Abstract
Objective:
Irreversible hypovolemia remains a major clinical problem. Preliminary studies indicate that administration of adrenomedullin and adrenomedullin binding protein-1 in combination (AM/AMBP-1) after hemorrhage, improves cardiovascular function despite the increased levels of AM. Our aim was to determine whether vascular responsiveness to AM is reduced after hemorrhage and, if so, to elucidate the possible mechanism responsible for such hyporesponsiveness.
Methods:
Male rats were bled to and maintained at a mean arterial pressure of 40 mm Hg for 90 minutes. The animals were then resuscitated with 4 times the volume of shed blood with lactated Ringer's solution over 60 minutes. At 1.5 hours postresuscitation, vascular responses to AM and AMBP-1, plasma levels of AM and AMBP-1, AMBP-1 and AM receptor gene expression were measured. In additional animals, AM and AMBP-1 were administered intravenously at 15 minutes after resuscitation over 45 minutes. Serum levels of liver enzymes, lactate, creatinine, TNF-α, IL-6, and IL-10 were measured at 1.5 hours postresuscitation.
Results:
AM-induced vascular relaxation decreased significantly after hemorrhage and resuscitation, which was markedly improved by AMBP-1. However, AM receptor gene expression did not change under such conditions. Hemorrhage-induced AM hyporesponsiveness was accompanied by the decreased expression and release of AMBP-1. Moreover, AM/AMBP-1 treatment down-regulated TNF-α and IL-6, up-regulated IL-10, and attenuated organ injury.
Conclusions:
The decreased AMBP-1 levels rather than alterations in AM receptors are responsible for producing AM hyporesponsiveness after hemorrhage. Thus, administration of AMBP-1 in combination with AM can be useful to reduce organ injury after severe hypovolemia.
Vascular responsiveness to adrenomedullin (AM) is depressed after severe blood loss. The reduction of AM binding protein (ie, AMBP-1) appears to contribute to the vascular AM hyporesponsiveness after hemorrhage. Addition of AMBP-1 partially restores vascular AM responsiveness after hemorrhage and resuscitation. Moreover, AM/AMBP-1 treatment down-regulates proinflammatory cytokines, up-regulates anti-inflammatory cytokine, and attenuates organ injuries under such conditions.
Trauma is the leading cause of death in the population between ages 1 to 44 and one of the leading causes of death in those over age 65 in the United States. It results in approximately 150,000 deaths per year.1,2 Most trauma deaths result either from insufficient tissue perfusion due to excessive blood loss or the development of inflammation, infection, and vital organ damage following resuscitation.2 Current treatment plans for hypovolemic shock rely on massive and rapid infusion of crystalloid fluids to maintain blood pressure. However, the majority of victims with severe blood loss do not respond well to fluid restoration and/or the use of vasoactive agents. Recent laboratory investigations have also shown that cardiac output, oxygen utilization, tissue perfusion, and cell and organ functions decreased significantly after hemorrhage despite large volume crystalloid resuscitation.3–5 As such, the development of alternative strategies for the treatment of traumatic blood loss will be critical for the improvement of patient outcome following hypovolemic shock.
Adrenomedullin (AM) is a potent vasodilatory peptide that was first isolated from extracts of human pheochromocytoma by monitoring the elevating activity of platelet cAMP.6 It belongs to the calcitonin gene peptide superfamily based on its homology with calcitonin gene-related peptide, amylin, and calcitonin.7 The biologic effect of AM is mediated by AM receptors. AM receptors are formed by the association of 2 proteins: calcitonin receptor-like receptor (CRLR) and receptor activity-modifying protein (RAMP). CRLR is a 7-transmembrane domain protein and RAMP is a single transmembrane protein. There are 3 subtypes of RAMP, and CRLR associated with RAMP-2 or RAMP-3 to form AM receptors.8,9 Recently, a novel specific AM binding protein (ie, AMBP-1) was identified in human plasma and the purified AMBP-1 is identical to human complement factor H.10,11 AMBP-1 potentiates AM-induced vascular relaxation in the aorta under normal as well as pathophysiologic conditions.12
Studies have shown that circulating levels of AM increase significantly after hemorrhagic and cardiogenic shock.13,14 In our preliminary studies, we found that intravenous administration of AM in combination with AMBP-1 during crystalloid resuscitation significantly improved heart performance, cardiac output, and organ blood flow after hemorrhage and resuscitation. However, it remains unknown whether vascular responsiveness to AM is altered under such conditions. We therefore hypothesized that vascular responsiveness to AM is depressed after severe blood loss. The aim of this study was to determine whether vascular responsiveness to AM is reduced after hemorrhage and, if so, to elucidate the mechanism responsible for AM hyporesponsiveness.
MATERIALS AND METHODS
Experimental Animals
Male Sprague-Dawley rats (275–325 g, Charles River Laboratories, Wilmington, MA) were housed in a temperature-controlled room on a 12-hour light/dark cycle and fed on a standard Purina rat chow diet. Prior to the induction of hemorrhage shock, rats were fasted overnight but allowed water ad libitum. The experiments were performed in accordance with the National Institutes of Health guidelines for the use of experimental animals. This project was approved by the Institutional Animal Care and Use Committee of the North Shore-Long Island Jewish Research Institute.
Animal Model of Hemorrhage Shock
The model of hemorrhage shock used in this experiment was described in detail previously with minor modification.3,15,16 Briefly, rats were anesthetized with isoflurane inhalation. Catheters (PE-50 tubing) were placed in a femoral vein and artery after carefully separating the femoral nerve and blood vessels. The femoral artery on the opposite side was also catheterized. One arterial catheter was used for monitoring the mean arterial pressure (MAP) and heart rate (HR) via a blood pressure analyzer (Digi-Med, Louisville, KY), the other was for blood withdrawal and the venous catheter was used for fluid resuscitation. The rat was bled to an MAP of 40 mm Hg within 10 minutes. This pressure was maintained for 90 minutes by further withdrawal of small volumes of blood or provision of small volumes of lactated Ringer's solution. At the end of this hypotensive period, the rats were then resuscitated with lactated Ringer's solution (equivalent 4 times the maximum bleed-out volume, which was approximately 60% of calculated blood volume) over a 60-minute period. The shed blood was not used for resuscitation and the animals were not heparinized prior to, during, and following hemorrhage. Sham-operated animals underwent the same surgical procedure but were neither bled nor resuscitated.
Isolation of the Small Intestine and Determination of AM-Induced Vascular Relaxation
An isolated intestinal preparation was used as previously described.17,18 At 1.5 hours after the completion of resuscitation, the abdomen was opened and a section (≈12 cm) of the jejunum and ileum was ligated on either side with a 4-0 silk suture. The duodenum was ligated adjacent to the pyloric sphincter and the sigmoid colon was ligated adjacent to the rectum. The intestinal vessels on either side of the small intestine were then ligated, leaving only the vessels sustaining the isolated small intestine unobstructed. The large intestine and the small intestine proximal to the isolated segment were removed. Animals were given 0.5 mL heparin sodium solution (500 U) intravenously. The superior mesenteric artery was cannulated with PE-50 tubing and perfused with aerated Krebs-Ringer HCO3 buffer (composition in mmol/L: 118.3 NaCl, 4.7 KCl, 2.5 CaCl2, 1.2 MgSO4, 1.2 KH2PO4, 25.0 NaHCO3, 0.026 Ca-EDTA, and 11.1 glucose), which was aerated with 95% O2:5% CO2 at 37°C. A constant perfusion rate (2.5 mL/min) was maintained by a pump. Vascular resistance was measured with a blood pressure analyzer (Micro-Med, Louisville, KY) coupled to the mesenteric arterial catheter. After 15 minutes of equilibration and pressure stabilization at the 2.5 mL/min perfusion rate, blood vessels in the isolated gut were constricted with norepinephrine (2 × 10−6 mol/L) added to the buffer reservoir of the organ chamber. When pressure was stabilized, rat AM was applied and the vascular response was measured by determining the change in perfusion pressure to the isolated small intestine. The intestinal preparation remained viable throughout the experiment as there was no significant change in norepinephrine-induced increase in perfusion pressure. The alterations in intestinal vascular responsiveness to AM (10−7 mol/L) and AMBP-1 (2 × 10−8 mol/L) stimulation after hemorrhage and resuscitation was assessed.
RNA Extraction and Determination of AM Receptor, AMBP-1, and AM Genes
Total RNA was extracted from the small intestine and liver by Tri-Reagent (Molecular Research Center, Cincinnati, OH); 150-mg tissue was homogenized in 1.5 mL Tri-Reagent and the homogenate was separated into aqueous and organic phases by chloroform addition and centrifugation. RNA was precipitated from the aqueous phase by addition of isopropanol, and washed with ethanol. The pellet was dissolved in 0.1% DEPC-treated, deionized distilled water. RNA concentration and purity were determined by measuring the absorbance at 260 and 280 nm; 5 μg of RNA from each tissue was reverse-transcribed in a 20-μL reaction volume containing 50mmol/L KCl, 10 mmol/L Tris-HCl, 5 mmol/L MgCl2, 1 mmol/L dNTP, 20 U RNase inhibitor, 2.5 mmol/L oligo d(T)16 primer, and 50 U reverse transcription. The reverse transcription reaction solution was incubated at 42°C for 1 hour, followed by heating at 95°C for 5 minutes; 1 μL cDNA was amplified with 0.15 μmol/L each of 3′ and 5′ primers, specific for the rat AM receptor subunits calcitonin receptor-like receptor (CRLR), receptor activity-modifying protein (RAMP)-2, and RAMP-3, as well as AMBP-1 and AM. Rat glyceraldehyde 3-phosphate dehydrogenase (G3PDH; Clontech, Palo Alto, CA) was used as the housekeeping gene. The primers are as follows: 5′-CCA AAC AGA CTT GGG AGT CAC TAG G-3′ (forward) and 5′-GCT GTC TTC TCT TTC TCA TGC GTG C-3′ (reverse) for CRLR (L27487), 5′-AGG TAT TAC AGC AAC CTG CGG T-3′ (forward) and 5′-ACA TCC TCT GGG GGA TCG GAG A-3′ (reverse) for RAMP-2 (AB028934), and 5′-ACC TGT CGG AGT TCA TCG TG-3′ (forward) and 5′-ACT TCA TCC GGG GGG TCT TC-3′ (reverse) for RAMP-3 (AB028935), 5′-CAC TTC CTT TTG CCT TGC TT-3′(forward), 5′-TCA ATT ATC CCA CCT GCT CA-3′ (reverse) for AMBP-1 (AA819055), 5′-TGG GTT CGC TCG CCG TTC TCG-3′(forward) and 5′-CGT CCT TGT CTT TGT CTG TAA-3′ (reverse) for AM (BC061775), 5′ TGA AGG TCG GTG TCA ACG GAT TTG GC 3′ (forward), 5′ CAT GTA GGC CAT GAG GTC CAC CAC 3′ (reverse) for G3PDH (M17701). The PCR was conducted at 30 cycles for G3PDH, CRLR, and RAMP-3, 25 cycles for RAMP-2, 28 (small intestine) and 18 (liver) cycles for AMBP-1, and 30 cycles for AM. Each cycle consisted of 30 seconds at 94°C, 30 seconds at 60°C, and 45 seconds at 72°C. After the RT-PCR procedure, the PCR amplification products were electrophoresed by using a 1.6% agarose gel containing 0.22 μg/mL ethidium bromide. The gel was then developed and band intensities were normalized by G3PDH using the Bio-Rad image system (Hercules, CA).
Western Blotting Analysis of AMBP-1
Tissue samples (0.1 g) were lysed and homogenized in 1 mL lysis buffer (10 mmol/L TBS, 1 mmol/L EDTA, 1 mmol/L EGTA, 2 mmol/L Na orthovanadate, 0.2 mmol/L PMSF, 2 μg/mL leupeptin, 2 μg/mL aprotinin, 1% Triton-X 100) for 30 minutes on ice, and cleared by centrifugation at 14,000 rpm for 15 minutes at 4°C; 200 μg tissue protein or 400 μg plasma protein was fractionated on 4% to 12% Bis-Tris gel and transferred to 0.2-μm nitrocellulose membrane. Nitrocellulose blots were blocked by incubation in TBST (10 mmol/L Tris-HCl, pH 7.5, 150 mmol/L NaCl, 0.1% Tween 20) containing 5% milk for 1 hour. Blots were incubated with rabbit antihuman complement factor H polyclonal antibodies (1:2000, Accurate Chemical, Westbury, NY) overnight at 4°C. The blots were then washed in TBST 5 times for 10 minutes. Blots were incubated with horseradish peroxidase-linked anti-rabbit IgG (Cell Signaling Technology, Beverly, MA) for 1 hour at room temperature, and then washed 5 times in TBST for 10 minutes. A chemiluminescent peroxidase substrate (ECL, Amersham Biosciences, Piscataway, NJ) was applied according to the manufacturer's instructions, and the membranes were exposed briefly to x-ray film. The band densities were determined using a Bio-Rad image system. Our preliminary results indicate that the anti-human complement factor H antibodies recognize rat AMBP-1. We used such antibodies, since antirat AMBP-1 antibodies are not commercially available.
Measurement of Plasma AM Levels
Plasma levels of AM were assayed according to the procedure provided by the manufacturer, using a radioimmunoassay kit specific for rat AM from Peninsula Labs (Belmont, CA). Briefly, 1.5-mL blood samples were collected into a polypropylene tube containing EDTA (1 mg/mL) and aprotinin (500 KIU/mL) at 1.5 hours after hemorrhage and resuscitation, and plasma was separated immediately. The collected plasma was used for AM extraction by C18 Sep-Column. Each RIA incubation mixture was composed of 100 μL standard AM or an unknown sample, and 200 μL antiserum diluted with RIA buffer containing 0.5% normal rabbit serum. After 16 hours incubation, 100 μL 125I-labeled tracers (15,000 cpm) were added. After additional 24 hours incubation, 100 μL antirabbit IgG goat serums were added. Free and bound tracers were separated after 90 minutes incubation by centrifugation at 3000 rpm for 30 minutes. After aspiration of the supernatant, radioactivity in the pellet was counted with a γ-counter. AM levels were assessed according to the manufacturer's instructions.
Administration of AM and AMBP-1
In additional groups of animals, AM (12 μg/kg BW) and AMBP-1 (40 μg/kg BW) or vehicle (PBS, 1 mL) were administered at 15 minutes after the beginning of resuscitation in hemorrhaged animals via the femoral venous catheter over a period of 45 minutes. At 1.5 hours after the completion of treatment, blood samples (3 mL) were collected and placed on ice to allow clotting. The samples then were centrifuged at 1200 g for 10 minutes at 4°C, and the serum samples were stored at −80°C until assayed.
Determination of Serum Levels of Transaminases, Lactate, and Creatinine
Serum concentrations of alanine aminotransferase (ALT), aspartate aminotransferase (AST), lactate, and creatinine were determined by using assay kits according to the manufacturer's instructions (Pointe Scientific, Lincoln Park, MI).
Determination of Serum Levels of TNF-α, IL-6, and IL-10
The concentrations of TNF-α, IL-6, and IL-10 in the serum were quantified by using commercially obtained enzyme-linked immunosorbent assay kits specific for rat-TNF-α, IL-6, and IL-10 (Pharmingen, San Diego, CA). The assay was carried out according to the instructions provided by the manufacturer.
Statistical Analysis
All data are expressed as mean ± SE and compared by one-way analysis of variance (ANOVA) and Tukey's test or Student t test. Differences in values were considered significant if P < 0.05.
RESULTS
Alterations in Intestinal Vascular Responsiveness to AM Stimulation After Hemorrhage and Resuscitation
As indicated in Figure 1, AM at the concentration of 10−7 mol/L induced 83 ± 3.5% relaxation in the norepinephrine-preconstricted resistance blood vessels in the isolated small intestine in sham-operated animals. However, AM-induced vascular relaxation was significantly decreased to 53 ± 2.3% in hemorrhage animals at 1.5 hours after the completion of resuscitation (n = 6, P < 0.05, Fig. 1). The reduced AM responsiveness in the resistance blood vessels of the gut was marked improved by the addition of 2 × 10−8 mol/L AMBP-1 (n = 6, P < 0.05, Fig. 1).
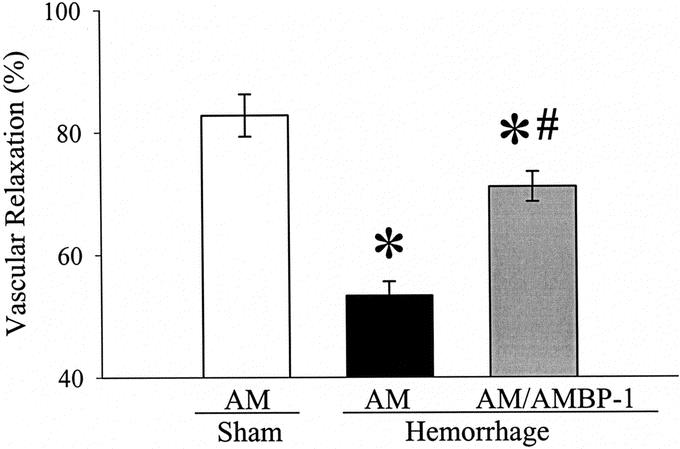
FIGURE 1. Alterations in AM (10−7 mol/L)-induced vascular relaxation in the isolated small intestine at 1.5 hours after the completion of hemorrhage and resuscitation (Hemorrhage) with or without the addition of AMBP-1 (2 × 10−8 mol/L) or sham operation (Sham). Data (percent relaxation from constriction induced by 2 × 6−6 mol/L norepinephrine) are expressed as mean ± SE (n = 6/group) and compared by one-way analysis of variance (ANOVA) and Tukey's test: *P < 0.05 versus Sham group. #P < 0.05 versus AM alone group.
Alterations in AM Receptor Complex Gene Expression After Hemorrhage and Resuscitation
As shown in Figure 2, the gene expression of AM receptor complex CRLR, RAMP2, and RAMP3 in the small intestine did not change significantly after hemorrhage and resuscitation (n = 6).
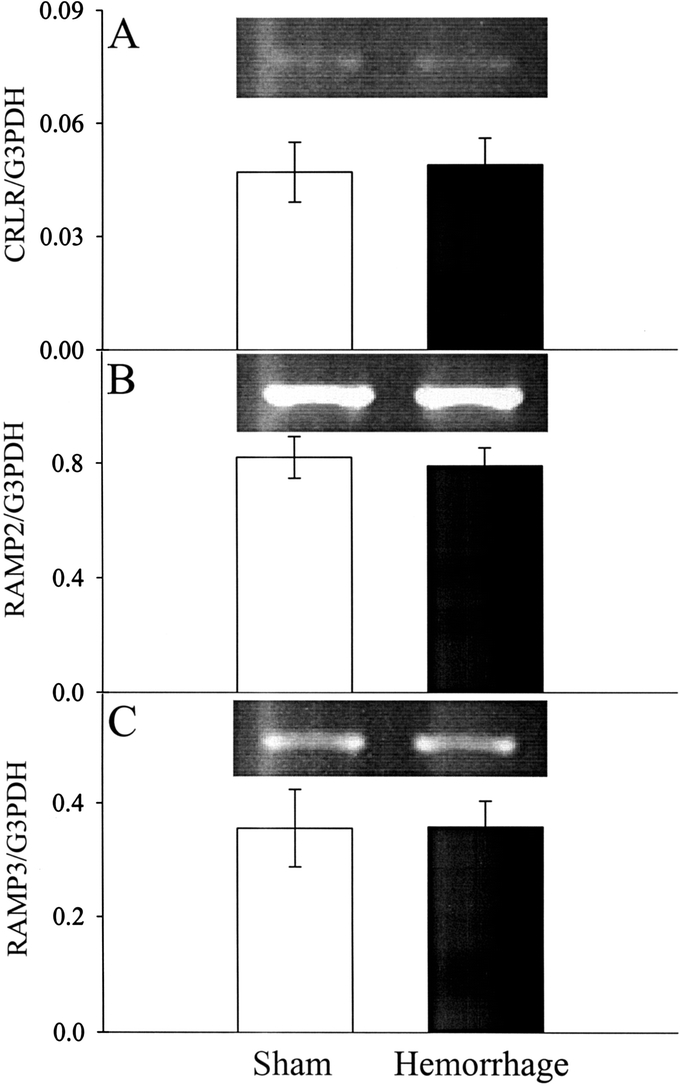
FIGURE 2. Alterations in AM receptor complex CRLR (A), RAMP2 (B), and RAMP3 (C) mRNA expressions in the small intestine at 1.5 hours after the completion of hemorrhage and resuscitation (Hemorrhage) or sham operation (Sham). Representative blots are also presented. Data are expressed as mean ± SE (n = 6/group). Student t test did not show any statistical differences between Hemorrhage and Sham groups.
Alterations in AMBP-1 Levels After Hemorrhage and Resuscitation
The results in Figure 3 indicate that, although the plasma levels of AMBP-1 did not change at the end of 90 minutes of hemorrhage, it decreased dramatically at 1.5 hours after the completion of resuscitation (n = 6, P < 0.05). The density of the bands represents AMBP-1's concentrations with the loading of equal amount of plasma proteins (Fig. 3). Similarly, at 1.5 hours after the completion of hemorrhage and resuscitation, the expression of AMBP-1 in the small intestine decreased significantly at both mRNA (n = 6, P < 0.05, Fig. 4A) and protein (n = 6, P < 0.05, Fig. 4B) levels. AMBP-1's gene expression in the liver also decreased significantly at 1.5 hours after the completion of hemorrhage and resuscitation (n = 6, P < 0.05, Fig. 5).
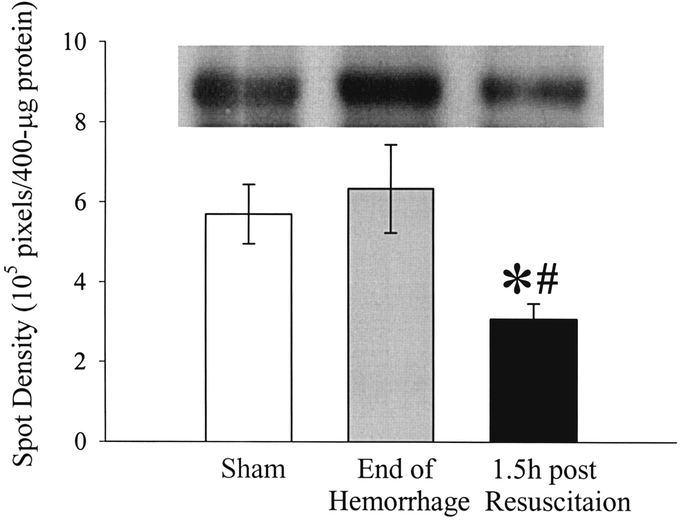
FIGURE 3. Alterations in plasma levels of AMBP-1 at the end of 90 minutes of hemorrhage (End of Hemorrhage), 1.5 hours after the completion of resuscitation (1.5 hours post Resuscitation), or sham operation (Sham). A representative blot is also presented. Data are expressed as mean ± SE (n = 6/group) and compared by one-way analysis of variance (ANOVA) and Tukey's test: *P < 0.05 versus Sham group. #P < 0.05 versus End of Hemorrhage group.
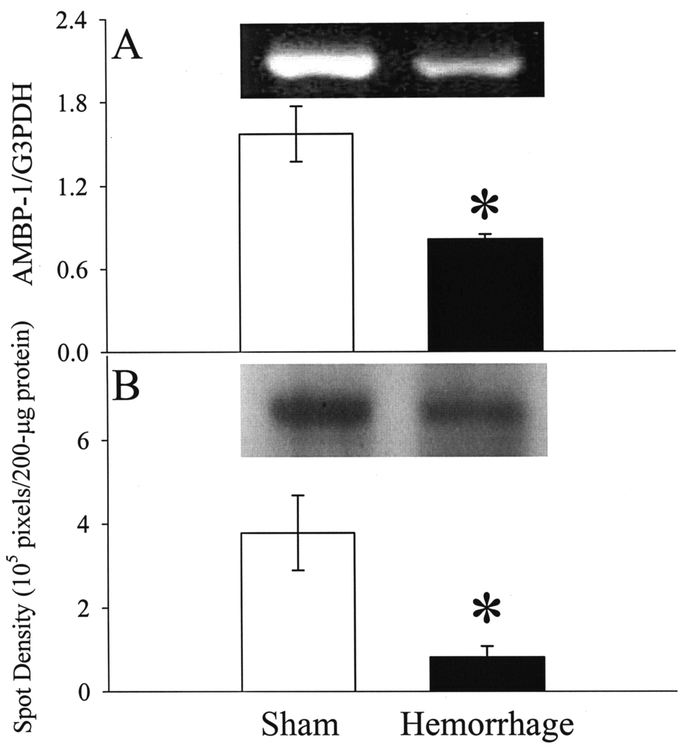
FIGURE 4. Alterations in AMBP-1 mRNA expression (A) and protein levels (B) in the small intestine at 1.5 hours after the completion of hemorrhage and resuscitation (Hemorrhage) or sham operation (Sham). Representative blots are also presented. Data are expressed as mean ± SE (n = 6/group) and compared by Student t test: *P < 0.05 versus Sham group.
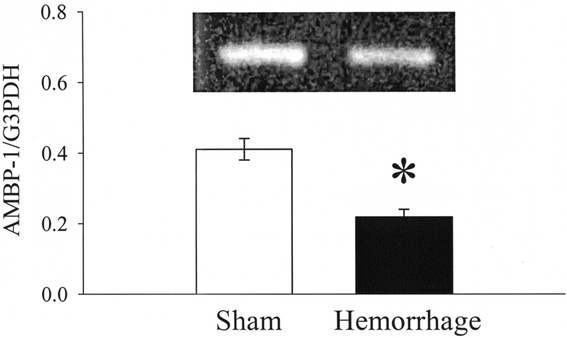
FIGURE 5. Alterations in AMBP-1 mRNA expression in the liver at 1.5 hours after the completion of hemorrhage and resuscitation (Hemorrhage) or sham operation (Sham). A representative blot is also presented. Data are expressed as mean ± SE (n = 6/group) and compared by Student t test: *P < 0.05 versus Sham group.
Alterations in AM Expression After Hemorrhage and Resuscitation
Unlike AMBP-1, the gene expression of AM in the small intestine increased significantly at 1.5 hours after the completion of hemorrhage and resuscitation as shown in Figure 6A (n = 6, P < 0.05). Although circulating levels of AM (pg/mL) increased by 19% at 1.5 hours after resuscitation, this increase was not statistically significant (Fig. 6B). This appears to be due to the hemodilutory effects of large volume crystalloid resuscitation. Taken consideration of hemodilution, the indexed concentration of AM (pg/mg plasma protein) increased by 89% at 1.5 hours after resuscitation (n= 4–6, P < 0.05, Fig. 6C).
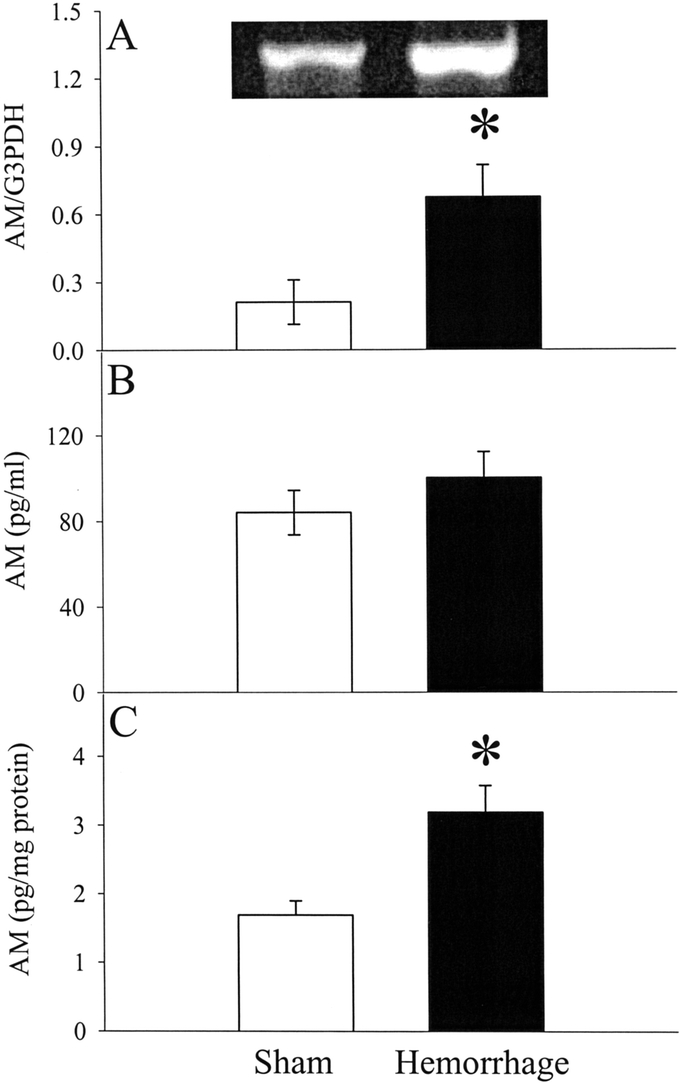
FIGURE 6. Alterations in AM mRNA expression in the small intestine (A), plasma levels of AM (B), and indexed plasma concentrations of AM (C) at 1.5 hours after the completion of hemorrhage and resuscitation (Hemorrhage) or sham operation (Sham). A representative blot is also presented for AM's mRNA expression. Data are expressed as mean ± SE (n = 4–6/group) and compared by Student t test: *P < 0.05 versus Sham group.
Effects of AM/AMBP-1 Administration After Hemorrhage and Resuscitation
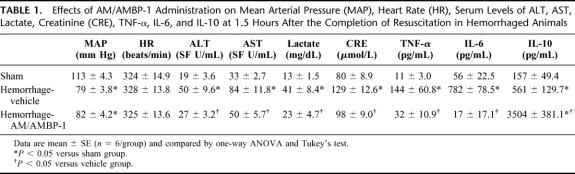
As indicated in Table 1, MAP in hemorrhage vehicle-treated animals was 30% lower than that in sham-operated animals at 1.5 hours after resuscitation, irrespective of large-volume crystalloid resuscitation (n = 6, P < 0.05). Treatment with AM/AMBP-1 did not further alter MAP. No changes in heart rate were found in hemorrhage-vehicle and AM/AMBP-1-treated animals as compared with that in sham-operated animals. Significant increases in hepatic enzymes (AST, ALT) were noted after hemorrhage, indicating liver injury. AM/AMBP-1 treatment during fluid resuscitation, however, attenuated the increased levels of AST and ALT (n = 6, P < 0.05, Tab. 1). Similarly, serum levels of lactate and creatinine were elevated significantly at 1.5 hours after the completion of resuscitation, and AM/AMBP-1 decreased lactate and creatinine levels significantly (n = 6, P < 0.05, Table 1), indicating improvement in tissue perfusion and renal function after treatment. At 1.5 hours after resuscitation, serum levels of TNF-α increased by 13-fold in vehicle-treated animals as compared with those in sham-operated animals. Administration of AM/AMBP-1, however, decreased TNF-α levels by 78% (n = 6, P < 0.05, Table 1). A marked increase in serum levels of IL-6 was also found at 1.5 hours after hemorrhage and resuscitation. Treatment with AM/AMBP-1, however, reduced the concentrations of IL-6 to sham levels (n = 6, P< 0.05, Table 1). Unlike TNF-α and IL-6, approximately 4-fold increase in IL-10 levels was found at 1.5 hours after resuscitation. Treatment with AM/AMBP-1 further increased the already elevated IL-10 levels by 6 fold (n = 6, P < 0.05, Table 1).
TABLE 1. Effects of AM/AMBP-1 Administration on Mean Arterial Pressure (MAP), Heart Rate (HR), Serum Levels of ALT, AST, Lactate, Creatinine (CRE), TNF-α, IL-6, and IL-10 at 1.5 Hours After the Completion of Resuscitation in Hemorrhaged Animals
DISCUSSION
Adrenomedullin is a potent vasodilator peptide, which was originally isolated from a human pheochromocytoma and reported by Kitamura et al in 1993.6 Subsequent studies have shown that AM is expressed in a large number of tissues and exhibits various properties, including the cardiovascular and fluid regulation, regulation of growth and differentiation, and secretions of other hormones.19 It is implicated in a variety of disease states such as cardiovascular and renal disorders, sepsis, cancer, and diabetes. AM acts as a circulating hormone, which elicits various biologic activities in a paracrine or autocrine manner. The primary activity of AM is to maintain cardiovascular and renal homeostasis through vasodilation, diuresis, and natriuresis.20–22 AM dilates blood vessels of different vascular beds in different animal species.23 Our present results indicate that vascular responsiveness to AM is depressed after severe blood loss. The pathophysiology of hemorrhagic shock consists of redistribution of body fluids and electrolytes, systemic inflammatory responses, deleterious intestinal vasoconstriction, and disproportionate splanchnic hypoperfusion, which persist even after seemingly adequate fluid replacement.24–26 These pathophysiologic events cause local and remote tissue injury that culminates in multiple organ failure and death. The present study shows that AM gene expression increased significantly after hemorrhagic shock, similar to that reported by others.13 However, because of the hemodilutory effects of large-volume crystalloid resuscitation, we did not find increased circulating levels of AM at 1.5 hours after resuscitation. The most important finding of this study is that vascular responsiveness to AM decreased significantly under such conditions. The reduced vascular responsiveness to AM may promote vasoconstriction in the splanchnic organs, leading to persistent hypoperfusion and circulatory collapse after hemorrhage and resuscitation.
The spectrum of AM functions is thought to be achieved by interaction with its receptors, which are coupled to adenylate cyclase via guanine nucleotide-binding regulatory proteins (G-protein).27 The mechanism of AM's vasodilatory effect has been addressed in several studies, and the results differ depending on the experimental models used.23 In general, AM induces endothelium-independent relaxation by elevating cAMP levels in vascular smooth muscle cells, and endothelium-dependent relaxation by increasing endothelium-derived nitric oxide (NO) release. Stimulation of AM receptors in vascular smooth muscle cells increases intracellular cAMP levels,28 which in turn activate cAMP-dependent protein kinase A (PKA).19 PKA converts myosin light chain kinase to its less active phosphorylated form, leading to smooth muscle relaxation and vasodilation.29 In addition to the adenylate cyclase-cAMP pathway, AM increases Ca2+ mobilization in vascular endothelial cells, resulting in an activation of endothelial constitutive nitric oxide synthase (ecNOS).29 With regard to the mechanism responsible for AM hyporesponsiveness after hemorrhage, our results indicate that the gene expression of 3 components of AM receptors (ie, CRLR, RAMP2 and RAMP3) was not altered after hemorrhage and resuscitation despite a decreased vascular responsiveness. Although we did not confirm the gene expression data by Western blot or receptor binding assay, the fact that the addition of AMBP-1 partially restored the reduced AM responsiveness in the resistance blood vessels of the gut supports the notion that AMBP-1, not AM receptors, plays an important role in producing AM hyporesponsiveness after hemorrhage and resuscitation.
AMBP-1 is a novel specific AM-binding protein, which was first reported by Elsasser et al in 1999.10 Recent studies have indicated that AMBP-1 is identical to human complement factor H.11 The liver is considered to be the main source of AMBP-1, although it is also synthesized by extrahepatic cells, such as mononuclear phagocytes, fibroblasts, endothelial cells, mesangial cells, astrocytes, oligodendrocytes, and neurons.30 The presence of AMBP-1 in tissues may affect the autocrine/paracrine actions of AM. Binding to AMBP-1 increases receptor-mediated effects of AM but suppresses its receptor-independent antimicrobial activity.23 AMBP-1 does not change the affinity of AM receptors for AM but has sequences that may bind to cell surface adhesion molecules and could therefore bring AM near its receptors and raise the effective concentration of AM.11 AM binding with AMBP-1 may also create a locally contained reservoir of AM at high concentrations. In this regard, AMBP-1 may increase the AM effectiveness without modifying the affinity of its receptor. It is also possible that AMBP-1 inhibits the degradation of the biologically active AM. In this study, we found that AMBP-1 expression decreased significantly at both mRNA and protein levels after hemorrhage and resuscitation. Since the gene expression of AM receptors does not change under such conditions, the vascular AM hyporesponsiveness observed after hemorrhage and resuscitation is, at least in part, caused by the altered AM/AMBP-1 interaction due to AMBP-1 down-regulation. Since AMBP-1 enhances AM-induced vascular relaxation under normal as well as phathophysiologic conditions,12 additional AMBP-1 is needed to restore vascular responsiveness to AM to maintain tissue perfusion after hemorrhage and resuscitation.
A combination of AM and AMBP-1 was administered after hemorrhage to determine their effects under in vivo conditions. Since AM is a potent vasodilator, AM/AMBP-1 was administered at 15 minutes after the onset of resuscitation in this study. At that time, one volume of shed blood in the form of lactated Ringer's solution has been injected, the average MAP are over 90 mm Hg (data not shown). It appears to be safe to use a low dosage of vasodilator at this blood pressure. Moreover, AM (12 μg/kg BW) and AMBP-1 (40 μg/kg BW) were administered over a period of 45 minutes. No significant adverse effect of AM/AMBP-1 on heart rate and MAP has been noted during and following the administration. The data presented in this study show that AM/AMBP-1 treatment significantly down-regulated proinflammatory cytokines (ie, TNF-α and IL-6), up-regulated anti-inflammatory cytokine (IL-10), and attenuated organ injury after hemorrhagic shock and resuscitation. In addition, our preliminary results have shown that treatment with AM/AMBP-1 during crystalloid resuscitation significantly improves heart performance, cardiac output, and organ blood flow after severe hemorrhage. AM is a peptide possessing multiple functions. When AMBP-1 is administered together with AM, the vascular sensitivity to AM is improved, which in turn leads to better organ perfusions. In addition to its well-known vasodilatory effects, AM also modulates production of inflammatory cytokines. Studies have shown that AM suppresses proinflammatory cytokine expression in various cell types.31,32 Our recent studies indicate that AMBP-1 can enhance the inhibitory effect of AM on TNF-α release from RAW264.7 cells (a murine macrophage cell line) as well as Kupffer cells isolated from normal rats.33 In addition, AMBP-1 significantly enhances AM-induced vascular relaxation in an aortic ring preparation.12 Our recent results also show that sepsis-induced vascular hyporesponsiveness to AM was restored by the addition of AMBP-1 ex vivo using the isolated aortic ring preparation.12 Taken together, these results suggest that the availability of AMBP-1 is a critically important factor for AM activity under normal as well as pathophysiologic conditions. Moreover, administration of AM/AMBP-1 significantly reduced circulating concentrations of TNF-α, IL-1β, and IL-6 in a rat model of polymicrobial sepsis induced by cecal ligation and puncture.34 In this study, we also found that AM/AMBP-1 treatment increases IL-10's levels after hemorrhage and resuscitation, which appears to be a novel pathway for AM/AMBP-1's anti-inflammatory effects.
CONCLUSION
Vascular responsiveness to AM is depressed after severe blood loss, and AM hyporesponsiveness may play an important role in the transition from reversible hypovolemia to circulatory collapse after severe blood loss. The reduction of AMBP-1 appears to contribute to the vascular hyporesponsiveness to AM after hemorrhage. Addition of AMBP-1 partially restores vascular AM responsiveness after hemorrhage. Moreover, AM/AMBP-1 treatment down-regulates proinflammatory cytokines, up-regulates anti-inflammatory cytokine, and attenuates organ injuries. Thus, AM/AMBP-1 should be considered as a novel approach to maintain cardiovascular stability and prevent cell and organ damage after severe hypovolemia.
Footnotes
Supported by NIH grants R01 HL076179 and R01 GM057468 (to P.W.). R.W. is supported by a Postdoctoral Fellowship from the American Heart Association (the Heritage Affiliate) (no. 0325802T).
Reprints: Ping Wang, MD, Division of Surgical Research, North Shore-Long Island Jewish Research Institute, 350 Community Drive, Manhasset, NY 11030. E-mail: pwang@nshs.edu.
REFERENCES
- 1.Anderson RN, Smith BL. Deaths: leading causes for 2001. Natl Vital Stat Rep. 2003;52:1–85. [PubMed] [Google Scholar]
- 2.Carrico CJ, Holcomb JB, Chaudry IH. Scientific priorities and strategic planning for resuscitation research and life saving therapy following traumatic injury: report of the PULSE Trauma Work Group: Post Resuscitative and Initial Utility of Life Saving Efforts. Acad Emerg Med. 2002;9:621–626. [DOI] [PubMed] [Google Scholar]
- 3.Wang P, Ba ZF, Chaudry IH. Chemically modified heparin improves hepatocellular function, cardiac output, and microcirculation after trauma-hemorrhage and resuscitation. Surgery. 1994;116:169–176. [DOI] [PubMed] [Google Scholar]
- 4.Wang P, Ba ZF, Biondo A, et al. Liver endothelial cell dysfunction occurs early following hemorrhagic shock and persists despite crystalloid resuscitation. J Surg Res. 1996;63:241–247. [DOI] [PubMed] [Google Scholar]
- 5.Fruchterman TM, Spain DA, Wilson MA, et al. Selective microvascular endothelial cell dysfunction in the small intestine following resuscitated hemorrhagic shock. Shock. 1998;10:417–422. [DOI] [PubMed] [Google Scholar]
- 6.Kitamura K, Kangawa K, Kawamoto M, et al. Adrenomedullin: a novel hypotensive peptide isolated from human pheochromocytoma. Biochem Biophys Res Commun. 1993;192:553–560. [DOI] [PubMed] [Google Scholar]
- 7.Eto T. A review of the biological properties and clinical implications of adrenomedullin and proadrenomedullin N-terminal 20 peptide (PAMP), hypotensive and vasodilating peptides. Peptides. 2001;22:1693–1711. [DOI] [PubMed] [Google Scholar]
- 8.McLatchie LM, Fraser NJ, Main MJ, et al. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature. 1998;393:333–339. [DOI] [PubMed] [Google Scholar]
- 9.Kamitani S, Asakawa M, Shimekake Y, et al. The RAMP2/CRLR complex is a functional adrenomedullin receptor in human endothelial and vascular smooth muscle cells. FEBS Lett. 1999;448:111–114. [DOI] [PubMed] [Google Scholar]
- 10.Elsasser TH, Kahl S, Martinez A, et al. Adrenomedullin binding protein in the plasma of multiple species: characterization by radioligand blotting. Endocrinology. 1999;140:4908–4911. [DOI] [PubMed] [Google Scholar]
- 11.Pio R, Martinez A, Unsworth EJ, et al. Complement factor H is a serum binding protein for adrenomedullin: the resulting complex modulates the bioactivities of both partners. J Biol Chem. 2001;276:12292–12300. [DOI] [PubMed] [Google Scholar]
- 12.Zhou M, Ba ZF, Chaudry IH, et al. Adrenomedullin binding protein-1 modulates vascular responsiveness to adrenomedullin in late sepsis. Am J Physiol Regul Integr Comp Physiol. 2002;283:R553–R560. [DOI] [PubMed] [Google Scholar]
- 13.Ehlenz K, Koch B, Preuss P, et al. High levels of circulating adrenomedullin in severe illness: correlation with C-reactive protein and evidence against the adrenal medulla as site of origin. Exp Clin Endocrinol Diabetes. 1997;105:156–162. [DOI] [PubMed] [Google Scholar]
- 14.Fujioka S, Ono Y, Kangawa K, et al. Plasma concentration of adrenomedullin is increased in hemorrhagic shock in dogs. Anesth Analg. 1999;88:326–328. [DOI] [PubMed] [Google Scholar]
- 15.Ba ZF, Kuebler JF, Rue LW, et al. Gender dimorphic tissue perfusion response after acute hemorrhage and resuscitation: role of vascular endothelial cell function. Am J Physiol Heart Circ Physiol. 2003;284:H2162–H2169. [DOI] [PubMed] [Google Scholar]
- 16.Ma Y, Wang P, Kuebler JF, et al. Hemorrhage induces the rapid development of hepatic insulin resistance. Am J Physiol Gastrointest Liver Physiol. 2003;284:G107–G115. [DOI] [PubMed] [Google Scholar]
- 17.Wang P, Ba ZF, Chaudry IH. Endothelium-dependent relaxation is depressed at the macro- and microcirculatory levels during sepsis. Am J Physiol. 1995;269:R988–R994. [DOI] [PubMed] [Google Scholar]
- 18.Wu R, Zhou M, Cui X, et al. Upregulation of cardiovascular ghrelin receptor occurs in the hyperdynamic phase of sepsis. Am J Physiol Heart Circ Physiol. 2004;287:H1296–H1302. [DOI] [PubMed] [Google Scholar]
- 19.Hinson JP, Kapas S, Smith DM. Adrenomedullin, a multifunctional regulatory peptide. Endocr Rev. 2000;21:138–167. [DOI] [PubMed] [Google Scholar]
- 20.Caron KM, Smithies O. Extreme hydrops fetalis and cardiovascular abnormalities in mice lacking a functional Adrenomedullin gene. Proc Natl Acad Sci USA. 2001;98:615–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taylor MM, Samson WK. Adrenomedullin and central cardiovascular regulation. Peptides. 2001;22:1803–1807. [DOI] [PubMed] [Google Scholar]
- 22.Shindo T, Kurihara Y, Nishimatsu H, et al. Vascular abnormalities and elevated blood pressure in mice lacking adrenomedullin gene. Circulation. 2001;104:1964–1971. [DOI] [PubMed] [Google Scholar]
- 23.Beltowski J, Jamroz A. Adrenomedullin: what we know 10 years since its discovery? Pol J Pharmacol. 2004;56:5–27. [PubMed] [Google Scholar]
- 24.Abraham E, Chang YH. Haemorrhage-induced alterations in function and cytokine production of T-cells and T-cell subpopulations. Clin Exp Immunol. 1992;90:497–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fruchterman TM, Spain DA, Wilson MA, et al. Complement inhibition prevents gut ischemia and endothelial cell dysfunction after hemorrhage/resuscitation. Surgery. 1998;124:782–791. [DOI] [PubMed] [Google Scholar]
- 26.Caruso JM, Deitch EA, Xu DZ, et al. Gut injury and gut-induced lung injury after trauma hemorrhagic shock is gender and estrus cycle specific in the rat. J Trauma. 2003;55:531–539. [DOI] [PubMed] [Google Scholar]
- 27.Montuenga LM, Martinez A, Miller MA, et al. Expression of adrenomedullin and its receptor during embryogenesis suggests autocrine or paracrine modes of action. Endocrinol. 1997;138:440–451. [DOI] [PubMed] [Google Scholar]
- 28.Ishizaka Y, Tanaka M, Kitamura K, et al. Adrenomedullin stimulates cyclic AMP formation in rat vascular smooth muscle cells. Biochem Biophys Res Commun. 1994;200:642–646. [DOI] [PubMed] [Google Scholar]
- 29.Shimekake Y, Nagata K, Ohta S, et al. Adrenomedullin stimulated two signal transduction pathways, cAMP accumulation and Ca2+ mobilization, in bovine aortic endothelial cells. J Biol Chem. 1995;270:4412–4417. [DOI] [PubMed] [Google Scholar]
- 30.Friese MA, Hellwage J, Jokiranta TS, et al. Different regulation of factor H and FHL-1/reconectin by inflammatory mediators and expression of the two proteins in rheumatoid arthritis (RA). Clin Exp Immunol. 2000;121:406–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Isumi Y, Kubo A, Katafuchi T, et al. Adrenomedullin suppresses interleukin-1β-induced tumor necrosis factor-α production in Swiss 3T3 cells. FEBS Lett. 1999;463:110–114. [DOI] [PubMed] [Google Scholar]
- 32.Kubo A, Minamino N, Isumi Y, et al. Production of adrenomedullin in macrophage cell line and peritoneal macrophage. J Biol Chem. 1998;273:16730–16738. [DOI] [PubMed] [Google Scholar]
- 33.Wu R, Zhou M, Wang P. Adrenomedullin and adrenomedullin binding protein-1 downregulate TNF-alpha in macrophage cell line and rat Kupffer cells. Regul Pept. 2003;112:19–26. [DOI] [PubMed] [Google Scholar]
- 34.Yang S, Zhou M, Fowler DE, et al. Mechanisms of the beneficial effect of adrenomedullin and adrenomedullin-binding protein-1 in sepsis: down-regulation of proinflammatory cytokines. Crit Care Med. 2002;30:2729–2735. [DOI] [PubMed] [Google Scholar]