) survived more than 5 years and 31 patients (15.6%) more than 10 years.
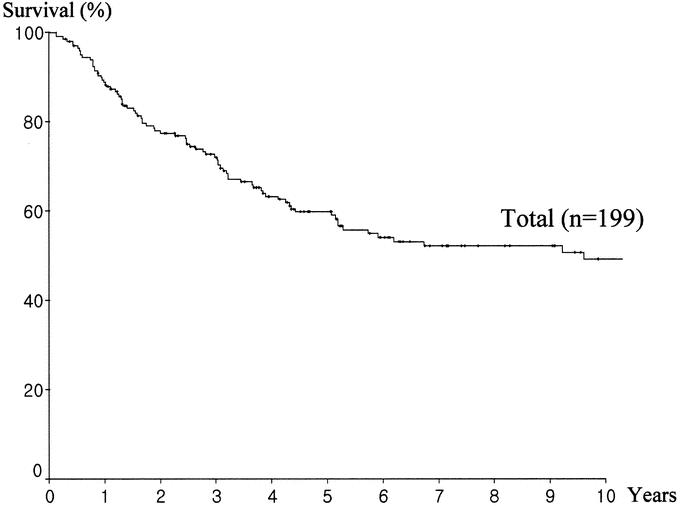
FIGURE 2. Overall survival of 199 patients with ampullary cancer after pancreatoduodenectomy (analysis excluded 2 operative mortality cases).
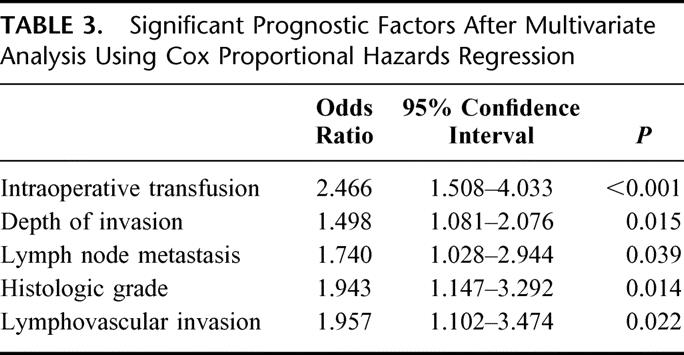
Factors that were significantly correlated with survival by univariate analysis were: jaundice, intraoperative transfusion, tumor size, gross morphology, histologic differentiation, NAC, perineural invasion, lymphovascular invasion, depth of invasion, and lymph node metastasis (Table 2). When multivariate analysis was performed using these significant factors, intraoperative transfusion, depth of invasion, lymph node metastasis, histologic differentiation, and lymphovascular invasion turned out to be independent significant prognostic factors (Table 3). Gross morphology (P = 0.059) and tumor size (P = 0.072) showed marginal significance.
TABLE 2. Univariate Analysis of Prognostic Factors Affecting Survival
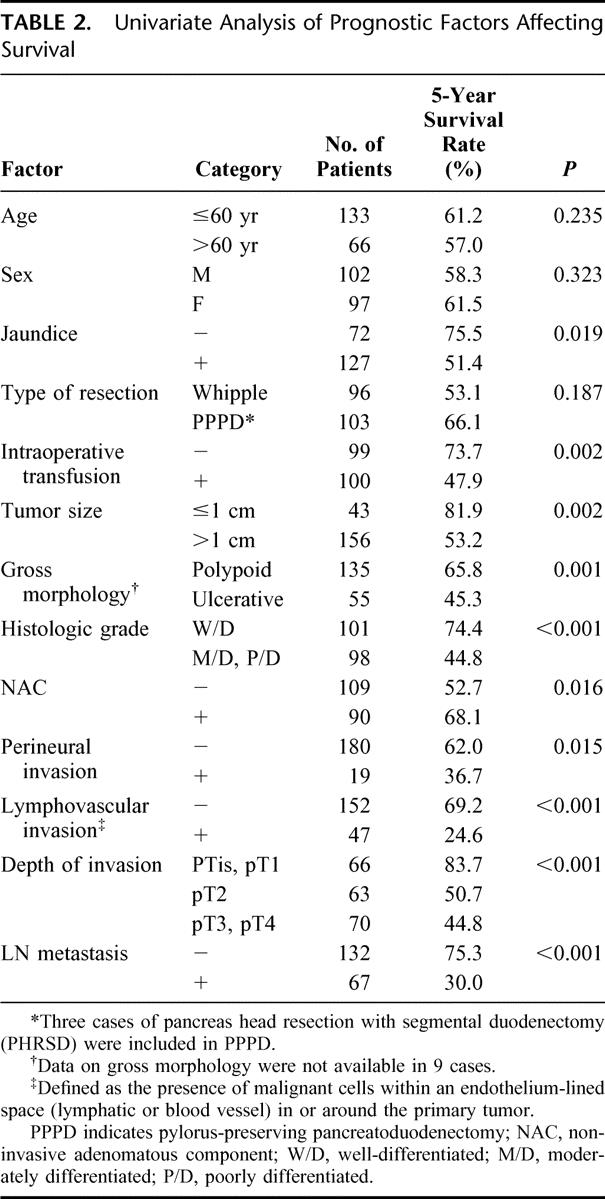
TABLE 3. Significant Prognostic Factors After Multivariate Analysis Using Cox Proportional Hazards Regression
Predictability of Lymph Node Metastasis
Of the above prognostic factors, lymph node metastasis is a key factor that makes local resection inappropriate (Table 4). Therefore, we investigated the relationship between lymph node metastasis and other preoperatively assessable pathologic prognostic factors. Lymph node metastasis was identified in 67 (33.3%) of 201 patients, and the rate of lymph node metastasis was lowest in pTis or pT1 cancer (9%), whereas it rose to about 50% in pT2 cancer. Tumors of size 1 cm or less also had a relatively low lymph node metastasis rate (11.6%). However, tumors with a well-differentiated or polypoid appearance without ulceration showed a high rate of positive lymph nodes (23.5% and 29.9%, respectively).
TABLE 4. Correlation Between Lymph Node Metastasis and Other Factors Assessable Preoperatively
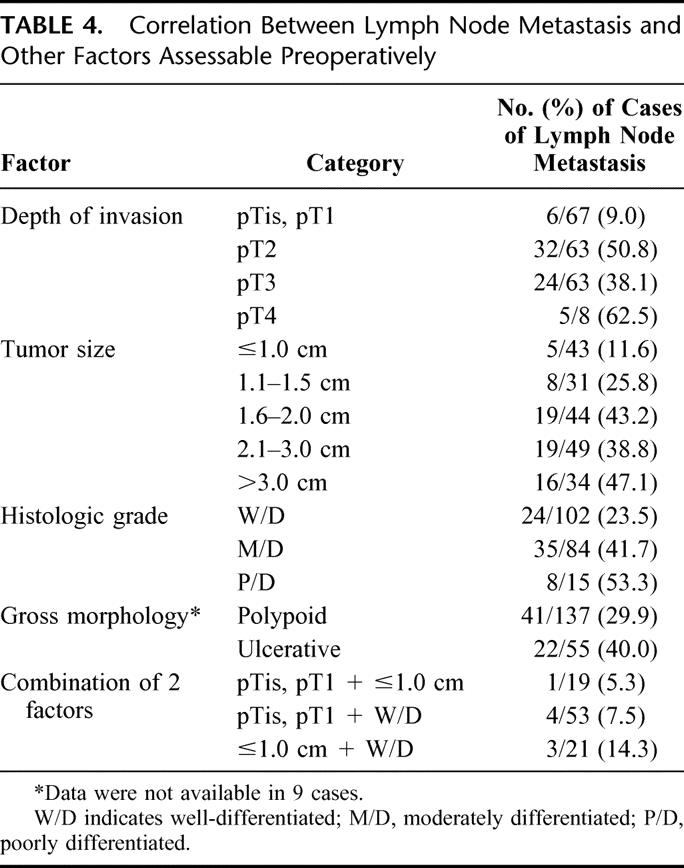
When the above factors were combined, concomitant pTis, pT1, and tumor size of 1 cm or less, and pTis, pT1 with a well-differentiated histology showed lymph node metastasis rates of 5.3% (1 of 19) and 7.5% (4 of 53), respectively. By comparison, a well-differentiated histology and tumor size of 1 cm or less had a lymph node positive rate of 14.3% (3 of/21).
Clinical Outcome of Early Ampullary Cancer
The overall 5-year survival rate of the 66 early ampullary cancer cases (excluding one mortality in pT1) was 83.7%, which was significantly higher than that of pT2 (n = 63, 50.7%), pT3 (n = 62, 46.5%) or pT4 (n = 8, 29.2%) (P< 0.001) (Fig. 3). In those with early ampullary cancer, patients with pTis (n = 5) and pT1 (n = 61) cancers showed 5-year survival rates of 100% and 82.9%, respectively, although these were not significantly different (P = 0.426).
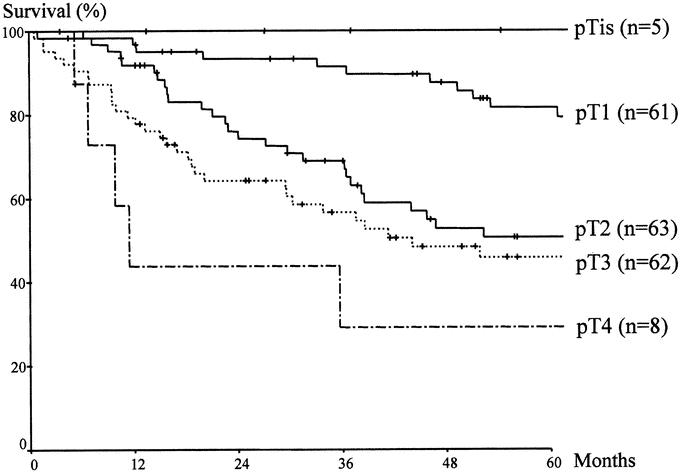
FIGURE 3. Comparison of survival according to the depth of invasion. Significant differences were found between early ampullary cancers (pTis, pT1) and other T stage cancers (pT2, pT3, pT4) (P < 0.001).
Recurrence was detected in 12 (18.2%) of 66 early ampullary cancers during the follow-up period. All recurrences occurred in pT1 cancer patients. Locoregional and systemic recurrence occurred in 4 patients and systemic recurrence alone in 8 patients. The most frequent site of systemic recurrence was the liver (n = 10), followed by lung (n = 2) and peritoneal seeding (n = 2). The mean period from operation to recurrence was 30.3 ± 5.5 months (range, 10.5–68.0 months). Finally, all 12 patients with recurrence died of tumor progression after a mean period of 10.1 ± 2.3 months (range, 1.0–24.1 months) from the detection of recurrence.
Pathologic Features of Early Ampullary Cancer
The mean size of the 67 resected early ampullary cancers was 1.8 ± 0.1 cm, ranging from 0.2 to 4.0 cm. Tumor were classified as well differentiated in 53 cases (79.1%) and moderately differentiated in 14 cases (20.8%); there was no poorly differentiated tumor. NAC was identified in 42 patients (62.7%). Table 5 shows the risk factors with the possibility of failure after ampullectomy in early ampullary cancer. Lymph node metastasis was identified in 6 (9.0%) of the 67 patients. Perineural and lymphovascular invasion were rare; only 1 patient had perineural invasion. CBD or P-duct mucosal involvement was observed in 15 cases (22.4%) (CBD, 14 cases; P-duct, 4 cases). The mean length of CBD mucosal involvement was 7.7 mm beyond the ampulla of Vater, ranging from 1 to 25 mm, and the mean length of P-duct mucosal involvement was 6.3 mm, ranging from 2 to 18 mm. Figure 4 shows an ampullary cancer that superficially spread into a CBD. As a result, 22 (32.8%) of the 67 patients with early ampullary cancer had at least one risk factor for failure after ampullectomy (lymph node metastasis, perineural invasion, lymphovascular invasion, CBD, or P-duct mucosal involvement). However, among those patients with early ampullary cancer, 5 patients with pTis cancer showed no risk factor for failure after ampullectomy.
TABLE 5. Risk Factors for Failure After Ampullectomy in Early Ampullary Cancer
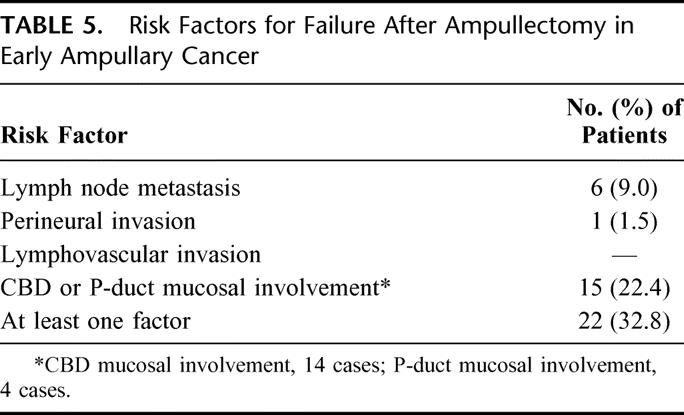
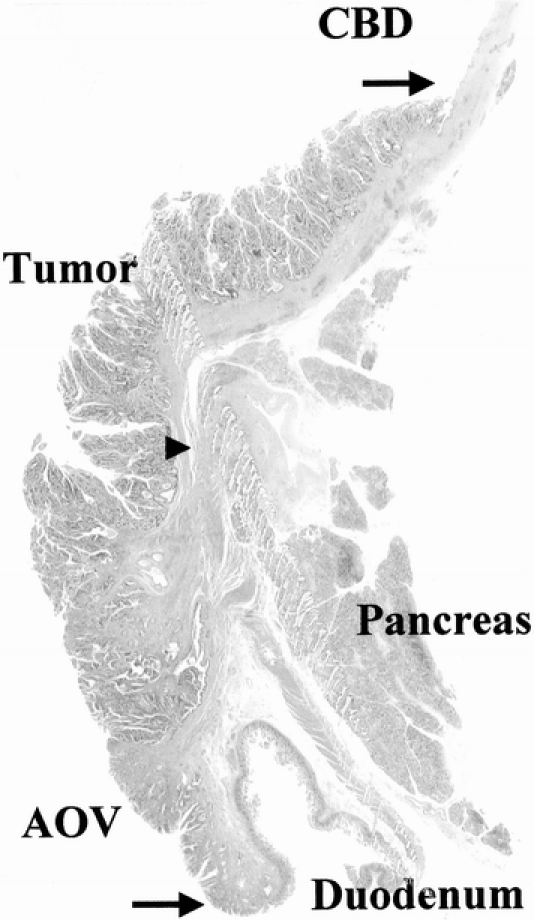
FIGURE 4. Photomicrograph showing pT1 ampullary cancer extending proximally along the mucosal layer of the CBD. Arrows indicate the extent of tumor infiltration and the arrowhead shows the end of the sphincter of Oddi.
Correlations Between the Risk Factors for Failure After Ampullectomy and the Indication Factors for Ampullectomy
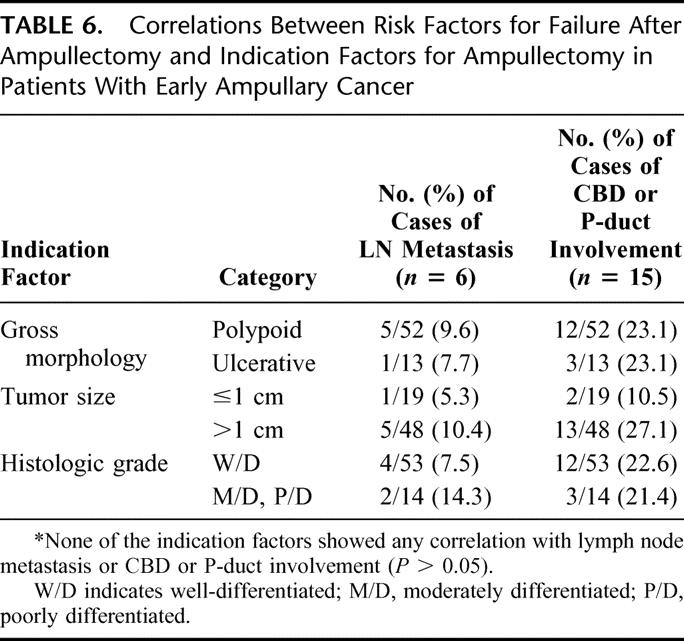
We evaluated whether any of the risk factors was correlated with the indication factors for ampullectomy in early ampullary cancer (tumor size, histologic differentiation, and gross morphology). Perineural invasion and lymphovascular invasion were excluded because they were positive in only one patient. Although the number of cases with lymph node metastasis or CBD or P-duct involvement was too small to accurately define a correlation, none of the indication factors showed a correlation with lymph node metastasis or with CBD or P-duct involvement (P > 0.05) (Table 6). However, the possibility of the presence of both risk factors was least in tumors sized 1 cm or less (lymph node metastasis: 5.3%, CBD or P duct involvement: 10.5%).
TABLE 6. Correlations Between Risk Factors for Failure After Ampullectomy and Indication Factors for Ampullectomy in Patients With Early Ampullary Cancer
DISCUSSION
The survival outcome for resected ampullary cancer in this series is one of the best results ever reported; 3-, 5-, and 10- year survival rates were 72.1%, 59.8%, and 49.2%, respectively. In particular, the actual number of 5-year survivors almost reached 40% of all patients that underwent resection for ampullary cancer, and the curative resection rate was 95.5%. The prognosis of ampullary cancer seems to have improved since the 1980s. Most series,3,5,9,10 including patients enrolled after the 1980s alone, have reported 5-year survival rates of more than 40%, whereas those including patients before the 1980s reported the 5-year survival rates of less than 40%.6–8 Although the reasons for this improved survival are unclear, increased curative resectability resulting from improved diagnostic technique and early diagnosis, and improvement in operative technique and postoperative care are proposed as likely causes.
Various clinicopathologic factors have been proposed to be significantly correlated with survival after PD for ampullary cancer; lymph node metastasis,2–6,8,21,22,24 depth of invasion,7,17,23 tumor size,7,8 histologic grade,4,6–8 resection margin,3,5 perineural invasion,10,23 lymphovascular invasion,4 and intraoperative transfusion.6 In our series, the absence of an intraoperative transfusion, an early cancer (pTis, pT1), negative lymph nodes, a well-differentiated tumor, and the absence of lymphovascular invasion were found to favor prolonged survival by multivariate analysis. In particular, of those factors, the absence of an intraoperative transfusion was found to be most significantly related to prolonged survival (P < 0.001, odds ratio = 2.466). This observation reconfirmed the finding of Talamini et al6 that intraoperative transfusions was the best predictor of prognosis in patients undergoing resection for ampullary cancer and thus blood transfusion was more important to the prognosis than was tumor biology. Previously, we evaluated the effect of blood transfusion on survival after PD for periampullary cancers24 and found that an intraoperative transfusion was a significant prognostic factor in ampullary cancer, not in pancreatic or bile duct cancer. We are unable to put forward a good explanation for this finding, but a possible reason is that because ampullary cancer has the least aggressive biology, the adverse prognostic impact of a transfusion could be seen only for ampullary cancer. Based on this finding, efforts should be made to avoid unnecessary bleeding during PD, and transfusion should be given only when essential. However, further study of the mechanism and clinical impact of transfusion in ampullary cancers is needed.
Of the ampullary cancers, early ampullary cancers (pTis, pT1) limited to the ampulla of Vater showed a fairly good prognosis. These tumors were radically resectable in all cases and showed a 5-year survival rate of 83.7%. These results are compatible with other reports showing 5-year survival rates of 80% to 100% in T1 cancer.25–27 However, once a cancer had invaded other organs beyond the ampulla of Vater, the 5-year survival rate declined sharply to about 50%.
To achieve such a good prognosis in early ampullary cancer, complete resection is mandatory.4,5,28 In terms of curative resection, there is no doubt that the standard operation for ampullary cancer should be PD. However, morbidity after PD remains significant, which was confirmed by the present study with a complication rate of 33.8%; in particular, this was about 50% in early cancer with one mortality. For this reason, as for other early gastrointestinal cancers, local resection has been attempted for early ampullary cancer. With regard to the indicators for ampullectomy in ampullary cancer, Rattner et al29 recommended ampullectomy for T1 cancer and Beger et al27 for Tis or T1 cancer with well or moderate differentiation. However, a general consensus is still lacking with regard to the indications and the extent of local resection with curative intent in ampullary cancer because of the lack of a large clinical series. Indeed, we do not perform ampullectomy for ampullary cancer, although we experienced 3 cases of ampullectomy in the patients with ampullary adenoma, who remain alive without recurrence after the procedure. So we have no direct evidence as to whether ampullectomy can offer operative curability in ampullary cancer. Instead, in the present study, we examined the detailed pathologic features of ampullary cancers by reviewing PD specimens to evaluate the feasibility of ampullectomy from the oncologic aspect.
For local resection to be a successful curative resection in early ampullary cancer, the following factors should be taken into consideration: 1) the mortality and morbidity of the procedure, 2) operative curability, and 3) accuracy of the preoperative and intraoperative diagnosis (ERCP biopsy, EUS, and intraoperative frozen biopsy). With regard to mortality and morbidity associated with ampullectomy, many reports have demonstrated lower postoperative mortality and morbidity compared with PD.16,29–34 Thus, if ampullectomy guarantees operative curability, it would be more attractive than PD in early ampullary cancer. Indeed, some reports15,35 have shown better or comparable survival versus PD, although the number of ampullectomy cases involved was limited. However, a high rate of local recurrence is a major problem of ampullectomy. Branum et al16 reported that 6 of 8 patients with a malignant lesion experienced recurrent disease after local resection, and Tarazi et al15 documented that 3 of 11 patients recurred after local resection. Although Knox et al35 reported that local resection in 25 patients provided better survival than PD, 4 of their patients with local resection died of metastatic disease and 2 patients died of local recurrence. When one considers that the initial operation offers the best opportunity to perform a safe and complete resection, the failure of a local resection may delay the optimal time for curative resection and finally cause a dismal result. Thus, the assurance of operative curability is a requisite in the planning of ampullectomy.
For ampullectomy to achieve operative curability, 2 criteria should be fulfilled: no lymph node metastasis and a free resection margin. In the present study, we defined lymph node metastasis, perineural invasion, lymphovascular invasion, and CBD or P-duct mucosal involvement as risk factors for failure after ampullectomy. This was done for the following reasons: First, lymph node metastasis,2–4,6,8,21–23 perineural invasion,10,24 and lymphovascular invasion4 have been identified as important factors affecting survival and recurrence in patients with radically resected ampullary cancer, and the effect of these factors on survival or recurrence after local resection is presumed to be no less than after PD. Moreover, the presence of lymph node metastasis, if only local resection is performed, would invariably result in a treatment failure. Second, theoretically perineural or lymphovascular invasion may lead to tumor emboli infiltrating beyond the main tumor of the ampulla of Vater along the perineural or lymphovascular space. Also, mucosal tumor infiltration along the CBD or P-duct may extend beyond the range of ampullary resection. After all, these factors potentially compromise a free resection margin.
Although Beger et al27 recommended local lymph node dissection with ampullectomy for curative resection, it would be difficult to complete lymph node dissection during ampullectomy. Therefore, the selection of ampullary cancers without lymph node metastasis prior to resection is an essential part of achieving curative local resection. However, preoperative imaging modalities have limitations in terms of diagnosing regional lymph node metastasis from ampullary cancer. Even EUS, although it can accurately define the depth of invasion in 75% to 83% of cases, is less helpful at accurately predicting the presence or absence of lymph node metastasis. The reported accuracy of EUS for detecting lymph node metastasis ranges from 54% to 68%.36–40 Accordingly, the identification of clinicopathologic factors capable of predicting lymph node metastasis before operation is important for the selection of ampullary cancers suitable for local resection. Böttger and Junginger12 reported that lymph node metastases were not found in T1, or in tumors smaller than 0.6 cm, or in tumors with a well-differentiated histology, so local resection for ampullary cancer may be indicated in such cases. Yamaguchi and Enjoji39 reported that, in the 12 ampullary cancer cases confined within SoO, there was no lymph node metastasis. In the present study, patients with pTis or pT1 cancer had the lowest rate of lymph node metastasis (9%), but the lymph node positive rate in patients with pT2 cancer was up to about 50%. In comparison, lymph node positive rates in patients with a well-differentiated histology or a polypoid tumor were 23.5% and 29.9%, respectively, which means that a well-differentiated histology or a polypoid appearance cannot satisfy the required criteria for ampullary resection. These findings show that Tis or T1 cancer appears to be an essential condition for localized disease.
Therefore, we examined the histopathologic features of early ampullary cancer with special focus on its locoregional spread pattern, to evaluate whether ampullectomy actually guarantees a free resection margin in early ampullary cancer. A noteworthy observation in the present study was that as many as 22 (32.8%) of the 67 early ampullary cancer cases had risk factors for failure after ampullectomy: lymph node metastasis (n = 6), perineural invasion (n = 1), and CBD or P-duct mucosal involvement beyond SoO (n = 15). Of these factors, perineural invasion and CBD or P-duct mucosal involvement are considered as limitations for a free resection margin. Although there was only 1 case of perineural invasion, mucosal tumor infiltration along the CBD or P-duct was present in 15 (22.4%) of 67 early ampullary cancers. The mean lengths of mucosal tumor infiltration into the CBD or P-duct were 7.7 mm and 6.3 mm, respectively, and some extended more than 2 cm from the SoO. Our results are consistent with those of Futakawa et al,17 who reported that in all 7 cases with local resection, cancer was found histologically at the cut surface of the tumor as a result of interstitial infiltration or mucosal spread into the CBD or P-duct. They also revealed that, among 12 cases with early ampullary cancer, cancer was found some distance along the CBD or main P-duct due to interstitial invasion in 3 cases or mucosal spread in 6 cases. This finding limits the indiscreet application of ampullectomy to early ampullary cancer, since preoperative diagnostic modalities such as ERCP or EUS may not be able to accurately predict the presence or extent of mucosal tumor infiltration into the CBD or P-duct.
Frozen section examination of the resection margin during ampullectomy may help to obtain a free resection margin, but its accuracy is somewhat questionable; furthermore, negative findings cannot definitely exclude the presence of cancer in the resection margin.29,32 Although a recent study34 reported upon the accuracy of intraoperative frozen section biopsy for ampullary adenocarcinoma with a sensitivity of 85% and a specificity of 100%, their study mainly focused on differentiating adenoma and adenocarcinoma, and did not produce detailed results on the resection margin. In the case of bile duct cancer, the accuracy of determining the bile duct resection margin has been reported to be low.41,42 Furthermore, the catheter used for preoperatively biliary drainage, which is frequently performed for obstructive jaundice from ampullary cancer, may cause inflammation and thus make the interpretation of frozen section biopsy more difficult. Another possible problem of tumor infiltration along the duct is that it would be technically difficult to perform a local resection with a safe resection margin for tumors infiltrating a considerable distance along the duct, and to reconstruct the large defect left after resection.
Based on these results, ampullectomy should not be chosen based on depth of invasion alone, and other factors that could increase the failure of local resection should be considered. From this viewpoint, early ampullary cancers without these risk factors for failure after ampullectomy should be selected for a successful local resection. Therefore, we evaluated whether factors that can be assessed preoperatively and have been proposed as indication factors for ampullectomy (eg, tumor size, histologic differentiation, and gross morphology) can predict the risk factors for failure after ampullectomy. However, as demonstrated in the present study (Table 6), none of these indication factors showed any correlation with the presence of lymph node metastasis or CBD or P-duct involvement. This suggests that there are no reliable criteria even in early cancer to indicate ampullectomy with curative resection. In particular, well-differentiated early ampullary cancer, which has been proposed as an indicator of local resection by other reports,27,30 had a lymph node metastasis rate of 7.5% and a CBD or P-duct mucosal involvement rate of 22.6%. Even in small (≤1 cm) early cancers, lymph node metastasis and CBD or P-duct mucosal involvement were present in 5.3% and 10.5%, respectively. Therefore, our results on the extent of tumor infiltration of early ampullary cancer, and the low mortality rates and favorable survival outcomes following PD, lead us to prefer PD rather than local resection unless patients have unacceptable medical condition for PD.
Additionally the present study reveals that early ampullary cancer is somewhat aggressive despite a reasonable prognosis. Recurrence occurred in a significant proportion of patients (18.2%) even after PD. All patients with recurrence presented with systemic recurrence, although locoregional recurrence was also observed, and finally died due to cancer progression. This implies that early ampullary cancer has an unpredictable tumor biology despite a good prognosis, which cannot be well explained. Furthermore, the possibility of recurrence, particularly of locoregional recurrence after ampullectomy would be higher than after PD.15,16,35 Therefore, until further information on the tumor biology of early ampullary cancer becomes available, local resection of ampullary cancer should be limited if conducted with curative intent.
However, among those patients with early ampullary cancer, all patients with pTis cancer (5 of 67, 7.5%) survived without recurrence and, furthermore, showed none of risk factors for failure after ampullectomy. This finding seems to indicate that ampullectomy is indicated in patients with pTis cancers. However, not only do pTis cancers account for small proportion of early ampullary cancers, but also their accurate distinction from pT1 cancers using preoperative diagnostic method is believed nearly impossible. So it would be both clinically difficult and meaningless to select patients with Tis cancer from all patients with ampullary cancer for ampullectomy.
CONCLUSION
This study suggests that, despite a fairly good prognosis for early ampullary cancer, ampullectomy does not offer an alternative to PD even in early ampullary cancer, due to the high possibility of local recurrence. PD should be preferably performed for adequate radical resection, and ampullectomy should be reserved for pTis cancers or pT1 cancers sized 1.0 cm or less in patients with a high operative risk; however, operative curability cannot be guaranteed.
Footnotes
Reprints: Sun-Whe Kim, MD, FACS, Department of Surgery, Seoul National University Hospital, 28 Yongon-dong, Chongno-gu, Seoul 110-744, Korea. E-mail: sunkim@plaza.snu.ac.kr.
REFERENCES
- 1.Michelassi F, Erroi F, Dawson PJ, et al. Experience with 647 consecutive tumors of the duodenum, ampulla, head of the pancreas, and distal common bile duct. Ann Surg. 1989;210:544–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trede M, Schwall G, Saeger HD. Survival after pancreatoduodenectomy: 118 consecutive resections without an operative mortality. Ann Surg. 1990;211:447–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Howe JR, Klimstra DS, Moccia RD, et al. Factors predictive of survival in ampullary carcinoma. Ann Surg. 1998;228:87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Monson JRT, Donohue JH, McEntee GP, et al. Radical resection for carcinoma of the ampulla of Vater. Arch Surg. 1991;126:353–357. [DOI] [PubMed] [Google Scholar]
- 5.Allema JH, Reinders ME, van Gulik TM, et al. Results of pancreaticoduodenectomy for ampullary carcinoma and analysis of prognostic factors for survival. Surgery. 1995;117:247–253. [DOI] [PubMed] [Google Scholar]
- 6.Talamini MA, Moesinger RC, Pitt HA, et al. Adenocarcinoma of the ampulla of Vater: a 28-year experience. Ann Surg. 1997;225:590–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klempnauer J, Ridder GJ, Maschek H, et al. Carcinoma of the ampulla of Vater: determinants of long-term survival in 94 resected patients. HPB Surg. 1998;11:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Su CH, Shyr YM, Lui WY, et al. Factors affecting morbidity, mortality and survival after pancreaticoduodenectomy for carcinoma of the ampulla of Vater. Hepatogastroenterology. 1999;46:1973–1979. [PubMed] [Google Scholar]
- 9.Bottger TC, Boddin J, Heintz A, et al. Clinicopathologic study for the assessment of resection for ampullary carcinoma. World J Surg. 1997;21:379–383. [DOI] [PubMed] [Google Scholar]
- 10.Duffy JP, Hines OJ, Liu JH, et al. Improved survival for adenocarcinoma of the ampulla of Vater: fifty-five consecutive resections. Arch Surg. 2003;138:941–948. [DOI] [PubMed] [Google Scholar]
- 11.Yeo CJ, Cameron JL, Sohn TA, et al. Six hundred fifty consecutive pancreaticoduodenectomies in the 1990s: pathology, complications, and outcomes. Ann Surg. 1997;226:248–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Böttger TC, Junginger T. Factors influencing morbidity and mortality after pancreaticoduodenectomy: critical analysis of 221 resections. World J Surg. 1999;23:164–172. [DOI] [PubMed] [Google Scholar]
- 13.Castillo CF, Rattner DW, Warshaw AL. Standards for pancreatic resection in the 1990s. Arch Surg. 1995;130:295–300. [DOI] [PubMed] [Google Scholar]
- 14.Halsted WS. Contributions to the surgery of the bile duct passages, especially of the common bile duct. Boston Med Surg J. 1899;141:645–654. [Google Scholar]
- 15.Tarazi RY, Hermann RE, Vogt DP, et al. Results of surgical treatment of periampullary tumors: a thirty-five-year experience. Surgery. 1986;100:716–723. [PubMed] [Google Scholar]
- 16.Branum GD, Pappas TN, Meyers WC. The management of tumors of the ampulla of Vater by local resection. Ann Surg. 1996;224:621–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Futakawa N, Kimura W, Wada Y, et al. Clinicopathological characteristics and surgical procedures for carcinoma of the papilla of Vater. Hepatogastroenterology. 1996;43:260–267. [PubMed] [Google Scholar]
- 18.van Gulik TM, Gerhards M, de Vries J, et al. Local resection of biliopancreatic cancer. Ann Oncol. 1999;10:243–246. [PubMed] [Google Scholar]
- 19.Alstrup N, Burcharth F, Hauge C, et al. Transduodenal excision of tumours of the ampulla of Vater. Eur J Surg. 1996;162:961–967. [PubMed] [Google Scholar]
- 20.Greene FL, Page DL, Fleming IV, et al. AJCC Cancer Staging Manual. New York: Springer-Verlag, 2002:139–149. [Google Scholar]
- 21.Roder JD, Schneider PM, Stein HJ, et al. Number of lymph node metastases is significantly associated with survival in patients with radically resected carcinoma of the ampulla of Vater. Br J Surg. 1995;82:1693–1696. [DOI] [PubMed] [Google Scholar]
- 22.Kayahara M, Nagakawa T, Ohta T, et al. Surgical strategy for carcinoma of the papilla of Vater on the basis of lymphatic spread and mode of recurrence. Surgery. 1997;121:611–617. [DOI] [PubMed] [Google Scholar]
- 23.Chan C, Herrera MF, de la Garza L, et al. Clinical behavior and prognostic factors of periampullary adenocarcinoma. Ann Surg. 1995;222:632–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park SJ, Kim SW, Jang JY, et al. Intraoperative transfusion: is it a real prognostic factor of periampullary cancer following pancreatoduodenectomy? World J Surg. 2002;26:487–492. [DOI] [PubMed] [Google Scholar]
- 25.Mori K, Ikei S, Yamane T, et al. Pathological factors influencing survival of carcinoma of the ampulla of Vater. Eur J Surg Oncol. 1990;16:183–188. [PubMed] [Google Scholar]
- 26.Shirai Y, Tsukada K, Ohtani T, et al. Carcinoma of the ampulla of Vater: is radical lymphadenectomy beneficial to patients with nodal disease? J Surg Oncol. 1996;61:190–194. [DOI] [PubMed] [Google Scholar]
- 27.Beger HG, Treitschke F, Gansauge F, et al. Tumor of the ampulla of Vater: experience with local or radical resection in 171 consecutively treated patients. Arch Surg. 1999;134:526–532. [DOI] [PubMed] [Google Scholar]
- 28.Matory YL, Gaynor J, Brennan M. Carcinoma of the ampulla of Vater. Surg Gynecol Obstet. 1993;177:366–370. [PubMed] [Google Scholar]
- 29.Rattner DW, Fernandez-del Castillo C, et al. Defining the criteria for local resection of ampullary neoplams. Arch Surg. 1996;131:366–371. [DOI] [PubMed] [Google Scholar]
- 30.Klein P, Reingruber B, Kastl S, et al. Is local excision of pT1-ampullary carcinomas justified? Eur J Surg Oncol. 1996;22:366–371. [DOI] [PubMed] [Google Scholar]
- 31.Lindell G, Borch K, Tingstedt B, et al. Management of cancer of the ampulla of Vater: does local resection play a role? Dig Surg. 2003;20:511–515. [DOI] [PubMed] [Google Scholar]
- 32.Asbun HJ, Rossi RL, Munson JL. Local resection for ampullary tumors: is there a place for it? Arch Surg. 1993;128:515–520. [DOI] [PubMed] [Google Scholar]
- 33.Farouk M, Niotis M, Branum GD, et al. Indications for and the technique of local resection of tumors of the papilla of Vater. Arch Surg. 1991;126:650–652. [DOI] [PubMed] [Google Scholar]
- 34.Clary BM, Tyler DS, Dematos P, et al. Local ampullary resection with careful intraoperative frozen section evaluation for presumed benign ampullary neoplasms. Surgery. 2000;127:628–633. [DOI] [PubMed] [Google Scholar]
- 35.Knox RA, Kingston RD. Carcinoma of the ampulla of Vater. Br J Surg. 1986;73:72–73. [DOI] [PubMed] [Google Scholar]
- 36.Tio TL, Tytgat GN, Cikot RJ, et al. Ampullopancreatic carcinoma: preoperative TNM classification with endosonography. Radiology. 1990;175:455–461. [DOI] [PubMed] [Google Scholar]
- 37.Cannon ME, Carpenter SL, Elta GH, et al. EUS compared with CT, magnetic resonance imaging, and angiography and the influence of biliary stenting on staging accuracy of ampullary neoplasms. Gastrointest Endosc. 1999;50:27–33. [DOI] [PubMed] [Google Scholar]
- 38.Shoup M, Hodul P, Aranha GV, et al. Defining a role for endoscopic ultrasound in staging periampullary tumors. Am J Surg. 2000;179:453–456. [DOI] [PubMed] [Google Scholar]
- 39.Yamaguchi K, Enjoji M. Carcinoma of the ampulla of Vater: a clinicopathologic study and pathologic staging of 109 cases of carcinoma and 5 cases of adenoma. Cancer. 1987;59:506–515. [DOI] [PubMed] [Google Scholar]
- 40.Chen CH, Tseng LJ, Yang CC, et al. The accuracy of endoscopic ultrasound, endoscopic retrograde cholangiopancreatography, computed tomography, and transabdominal ultrasound in the detection and staging of primary ampullary tumors. Hepatogastroenterology. 2001;48:1750–1753. [PubMed] [Google Scholar]
- 41.Okazaki Y, Horimi T, Kotaka M, et al. Study of the intrahepatic surgical margin of hilar bile duct carcinoma. Hepatogastroenterology. 2002;49:625–627. [PubMed] [Google Scholar]
- 42.Strong RW, Lynch SV. Surgical resection of hilar cholangiocarcinoma. J Hepatobiliary Pancreat Surg. 1995;2:233–238. [Google Scholar]


