Abstract
Objective:
To determine the effects of COX-2 specific inhibitors on postoperative adhesion formation.
Summary and Background Data:
Intra-abdominal adhesions are the major cause of intestinal obstruction and secondary infertility after surgical procedures. Because adhesion synthesis requires angiogenesis, and cyclooxygenase-2 enzyme (COX-2) inhibitors have antiendothelial activity, we tested COX-2 inhibitors in a murine model of intra-abdominal adhesion formation.
Methods:
A silicone patch was secured to the lateral abdominal wall of groups of C57BL/6 mice, followed by cecal abrasion to promote adhesion formation. Beginning on the day of surgery, mice were treated with the selective COX-2 agents, celecoxib or rofecoxib, and the nonspecific COX inhibitors, aspirin, naproxen, ibuprofen, or indomethacin. Animals were treated for 10 days and killed. A second group (celecoxib, rofecoxib, aspirin) was treated for 10 days and observed for an additional 25 days. After treatment, intra-abdominal adhesions were scored using a standard method. The patch was subjected to immunohistochemistry with the endothelial-specific marker, CD31.
Results:
Animals treated with selective and nonselective COX-2 inhibitors, except aspirin, had significantly fewer adhesions than control animals. Celecoxib produced a maximal reduction in adhesion formation compared with rofecoxib and the nonselective COX-2 inhibitors at 10 days. After 25 days, celecoxib and rofecoxib, but not aspirin, had fewer adhesions than control mice. Adhesions from mice treated with celecoxib had reduced microvessel density compared with rofecoxib, the nonselective COX inhibitors, and control animals.
Conclusions:
Selective COX-2 inhibitors, in particular celecoxib, provide durable inhibition of intra-abdominal adhesions through an antiangiogenic mechanism.
Adhesion formation is a major cause of complications following surgery. Celecoxib, a COX-2 inhibitor with additional antiangiogenic and antifibroblastic properties, provides durable inhibition of intra-abdominal adhesions in a murine model.
Adhesion formation, the joining of 2 normally separate surfaces due to trauma or inflammation, can result in complications following surgical procedures. Although adhesions aid in healing, they frequently cause postoperative pain, blockage of intestines, and infertility.1–3 For example, 16% of patients with bowel obstructions caused by adhesions require adhesiolysis, with a resulting mortality of 5% to 20%.4,5 Adhesions also are the leading cause of secondary infertility, responsible for up to 20% of infertility cases.2,6
After injury to the peritoneal lining, the entire surface becomes re-epithelialized with mesothelial cells within 5 to 6 days. Peritoneal injury may result in local ischemia, with a subsequent inflammatory response consisting of polymorphonuclear leukocytes, macrophages, fibrin, fibroblasts, and new blood vessels. These processes may lead to the development of mature, fibrous adhesions covered by mesothelium.7 Fibroblasts from these adhesions express the COX-2 enzyme while fibroblasts in nonadhesion bearing areas do not express this enzyme.8
Adhesion formation, like all types of wound healing, is dependent on angiogenesis.9–11 An experimental antiangiogenic agent, TNP-470, has been shown to inhibit intra-abdominal adhesions in mice,12 suggesting that antiendothelial agents may be efficacious in blunting adhesion formation. However, side effects of TNP-470, such as neurotoxicity and delayed wound healing, precluded further investigations of this drug.
Because most antiangiogenic agents are not approved for clinical use, we tested an approved class of angiogenesis inhibitors, COX-2 specific drugs,13–16 for their ability to modify adhesion formation. Cyclooxygenase-1 enzyme (COX-1) is expressed on normal blood vessels while the cyclooxygenase-2 enzyme (COX-2) is present on new angiogenic endothelial cells13 as well as fibroblasts associated with surgical adhesions.8 Thus, the COX-2 inhibitors, celecoxib and rofecoxib, may selectively inhibit angiogenesis associated with newly forming adhesions. To determine the effects of COX-2 specific inhibitors, adhesions in control animals, animals treated with nonselective COX inhibitors, and COX-2-treated groups were compared. Furthermore, adhesions were assessed by immunohistochemistry to determine the effects of the different drugs on angiogenesis.
METHODS
Adhesion Model
Seven to 8-week-old male C57BL/6 mice (Jackson Laboratories, Bar Harbor, ME) were housed 5 animals to a cage in a barrier room. Mice were acclimated to their environment for at least 72 hours prior to the initiation of each experiment and allowed food and water ad libitum. Animal protocols complied with the NIH Animal Research Advisory Committee guidelines and were approved by the Children's Hospital Institutional Animal Care and Use Committee. A standard adhesion model was performed as previously described.12 Under isofluorane anesthesia, a 5.0 mm × 5.0 mm piece of sterile silicone (Dow Corning, Midland, MI) was attached to the right abdominal wall with two 7-0 prolene sutures, lateral to the epigastric vessels. The cecum then was gently rubbed with 2 cotton swabs to promote adhesion formation.17
Most murine adhesion models use a foreign material, not to generate adhesions but to improve objectivity. By using a patch, the investigator can measure the percentage of the patch covered, the characteristics of the adhesions to the patch, and subject the patch to immunohistochemistry. Without using a patch, quantification of intra-abdominal adhesions, as well as the assessment of microvessel density and the role of angiogenesis, becomes more subjective.
Drug Treatment
After recovering from anesthesia, groups of mice were randomly treated with either methylcellulose (control, 100 μL orally once a day) or an experimental drug mixed in 100 μL methylcellulose beginning on the day of surgery for 10 days. Groups of 6 to 18 mice treated with nonselective COX inhibitors received ibuprofen (30 mg/kg orally once a day),18 naproxen (10 mg/kg orally once a day),19 aspirin (100 mg/kg orally once a day),20 or indomethacin (1 mg/kg orally once a day).21 Mice treated with COX-2 selective inhibitors received either celecoxib (68 mg/kg orally twice a day) or rofecoxib (40 mg/kg orally once a day).22
Quantification of Intra-abdominal Adhesions
Ten and 35 days after surgery, mice were killed, and adhesion formation over the silicone patch was measured by 2 blinded individuals based on a previously described scoring system.12 The tenacity and extent of adhesions to the patch, as well as the tenacity of adhesions to the cecum, were graded on a scale of 0 to 4. The total adhesion score was the sum of the 3 individual scores. A minimum score was 0 and the maximum score was 12. Tenacity was rated as none (0), adhesions fell apart (1), lysed with traction (2), lysed with blunt dissection (3), and lysed with sharp dissection (4). Extent was measured as the percent of the patch covered by adhesion: 0% (0), <25% (1), 25% to 50% (2), 50% to 75% (3), and >75% (4). Cecal adhesions were graded as none (0), adhesions fell apart (1), lysed with traction (2), lysed with blunt dissection (3), and lysed with sharp dissection (4).
Immunohistochemistry
Silicone patches and adjacent abdominal wall were fixed at 4°C in 10% formalin overnight and washed with phosphate-buffered saline. The specimens were then embedded in paraffin and 5 ìm sections were cut. Endothelial cells were identified by antibody staining against the endothelial cell surface antigen CD31 (PECAM-1) using a diaminobenzidine chromogen (BD Biosciences Pharmingen, San Diego, CA). After deparaffinization and rehydration, sections were incubated with proteinase K (Roche, Basel, Switzerland) for 30 minutes at 37°C. Nonspecific binding sites were blocked for 30 minutes at room temperature with TNB blocking buffer (NEN Life Sciences, Boston, MA).
Primary CD31 antiserum (BD Biosciences Pharmingen) was added to the sections overnight at 4°C. Slides were washed twice with phosphate-buffered saline and secondary goat anti-mouse IgG antibody (Vector Laboratories, Burlingame, CA) was added for 30 minutes at room temperature. After again washing twice with phosphate-buffered saline, slides were incubated with streptavidin alkaline phosphatase solution (1:100) (Perkin-Elmer Life Sciences, Boston, MA) for 30 minutes at room temperature followed by biotinyl tyramide amplification diluent (1:50) (Perkin-Elmer Life Sciences) for 10 minutes. After repeat incubation with alkaline phosphatase solution (1:100) for 30 minutes at room temperature, the chromagen Vector Novar substrate kit (Vector Laboratories) was added. Slides were counterstained with hematoxylin for imaging.
Imaging and Microvessel Density
Sections were taken from the abdominal wall and patch at death on day 10. To quantitate vessel density, CD31 immunostained sections underwent digital image analysis using a Nikon Eclipse TE300 inverting photomicroscope with an attached Spot RT (Diagnostic Instruments Inc., Sterling Heights, MI) video camera linked to a personal computer. Images were imported directly to the image analysis program IP Laboratory (Scanalytics Inc., Fairfax, VA). Sequential images were grabbed at 200× for the CD31 sections without overlapping. The adhesion tissue between the silicone patch and the abdominal wall was assessed to provide consistency and reproducibility between the samples. To calculate the volume fraction of stained regions, a color threshold was chosen for each image that distinguished between the stained areas and background. The proportion of the selected color was calculated as a percentage of total area and used as a measure of vessel density.
Each experimental group of mice was housed in separate cages of up to 10 mice. The primary and senior authors randomly selected the first 3 mice that were able to be removed from each cage for immunohistochemical study. The sections from the 3 randomly chosen mice for each of the study groups were then given to a blinded coinvestigator (D.S.) for quantification of microvessel density. Specifically, this blinded coinvestigator quantified the microvessel density of the first 5 fields that he found containing the area under the patch for each slide. The scores of the 5 fields were then averaged to give a final score for each section.
Statistical Analysis
Adhesion scores were analyzed using the nonparametric Wilcoxon Rank Sum test. A Fisher exact test then was used to assess differences in the percentage of mice in each group that were adhesion-free compared with control. Because several treatment groups were included in this study, a Bonferroni adjustment was used to correct for multiple group comparisons. The conventional 2-tailed P value of 0.05 was divided by 5 to yield a more stringent cutoff value of P < 0.01 for significance. The rationale for using a more conservative P value was to protect against the likelihood of reporting a difference between groups that was really due to chance. These results are presented as median, interquartile range (IQR: 25th–75th percentile), and range (minimum–maximum).
Microvessel density data, with a normal variation, are presented as mean ± standard error of the mean. Since the numbers of mice evaluated were small, statistical analysis of these results was performed with the Student 2-tailed, unpaired t test for comparisons between groups. Differences were considered significant with a P value of <0.05. It was thought that this approach was appropriate given the microvessel density data conformed very closely to a normal distribution, and using a 2-tailed significance level of 0.05 would obviate any important difference that would otherwise go undetected if more stringent Bonferroni rules were applied. The statistical computer program used was SigmaStat (SPSS, Chicago, IL).
RESULTS
Gross Animal Findings
All animals gained weight and appeared healthy. There were no signs of impaired wound healing, bleeding, gastrointestinal complications, or capsule formation to the silicone patch. The control animals developed marked intra-abdominal adhesions involving adhesions from bowel-to-patch, bowel-to-bowel, omentum-to-patch, and omentum-to-bowel. In contrast, the animals treated with nonselective COX inhibitors, except for aspirin, had less intra-abdominal adhesions. Even fewer intra-abdominal adhesions were appreciated in the animals treated with the COX-2 selective inhibitors, rofecoxib and celecoxib.
Adhesion Score
The nonselective COX inhibitors, except for aspirin, decreased intra-abdominal adhesions, compared with control animals (Table 1; Fig. 1). The intra-abdominal adhesion score in control mice was 10.0 (IQR, 9.0–10.0; range, 8.0–10.0). Aspirin treated mice had a score of 10.0 (IQR, 9.0–11.0; range, 9.0–11.0, P = 0.46). Animals treated with naproxen, ibuprofen, and indomethacin had scores of 6.5 (IQR, 2.0–9.0; range, 0.0–10.0, P = 0.007), 3.5 (IQR, 2.0–8.0; range, 0.0–9.0, P < 0.001), and 4.0 (IQR, 2.0–7.8; range, 2.0–10.9, P = 0.004), respectively.
TABLE 1. Adhesion Scores of Control and Treated Animals
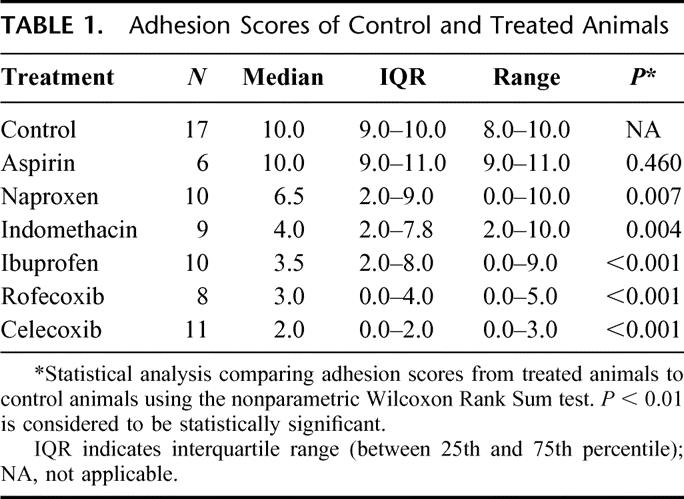
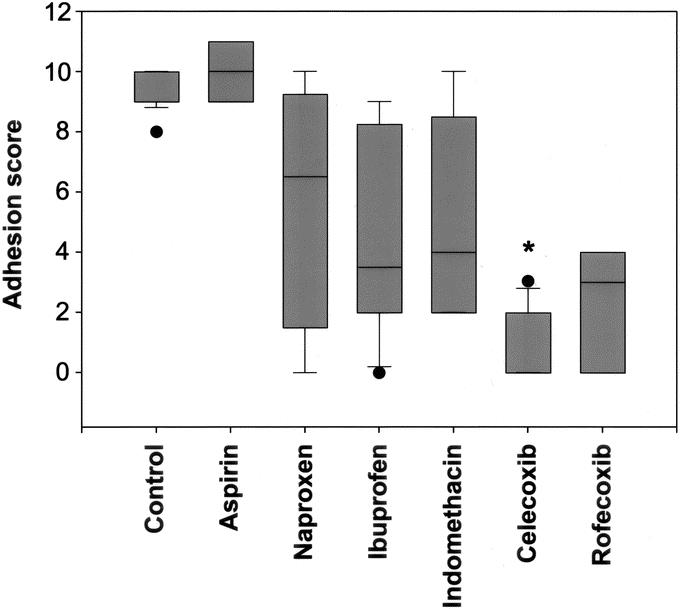
FIGURE 1. Effects of COX inhibitors on intra-abdominal adhesion formation. All drugs, except for aspirin, a nonselective COX-1 and COX-2 inhibitor, significantly decreased intra-abdominal adhesion formation. The most dramatic reduction of intra-abdominal adhesion formation was observed with celecoxib (*), the selective COX-2 inhibitor with antifibroblastic and antiangiogenic properties.
Selective COX-2 inhibitors decreased intra-abdominal adhesion formation compared with control animals as well as to most animals treated with nonselective COX inhibitors (Table 1; Fig. 1). The adhesion score for rofecoxib treated animals was 3.0 (IQR, 0.0–4.0; range, 0.0–5.0, P < 0.001, compared with control and aspirin treated animals). There was no difference between rofecoxib and naproxen, ibuprofen, or indomethacin treated animals. Adhesions in mice that received celecoxib were graded 2.0 (IQR, 0.0–2.0; range, 0.0–3.0). This was lower than control animals (P < 0.001), and most animals treated with nonselective COX inhibitors [aspirin (P = 0.001), ibuprofen (P = 0.005), indomethacin (P=0.004), and naproxen (P = 0.031)].
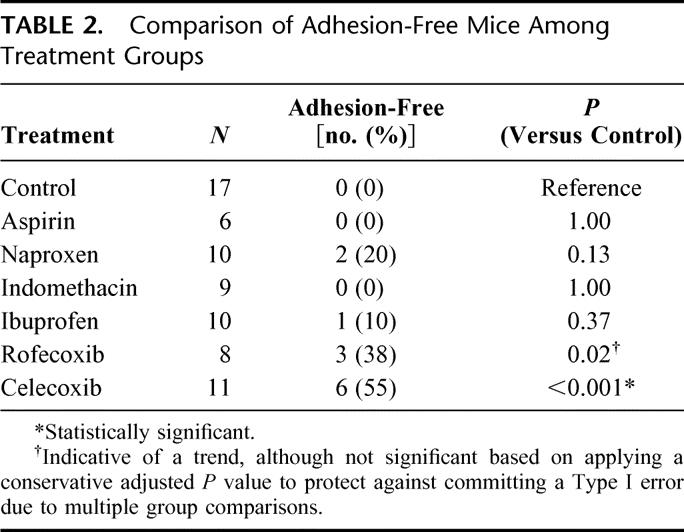
Because 6 of the 11 (55%) celecoxib treated animals did not have any adhesions at all, a reviewer suggested a post hoc analysis to determine differences in adhesion-free mice among treatment groups (Table 2). Celecoxib was able to completely prevent adhesion formation, compared with control (P < 0.001). Rofecoxib showed a trend toward keeping mice adhesion-free (P = 0.02). The nonselective COX inhibitors, aspirin, ibuprofen, indomethacin, and naproxen, in contrast, did not result in adhesion-free mice.
TABLE 2. Comparison of Adhesion-Free Mice Among Treatment Groups
To determine whether the prevention of adhesions was durable, mice treated for 10 days with celecoxib, rofecoxib, and aspirin were observed for an additional 25 days before death. Celecoxib and rofecoxib groups had fewer adhesions [1.0 (IQR, 0.0–2.0; range, 0.0–2.0) and 5.0 (IQR, 3.0–7.0; range, 2.0–8.0)] than control mice [11.0 (IQR, 8.0–11.0; range, 8.0–11.0), P=0.016 and P = 0.032, respectively] and aspirin treated mice [8.0 (IQR, 7.0–11.0; range, 7.0–11.0), P = 0.016 and P = 0.063]. No differences in adhesion scores between aspirin treated and control animals were found (P=0.42).
Immunohistochemistry and Microvessel Density
Microvessel density in celecoxib treated (2.0 ± 0.6) (P=0.014) but not rofecoxib (5.8 ± 0.7) (P = 0.94) treated mice was less than that of control mice (5.7 ± 1.1) (Fig. 2). The microvessel density for mice treated with the selective COX-2 antagonist celecoxib was significantly less than aspirin (6.7 ± 0.8, P < 0.001), naproxen (7.8 ± 0.9, P < 0.001), ibuprofen (7.8 ± 1.0, P < 0.001), and indomethacin (8.1 ± 1.4, P = 0.005). Animals treated with rofecoxib demonstrated no differences in microvessel density compared with mice treated with the nonselective COX-2 inhibitors. Moreover, celecoxib treated animals had lower microvessel density scores than rofecoxib treated groups (P = 0.003). The microvessel density of all nonselective COX inhibitors was not different [aspirin (P = 0.50), naproxen (P = 0.16), ibuprofen (P = 0.32), and indomethacin (P = 0.19)] from that of control animals. Sections from representative mice stained with the endothelium-specific marker CD31 are depicted in Figure 3.
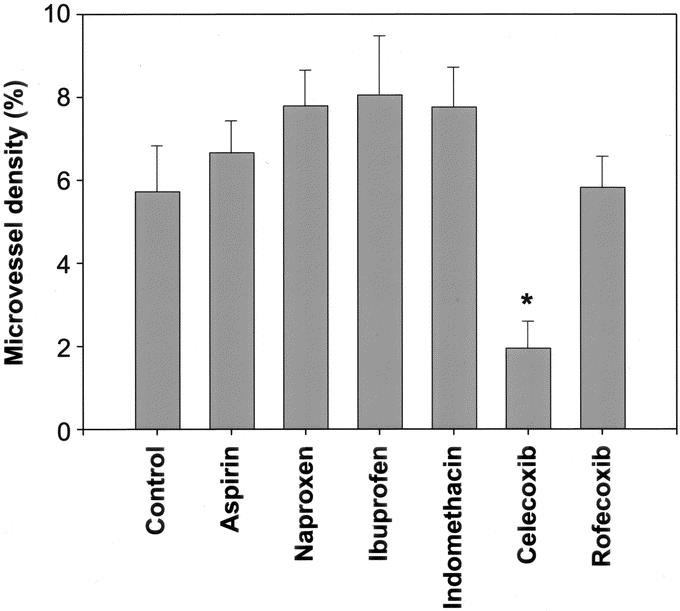
FIGURE 2. Microvessel density of adhesion tissue between the abdominal wall and the silicone patch. Celecoxib-treated animals had significantly lower microvessel density (*) than control animals, aspirin, naproxen, ibuprofen, indomethacin, and rofecoxib.
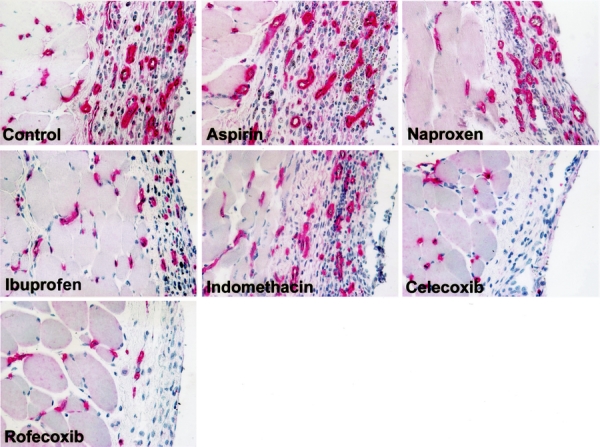
FIGURE 3. CD31-immunostained sections of adhesion tissue between the abdominal wall and the silicone patch from representative mice of each group. The left portion of each image consists of the abdominal wall muscle; the right portion is adhesion tissue between the abdominal wall and the silicone patch. The endothelium is stained red using a mAb specific for mouse CD31 (PECAM-1). Less endothelium is present in celecoxib-treated animals compared with control.
DISCUSSION
Several approaches have been used to inhibit intra-abdominal adhesion formation, most with limited success.23–25 Intra-abdominal crystalloids and dextran have not been shown to be effective,27,28 while corticosteroids and anti-inflammatory agents have given inconsistent results.26 LeGrand et al demonstrated encouraging results with nonsteroidal anti-inflammatory drugs in rodent and rabbit models.29,30 In these and other studies, however, the drugs were administered intraperitoneally as an injectable fluid, thereby reducing the clinical applicability of these agents due to uncertainties about dosing and delivery.31 We attempted to limit these concerns by using a clinically applicable dosing regimen of the tested drugs. Furthermore, we administered these drugs via orogastric gavage on a daily basis for 10 days, further simulating the clinical setting. Using an oral daily dosing schedule, we also were able to demonstrate that most NSAIDs could reduce the formation of intra-abdominal adhesions, albeit not to the extent that was observed with the specific COX-2 inhibitors.
The most commonly used method of preventing postoperative adhesions uses a barrier agent or gel to separate damaged peritoneal surfaces for a minimum of 5 to 7 days. Hyaluronic acid and polyethylene glycol/polylactic acid barrier films reduce adhesion formation experimentally as well as clinically.32–35 However, concerns about immune suppression, infection, intra-abdominal placement,26 and impaired healing of bowel anastomoses remain.26,36
In an effort to reduce the unwanted side effects of common nonsteroidal anti-inflammatory drugs, it was discovered that 2 cyclo-oxygenases are involved in the transformation of arachidonic acid as the first step in the prostaglandin synthesis pathway. These enzymes have been termed cyclooxygenase-1 (COX-1) and cyclooxygenase-2 (COX-2).37,38 Inflammation causes the induction of COX-2, leading to the release of prostanoids and localized pain hypersensitivity,39 while COX-1 is involved with the noninflammatory response of prostaglandins such as platelet aggregation. Recently, compounds that selectively inhibit COX-2 rather than COX-1 activity have been discovered. These selective COX-2 inhibitors are thought to offer advantages that include the capacity to prevent or reduce inflammation while diminishing harmful side effects associated with the inhibition of COX-1, such as gastrointestinal and renal side effects, as well as bleeding from the inhibition of platelet aggregation. Furthermore, COX-2 inhibitors have been implicated in regulating angiogenesis in a variety of in vitro and in vivo models.16,40–42
While most studies into the prevention of adhesion formation have been focused on promoting fibrinolysis and inhibiting re-epithelialization of peritoneal surfaces, several investigators have implicated mediators of angiogenesis in the formation of adhesions. Vascular endothelial growth factor, fibroblast growth factor, and transforming growth factor beta are up-regulated during adhesion formation.9–11 Because newly forming adhesions produce angiogenic factors that recruit new endothelium, COX-2 inhibitors are likely to target the expressed COX-2 enzyme on the newly proliferating vasculature. The significant decrease in microvessel density in the celecoxib treated animals support the additional mechanism of inhibition of endothelial-mediated adhesion formation. Furthermore, these results demonstrate that celecoxib led to a more complete reduction of adhesions than rofecoxib. In addition to its antiangiogenic effect, celecoxib, unlike rofecoxib, possesses additional antifibroblastic activity.43 This extra property could contribute to the greater efficiency of celecoxib in reducing adhesion formation than rofecoxib.
Previously, Guvenal et al reported the reduction of adhesions in a rat uterine horn model of adhesions with the selective COX-2 inhibitor nimesulide.44 However, the proposed mechanism of action in this model was the anti-inflammatory effect and not the potential antiangiogenic or antifibroblastic properties of nimesulide. Similarly, haloguginone, a collagen type I and MMP2 inhibitor, has been shown to reduce postoperative adhesions presumably through its antifibrotic activity.45 However, haloguginone has recently been shown to be an angiogenesis inhibitor.46 Consequently, like nimesulide, the mechanism of action of haloguginone may include both antifibrotic and antiangiogenic activity.
One of the major shortcomings of our experiments is that they were performed in small animals. We acknowledge that there are major pharmacodynamic differences between animals and humans.47 Nonetheless, clinical trials need to be performed to confirm the beneficial properties of celecoxib in reducing intra-abdominal adhesions. Celecoxib and rofecoxib offer several advantages over other angiogenesis inhibitors for the treatment of adhesions. Unlike the majority of angiogenesis inhibitors, these COX-2 inhibitors are approved drugs. Celecoxib and rofecoxib are known to be safe, even when taken in high doses chronically, as illustrated by patients taking these drugs for arthritis. Thus, patients with a high risk of intra-abdominal adhesion formation may be given COX-2 inhibitors in the immediate postoperative period for a relatively short period of time. COX-2 drugs are presently the only antiangiogenic agents that also are pharmacologically effective against pain. In conclusion, COX-2 inhibitors, in particular celecoxib, can provide durable prevention of surgical adhesion formation in a murine model.
Footnotes
A.K.G. was supported by a Howard Hughes Postdoctoral Fellowship award. I.P.J.A. was supported by the Dutch Cancer Society.
Reprints: Mark Puder, MD, PhD, Department of Surgery, Children's Hospital Boston, 300 Longwood Ave, Boston, MA 02115. E-mail: mark.puder@childrens.harvard.edu.
REFERENCES
- 1.Ellis H. The clinical significance of adhesions: focus on intestinal obstruction. Eur J Surg Suppl. 1997;577:5–9. [PubMed] [Google Scholar]
- 2.Ellis H, Moran BJ, Thompson JN, et al. Adhesion-related hospital readmissions after abdominal and pelvic surgery: a retrospective cohort study. Lancet. 1999;353:1476–1480. [DOI] [PubMed] [Google Scholar]
- 3.Kossi J. Population-based study of the surgical workload and economic impact of bowel obstruction caused by postoperative adhesions. Br J Surg. 2003;90:1441–1444. [DOI] [PubMed] [Google Scholar]
- 4.Menzies D, Ellis H. Intestinal obstruction from adhesions: how big is the problem? Ann R Coll Surg Engl. 1990;72:60–63. [PMC free article] [PubMed] [Google Scholar]
- 5.Menzies D, Parker M, Hoare R, et al. Small bowel obstruction due to postoperative adhesions: treatment patterns and associated costs in 110 hospital admissions. Ann R Coll Surg Engl. 2001;83:40–46. [PMC free article] [PubMed] [Google Scholar]
- 6.Ray NF, Denton WG, Thamer M, et al. Abdominal adhesiolysis: inpatient care and expenditures in the United States in 1994. J Am Coll Surg. 1998;186:1–9. [DOI] [PubMed] [Google Scholar]
- 7.DiZerega GS, Campeau JD. Peritoneal repair and post-surgical adhesion formation. Hum Reprod Update. 2001;7:547–555. [DOI] [PubMed] [Google Scholar]
- 8.Saed GM, Munkarah AR, Diamond MP. Cyclooxygenase-2 is expressed in human fibroblasts isolated from intraperitoneal adhesions but not from normal peritoneal tissues. Fertil Steril. 2003;79:1404–1408. [DOI] [PubMed] [Google Scholar]
- 9.Wiczyk HP, Grow DR, Adams LA, et al. Pelvic adhesions contain sex steroid receptors and produce angiogenesis growth factors. Fertil Steril. 1998;69:511–516. [DOI] [PubMed] [Google Scholar]
- 10.Chegini N. The role of growth factors in peritoneal healing: transforming growth factor beta (TGF-beta). Eur J Surg Suppl. 1997;577:17–23. [PubMed] [Google Scholar]
- 11.Rout UK, Oommen K, Diamond MP. Altered expressions of VEGF mRNA splice variants during progression of uterine-peritoneal adhesions in the rat. Am J Reprod Immunol. 2000;43:299–304. [DOI] [PubMed] [Google Scholar]
- 12.Chiang SC, Cheng CH, Moulton KS, et al. TNP-470 inhibits intraabdominal adhesion formation. J Pediatr Surg. 2000;35:189–196. [DOI] [PubMed] [Google Scholar]
- 13.Masferrer JL, Koki A, Seibert K. COX-2 inhibitors. A new class of antiangiogenic agents. Ann NY Acad Sci. 1999;889:84–86. [DOI] [PubMed] [Google Scholar]
- 14.Masferrer JL, Leahy KM, Koki AT, et al. Antiangiogenic and antitumor activities of cyclooxygenase-2 inhibitors. Cancer Res. 2000;60:1306–1311. [PubMed] [Google Scholar]
- 15.Dicker AP, Williams TL, Grant DS. Targeting angiogenic processes by combination rofecoxib and ionizing radiation. Am J Clin Oncol. 2001;24:438–442. [DOI] [PubMed] [Google Scholar]
- 16.Leahy KM, Ornberg RL, Wang Y, et al. Cyclooxygenase-2 inhibition by celecoxib reduces proliferation and induces apoptosis in angiogenic endothelial cells in vivo. Cancer Res. 2002;62:625–631. [PubMed] [Google Scholar]
- 17.DeVirgilio C. Fibrin glue reduces the severity of intra-abdominal adhesions in a rat model. Am J Surgeons. 1999;178:577–580. [DOI] [PubMed] [Google Scholar]
- 18.Farag MM, Salama MA, Abou-Basha L. Experimental murine schistosomiasis: reduced hepatic morbidity after pre- and/or post-infection treatment with ibuprofen or diclofenac sodium. Ann Trop Med Parasitol. 1995;89:497–504. [DOI] [PubMed] [Google Scholar]
- 19.Cicala C, Ianaro A, Fiorucci S, et al. NO-naproxen modulates inflammation, nociception and downregulates T cell response in rat Freund's adjuvant arthritis. Br J Pharmacol. 2000;130:1399–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jablonski P, Howden BO. Oral buprenorphine and aspirin analgesia in rats undergoing liver transplantation. Lab Anim. 2002;36:134–143. [DOI] [PubMed] [Google Scholar]
- 21.Yamamoto T, Sakashita Y, Nozaki-Taguchi N. Anti-allodynic effects of oral COX-2 selective inhibitor on postoperative pain in the rat. Can J Anaesth. 2000;47:354–360. [DOI] [PubMed] [Google Scholar]
- 22.Laudanno OM, Cesolari JA, Esnarriaga J, et al. Gastrointestinal damage induced by celecoxib and rofecoxib in rats. Dig Dis Sci. 2001;46:779–784. [DOI] [PubMed] [Google Scholar]
- 23.Montz FJ, Holschneider CH, Bozuk M, et al. Interleukin 10: ability to minimize postoperative intraperitoneal adhesion formation in a murine model. Fertil Steril. 1994;61:1136–1140. [DOI] [PubMed] [Google Scholar]
- 24.DiZerega G. Use of Instilates to prevent intraperitoneal adhesions; crystalloid and dextran. Infert Reprod Med Clin. 1994;5:463. [Google Scholar]
- 25.Rodgers KE, Johns DB, Girgis W, et al. Reduction of adhesion formation with hyaluronic acid after peritoneal surgery in rabbits. Fertil Steril. 1997;67:553–558. [DOI] [PubMed] [Google Scholar]
- 26.Panay N, Lower AM. New directions in the prevention of adhesion in laparoscopic surgery. Curr Opin Obstet Gynecol. 1999;11:379–385. [DOI] [PubMed] [Google Scholar]
- 27.Soules MR, Dennis L, Bosarge A, et al. The prevention of postoperative pelvic adhesions: an animal study comparing barrier methods with dextran 70. Am J Obstet Gynecol. 1982;143:829–834. [DOI] [PubMed] [Google Scholar]
- 28.Wiseman DM, Trout JR, Diamond MP. The rates of adhesion development and the effects of crystalloid solutions on adhesion development in pelvic surgery. Fertil Steril. 1998;70:702–711. [DOI] [PubMed] [Google Scholar]
- 29.LeGrand EK, Rodgers KE, Girgis W, et al. Efficacy of tolmetin sodium for adhesion prevention in rabbit and rat models. J Surg Res. 1994;56:67–71. [DOI] [PubMed] [Google Scholar]
- 30.LeGrand EK, Rodgers KE, Girgis W, et al. Comparative efficacy of nonsteroidal anti-inflammatory drugs and anti-thromboxane agents in a rabbit adhesion-prevention model. J Invest Surg. 1995;8:187–194. [DOI] [PubMed] [Google Scholar]
- 31.Wiseman DM, Huang WJ, Johns DB, et al. Time-dependent effect of tolmetin sodium in a rabbit uterine adhesion model. J Invest Surg. 1994;7:527–532. [DOI] [PubMed] [Google Scholar]
- 32.Haney AF, Doty E. A barrier composed of chemically cross-linked hyaluronic acid (Incert) reduces postoperative adhesion formation. Fertil Steril. 1998;70:145–151. [DOI] [PubMed] [Google Scholar]
- 33.Wallwiener D, Meyer A, Bastert G. Adhesion formation of the parietal and visceral peritoneum: an explanation for the controversy on the use of autologous and alloplastic barriers? Fertil Steril. 1998;69:132–137. [DOI] [PubMed] [Google Scholar]
- 34.Rodgers K, Cohn D, Hotovely A, et al. Evaluation of polyethylene glycol/polylactic acid films in the prevention of adhesions in the rabbit adhesion formation and reformation sidewall models. Fertil Steril. 1998;69:403–408. [DOI] [PubMed] [Google Scholar]
- 35.Diamond MP. Reduction of de novo postsurgical adhesions by intraoperative precoating with Sepracoat (HAL-C) solution: a prospective, randomized, blinded, placebo-controlled multicenter study. The Sepracoat Adhesion Study Group. Fertil Steril. 1998;69:1067–1074. [DOI] [PubMed] [Google Scholar]
- 36.Bowers D, Raybon RB, Wheeless CR Jr. Hyaluronic acid-carboxymethylcellulose film and perianastomotic adhesions in previously irradiated rats. Am J Obstet Gynecol. 1999;181:1335–1337; discussion 1137–1138. [DOI] [PubMed]
- 37.Needleman P, Isakson PC. The discovery and function of COX-2. J Rheumatol. 1997;24(suppl):6–8. [PubMed] [Google Scholar]
- 38.Fu JY, Masferrer JL, Seibert K, et al. The induction and suppression of prostaglandin H2 synthase (cyclooxygenase) in human monocytes. J Biol Chem. 1990;265:16737–16740. [PubMed] [Google Scholar]
- 39.Samad TA, Moore KA, Sapirstein A, et al. Interleukin-1beta-mediated induction of Cox-2 in the CNS contributes to inflammatory pain hypersensitivity. Nature. 2001;410:471–475. [DOI] [PubMed] [Google Scholar]
- 40.Wilkinson-Berka JL, Alousis NS, Kelly DJ, et al. COX-2 inhibition and retinal angiogenesis in a mouse model of retinopathy of prematurity. Invest Ophthalmol Vis Sci. 2003;44:974–979. [DOI] [PubMed] [Google Scholar]
- 41.Tsujii M, Kawano S, Tsuji S, et al. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell. 1998;93:705–716. [DOI] [PubMed] [Google Scholar]
- 42.Ghosh AK, Hirasawa N, Niki H, et al. Cyclooxygenase-2-mediated angiogenesis in carrageenin-induced granulation tissue in rats. J Pharmacol Exp Ther. 2000;295:802–809. [PubMed] [Google Scholar]
- 43.Kusunoki N, Yamazaki R, Kawai S. Induction of apoptosis in rheumatoid synovial fibroblasts by celecoxib, but not by other selective cyclooxygenase 2 inhibitors. Arthritis Rheum. 2002;46:3159–3167. [DOI] [PubMed] [Google Scholar]
- 44.Guvenal T, Cetin A, Ozdemir H, et al. Prevention of postoperative adhesion formation in rat uterine horn model by nimesulide: a selective COX-2 inhibitor. Hum Reprod. 2001;16:1732–1735. [DOI] [PubMed] [Google Scholar]
- 45.Nagler A, Genina O, Lavelin I, et al. Haloguginone, an inhibitor of collagen type I synthesis, prevents postoperative adhesion formation in the rat uterine horn model. Am J Obstet Gynecol. 1999;180:558–563. [DOI] [PubMed] [Google Scholar]
- 46.Elkin M, Miao HQ, Nagler A, et al. Halofuginone: a potent inhibitor of critical steps in angiogenesis progression. FASEB J. 2000;15:2477–2485. [DOI] [PubMed] [Google Scholar]
- 47.Wiseman D. Animal adhesion models: design, variables and relevance. In: diZerega et al, eds. Peritoneal Surgery. New York: Springer-Verlag, 2000:459–478. [Google Scholar]


