Abstract
Objective:
Based on development of stem cell technology, newborn tissue, even undergoing cryopreservation, possesses promising potential as a donor source in the field of organ transplantation. However, the precise regeneration processes remains unclear. This study was designed to investigate the regenerative potential of newborn intestine with or without cryopreservation in the transplantation.
Methods:
Newborn rat intestines with or without cryopreservation were transplanted subcutaneously into the syngeneic host, and specimens were evaluated by histology, multiple immunostaining, and comprehensive gene expression analysis.
Results:
We determined that newborn rat intestine possessed regenerative potential in the syngeneic host even after cryopreservation, where angiogenesis was induced early in the submucosa with subsequent maturation in the crypts. Furthermore, newborn intestinal graft could facilitate the survival of maturation-incompetent 10-day-old graft that lacked regenerating activity (P < 0.01, n = 13). Tissue aggregates from the maturation-incompetent graft underwentreconstitution of their histologic configuration in the presence of newborn intestinal aggregates. Comprehensive gene expression analysis showed that 37 genes were preferentially up-regulated, while 19 genes were down-regulated in the regenerating 10-day-old graft (supported by the newborn graft).
Conclusions:
Regeneration of newborn intestine is implicated in neo-angiogenesis in the host, and the newborn intestinal graft is capable of mediating the survival of the maturation-incompetent 10-day-old graft. Notwithstanding ethical and legal limitations in the clinic, these results may provide new insights into the regenerative role of newborn grafts.
Based on development of stem cell technology, we demonstrated the regenerative potential of newborn intestine with (or without) cryopreservation in the transplantation. Further, we found that the newborn graft was capable of promoting the survival of a 10-day-old graft that lacked regenerating potential. These results may provide new insights into the regenerative role of newborn intestinal grafts.
Recent interest in the use of cells from a fetus or a newborn as a source of somatic stem cells has increased in the area of regeneration medicine. The fetus and newborns possess active regenerative capability, and their immune function is premature compared with adults. For example, postnatal rodent animals have stem cells in their small intestine, which can generate new small intestinal mucosa.1,2 Application of recent cryopreservation techniques might enhance the viability of the newborn intestine.
On the other hand, severe impairment of nutrient absorption results in clinical morbidity and mortality in the majority of patients with small intestinal failure such as short bowel syndrome.3–5 Small intestinal transplantation is an optional treatment of patients with intestinal failure in an effort to sustain their life.4 However, given chronic donor shortages, cadavaric intestinal transplantation remains limited, and the clinical results of intestinal transplantation do not always compare well with other organ transplantations. Since the small intestine is a high immunogenic organ, it is difficult to control acute and chronic rejection. Subsequently, issues concerning the immunologic unresponsiveness of recipients still need to be addressed, despite advances in the immunosuppressive regimen.
In our previous study, we demonstrated prolonged survival and subsequent development of the cryopreserved newborn intestine when the grafts were transplanted into the subcutaneous space of adult rats without vascular anastomosis between the graft and the recipient.6,7 Thus, newborn intestine possesses promising potential as a donor graft in the field of intestinal transplantation. To ensure the generation of a fully mature intestine from an avascular newborn intestinal graft, neo-angiogenesis is thought to be essential between the graft and the recipient.8 Given that the maturation ability of a fetal intestinal graft gradually decreases with time, angiogenesis should specifically occur shortly after birth.9 Many uncharacterized factors, however, are implicated in the enhanced survival of a newborn intestinal graft, the precise mechanisms of which remain unclear. In this study, we addressed the regenerative potential of newborn intestine with or without cryopreservation in the transplantation. Interestingly, we found that the newborn intestinal graft was capable of promoting the survival of a 10-day-old intestinal graft that lacked regenerating potential when both grafts were adhered to each other in parallel. Furthermore, we comprehensively analyzed the differential gene expression pattern between the competent and the incompetent 10-day-old intestine using a high-throughput microarray technique. These results may provide new insights into the regenerative role of newborn intestinal grafts.
MATERIALS AND METHODS
Animals
Inbred rat strains, Lewis (MHC haplotype: RT1l) and Brown Norway (MHC haplotype: RT1n), were purchased from CREA Japan, Inc. (Tokyo, Japan). PVG congenic rat strains (MHC haplotype: RT1c-7.1 and 7.2) were mated and maintained in our animal center. All experiments in this study were performed in accordance with the Jichi Medical School Guide for Laboratory Animals.
Donor newborn small intestines were obtained from neonatal Lewis and PVG RT7.1 rats within 24 hours after birth. Grafts of small intestine were isolated from 10-day-old rats as donors. Seven- to 10-week-old male Lewis and PVG RT7.2 rats were used as recipients.
For 5-bromo-2′-deoxyuridine (BrdU)-labeling, BrdU (20 mg/kg of body weight) was intravenously administrated into newborn rats 1 hour before killing them.
To establish green fluorescent protein (GFP)-expressing transgenic Lewis rats, the authentic microinjection technique was used as described previously.10 Briefly, enhanced GFP cDNA from pEGFP vector (Clontech, Palo Alto, CA) was inserted into the pCAGGS expression plasmid,11 and the HindIII–SalI fragment containing the promoter and coding sequence was injected into the fertilized Lewis rat (RT1l) egg. The transgene expression was confirmed under an excitation light (489 nm). The small intestine was strongly GFP-positive in the expression analysis.
Newborn Intestinal Transplantation
Newborn intestinal transplantation was performed as previously described.7,9,12 Briefly, following cervical decapitation, a transverse incision was made in the newborn rat andthe entire small intestine was excised, free of mesenteric tissue. The small intestine was cut into 20-mm pieces for grafting. Each lumen of the intestinal graft was flushed gently with 10 mL of cold saline containing 10 mg/mL cefazolin sodium (Fujisawa Phramaceutical Co., Osaka, Japan) using a 24-gauge needle. A nonabsorbable 4–0 nylon suture (Keisei Medical, Tokyo, Japan) was inserted into the intestinal lumen to avoid stoppage and kinking of the graft and to distinguish between the explanted sources.
For the grafting operation, a median incision was made in the recipient under ether anesthesia. A subcutaneous pocket was made large enough to receive the graft. For the purposes of twin grafting, a newborn and a 10-day-old graft were attached to each other side-by-side (in parallel) using 3 sutures and was subsequently placed into the subcutaneous pocket. In the single graft experiments, newborn and 10-day-old grafts were transplanted separately into the subcutaneous space on either side of the median incision. Following these procedures, standard rat food and water were given to the recipients.
Cryopreservation
The cryopreservation method used was as previously described.7 Briefly, grafts were immersed in a cold (4°C) preservation solution for 30 minutes and then placed into a freezing chamber (NALGENE Cryo 1°C Freezing Container). After cooling to −80°C at −1°C /min for 12 hours, samples were quenched to −180°C in liquid nitrogen for 7 days prior to transplantation.
Antibodies and Immunohistochemistry
The monoclonal antibody (mAb), HIS 41, which recognizes the rat RT7.2 alloantigen, and the OX42 mAb (raised against activated macrophages and neutrophils) were purchased from Serotec (Oxford, UK). A mAb against BrdU was purchased from Novocastra Laboratories (Newcastle upon Tyne, UK). A rabbit polyclonal antibody against mouse type IV collagen, which was used to outline the tissue framework,13 was purchased from Cosmobio (Tokyo, Japan). Thealkaline phosphatase (ALP)-labeled donkey F(ab′)2 to ratimmunoglobulin (Ig) (Jackson ImmunoResearch, West Grove, PA), and horseradish peroxidase (HRP)-labeled goat F(ab′)2 to rabbit Ig (55693; Cappel, Aurora, OH) were used as secondary antibodies.
For the purposes of double- or triple-immunostaining,13 fresh 4- to 6-μm-thick cryosections were stained with mAb(s) by repetition of either the indirect immunoperuoxidase technique with a diaminobenzidine substrate (brown), or the immuno-ALP technique with a Vector Blue substrate kit (Vector Laboratories, Burlingame, CA) and New Fuchsin substrate kit (Dako, Santa Barbara, CA).
Evaluation of the Grafts
Laparotomy was performed at 14 days following transplantation, and grafts were removed for histologic analysis. Grafts were evaluated according to the histologic grade using the following definitions as previously reported:9 “good,” the structure of the mucosa was almost normal; “fair,” the villi could be identified but were partially flattened; “poor,” mucosal deformity was severe or the mucosa could not be found. Cases that were classified as “good” or “fair” were judged histologically as successful transplantations and were considered as mature grafts. In an effort to quantify these histologic events, each grade was scored as 2, 1, and 0, respectively, and grafts were evaluated with an average of these scores.
RNA Isolation and Microarray Analysis
Twin grafts (10-day-old newborn) and 10-day-old single grafts were removed at 14 days following transplantation. The 10-day-old twin grafts were removed microscopically, free from surrounding tissue. All grafts were pooled at −80°C until their histologic evaluation was determined.
Total RNA was isolated from pooled grafts using the Atlas Glass Total RNA Isolation Kit (Clontech). Twenty micrograms of RNA was used for Cy3-fluorescent labeling using the Atlas Glass Fluorescent Labeling Kit (Clontech) according to the manufacturer's instruction. Synthesized cDNA probes were purified using a NucleoSpin Extraction Spin Column (Clontech). Atlas Glass Rat 1.0 array (1081 rat genes; Clontech) was hybridized with the Cy3-labeled probes in the GlassHyb Hybridization Solution (Clontech) at 50°C overnight (more than 16 hours). Hybridized arrays were washed 4 times with GlassHyb Wash Solution (Clontech) according to the manufacturer's instruction, briefly followed by distilled water. After drying, the fluorescent images of the array were digitized with a scanner and analyzed using AtlasImage software (Clontech). A 3-fold difference in signal intensity between the samples was considered to represent a significant difference in gene expression. A complete listing of genes included in the Atlas Glass Rat 1.0 array can be obtained at the Clontech web site (http://www.bdbiosciences.com/clontech/atlas/genelists/index.shtml).
Statistical Analysis
Mann-Whitney U nonparametric analysis was used for the statistical evaluation. Differences between groups were considered significant if the P value was less than 0.05.
RESULTS
Cryopreserved Newborn Intestine Possesses Regenerative Ability
In an effort to examine the regenerative potential of cryopreserved newborn intestine, grafts were transplanted into the subcutaneous space of syngeneic rats. Newborn intestines were isolated from Lewis rats and were preserved in liquid nitrogen for at least 30 days. Cryopreserved newborn intestine showed severe damage at 1 postoperation day (POD). Compared with intestine prior to cryopreservation (Fig. 1A), the mucosal epithelium and villi were stripped away, and crypts were not observed (Fig. 1B). However, the cryopreserved graft was capable of reestablishing a mature configuration at 14 POD (Fig. 1C). Histologic scoring of this graft (see Materials and Methods) yielded 5 good samples, 4 fair samples, and 1 poor sample. Experiments were performed 2 to 4 times each with similar results. Thus, these results suggest that cryopreserved newborn intestine possesses regenerative potential in the syngeneic host.
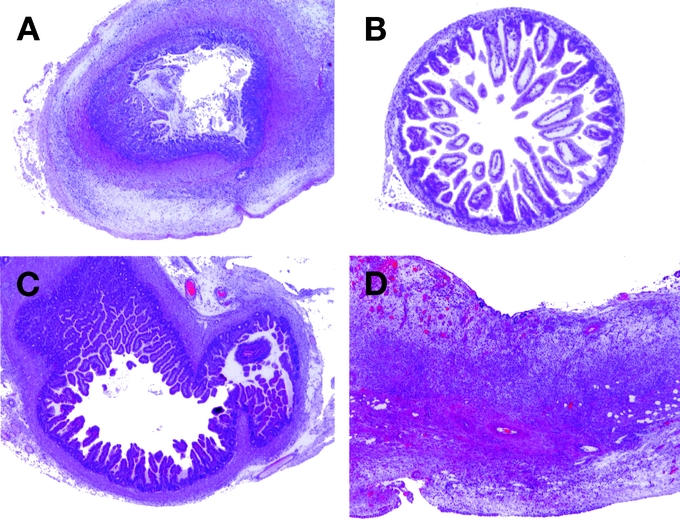
FIGURE 1. Regeneration of cryopreserved newborn intestine. A, A representative newborn intestine (hematoxylin and eosin [H&E], original magnification ×20). B, A newborn intestine following cryopreservation (for 30 days) was transplanted subcutaneously into a syngeneic Lewis rat. It was examined at 1 POD (H&E, original magnification ×20). C, A newborn intestine following subcutaneous transplantation at 14 POD (H&E, original magnification ×20). D, A cryopreserved newborn intestine (Lewis; RT1l) was transplanted into the allogeneic Brown Norway rat (RT1n), followed by treatment with the immunosuppressant drug, FK506 (tacrolimus, 0.64 mg/kg per day, intramuscularly) for 14 days (H&E, original magnification ×40).
Since it is known that cryopreserved organs become poorly immunogenic,14 we tested whether cryopreserved newborn intestine could be readily accepted in allogeneic recipients. Cryopreserved newborn intestines from Brown Norway rats were transplanted subcutaneously into Lewis rats, followed by tacrolimus (0.64 mg/kg per day, intramuscularly). As shown in Figure 1D, massive inflammatory mononuclear cells were infiltrated and the intestinal graft was almost completely destroyed. Histologic scoring designated all 11 samples as poor. This low histologic scoring was not due to cryopreservation since fresh intestinal graft was similarly damaged by allogeneic transplantation using the same immunosuppressive regimen.7 Thus, cryopreservation did not enhance the survival of newborn intestine in the allogeneic recipients, suggesting that newborn intestines, including those that were cryopreserved, are still highly immunogenic.
Regeneration of Newborn Intestine Is Implicated in Neo-Angiogenesis in the Host
We then attempted to determine the cellular processes that are associated with the regenerative potential of newborn intestine. In an effort to distinguish cells between the donor and the recipient, we used an RVG congenic rat strain (MHC haplotypes are RT1c-7.1 and 7.2). Fresh RT7.1-newborn intestinal grafts were transplanted subcutaneously into RT7.2-recipient rats. Serial immunohistochemical studies were performed following transplantation. Concomitant with histologic maturation in the graft, BrdU-positive cells emerged around the vasculature in the submucosa at 2 POD (Fig. 2A) and in the crypts at 3 POD (Fig. 2B). HIS41 (RT7.2)-positive recipient cells were also observed in the vascular lumen of the graft. The number of BrdU-positive cells increased in the crypts at 5 to 6 POD (data not shown), with histologic maturation being almost complete at 14 POD (Fig. 2C). Thus, these results suggest that neo-angiogenesis in submucosa and crypts is associated with histologic maturation in fresh newborn intestinal grafts.
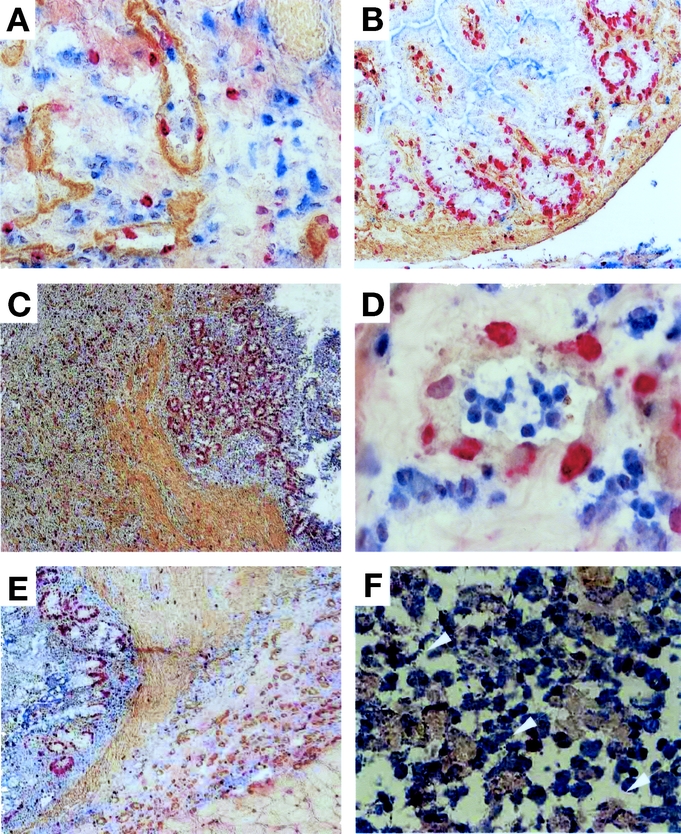
FIGURE 2. Regeneration process of rat newborn intestine. Either fresh (A–C) or 30-day-cryopreserved (D–F) newborn intestines of RT1c-7.1 rat were transplanted into RT1c-7.2 congenic rats, and specimens were multiply stained with anti-RT1c-7.2 (HIS41) mAb (blue), antitype IV-collagen mAb (brown), and anti-BrdU mAb (red). A, Blood vessels in the submucosa at 2 POD (original magnification ×100). The basement membrane was stained with antitype IV collagen mAb (brown). B, Crypts of the graft at 3 POD (original magnification ×40). C, The mucosal, submucosal and muscular layers developed at 14 POD, and numerous BrdU-positive cells were found in the crypts (original magnification ×40). D, Blood vessels in the submucosa of the cryopreserved graft at 2 POD (original magnification ×100). E, The mucosal, submucosal and muscular layers developed at 11 POD. F, Arrowheads indicate HIS41- and BrdU-double cells in the submucosal layer at 11 POD (original magnification ×100). Experiments were performed 2 times with similar results.
We also examined the aforementioned cellular process using cryo-preserved newborn intestine. RT7.1-newborn intestinal grafts were preserved in liquid nitrogen and then transplanted subcutaneously into RT7.2-recipient rats. Cryopreserved newborn intestines were also regenerated toward maturation in a similar time course to the fresh graft. BrdU-positive cells emerged in the vascular endothelial cells, and recipient-derived cells migrated to the vascular lumen (Fig. 2D). At 11 POD, the transplanted graft had matured histologically, and increased numbers of BrdU-positive cells were clearly seen in the crypts (Fig. 2E). Some of the BrdU-positive cells were derived from the RT7.2-recipient (Fig. 2F, arrowhead). Proliferation of vascular endothelial cells had also progressed in parallel with histologic maturation in the cryopreserved neonatal intestinal grafts. Neovascularization appears to be essential for the maturation of the intestinal graft in the recipient.
Newborn Graft Potentiates the Maturation-Incompetent 10-Day-Old Graft Toward Their Survival
There is striking evidence to suggest that newborn intestinal grafts impart survival potential in a rat organ transplantation model. While the graft survival decreased in the 10-day-old graft following subcutaneous transplantation, the newborn graft that underwent the same procedure as the 10-day-old graft clearly survived at 14 POD (Fig. 3A, B). We therefore hypothesized that the survival potential of newborn grafts might facilitate 10-day-old graft survival. Newborn and 10-day-old grafts were adhered to each other in parallel (twin grafting) and transplanted subcutaneously into syngeneic rats. As shown in Figure 3C, the 10-day-old graft morphologically regenerated into mature intestine at 14 POD. Twin grafting resulted in a high histologic score, even in the 10-day-old graft, which was equivalent to that of the newborn graft transplanted separately (Fig. 3D; P < 0.01, n = 13). Thus, these results demonstrate that the newborn graft is capable of mediating the survival of maturation-incompetent 10-day-old grafts.
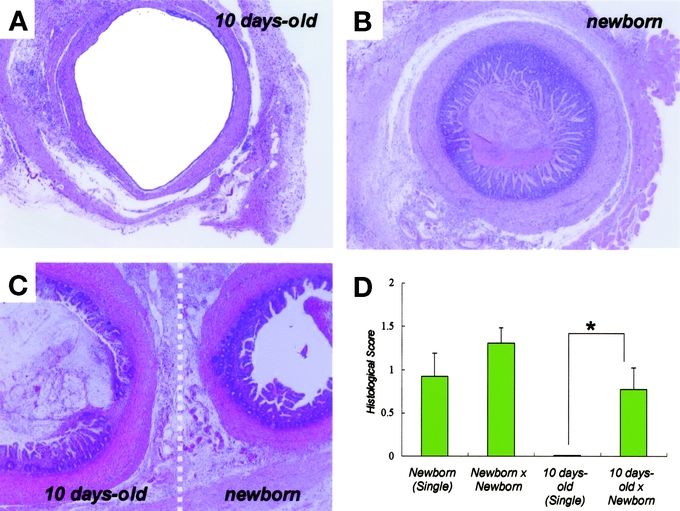
FIGURE 3. Maturation-incompetent 10-day-old intestinal graft was recovered in the presence of newborn graft. A, A fresh 10-day-old intestinal graft was transplanted in the subcutaneous space of syngeneic rats, and then removed at 14 POD for inspection (H&E, original magnification ×20). B, A fresh newborn graft at 14 POD (H&E, original magnification ×20). C, Histologic recovery of the 10-day-old intestinal graft in the presence of a newborn graft (twin grafting: H&E, original magnification ×20). D, Average histologic score of graft. Datarepresent mean ± SED (n = 13). *P = 0.006 versus 10-day-old× Newborn. One of 3 independent experiments with similar results.
We further examined whether the survival of maturation-incompetent 10-day-old grafts was associated with neovacularization, by which newborn intestinal grafts induced their own tissue maturation. BrdU-positive cells emerged in the vasculature within the graft at 2 POD (Fig. 4A), and also increased in the crypts after 3 POD (Fig. 4B). At 10 POD, the 10-day-old graft had morphologically matured, coupled with the adjacent newborn graft (Fig. 4C). Similar phenotypes wereobserved in 3 or more independent experiments. Thus, primaryangiogenesis associated with the newborn graft appearedto determine the survival of the maturation-incompetent 10-day-old graft.
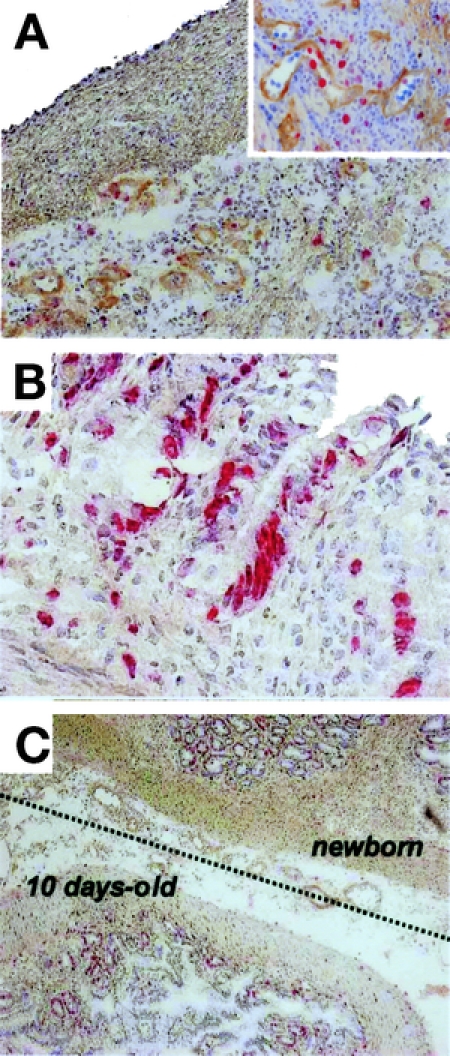
FIGURE 4. Regeneration process of 10-day-old intestinal graft in the presence of newborn graft. A fresh 10-day-old intestinal graft was transplanted subcutaneously as a twin graft in the presence of a newborn intestine. Specimens of 10-day-old grafts were multiply stained with antitype IV collagen mAb (brown) and anti-BrdU mAb (red). The counterstaining is hematoxylin. A, BrdU-positive cells were distributed in the submucosa at 2 POD (original magnification ×20). The upper-right corner inset represents a close-up view of blood vessels (original magnification ×100). B, BrdU-positive cells in the crypts at 3 POD (original magnification ×100). C, Lower view of 10-day-old intestinal graft at 10 POD (original magnification ×20). The dotted line represents the border between 10-day-old and newborn grafts.
Histologic Reconstitution of 10-Day-Old Grafts From Tissue Aggregates
In an effort to determine whether maturation-incompetent 10-day-old grafts could survive even in the tissue aggregates,10-day-old intestinal grafts of ubiquitously GFP-expressing transgenic Lewis rats were processed by chopping, followed by transplanting subcutaneously together with processed newborn grafts of wild-type Lewis rats. As shown in Figure 5A and B, the control GFP-positive newborn grafts showed morphologic reconstitution following transplantation, and regenerated epithelium gave rise to a green fluorescence under excitation light at 14 POD. The 10-day-old intestinal grafts from GFP rats were able to survive in the presence of chopped newborn grafts, and some mucus had accumulated in the lumen (Fig. 5C, D). Substantial GFP signal was also confirmed by immunohistochemistry using specific antibodies against GFP (data not shown). These findings demonstrate that the incompetent intestinal graft from tissue aggregates could reconstitute its own tissue in the presence of newborn grafts.
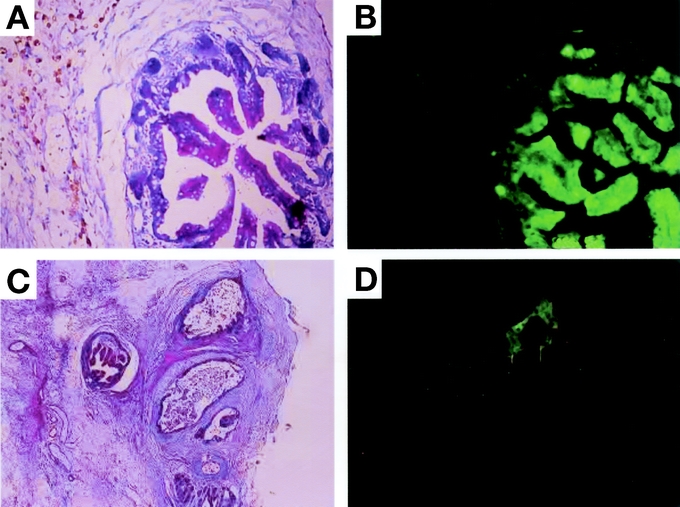
FIGURE 5. Histologic reconstitution of a 10-day-old graft from tissue aggregates. A, Neonatal intestinal grafts from GFP-transgenic Lewis rats were chopped at random and transplanted into the subcutaneous space of wild-type Lewis rats. A representative specimen is shown at 14 POD (original magnification H&E, ×40). B, GFP expression of the specimen in a (under a 489-nm excitation light, original magnification ×40). C, 10-day-old intestinal grafts from GFP-transgenic Lewis rats were chopped at random and transplanted subcutaneously together with similarly chopped newborn grafts from wild-type Lewis rats. A representative specimen is shown at 14 POD (H&E, original magnification ×20). D, GFP expression of the specimen in c (under a 489-nm excitation light, original magnification ×20). Note the substantial expression of GFP from the 10-day-old intestinal graft. One of 2 independent experiments with similar results.
Gene Expression Pattern in Regenerating 10-Day-Old Intestinal Grafts
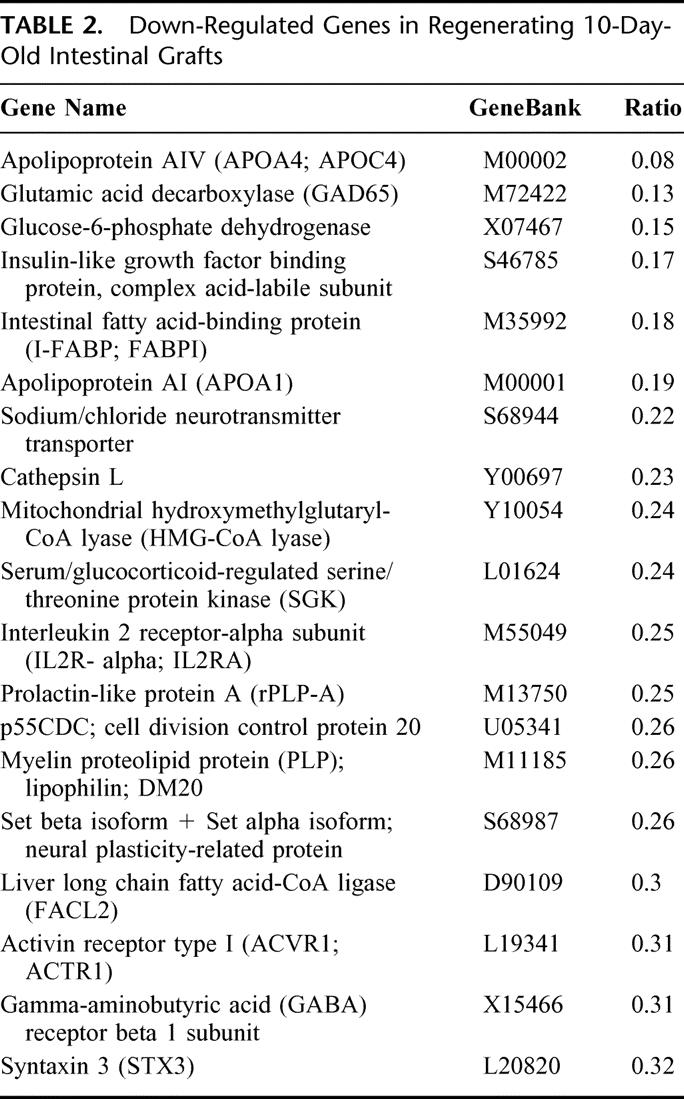
Comprehensive gene expression analysis is a powerful strategy that can be used to predict the factors associated with a particular biologic event. In an effort to determine which genes are activated (or suppressed) in 10-day-old grafts, the differential gene expression pattern of the regenerating 10-day-old graft (by twin grafting) and the maturation-incompetent single graft was examined using a DNA microarray containing 1081 rat genes. In this assay, we excluded data with less than a 3-fold difference in the signal ratio since a low level of expression could often be below the level of detection of the microarray system. Analysis of the hybridization signals revealed that 37 genes were up-regulated in the regenerating graft, including some angiogenic and growth factors, which are associated with intestinal development (eg, CSF-1R, PKC-delta, and IGF-binding protein 5) (Table 1). In contrast, 19 genes were down-regulated, including apolipoprotein A and glucose-6-phospahte dehydrogenase (Table 2). Furthermore, semi-quantitative RT-PCR analysis of several transcripts correlated well with the differential expression level from the microarray analysis (data not shown). This suggests that the microarray data correctly reflected the magnitude and direction of the change. Thus, these genes may be worthy of note due to their association with intestinal regeneration and their known roles in cellular regulatory pathways.
TABLE 1. Most Activated Transcripts in Regenerating 10-Day-Old Intestinal Grafts
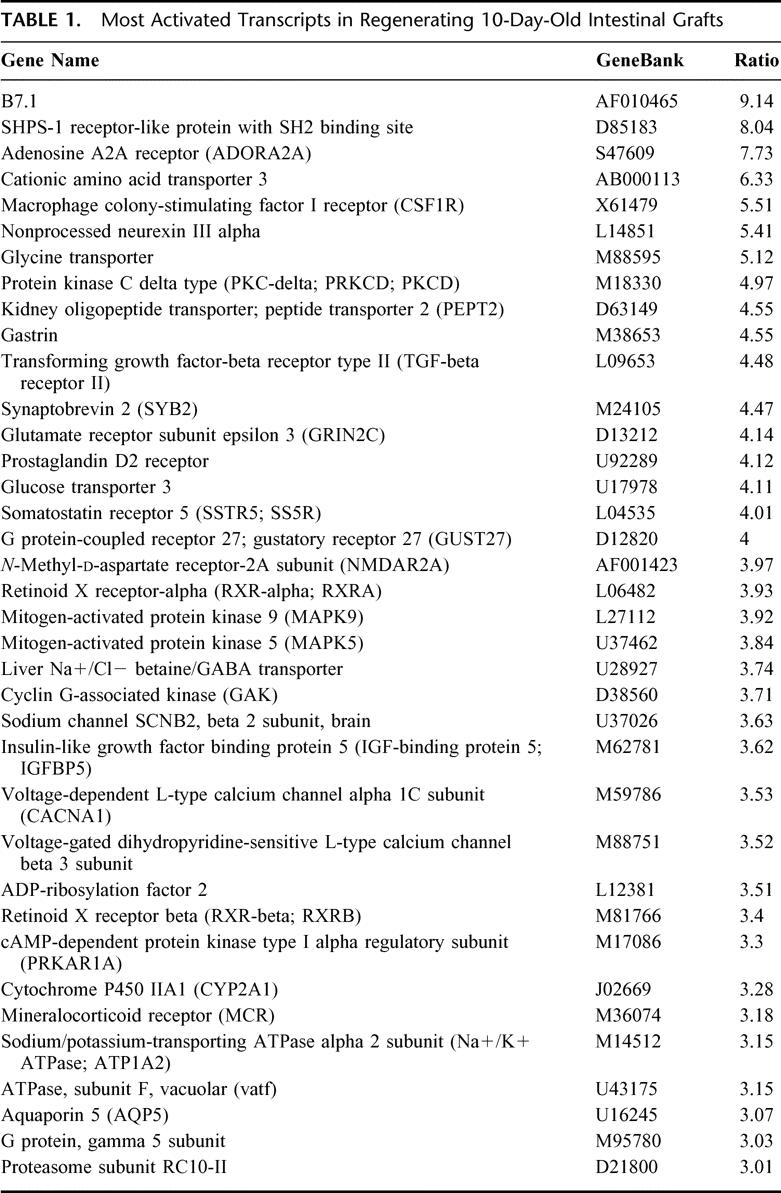
TABLE 2. Down-Regulated Genes in Regenerating 10-Day-Old Intestinal Grafts
DISCUSSION
Herein, we demonstrated that the newborn intestine possesses regenerative potential in the syngeneic host even after it had been preserved in liquid nitrogen. Two remarkable features were discovered in our investigations: (1) neo-angiogenesis was induced early in the submucosa followed by subsequent maturation in the crypts, and (2) the newborn intestinal graft was capable of mediating the survival of the maturation-incompetent 10-day-old graft. Furthermore, we investigated the genes that were preferentially associated with the survival of 10-day-old grafts in the newborn intestine.
Fetal and newborn tissues served as potential sources for the transplantation because of their immature immunogenic property. Small bowel transplantation had successfully been performed using a neonate donor.15 Furthermore, Kato et al16 demonstrated that specific acceptance for fetal bowel allografts in a mouse model could be introduced by a short-term treatment with monoclonal antibodies against intercellular adhesion molecule-1 and leukocyte function-associated antigen. While it is known that tissue cryopreservation is associated with a reduction in antigenicity,14 our observations suggest that the small intestine still possesses immunogenic potential, even after undergoing cryopreservation (Fig. 1D). Given that it has been reported that even tissue-engineered intestine has the capacity to develop a mucosal immune system,17 it is likely that the regenerative ability of newborn intestine leads to the development of the mucosal immune system, thereby resulting in immunologic conflict.
Although recent advances in the cryopreservation technique has made it possible to preserve many cell types, its application to complicated whole organs, including the small intestine, remains problematic given that the procedure can inflict damage on the tissue. Notwithstanding the hope that the tissue preservation technique is expected to resolve the donor shortage problem in transplantation, it is still limited to use with homogeneous tissues such as the aortic valve and skin for providing superior viability.18,19 As demonstrated in our current work, the newborn small intestine has the capacity to regenerate, while its maturation potential is not significantly inhibited by cryopreservation. Therefore, we propose that newborn intestine is a suitable tissue for cryopreservation and possible transplantation.
It has long been known that newborn, as well as fetal, cells possess great potential for proliferation and development. In this study, BrdU-positive cells were observed mainly in the endothelium of the blood vessel at 2 days following transplantation of newborn intestine into syngenic rats, indicating that neovascularization had occurred. Subsequently, BrdU-positive cells emerged in the crypts of the graft, where intestinal stem cells had generated including midcrypt columnar,oligomucous, young entero-endocrine and Paneth cells.4,20–22 Tait et al have shown that transplantation of corresponding crypt stem cells was able to generate neomucosa with normal morphology.23 Thus, the presence and protection of these stem cells seem to be essential for successful newborn intestinal transplantation.
How are the intestinal stem cells of the graft activated in the recipient (host)? There are 2 possible explanations that account for the graft and host interaction: (1) host-derived enterocytes are stimulated in the intestinal graft,24 and (2) bone marrow-derived cells repair damaged epithelia of the graft.25 Although we observed some cellular migration of the host cells (Fig. 2F), we were unable to determine which host-derived cells contributed to the tissue reconstruction. We speculate that an initial neoangiogenic event at early POD may stimulate the stem cells toward their maturation.
Newborn intestine can revascularize and mature following subcutaneous transplantation without surgical vascular anastomosis. This phenomenon was specifically observed shortly after birth, where the maturation ability of newborn intestinal grafts gradually decreased with time (this regeneration ability peaked within 24 hours after birth). In our previous study, electromyography of the newborn intestinal graft showed a similar pattern to that of the native intestine,12 suggesting functional graft maturation. Surprisingly, the regeneration-incompetent 10-day-old graft was rescued by twin grafting in the presence of the newborn graft. The histologic characteristics of both specimens were equivalent, and there was no difference in the restructuring of the newborn and 10-day-old grafts. In our microarray trial (on 1081 rat genes), the RNA levels of 37 transcripts preferentially increased in the regenerating 10-day-old graft following twin grafting with the newborn graft. Interestingly, some of the up-regulated genes included growth factors and intracellular signaling molecules such as colony-stimulating factor-1, which can promote angiogenesis in rats,26 and insulin-like growth factor (IGF) binding protein 5, which is capable of enhancing the IGF-mediated survival of small intestinal cells.27 Activation of protein kinase C-delta is required for basic FGF-induced angiogenesis.28 The family of mitogen-activated protein kinases has been shown to enhance cell growth, differentiation, and survival.29–31 Thus, these molecules induced by the newborn intestine graft appear to contribute to neovasclarization and enhance the maturation of the old intestine graft, although further functional linkages are needed.
CONCLUSION
These results provide new insights into the regenerative process of newborn intestinal grafts and suggest new molecules for future studies in the area of small intestinal transplantation. Although ethical and legal limitations in the clinic should be further discussed, transplantation, coupled with advanced regenerative studies, may reduce the clinical morbidity and mortality in patients with intestinal failure in the near future.
ACKNOWLEDGMENTS
The authors thank Dr. Masatsugu Udeda (YS New Technology Institute, Inc., Tochigi, Japan) for kindly providing GFP transgenic Lewis rats; Dr. Yoji Hakamata (Animal Resource Project, Center for Molecular Medicine, Jichi Medical School, Tochigi, Japan) for helpful suggestions; Dr. Jun-ichi Miyazaki (Osaka University, Graduate School of Medicine, Osaka, Japan) for providing pCAGGS plasmid; and Ms. Yasuko Sakuma, Ms. Megumi Hata, and Ms. Harumi Kawana for their skillful technical assistance. The transgenic rat embryo is available from the Health Science Research Resources Bank at hsrrb@osa.jhsf.or.jp.
Footnotes
Supported by grants to E.K. from the Research on Health Sciences focusing on Drug Innovation program of the Japan Heath Science Foundation (2004), the “High-Tech Research Center” Project for Private Universities: matching fund subsidy, and the COE program from MEXT (2003∼).
Reprints: Eiji Kobayashi, MD, PhD, Division of Organ Replacement, Research Center for Molecular Medicine, Jichi Medical School, 3311-1 Yakushiji Minamikawachi, Kawachi-gun Tochigi 329-0498, Japan. E-mail: eijikoba@jichi.ac.jp.
REFERENCES
- 1.Cheng H, Leblond CP. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine: I. Columnar cell. Am J Anat. 1974;141:461–479. [DOI] [PubMed] [Google Scholar]
- 2.Tait IS, Flint N, Campbell FC, et al. Generation of neomucosa in vivo by transplantation of dissociated rat postnatal small intestinal epithelium. Differentiation. 1994;56:91–100. [DOI] [PubMed] [Google Scholar]
- 3.Roberts JP, Brown RS Jr, Edwards EB, et al. Liver and intestine transplantation. Am J Transplant. 2003;3(suppl 4):78–90. [DOI] [PubMed] [Google Scholar]
- 4.Vanderhoof JA, Young RJ, Thompson JS. New and emerging therapies for short bowel syndrome in children. Paediatr Drugs. 2003;5:525–531. [DOI] [PubMed] [Google Scholar]
- 5.Fishbein TM, Gondolesi GE, Kaufman SS. Intestinal transplantation for gut failure. Gastroenterology. 2003;124:1615–1628. [DOI] [PubMed] [Google Scholar]
- 6.Uchida H, Tahara K, Takizawa T, et al. Experimental small bowel transplantation using a newborn intestine in rats: IV. Effect of cold preservation on graft neovascularization. J Pediatr Surg. 2001;36:1805–1810. [DOI] [PubMed] [Google Scholar]
- 7.Tahara K, Uchida H, Kawarasaki H, et al. Experimental small bowel transplantation using newborn intestine in rats: III. Long-term cryopreservation of rat newborn intestine. J Pediatr Surg. 2001;36:602–604. [DOI] [PubMed] [Google Scholar]
- 8.Rafii S, Lyden D. Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat Med. 2003;9:702–712. [DOI] [PubMed] [Google Scholar]
- 9.Uchida H, Kobayashi E, Yanagisawa K, et al. Experimental small bowel transplantation using newborn intestine in rats: II. Revascularization of newborn intestine is independent of vascular endothelial growth factor. J Pediatr Surg. 1999;34:1396–1400. [DOI] [PubMed] [Google Scholar]
- 10.Hakamata Y, Tahara K, Uchida H, et al. Green fluorescent protein-transgenic rat: a tool for organ transplantation research. Biochem Biophys Res Commun. 2001;286:779–785. [DOI] [PubMed] [Google Scholar]
- 11.Niwa H, Yamamura K, Miyazaki J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 1991;108:193–199. [DOI] [PubMed] [Google Scholar]
- 12.Uchida H, Yoshida T, Kobayashi E, et al. Experimental small bowel transplantation using newborn intestine in rats: I. Lipid absorption restored after transplantation of nonvascularized graft. J Pediatr Surg. 1999;34:1007–1011. [DOI] [PubMed] [Google Scholar]
- 13.Matsuno K, Ezaki T, Kudo S, et al. A life stage of particle-laden rat dendritic cells in vivo: their terminal division, active phagocytosis, and translocation from the liver to the draining lymph. J Exp Med. 1996;183:1865–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boren CH, Roon AJ, Moore WS. Maintenance of viable arterial allografts by cryopreservation. Surgery. 1978;83:382–391. [PubMed] [Google Scholar]
- 15.Goulet O, Revillon Y, Brousse N, et al. Successful small bowel transplantation in an infant. Transplantation. 1992;53:940–943. [DOI] [PubMed] [Google Scholar]
- 16.Kato Y, Yamataka A, Yagita H, et al. Specific acceptance of fetal bowel allograft in mice after combined treatment with anti-intercellular adhesion molecule-1 and leukocyte function-associated antigen-1 antibodies. Ann Surg. 1996;223:94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perez A, Grikscheit TC, Blumberg RS, et al. Tissue-engineered small intestine: ontogeny of the immune system. Transplantation. 2002;74:619–623. [DOI] [PubMed] [Google Scholar]
- 18.Kirklin JK, Smith D, Novick W, et al. Long-term function of cryopreserved aortic homografts: a ten-year study. J Thorac Cardiovasc Surg. 1993;106:154–165; discussion 165–166. [PubMed]
- 19.Sipe JD. Tissue engineering and reparative medicine. Ann NY Acad Sci. 2002;961:1–9. [DOI] [PubMed] [Google Scholar]
- 20.Cheng H. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine: II. Mucous cells. Am J Anat. 1974;141:481–501. [DOI] [PubMed] [Google Scholar]
- 21.Cheng H, Leblond CP. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine: III. Entero-endocrine cells. Am J Anat. 1974;141:503–519. [DOI] [PubMed] [Google Scholar]
- 22.Cheng H. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. IV. Paneth cells. Am J Anat. 1974;141:521–535. [DOI] [PubMed] [Google Scholar]
- 23.Tait IS, Penny JI, Campbell FC. Does neomucosa induced by small bowel stem cell transplantation have adequate function? Am J Surg. 1995;169:120–125. [DOI] [PubMed] [Google Scholar]
- 24.Tryphonopoulos P, Icardi M, Salgar S, et al. Host-derived enterocytes in intestinal grafts. Transplantation. 2002;74:120–138. [DOI] [PubMed] [Google Scholar]
- 25.Okamoto R, Yajima T, Yamazaki M, et al. Damaged epithelia regenerated by bone marrow-derived cells in the human gastrointestinal tract. Nat Med. 2002;8:1011–1017. [DOI] [PubMed] [Google Scholar]
- 26.Aharinejad S, Marks SC Jr, Bock P, et al. CSF-1 treatment promotes angiogenesis in the metaphysis of osteopetrotic (toothless, tl) rats. Bone. 1995;16:315–324. [DOI] [PubMed] [Google Scholar]
- 27.Wilkins HR, Ohneda K, Keku TO, et al. Reduction of spontaneous and irradiation-induced apoptosis in small intestine of IGF-I transgenic mice. Am J Physiol Gastrointest Liver Physiol. 2002;283:457–464. [DOI] [PubMed] [Google Scholar]
- 28.Kent KC, Mii S, Harrington EO, et al. Requirement for protein kinase C activation in basic fibroblast growth factor-induced human endothelial cell proliferation. Circ Res. 1995;77:231–238. [DOI] [PubMed] [Google Scholar]
- 29.Ding Q, Wang Q, Evers BM. Alterations of MAPK activities associated with intestinal cell differentiation. Biochem Biophys Res Commun. 2001;284:282–288. [DOI] [PubMed] [Google Scholar]
- 30.Gauthier R, Harnois C, Drolet JF, et al. Human intestinal epithelial cell survival: differentiation state-specific control mechanisms. Am J Physiol Cell Physiol. 2001;280:1540–1554. [DOI] [PubMed] [Google Scholar]
- 31.Houde M, Laprise P, Jean D, et al. Intestinal epithelial cell differentiation involves activation of p38 mitogen-activated protein kinase that regulates the homeobox transcription factor CDX2. J Biol Chem. 2001;276:21885–21894. [DOI] [PubMed] [Google Scholar]


