Abstract
Objective:
The purpose of this study was to analyze the factors that influence local recurrence after radiofrequency coagulation of liver tumors.
Summary Background Data:
Local recurrence rate varies widely between 2% and 60%. Apart from tumor size as an important risk factor for local recurrence, little is known about the impact of other factors.
Methods:
An exhaustive literature search was carried out for the period from January 1, 1990 to January 1, 2004. Only series with a minimal follow-up of 6 months and/or mean follow-up of 12 months were included. Univariate and multivariate meta-analyses were carried out.
Results:
Ninety-five independent series were included, allowing the analysis of the local recurrence rate of 5224 treated liver tumors. In a univariate analysis, tumor-dependent factors with significantly less local recurrences were: smaller size, neuroendocrine metastases, nonsubcapsular location, and location away from large vessels. Physician-dependent favorable factors were: surgical (open or laparoscopic) approach, vascular occlusion, general anesthesia, a 1-cm intentional margin, and a greater physician experience. In a multivariate analysis, significantly less local recurrences were observed for small size (P < 0.001) and a surgical (versus percutaneous) approach (P < 0.001).
Conclusions:
Radiofrequency coagulation by laparoscopy or laparotomy results in superior local control, independent of tumor size. The percutaneous route should mainly be reserved for patients who cannot tolerate a laparoscopy or laparotomy. The short-term benefits of less invasiveness for the percutaneous route do not outweigh the longer-term higher risk of local recurrence.
A meta-analysis of 5224 hepatic tumors treated by radiofrequency coagulation was carried out. In a multivariate analysis, significantly less local recurrences were observed for small size (P < 0.001) and a surgical approach (P < 0.001). The percutaneous route should mainly be reserved for patients who cannot tolerate a laparoscopy or laparotomy.
Local recurrence rate after radiofrequency coagulation (RFC) of liver tumors varies widely between 2%1,2 and 60%.3 While nearly all authors agree that tumor size is an important risk factor for local recurrence, little is known about the impact of other factors, such as tumor pathology, tumor location, or approach. Two reasons account for this uncertainty. First, the number of tumors per series is limited, precluding a meaningful multivariate analysis. Second, length of follow-up is often not sufficient to allow local recurrences to surface. The purpose of this study was to identify and analyze the factors that may influence local recurrence in an exhaustive meta-analysis.
MATERIALS AND METHODS
Data Accrual
We carried out an exhaustive PubMed search of the world literature for the period from January 1, 1990 to January 1, 2004 using keywords (radiofrequency, radio-frequency or radio frequency) and (liver or hepatic or hepatocellular) in English, French, German, Italian, Spanish, Danish, and Dutch. All abstract supplements from the same period published in Radiology, American Journal of Radiology, Journal of Vascular and Interventional Radiology, European Radiology, and Surgical Endoscopy were searched manually. Relevant papers were also identified from the reference lists of the papers previously obtained through the search and from abstracts from recent international meetings. In case of overlap between 2 reports, only the most detailed report was included. Only series with a minimal follow-up of 6 months and/or or mean follow-up of 12 months were included. Reports about treatments obtained with noncommercial electrodes and treatments with palliative intent (intentional partial debulking) were excluded. When appropriate, authors were contacted to obtain more details about the cases they reported.
Definitions
Local recurrence was defined as radiologic (CT, MRI or contrast-enhanced ultrasound) and/or histologic (tumor cells with intact mitochondrial enzyme staining) detection of residual or recurrent viable tumor at the site of the original tumor, during follow-up and after completion of all (one or more) sessions. Our definition of local recurrence also includes tumors for which no complete coagulation could be obtained despite one or more RFC sessions.
Size
Tumors were classified as small (≤3 cm), medium (3–5 cm), and large (>5 cm) according to a recent international proposal.4
Subcapsular tumor was defined as a tumor 1 cm or less under the liver capsule.
Proximity of large vessel was defined as the situation where the tumor invades, abuts, or is situated within 5 mm of a vessel of at least 3 mm in diameter.1,5
Partial vascular occlusion was defined as a temporary or permanent occlusion of the hepatic artery or its branches by either an intravascular balloon during the procedure or a (chemo) embolization immediately or a few days before the procedure.
Full vascular occlusion was defined as the performance of a Pringle maneuver (clamping of both the hepatic artery and the portal vein) during the procedure.
Intentional margin was defined as the minimal margin of coagulation at each side of the tumor that was aimed for in each report.
Physician's experience was defined as the total number of tumors treated by RFC, included in the meta-analysis, performed by the same author. For the analysis of this variable, multicenter studies were excluded.
Statistical Analysis
In univariate analysis, recurrence rates were compared between groups by a χ2 or Fisher exact test when groups were nominal categories and by a Cochrane test when they were ordinal categories. A multivariate analysis was performed by logistic regression with Wald test for assessing the significance of any variable. All statistical tests are 2-tailed. Analysis was performed using SPSS statistical software (SPSS Inc., Chicago, IL).
RESULTS
Ninety-five independent series were included, allowing the analysis of the local recurrence rate of 5224 treated liver tumors.2,5–98
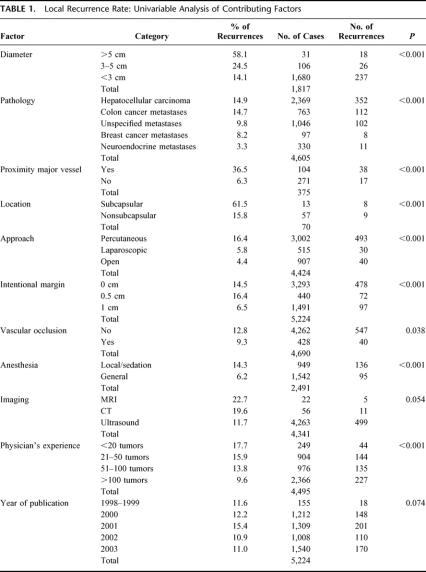
Tumors were coagulated percutaneously (67.9%), laparoscopically (11.6%), or by laparotomy (20.5%). Total local recurrence rate was 12.4% (647 of 5224). In a univariate analysis (Table 1), tumor-dependent factors with significantly less local recurrences were: small size, neuroendocrine metastases, nonsubcapsular location, and location away from large vessels. Physician-dependent favorable factors were: surgical (open or laparoscopic) approach, vascular occlusion, general anesthesia, a 1-cm intentional margin, and a greater physician experience.
TABLE 1. Local Recurrence Rate: Univariable Analysis of Contributing Factors
In a multivariate analysis, significantly less local recurrences were observed for small size (P < 0.001) and a surgical (versus percutaneous) approach (P < 0.001).
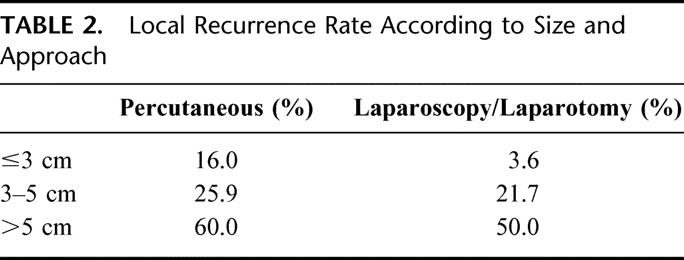
Local recurrence rates were lower after a surgical approach for each size category, even for small tumors (Table 2).
TABLE 2. Local Recurrence Rate According to Size and Approach
DISCUSSION
Meta-Analysis
Local recurrence rate after RFC of liver tumors varies widely between 2%1,2 and 60%.3 While nearly all authors agree that tumor size is an important risk factor for local recurrence, little is known about the impact of other factors, such as tumor pathology, tumor location, or approach. The few series that have looked at factors influencing local recurrences have produced conflicting results, probably because of a limited number of tumors in most of them, precluding a meaningful multivariate analysis.5,52,58,63,99–103
Awaiting multivariate analyses in large prospective trials, this meta-analysis comes as close as one can currently get to study the factors influencing local recurrence after RFC. As every meta-analysis, it has its strengths and weaknesses. As for its strengths, several measures have been taken to obtain the most correct reflection of daily RFC practice in the world (as opposed to the practice in a handful selected high-volume centers of excellence). Reports with a limited number of cases, including small centers and centers that have only recently started with this technique, were included. To have broader coverage, reports in 7 languages were included. To counter a publication bias (ie, that bad results are less likely to be published), not only peer-reviewed articles but also abstracts from international meetings were included.
As for its weaknesses, the individual series in this meta-analysis may not be entirely comparable. Definitions may differ slightly between reports. Some authors have collected their data retrospectively rather than prospectively. Follow-up duration varies, even if a minimum follow-up of 6 months was required. Electrode and generator technology have evolved rapidly and may be different between early and recent series. We found however no significant impact of the year of publication on local recurrence rate (Table 1).
A second weakness is that not all series reported the data on all of the analyzable factors or did not report them on an individual tumor basis. The lacking of some of the individual data did not hamper the univariate analysis as much as the multivariate analysis, in which all the data had to be present simultaneously. Only 2 of the 9 factors that had a statistically significant impact on local recurrence in the univariate analysis remained significant in the multivariate analysis. For the reason mentioned, this should not be interpreted as proof of absence of an independent impact of the 7 other factors. In other words, because of the lack of some data on an individual tumor basis, the multivariate analysis probably underestimates the number of factors with an independent influence on local recurrence.
Duration of Follow-up
In the present study, a minimum follow-up of 6 months for each tumor and/or a mean follow-up of at least 12 months for the whole series was required for inclusion. When applying a more strict inclusion criterion (minimum follow-up of 6 months for each tumor), only 3010 tumors were available for analysis, but the statistical results were the same. Extending the minimum follow-up period to 12 months or more did not allow inclusion of a sufficient number of tumors for a meaningful analysis.
A minimal follow-up of 6 months very probably underestimates the real local recurrence rate. Radiologic local recurrences have been reported after 12 months or longer,2,7,10,31,35,52,57,70,77 18 months or longer,2,31,52,77 and even up to 23 months.77 In one series, local recurrence rate at 12, 24, and 36 months was 3, 5, and 7 times, respectively, higher than after 6 months.101
Another indication that a 6-month follow-up period most likely underestimates the true local recurrence rate comes from reports of histologic examination after resection of tumors that had been treated by RFC. Eleven studies reported cases of microscopic residual viable tumor despite a negative CT.9,31,71,104–106,175 Only with time will these tumor nests grow sufficiently large to appear on imaging. For these reasons, future authors are encouraged to restrict reporting results after RFC to patients with a minimal follow-up of 12 months.
Importance of Local Recurrence
A local recurrence seriously jeopardizes the chances of cure, as re-treatment is often impossible or has a high risk of failure. In an exhaustive review of 153 reports on RFC up until January 1, 2004, we found only 18 authors (12%) who reported an attempt at local re-treatment.18,25,32,61,63,64,70,91,100,107–109,175 Of these reports, 13 were sufficiently detailed for analysis.18,25,32,61,63,64,70,107,108,175 In total, of 64 recurrent tumors, only 35 (55%) were re-treated and a complete coagulation was obtained in only 23 cases (36%). One report described 64 patients with local recurrences, of which only 34 received re-treatment.109 This report did not mention the success rate of re-treatment. Reasons for not considering re-treatment were unfavorable geometry108 and diffuse metastases.18,107
The poor local results of patients with established recurrent tumor during follow-up after completion of one or more sessions of RFC should not be confused with the better results of repeat RFC in cases where incomplete tumor coagulation is detected on immediate follow-up imaging, a common practice after a percutaneous approach. For example, in a series of 364 hepatocellular carcinomas (HCC) smaller than 3.5 cm, 1 percutaneous session achieved a complete coagulation at immediate post-RFC CT in 77% of tumors. Immediately after the second session in the incompletely treated cases, 99.7% of tumors appeared completely coagulated on CT.110
Importance of Number of Tumors
In this meta-analysis, we defined the local recurrence rate on a tumor base, for reasons of technical comparability. What counts for a patient, however, is the patient-based local recurrence rate. The more tumors a patient has, the more he is at risk for having at least one local recurrence. The deleterious effect of every factor that increases local recurrence rate on a single tumor level is being amplified in patients with multiple tumors.
Tumor-Dependent Factors
Size
Nearly all authors agree that size is an important factor determining local recurrence rate,3,12,22,24,31,37,49,52,54,58,63,70,80,83,94,99–102,105,108,109,111–114 with rare exceptions.5,103
Several factors may contribute to the higher local recurrence rate for larger tumors. First, the fact that the size of individual RFC lesions is limited. A single coagulation may be sufficient to cover a small tumor and its 1-cm safety margin at both sides, but not to cover a large tumor. Unfortunately, when more than one treatment session is needed to obtain a complete coagulation, a higher risk of local recurrence has been described.7
For large tumors, in mathematical models, a large number of precisely calculated overlapping coagulations is necessary.115 For example, to cover a 3-cm tumor and its safety margin with an electrode that produces a perfectly spherical coagulation of 3 cm, 14 overlapping coagulations are required.115 Many authors, however, restrict the number of overlapping coagulations to 2 or 3. The technique of overlap is not easy: using ultrasound, it is difficult to visualize the tumor after the first coagulation session due to the appearance of a hyperechogenic microbubble cloud.103 Performing the overlaps in a mathematically regular fashion is difficult, especially percutaneously. As a result, nests of viable tumor cells will remain in the clefts between the incompletely fused coagulation zones. As an alternative to overlapping coagulations, new electrodes that claim to produce larger coagulation zones in a single session have been introduced recently.116 Regrettably, no scientific data on size and geometry obtained by these electrodes116 were available by January 1, 2004.
A second factor is that larger tumors more frequently have irregular borders than small tumors.113 They also more frequently present satellite lesions that are at a greater distance from the main tumor. This is true for colorectal metastases117,118 as well as for HCC.119,120 These satellite lesions are often invisible on pre-RFC imaging.120 If the coagulation is restricted to the main tumor without safety margin, spiky irregular extensions and satellites will be left untreated.
Pathology
The impact of pathology on local recurrence rate is unclear in the literature.3,5,83,99,102,103 The univariate analysis of all cases shows that local control is best for neuroendocrine metastases, followed by breast cancer metastases. Local recurrence rates for HCC and colorectal metastases were similar.
Differences in local recurrence rate between various tumor types may be due to differences in the mean natural growth rate. In this hypothesis, local recurrences of slow-growing tumors (such as neuroendocrine metastases) will appear later than recurrences of fast-growing tumors. For medium (3–5 cm) and large (>5 cm) HCC, an infiltrating growth pattern (irregular margins, peripheral portal invasion, extranodal growth) is associated with a clearly higher risk of local recurrence than a noninfiltrating growth pattern (smooth, well-circumscribed margins or surrounded by a capsule).94,113 For small HCC (≤3 cm), however, presence or absence of a capsule did not influence risk of local recurrence.94,101
Proximity of Large Vessels
The literature is not clear about the influence of the proximity of large vessels on the risk of local recurrence. Residual or recurrent tumor near large vessels was reported by 20 authors30,32,35,47,49,70,80,108,111,112,114,121–123,175 and 3 comparative studies found an increased risk,5,12,63 but 2 other studies did not.52,101 This meta-analysis, however, clearly confirms the negative impact of the proximity of large vessels on the risk of local recurrence.
In animal experiments with perfused liver, a rim of viable tissue around the vessel is observed in 100% of vessels >5 mm; in 29% of vessels 3 to 5 mm, and in 3% of vessels <3 mm.124 After percutaneous RFC or laser therapy, residual tumor was observed in 100% of tumors adjacent to the vena cava, in 57% of tumors adjacent to the portal vein, and in 33% of tumors adjacent to the hepatic veins.121
Two strategies that may counter the perivascular heat-sink4 effect include the Pringle maneuver and manipulation of the electrode.
Pringle Maneuver.
Compared with RFC in perfused liver, the very common distortion near blood vessels disappears almost completely with a Pringle maneuver.116,125–127 Two biophysical phenomena govern the shape of an RFC lesion near a blood vessel. The first is the well-known heat-sink effect, in which the cooler blood carries away part of the generated heat and causes a type 1 distortion of the RFC lesion.116 The second is the much less known attraction of the RFC current to the vessel because of the higher electrical conductivity of blood.128 With preserved blood flow, the dominating heat-sink effect annihilates the effect of the second phenomenon. With interrupted blood flow only, the second phenomenon will play a role, and it will cause a preferential perivascular heating, which is exactly what is needed.
A Pringle maneuver has to be used with caution, as it may cause hepatic vessel thrombosis. In a recent review of complications of RFC, the clinical risk of portal vein thrombosis was 0.2% in 3227 patients with normal blood flow versus 4.2% in 96 patients with a Pringle maneuver throughout the whole RFC procedure.129 Interestingly, in a series of 123 patients treated with a short (2–3 minutes) Pringle maneuver, no patient developed a portal thrombosis despite the fact that 71.6% of the tumors were within 5 mm of large vessels1; and the local recurrence rate after a median follow-up of 15 months was only 1.8%. The risk of hepatic vein thrombosis seems to be much lower (2 in 3670 patients) than for portal vein thrombosis.129
Manipulation of the Electrode.
Several tips and tricks have been described to counter the heat-sink effect of perivascular tumors. The first tip is to apply the current first to the deepest (ie, most central) part of the tumor, which contains the afferent vessels, to enhance coagulation of the devascularized remaining part of the tumor. Preferably, using color Doppler, the electrode tip is positioned precisely near the feeding vessel.130,131 A second tip was recommended for the 4-prong model 30 RITA electrode, which is now less frequently used. When target temperatures were not reached at the 4 prongs due to the presence of a large vessel, the prongs were retracted after the first coagulation, the electrode was twisted 45°, and the prongs were redeployed to perform a second coagulation.114,123,131 A third tip is to deploy the prongs only halfway, to concentrate current and heating in a smaller area, and to overcome tissue cooling by the blood flow.58,132 This way, complete coagulation of 3 perivascular tumors has been reported.132 A drawback of all 3 tips and tricks is that experimental evidence of their efficacy is lacking, and proof of their clinical efficacy is only anecdotal.
Subcapsular Location
A subcapsular location was found to significantly increase local recurrence rate in 2 studies,52,101 which was confirmed in our meta-analysis. A third study, published after the deadline of inclusion in the meta-analysis, found no difference in local recurrence rate between subcapsular and nonsubcapsular tumors.133 All subcapsular tumors of the meta-analysis, including those of the first 2 studies,52,101 had been approached percutaneously under local anesthesia with or without sedation, whereas 75% of subcapsular tumors in the third study133 had been approached surgically under general anesthesia. Therefore, 2 factors may account for the different outcome between these studies. First, it is possible that in the percutaneous approach, subcapsular tumors have been undertreated for fear of burning adjacent organs, diaphragm, or the abdominal wall.52,101,112 Second, a percutaneous treatment of subcapsular tumors under local anesthesia with or without sedation can be painful,133 which may have prevented a correct complete coagulation.112 In conclusion, a subcapsular location is probably not a risk factor for local recurrence per se, but only if RFC is performed percutaneously. For this reason, as well as for the increased risk of bleeding and seeding when treated percutaneously,129 a laparoscopic or open approach is favored for subcapsular tumors.52,101,129
Physician-Dependent Factors
Approach
RFC was pioneered by interventional radiologists. They reported the first experiments of RFC on ex vivo liver134 and in vivo animal livers135 in 1990 and the first clinical results in 1992.136 Surgeons entered the field only in 1996.137 When RFC was first introduced clinically, it was entirely experimental and considered as a palliative treatment. In that context, the percutaneous route was justified as it was the least invasive and a less costly approach. Even today, the majority of RFC procedures are still performed percutaneously. In this meta-analysis, tumors were coagulated percutaneously in 67.9%, by laparotomy in 20.5%, and laparoscopically in 11.6%.
Given the absence of randomized trials, there is no consensus among experts about which approach is best.138 Some authors that used more than one approach found better local control after a surgical approach,3,5,11,102,103,122,139 while other reports found no statistically significant difference.1,12,22,54,63,111,114,140 One author99 found worse results after the percutaneous approach in the univariate analysis of his results, but not in the multivariate analysis.
In the present meta-analysis, a surgical approach (laparotomy or laparoscopy) clearly yielded statistically significantly (P < 0.001) superior results than a percutaneous approach, independent of the size of the tumors (Table 2).
Several factors may contribute to better results after a surgical approach. Intraoperative ultrasound greatly improves spatial resolution. The probe is placed directly on the liver surface, without sound attenuation by skin and subcutaneous tissue. Further, the acoustic window is much wider compared with external ultrasound, which is hampered by the interposition of ribs and bowel.141 Many studies have demonstrated a ±30% increase in tumor detection rate by intraoperative ultrasound during laparoscopy18,80,114,175 or laparotomy62,114,175 compared with preoperative imaging. Several authors have reported improved visibility of the tumor itself, although no firm literature data are available, probably because this is much more difficult to quantify. These authors claim better tumor visualization compared with external ultrasound, especially of tumors located in the superior right lobe of the liver.63,71,106,142,175 They also report better identification of tumor margins and small satellite nodules.3,7,80,111,143 Improved visibility will lead to a more correct insertion of the electrodes and an increased chance of complete covering of the tumor, including its irregular margins, satellites, and a 1-cm safety margin.
RFC by a surgical approach allows an easy access to tumors located in the superior right lobe of the liver, which are often hard to reach percutaneously.63,71,106,142,175 The surgical, especially the open approach, provides a larger degree of freedom for inserting the electrodes under an optimal angle, with mobilization of the liver if necessary.142,144 In the percutaneous approach, the electrodes have to be inserted through a narrow access window, between ribs or subcostally.3 In the laparoscopic approach, because of the pneumoperitoneum and the upward movement of the diaphragm, liver movement is minimal, facilitating precise electrode placement.145 The surgical route allows multiple parallel reinsertions of the electrode in cases where overlapping coagulations are necessary, which is difficult percutaneously.106 In the future, novel RFC electrodes that would allow a large and reliable coagulation zone with a single insertion could take away this current disadvantage of the percutaneous route.
Intraoperative RFC allows the use of a full Pringle maneuver, which has been shown to result in larger, more complete, and less distorted coagulation zones when compared with normal hepatic flow or interrupting the flow in the hepatic artery only.116 Even if the surgeon does not perform a Pringle maneuver during laparoscopy, a 12-mm Hg pneumoperitoneum by itself causes a 40% decrease of portal vein flow, with a subsequent increase in RFC size.146
For fear of burning adjacent organs, diaphragm, or the abdominal wall, subcapsular tumors are often undertreated by a percutaneous approach, leading to higher local recurrence rates compared with deeper tumors.52,101,112
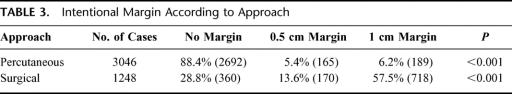
Finally, it appeared that an intended safety margin of 1cm, which was found to be associated with less local recurrence in our univariate analysis, was used much less in the percutaneous approach than in the surgical approach (Table 3). The surgical route is used mainly by surgeons (although in some centers an interventional radiologists scrubs and performs the RFC), while the percutaneous route is used mainly by radiologists (although surgeons also perform percutaneous RFC for selected indications). It is likely that surgeons more rigorously apply the 1-cm oncologic margin in RFC because they have been using it in hepatic surgery for over 20 years.
TABLE 3. Intentional Margin According to Approach
While formal proof of the therapeutic value of RFC awaits the results of randomized trials such as the recently opened CLOCC 40004 trial (chemotherapy + local ablation versus chemotherapy) of the European Organisation for Research and Treatment of Cancer, there are currently good indications that RFC may potentially lead to curative treatment of individual metastases. If cure is possible, then the most reliable treatment protocol should be chosen, even if it is more invasive and costly. This meta-analysis indicates that RFC by laparoscopy or laparotomy results in superior local control, independent of tumor size. While some authors argue that a percutaneous approach is acceptable for small tumors, our data indicate that the superiority of the surgical approach is even more evident for small than for larger tumors (Table 2). The surgical route is the first choice approach for any patient who can tolerate a laparoscopy or laparotomy. The short-term benefits of less invasiveness for the percutaneous route do not outweigh the longer-term higher risk of local recurrence, as a local recurrence may jeopardize cure.
The percutaneous route remains valuable for certain indications. First, it is indicated for patients that are too fragile to undergo laparoscopy or laparotomy. Second, tumors that are invisible on ultrasound imaging can be treated by a CT- or MRI-guided percutaneous procedure. Third, it is not excluded that some highly specialized centers through expertise and patient selection can produce equivalent results by the percutaneous approach to those obtained after a surgical route. Such centers, however, are rare. For percutaneously treated tumors ≤3 cm, only one of 5 reports with at least 50 tumors came close (0%) to the 3.6% local recurrence rate of the surgical approach.94 The other 4 series obtained local recurrence rates between 13% and 26%.52,77,83,84
Margin
The importance of a 1-cm oncologic safety margin in RFC of liver metastases2,12,18,46,62,63,67,70,74,87,97,103,112,114,116,175 and HCC12,18,22,46,63,70,87,97,103,112,114,116,133,175 has been stressed mainly by surgeons. Some authors, mainly in the radiologic literature, have lowered the proposed safety margin to 0.5 cm for metastases5,13,17,23,25,30,39,41,42,55,60,61,80,88,104,113,175 and HCC.5,8,11,17,23,25,39,41,42,57,80,88,94,96,104,175 A third group of authors explicitly state that a margin is not necessary for HCC.13,113,175
At present, there is only one study relating the local recurrence rate to the peritumoral coagulation margin. In this short-term follow-up study, much less local recurrences were observed after RFC of HCC with a margin of 5 mm versus no margin.147 Our meta-analysis found that local recurrence rates are higher when the physician does not aim at coagulating a peritumoral margin of 1 cm. Local recurrences at the edge of an initially complete radiologic coagulation have been reported by many authors.2,7,15–17,22,27,30,34,35,37,39,40,43,48,50,51,55–58,61,70,72–75,77,87–89,91,94,96 The underlying reason may be that the tumor can microscopically extend further than macroscopically suspected.
In HCC, viable satellite nodules were found in 57% of patients who underwent transplantation after RFC, despite a complete marginal necrosis of the main tumor.68 In medium (3–5 cm) and large (>5 cm) HCC, microscopic tumor extends more than 2 cm beyond macroscopic borders in 67%.119 In small HCC (≤3 cm), microscopic tumor extends more than 1 cm beyond macroscopic borders in 60%.119 Even in the most favorable subgroup, nodular-type HCC of less than 2 cm, satellites 10 mm from the nodule were observed in 10% of cases and microscopic portal invasion in up to 25% of cases.148
In resected colorectal metastases, microscopic bile duct, portal, or hepatic vein invasion or peritumoral micrometastases is found in 31% to 50%, up until 9 to 21 mm from the macroscopic tumor edge.118,149
The histologic data and the results of our meta-analysis provide the rationale to recommend a minimal safety margin of 1 cm for both primary and secondary liver tumors. A marginal coagulation of neuroendocrine metastases may be sufficient when the intent is purely palliative.10,18,40,54,93
Correctly obtaining a minimal 1-cm coagulation margin at all sides is not that easy. First, in the transverse plane perpendicular to the electrode, the electrode tip may not be placed perfectly in the center. In an experimental study, the mean distance between the center of the tumor and the center of the thermal coagulation was 3.8 ± 1.5 mm.150 In patients, after satisfactory placement of an electrode under 2-dimensional imaging, 3-dimensional imaging disclosed unacceptable eccentric device placement in 40% to 45%.151,152 Second, in the axial plane parallel to the electrode, the exact position of the electrode tip is not always easy to verify and a straight electrode may slide after placement.123 Moreover, there are no data in the literature on the precise position of the coagulation zone in relation to the electrode tip.116 Third, the diameter of coagulation lesions is rather variable, with a wide range between minimum and maximal diameter.116,153 Data on size and geometry obtained by current RFC electrodes have recently been published.116
The effect of the addition of all these small errors is illustrated by an experiment in which simulated 1-cm tumors were coagulated with an expandable electrode with an expected thermal coagulation diameter of 3 cm. Instead of obtaining a 1 cm safety margin around the tumor, the mean margin was only 0.16 cm with a positive margin in 23%.150
Reporting the extent of the resection margin is essential in any publication on hepatectomy for tumor. In the RFC literature, similar information on the peritumoral coagulation margin is inexistent, with a rare exception.147,154,155 By comparing pre- and post-RFC CT/MR images with superimposing of hepatic anatomic landmarks, the minimal coagulation margin can be measured.154 Some reports compared pre-RFC tumor volumes with post-RFC coagulation volumes or pre-RFC tumor diameter with post-RFC coagulation diameter. These surrogate measures are not recommended: the margin can be positive when the coagulation zone is asymmetric or eccentric, even when the coagulation diameter exceeds the tumor diameter or the coagulation volume triples the tumor volume.
Vascular Occlusion
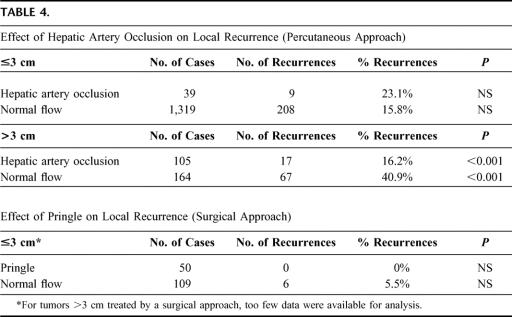
The clinical value of vascular occlusion has been regarded as controversial. For HCC treated percutaneously, 3 studies found better local control of HCC when RFC was combined with occlusion of the hepatic artery,8,96,156 while 2 did not.50,52 No studies were available that evaluate the influence of a Pringle maneuver on local recurrence for the surgical approach. In this meta-analysis, local control was better with vascular occlusion than with normal blood flow. The benefit of vascular occlusion is proven only for large tumors (Table 4).
TABLE 4. Effect of Hepatic Artery Occlusion on Local Recurrence (Percutaneous Approach)
The differences in local control rate can be explained by the negative effects of a perfusion-mediated tissue cooling on size and geometry of RFC lesions.4,116 Compared with RFC in perfused liver, thermal coagulations are larger and more regular when performing RFC during blood flow interruption. These findings are more pronounced in case of complete interruption of inflow (Pringle maneuver) or outflow (occlusion of hepatic veins), than with partial occlusion, ie, only the hepatic artery or the portal vein.104,116,125–127,157,158
A Pringle maneuver (clamping the hepatic artery and portal vein at the level of the porta hepatis) can be performed during laparotomy22,30,62,63,70,74,85,93,99,143 as well as during laparoscopy.36,127,175
For patients treated by a percutaneous approach, a hepatic artery occlusion can be performed by balloon catheter occlusion,33,72,96,159 by selective embolization,13,33,72,94 or by chemoembolization.8,27,50,156 Selective occlusion of a hepatic vein may in theory be as effective as a Pringle maneuver,116,126 but it is less frequently performed.25,33,175 Hypotensive anesthesia160 yields larger coagulation lesions than normotensive anesthesia, but the effect on local recurrence rates has not yet been reported. Percutaneous balloon occlusion of the portal vein is still experimental161 and unlikely to be of any benefit, given the predominantly arterial vascularization of liver tumors.162
Based on our current findings, vascular occlusion is recommended for the treatment of tumors >3 cm.
Anesthesia
We found no studies that looked at the effect of the type of anesthesia on local recurrence, although one author reported higher coagulation volumes after RFC under general anesthesia versus local anesthesia with or without sedation.112 Our meta-analysis of the whole series indicates that local control is superior for RFC performed during general anesthesia compared with local anesthesia with or without sedation (Table 1). As surgical cases are always done under general anesthesia, analysis was repeated for the percutaneous cases only, which showed no significant differences in local recurrence rate.
Procedures under local anesthesia with or without sedation are often painful,1,122 especially when the diameter of the thermal lesion exceeds 3 cm,163 when the tumor is superficial or in contact to pain-sensitive Glissonian structures,112,133,163 when treatment power exceeds 100 W, or when electric current exceeds 1 to 1.5 Amp.163 Pain may force the physician to lower the current intensity, to shorten coagulation duration, or to limit the number of overlapping coagulations. Incomplete tumor coagulation has been explicitly attributed to pain during a procedure under sedation.112
For a procedure under local anesthesia, the patient has to breathe in deeply and then hold his breath during electrode insertion.71 Under general anesthesia, inadvertent breathing movements while inserting the electrode are prevented. The anesthetist can be asked for a short-lasting apnea to facilitate a difficult electrode positioning.113
General anesthesia carries the additional advantage that systolic blood pressure can be lowered, aiming at a decreased liver blood flow and an increased coagulation diameter.157,160
Electrodes
At present, 6 companies have marketed RFC electrodes: Valleylab, Boulder, CO (formerly, Radionics); RITA Medical Systems, Mountain View, CA; Boston Scientific (formerly, Radiotherapeutics), Natick, MA; Berchtold, Tuttlingen, Germany; Invatec, Roncadelle, Italy; and Celon AG Medical Instruments, Teltow, Germany. The influence of electrodes on local recurrence rate is unknown. Several studies found equal results between electrodes.5,52,77,103 There are currently more than 28 RFC electrodes on the market,116 each of which can be used with a number of different protocols.116,164 Comparison of results with different electrodes in this meta-analysis was not possible.
A recent review116 showed that scientific data about size and geometry of coagulations obtained by current commercial electrodes are scarce. Data on the most basic parameter, ie, the transverse diameter in perfused pig liver, were available for only 10 of the 28 electrodes on the market. The paucity of data on size and geometry of the coagulation zone was incriminated explicitly as cause of several local recurrences in a recent study.103 The authors strongly recommend that new electrodes not be approved for release on the market without a complete set of experimental data on the size and geometry of the coagulation. Companies should also quickly provide data for those electrodes that are already for sale.116
For those electrodes for which experimental data are available, a substantial variability of coagulation diameters has been observed.116,153 An electrode used in the same experiment using the same treatment algorithms can yield coagulation lesions with a difference of up to 3.2 cm between minimal and maximal diameter. Overestimation of expected coagulation size may contribute to failure of local tumor control. Future research should focus on the development of novel electrodes that yield coagulations with more predictable diameters and shapes.
Imaging
In the meta-analysis, 98% of tumors have been treated under ultrasound guidance. Percutaneous RFC can also be performed under CT21,64,89,94,96,105 or MRI.43,48,88,112,175 Proponents of CT and MRI claim superiority in the treatment of tumors that are less conspicuous on ultrasound, particularly those in less accessible areas, such as the right hepatic dome.48,94,112 MRI holds the prospect of on-line monitoring of the thermocoagulation process.48,112 Contrast-enhanced color Doppler allows immediate recognition of remaining viable parts of HCC.20 There are no comparative studies on outcome after each of these imaging modalities. The meta-analysis found no significant differences.
Physician's Experience
A recent study found significantly more local recurrences in the first group of 50 patients versus the second group of 50 patients treated with RFC in the same center.103 The present meta-analysis confirms the importance of experience: authors who treated large numbers of tumors had significantly less local recurrences than authors who treated fewer tumors. Measures to shorten the learning curve may include appraisal of the literature, visiting specialized centers, and participating in training workshops with animal models.103 Physicians have an ethical obligation to acquire a thorough understanding of the physics, the possible complications, and the correct application of RFC prior to using it in patients.164 Regrettably, a minimally prepared physician who performs his first RFC directly on a patient, guided only by a company representative, is not an exception.
RFC Versus Surgery for Resectable Colorectal Metastases
The local recurrence rates obtained by RFC are encouraging in case of unresectable colorectal metastases. Nevertheless, no data from randomized trials are available evaluating the effect of RFC on overall survival when compared with chemotherapy alone. RFC for unresectable colorectal liver metastases seems justified only when such trials demonstrate a clear benefit on overall survival of RFC over chemotherapy. Currently such a trial (EORTC-CLOCC) is under way.
For patients with resectable colorectal metastases, current local recurrence rates are totally inacceptable, except maybe for the 3.6% local recurrence rate after RFC of small (≤3 cm) tumors by a surgical approach. Surgical RFC for such small resectable colorectal metastases could be acceptable in a randomized trial comparing resection with surgical RFC. For all other situations (percutaneous RFC and surgical RFC for medium and large tumors), RFC is contraindicated for resectable colorectal metastases. Sad cases of solitary, resectable, central lesions that were unsuccessfully treated with RFC and then progressed to incurability because of extension of tumor into major vasculature have been described.165
Yet the pressure on oncologists to refer their patients for minimally invasive techniques rather than for hepatic surgery becomes heavier.60,166–169 On the other hand, surgeons that have no experience with hepatic surgery start to perform RFC on an occasional basis to treat patients with resectable tumors, rather than referring these patients to a center where the resectability of the tumor can be evaluated. An alarming survey from Germany reported that 25.9% of patients undergoing RFC had a resectable tumor.170
The search for minimally invasive techniques is laudable. In oncology, however, the goal is not minimal invasiveness but cure.164,165,171–174 Innovative, less invasive techniques in oncology are welcome, but they should obtain at least the same results as traditional more invasive techniques.
ACKNOWLEDGMENTS
The authors thank William A. Wagle, MD, for reviewing the manuscript and the following authors who kindly provided more details about their reported cases: G. Bernardi,11 K. P. de Jong,92 D. Elias,30 R. J. Fontana,31 S. M. Lin,58 J. Machi,63 D. T. Nielsen,67 O. Risse,70 T. Shibata,75,77 and M. Zago.97
REFERENCES
- 1. Curley SA Izzo F Delrio P et al. Radiofrequency ablation of unresectable primary and metastatic hepatic malignancies: results in 123 patients. Ann Surg. 1999;230:1–8. doi: 10.1097/00000658-199907000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pawlik TM Izzo F Cohen DS et al. Combined resection and radiofrequency ablation for advanced hepatic malignancies: results in 172 patients. Ann Surg Oncol. 2003;10:1059–1069. doi: 10.1245/ASO.2003.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kuvshinoff BW Ota DM Radiofrequency ablation of liver tumors: influence of technique and tumor size. Surgery. 2002;132:605–611. doi: 10.1067/msy.2002.127545. [DOI] [PubMed] [Google Scholar]
- 4. Goldberg SN Charboneau JW Dodd GD et al. Image-guided tumor ablation: proposal for standardization of terms and reporting criteria. Radiology. 2003;228:335–345. doi: 10.1148/radiol.2282021787. [DOI] [PubMed] [Google Scholar]
- 5. Lu DS Raman SS Limanond P et al. Influence of large peritumoral vessels on outcome of radiofrequency ablation of liver tumors. J Vasc Interv Radiol. 2003;14:1267–1274. doi: 10.1097/01.rvi.0000092666.72261.6b. [DOI] [PubMed] [Google Scholar]
- 6. Abraham J Fojo T Wood BJ Radiofrequency ablation of metastatic lesions in adrenocortical cancer. Ann Intern Med. 2000;133:312–313. doi: 10.7326/0003-4819-133-4-200008150-00028. [DOI] [PubMed] [Google Scholar]
- 7. Adam R Hagopian EJ Linhares M et al. A comparison of percutaneous cryosurgery and percutaneous radiofrequency for unresectable hepatic malignancies. Arch Surg. 2002;137:1332–1339. doi: 10.1001/archsurg.137.12.1332. [DOI] [PubMed] [Google Scholar]
- 8. Anai H Yamakado K Kimura M et al. Radiofrequency ablation combined with lipiodol-transcatheter arterial embolization for hepatocellular carcinoma. J Vasc Interv Radiol. 2003;14:90. [Google Scholar]
- 9. Arch-Ferrer JE Smith JK Bynon S et al. Radio-frequency ablation in cirrhotic patients with hepatocellular carcinoma. Am Surg. 2003;69:1067–1071. [PubMed] [Google Scholar]
- 10. Berber E Flesher N Siperstein AE Laparoscopic radiofrequency ablation of neuroendocrine liver metastases. World J Surg. 2002;26:985–990. doi: 10.1007/s00268-002-6629-5. [DOI] [PubMed] [Google Scholar]
- 11. Bernardi G Roveda L Trotta F et al. Radio-frequency thermal ablation of primary hepatic tumours: comparison of different surgical approaches. Surg Endosc. 2002;16:185. [Google Scholar]
- 12. Bowles BJ Machi J Limm WM et al. Safety and efficacy of radiofrequency thermal ablation in advanced liver tumors. Arch Surg. 2001;136:864–869. doi: 10.1001/archsurg.136.8.864. [DOI] [PubMed] [Google Scholar]
- 13. Buscarini L Buscarini E Di Stasi M et al. Percutaneous radiofrequency thermal ablation combined with transcatheter arterial embolization in the treatment of large hepatocellular carcinoma. Ultraschall Med. 1999;20:47–53. doi: 10.1055/s-1999-14233. [DOI] [PubMed] [Google Scholar]
- 14. Buscarini L Buscarini E Di Stasi M et al. Percutaneous radiofrequency ablation of small hepatocellular carcinoma: long-term results. Eur Radiol. 2001;11:914–921. doi: 10.1007/s003300000659. [DOI] [PubMed] [Google Scholar]
- 15. Cabassa P Ghirardi C Simeone F et al. Radio-frequency ablation of hepatocellular carcinoma: postprocedural assessment with spiral CT: one month versus 4 months in the assessment of local therapeutic efficacy. Eur Radiol. 2003;13:108. [Google Scholar]
- 16. Chen Y Wu MC Chen H et al. Percutaneous hepatic puncture radio frequency ablation in the treatment of hepatocellular carcinoma. Zhonghua Zhong Liu Za Zhi. 2003;25:88–90. [PubMed] [Google Scholar]
- 17. Chopra S Dodd GD3rd Chanin MP et al. Radiofrequency ablation of hepatic tumors adjacent to the gallbladder: feasibility and safety. AJR Am J Roentgenol. 2003;180:697–701. doi: 10.2214/ajr.180.3.1800697. [DOI] [PubMed] [Google Scholar]
- 18. Chung MH Wood TF Tsioulias GJ et al. Laparoscopic radiofrequency ablation of unresectable hepatic malignancies: a phase 2 trial. Surg Endosc. 2001;15:1020–1026. doi: 10.1007/s00464-001-0026-2. [DOI] [PubMed] [Google Scholar]
- 19. Cioni D Lencioni R Neri E et al. Radiofrequency thermal ablation of liver malignancies: assessment of treatment outcome by volume-rendered spiral CT. Eur Radiol. 2001;11:172. [Google Scholar]
- 20. Cioni D Lencioni R Rossi S et al. Radiofrequency thermal ablation of hepatocellular carcinoma: using contrast-enhanced harmonic power doppler sonography to assess treatment outcome. AJR Am J Roentgenol. 2001;177:783–788. doi: 10.2214/ajr.177.4.1770783. [DOI] [PubMed] [Google Scholar]
- 21. Crocetti L Lencioni R Neri E et al. Volume-rendered spiral CT in the assessment of outcome of liver malignancies radiofrequency thermal ablation. Eur Radiol. 2001;11:C31. [Google Scholar]
- 22. Curley SA Izzo F Ellis LM et al. Radiofrequency ablation of hepatocellular cancer in 110 patients with cirrhosis. Ann Surg. 2000;232:381–391. doi: 10.1097/00000658-200009000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cuschieri A Bracken J Boni L Initial experience with laparoscopic ultrasound guided radiofrequency thermal ablation of hepatic tumours. Endoscopy. 1999;31:318–321. doi: 10.1055/s-1999-16. [DOI] [PubMed] [Google Scholar]
- 24. Danza F Rossi P Marino V et al. Radio frequency interstitial thermal ablation of hepatocellular carcinoma. Eur Radiol. 2002;12:122. [Google Scholar]
- 25. de Baere T Bessoud B Dromain C et al. Percutaneous radiofrequency ablation of hepatic tumors during temporary venous occlusion. AJR Am J Roentgenol. 2002;178:53–59. doi: 10.2214/ajr.178.1.1780053. [DOI] [PubMed] [Google Scholar]
- 26.de Baere TJ, Elias D, Ducreux M, et al. Percutaneous radiofrequency of liver metastases: single center experience over a five year period. ASCO Annual Meeting, May 31-June 3 2003, Chicago, Illinois. Abstract no 1387. Available at http://www.asco.org. Accessed January 30, 2004.
- 27. De Santis M Long-term follow-up of combined treatment by chemoembolization and radiofrequency thermal ablation of medium-large hepatocellular carcinoma. Eur Radiol. 2002;12:425. [Google Scholar]
- 28. Decker GA Gores GJ Roberts LR Tumor seeding complicating radiofrequency ablation of hepatocellular carcinoma. J Hepatol. 2003;38:692. doi: 10.1016/s0168-8278(03)00028-x. [DOI] [PubMed] [Google Scholar]
- 29. Diche T Boyer B Lipski M et al. MR imaging follow up of radio-frequency ablation of hepatocellular carcinoma: role of Mn-DPDP injection. Eur Radiol. 2000;10:D22. [Google Scholar]
- 30. Elias D Goharin A El Otmany A et al. Usefulness of intraoperative radiofrequency thermoablation of liver tumours associated or not with hepatectomy. Eur J Surg Oncol. 2000;26:763–769. doi: 10.1053/ejso.2000.1000. [DOI] [PubMed] [Google Scholar]
- 31. Fontana RJ Hamidullah H Nghiem H et al. Percutaneous radiofrequency thermal ablation of hepatocellular carcinoma: a safe and effective bridge to liver transplantation. Liver Transpl. 2002;8:1165–1174. doi: 10.1053/jlts.2002.36394. [DOI] [PubMed] [Google Scholar]
- 32. Francica G Marone G Ultrasound guided percutaneous treatment of hepatocellular carcinoma by radiofrequency hyperthermia with a ‘cooled tip needle’: a preliminary clinical experience. Eur J Ultrasound. 1999;9:145–153. doi: 10.1016/s0929-8266(99)00022-1. [DOI] [PubMed] [Google Scholar]
- 33. Gasparini D Sponza M Marzio A et al. Combined treatment, TACE and RF ablation, in HCC: preliminary results. Radiol Med (Torino) 2002;104:412–420. [PubMed] [Google Scholar]
- 34. Gervais DA Goldberg SN Arellano RS et al. Treatment of hepatocellular carcinoma in cirrhotic patients awaiting transplantation: a new and evolving use of percutaneous radiofrequency ablation. Radiology. 2000;217:606–607. [Google Scholar]
- 35. Giovannini M Moutardier V Danisi C et al. Treatment of hepatocellular carcinoma using percutaneous radiofrequency thermoablation: results and outcomes in 56 patients. J Gastrointest Surg. 2003;7:791–716. doi: 10.1016/s1091-255x(03)00112-4. [DOI] [PubMed] [Google Scholar]
- 36. Goletti O Lencioni R Armillotta N et al. Laparoscopic radiofrequency thermal ablation of hepatocarcinoma: preliminary experience. Surg Laparosc Endosc Percutan Tech. 2000;10:284–290. [PubMed] [Google Scholar]
- 37. Guglielmi A Ruzzenente A Battocchia A et al. Radiofrequency ablation of hepatocellular carcinoma in cirrhotic patients. Hepatogastroenterology. 2003;50:480–484. [PubMed] [Google Scholar]
- 38. Gulati MS Batra Y Paul SB et al. Radiofrequency ablation: a new therapeutic modality for the management of hepatocellular cancer. Trop Gastroenterol. 2002;23:183–185. [PubMed] [Google Scholar]
- 39. Hänsler J Witte A Strobel D et al. Radio-frequency-ablation (RFA) with wet electrodes in the treatment of primary and secondary liver tumours. Ultraschall Med. 2003;24:27–33. doi: 10.1055/s-2003-37413. [DOI] [PubMed] [Google Scholar]
- 40. Hellman P Ladjevardi S Skogseid B et al. Radiofrequency tissue ablation using cooled tip for liver metastases of endocrine tumors. World J Surg. 2002;26:1052–1056. doi: 10.1007/s00268-002-6663-3. [DOI] [PubMed] [Google Scholar]
- 41. Holtkamp W Müller W Ultrasonically controlled percutaneous high frequency thermotherapy of liver tumors with perfused needle electrodes. Z Gastroenterol. 2000;38:221–227. doi: 10.1055/s-2000-14861. [DOI] [PubMed] [Google Scholar]
- 42. Horigome H Nomura T Nakao H et al. Percutaneous radio-frequency ablation therapy using a clustered electrode for malignant liver tumors. J Clin Gastroenterol. 2001;32:418–422. doi: 10.1097/00004836-200105000-00012. [DOI] [PubMed] [Google Scholar]
- 43. Huppert PE Trubenbach J Schick F et al. MRI guided percutaneous radiofrequency ablation of hepatic neoplasms: technical and clinical experiences. Rofo Fortschr Geb Rontgenstr Neuen Bildgeb Verfahr. 2000;172:692–700. doi: 10.1055/s-2000-12045. [DOI] [PubMed] [Google Scholar]
- 44. Ishikawa T Kohno T Shibayama T et al. Thoracoscopic thermal ablation therapy for hepatocellular carcinoma located beneath the diaphragm. Endoscopy. 2001;33:697–702. doi: 10.1055/s-2001-16216. [DOI] [PubMed] [Google Scholar]
- 45. Jacobs IA Chang CK Salti G Hepatic radiofrequency ablation of metastatic ovarian granulosa cell tumors. Am Surg. 2003;69:416–418. [PubMed] [Google Scholar]
- 46. Jung EM Clevert DA Rupp N Contrast-enhanced ultrasound with Optison in percutaneous thermoablation of liver tumors. Rofo Fortschr Geb Rontgenstr Neuen Bildgeb Verfahr. 2003;175:1403–1412. doi: 10.1055/s-2003-42882. [DOI] [PubMed] [Google Scholar]
- 47. Kainuma O Asano T Aoyama H Recurrent hepatocellular carcinoma successfully treated with radiofrequency thermal ablation. J Hepatobiliary Pancreat Surg. 1999;6:190–194. doi: 10.1007/s005340050105. [DOI] [PubMed] [Google Scholar]
- 48. Kelekis AD Terraz S Roggan A et al. Percutaneous treatment of liver tumors with an adapted probe for cooled-tip, impedance-controlled radio-frequency ablation under open-magnet MR guidance: initial results. Eur Radiol. 2003;13:1100–1105. doi: 10.1007/s00330-003-1847-2. [DOI] [PubMed] [Google Scholar]
- 49. Kessler A Blank A Merhav H et al. Minimally invasive techniques in the treatment of liver tumors. Isr Med Assoc J. 2002;4:1106–1110. [PubMed] [Google Scholar]
- 50. Kitamoto M Imagawa M Yamada H et al. Radiofrequency ablation in the treatment of small hepatocellular carcinomas: comparison of the radiofrequency effect with and without chemoembolization. AJR Am J Roentgenol. 2003;181:997–1003. doi: 10.2214/ajr.181.4.1810997. [DOI] [PubMed] [Google Scholar]
- 51. Koda M Maeda Y Matsunaga Y et al. Hepatocellular carcinoma with sarcomatous change arising after radiofrequency ablation for well-differentiated hepatocellular carcinoma. Hepatol Res. 2003;27:163–167. doi: 10.1016/s1386-6346(03)00207-9. [DOI] [PubMed] [Google Scholar]
- 52. Komorizono Y Oketani M Sako K et al. Risk factors for local recurrence of small hepatocellular carcinoma tumors after a single session, single application of percutaneous radiofrequency ablation. Cancer. 2003;97:1253–1262. doi: 10.1002/cncr.11168. [DOI] [PubMed] [Google Scholar]
- 53. Kormann J Ockert D Bunk A Radiofrequency ablation of liver tumours. Zentralbl Chir. 2001;126:576–585. doi: 10.1055/s-2001-16572. [DOI] [PubMed] [Google Scholar]
- 54. Kosari K Gomes M Hunter D et al. Local, intrahepatic, and systemic recurrence patterns after radiofrequency ablation of hepatic malignancies. J Gastrointest Surg. 2002;6:255–263. doi: 10.1016/s1091-255x(02)00002-1. [DOI] [PubMed] [Google Scholar]
- 55. Langenhoff BS Oyen WJG Jager GJ et al. Efficacy of Fluorine-18-deoxyglucose Positron Emission Tomography in detecting tumor recurrence after local ablative therapy for liver metastases: a prospective study. J Clin Oncol. 2002;20:4453–4445. doi: 10.1200/JCO.2002.12.134. [DOI] [PubMed] [Google Scholar]
- 56. Lencioni RA Allgaier HP Cioni D et al. Small hepatocellular carcinoma in cirrhosis: randomized comparison of radio-frequency thermal ablation versus percutaneous ethanol injection. Radiology. 2003;228:235–240. doi: 10.1148/radiol.2281020718. [DOI] [PubMed] [Google Scholar]
- 57. Lim HK Choi D Lee WJ et al. Hepatocellular carcinoma treated with percutaneous radio-frequency ablation: evaluation with follow-up multiphase helical CT. Radiology. 2001;221:447–454. doi: 10.1148/radiol.2212010446. [DOI] [PubMed] [Google Scholar]
- 58. Lin SM Lin CJ Chung HJ et al. Power rolloff during interactive radiofrequency ablation can enhance necrosis when treating hepatocellular carcinoma. AJR Am J Roentgenol. 2003;180:151–157. doi: 10.2214/ajr.180.1.1800151. [DOI] [PubMed] [Google Scholar]
- 59. Liu C Frilling A Dereskewitz C et al. Tumor seeding after fine needle aspiration biopsy and percutaneous radiofrequency thermal ablation of hepatocellular carcinoma. Dig Surg. 2003;20:460–463. [Google Scholar]
- 60. Livraghi T Solbiati L Meloni F et al. Percutaneous radiofrequency ablation of liver metastases in potential candidates for resection: the ‘test-of-time approach.’. Cancer. 2003;97:3027–3035. doi: 10.1002/cncr.11426. [DOI] [PubMed] [Google Scholar]
- 61. Ludwig V Hopper OW Martin WH et al. [18F] Fluorodeoxyglucose positron emission tomography surveillance of hepatic metastases from prostate cancer following radiofrequency ablation: a case report. Am Surg. 2003;69:593–598. [PubMed] [Google Scholar]
- 62. Machi J Oishi AJ Morioka WK et al. Radiofrequency thermal ablation of synchronous metastatic liver tumors can be performed safely in conjunction with colorectal cancer resection. Cancer J. 2000;6:344–350. [Google Scholar]
- 63. Machi J Uchida S Sumida K et al. Ultrasound-guided radiofrequency thermal ablation of liver tumors: percutaneous, laparoscopic, and open surgical approaches. J Gastrointest Surg. 2001;5:477–489. doi: 10.1016/s1091-255x(01)80085-8. [DOI] [PubMed] [Google Scholar]
- 64. Mahnken AH Tacke J Bucker A et al. Percutaneous radiofrequency ablation of liver malignancies: first experience with a 200-W radiofrequency generator. Rofo Fortschr Geb Rontgenstr Neuen Bildgeb Verfahr. 2002;174:216–223. doi: 10.1055/s-2002-20138. [DOI] [PubMed] [Google Scholar]
- 65. Morimoto M Sugimori K Shirato K et al. Treatment of hepatocellular carcinoma with radiofrequency ablation: radiologic-histologic correlation during follow-up periods. Hepatology. 2002;35:1467–1475. doi: 10.1053/jhep.2002.33635. [DOI] [PubMed] [Google Scholar]
- 66. Neeman Z Libutti SK Patti JW et al. Percutaneous radiofrequency ablation of hepatocellular carcinoma in the presence of portal vein thrombosis. Clin Imaging. 2003;27:417–420. doi: 10.1016/S0899-7071(03)00015-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Nielsen DT Laursen HB Rokkjaer M et al. Radiofrequency ablation of malignant liver tumors. Ugeskr Laeger. 2002;164:4642–4645. [PubMed] [Google Scholar]
- 68. Pulvirenti A Garbagnati F Regalia E et al. Experience with radiofrequency ablation of small hepatocellular carcinomas before liver transplantation. Transplant Proc. 2001;33:1516–1517. doi: 10.1016/s0041-1345(00)02577-x. [DOI] [PubMed] [Google Scholar]
- 69. Rhim H Kim YS Intrahepatic regional portal blood flow modulation using percutaneous US-monitoring portal vein compression during radiofrequency thermal ablation. Cardiovasc Intervent Radiol. 2003;26:416–418. doi: 10.1007/s00270-003-0449-3. [DOI] [PubMed] [Google Scholar]
- 70. Risse O Sengel C Penillon S et al. Radiofrequency ablation of malignant hepatic tumors: preliminary experience apropos of 25 cases. Ann Chir. 2001;126:118–126. doi: 10.1016/s0003-3944(00)00474-0. [DOI] [PubMed] [Google Scholar]
- 71. Rossi S Buscarini E Gabargnati R et al. Percutaneous treatment of small hepatic tumors by an expandable RF needle electrode. AJR Am J Roentgenol. 1998;170:1015–1022. doi: 10.2214/ajr.170.4.9530052. [DOI] [PubMed] [Google Scholar]
- 72. Rossi S Garbagnati F Lencioni R et al. Percutaneous radio frequency thermal ablation of nonresectable hepatocellular carcinoma after occlusion of tumor blood supply. Radiology. 2000;217:119–126. doi: 10.1148/radiology.217.1.r00se02119. [DOI] [PubMed] [Google Scholar]
- 73. Rossi M Pepino D Bezzi M et al. Mid-term results alter RF thermal ablation of HCC by using cooled tip electrodes. Radiology. 2001;221:248. [Google Scholar]
- 74. Scaife CL Curley SA Izzo F et al. Feasibility of adjuvant hepatic arterial infusion of chemotherapy after radiofrequency ablation with or without resection in patients with hepatic metastases from colorectal cancer. Ann Surg Oncol. 2003;10:348–354. doi: 10.1245/aso.2003.08.019. [DOI] [PubMed] [Google Scholar]
- 75. Shibata T Iimuro Y Yamamoto Y et al. Small hepatocellular carcinoma: comparison of radio-frequency ablation and percutaneous microwave coagulation therapy. Radiology. 2002;223:331–337. doi: 10.1148/radiol.2232010775. [DOI] [PubMed] [Google Scholar]
- 76. Shibata T Iimuro Y Ikai I et al. Percutaneous radiofrequency ablation therapy after intrathoracic saline solution infusion for liver tumor in the hepatic dome. J Vasc Interv Radiol. 2002;13:313–315. doi: 10.1016/s1051-0443(07)61725-4. [DOI] [PubMed] [Google Scholar]
- 77. Shibata T Shibata T Maetani Y et al. RFA for hepatocellular carcinoma: comparison of retractable electrode and internally cooled- tip electrode. J Vasc Interv Radiol. 2003;14:50. [Google Scholar]
- 78. Shiozawa K Ishii K Mori T et al. Heterochronous development of intrahepatic cholangiocellular carcinoma following hepatocellular carcinoma in a hepatitis B virus carrier. Intern Med. 2001;40:624–630. doi: 10.2169/internalmedicine.40.624. [DOI] [PubMed] [Google Scholar]
- 79. Shirato K Morimoto M Tomita N et al. Hepatocellular carcinoma: percutaneous radiofrequency ablation using expandable needle electrodes and the double-insertion technique. Hepatogastroenterology. 2002;49:1481–1483. [PubMed] [Google Scholar]
- 80. Siperstein A Garland A Engle K et al. Local recurrence after laparoscopic radiofrequency thermal ablation of hepatic tumors. Ann Surg Oncol. 2000;7:106–113. doi: 10.1007/s10434-000-0106-x. [DOI] [PubMed] [Google Scholar]
- 81. Sironi S Livraghi T Meloni F et al. Small hepatocellular carcinoma treated with percutaneous RF ablation: MR imaging follow up. AJR Am J Roentgenol. 1999;173:1225–1229. doi: 10.2214/ajr.173.5.10541093. [DOI] [PubMed] [Google Scholar]
- 82. Slakey DP Radiofrequency ablation of recurrent cholangiocarcinoma. Am Surg. 2002;68:395–397. [PubMed] [Google Scholar]
- 83. Solbiati L Livraghi T Ierace T et al. Local control of focal liver malignancies treated with RF ablation: initial report of the Italian Multicenter Cooled tip RF Study Group. Radiology 2000. 217 228 11012449 [Google Scholar]
- 84. Solbiati L Ierace T Livraghi T et al. Outcome and long-term survival of patients with liver metastases from colorectal cancer treated with percutaneous cool-tip radiofrequency ablation. Radiology. 2001;221:625–626. [Google Scholar]
- 85. Stella M Minuto MN Pasqualini M et al. Intraoperative use of radiofrequency thermoablation of liver tumors: considerations on indications and related therapeutic aspects. Ann Ital Chir. 2002;73:511–516. [PubMed] [Google Scholar]
- 86. Tepetes K Tsamandas AC Ravazoula P et al. Survival for 5 years after repeat liver resections and multimodality treatment for metastatic intestinal leiomyosarcoma: report of a case. Surg Today. 2002;32:925–928. doi: 10.1007/s005950200184. [DOI] [PubMed] [Google Scholar]
- 87. Topal B Aerts R Penninckx F Laparoscopic radiofrequency ablation of unresectable liver malignancies: feasibility and clinical outcome. Surg Laparosc Endosc Percutan Tech. 2003;13:11–15. doi: 10.1097/00129689-200302000-00003. [DOI] [PubMed] [Google Scholar]
- 88. Trübenbach J Pereira PL Schick F et al. MRI guided radiofrequency ablation of liver tumours: a valuable and minimally invasive therapeutic option. Minim Invasive Ther Allied Technol. 1998;7:533–539. [Google Scholar]
- 89. Trübenbach J König CW Duda SH et al. Percutaneous radiofrequency ablation of hepatic neoplasms using a clustered electrode first clinical results. Rofo Fortschr Geb Rontgenstr Neuen Bildgeb Verfahr. 2000;172:905–910. doi: 10.1055/s-2000-8369. [DOI] [PubMed] [Google Scholar]
- 90. Veltri A Brunello F Hosseinollhai P et al. Percutaneous ethanol injection (PEI) and radiofrequency induced thermal ablation (RITA) for small hepatocellular carcinoma: comparative study. J Vasc Interv Radiol. 2001;12:25. [Google Scholar]
- 91. Veltri A Barisone F Falco M et al. Local efficacy of Radiofrequency Intersitial Thermal Ablation (RITA) for newly diagnosed vs recurrent hepatocellular carcinoma (HCC). Radiology. 2001;221:250. [Google Scholar]
- 92. Verhoeven BH Haagsma EB Appeltans BM et al. Hyperkalaemia after radiofrequency ablation of hepatocellular carcinoma. Eur J Gastroenterol Hepatol. 2002;14:1023–1024. doi: 10.1097/00042737-200209000-00015. [DOI] [PubMed] [Google Scholar]
- 93. Wessels FJ Schell SR Radiofrequency ablation treatment of refractory carcinoid hepatic metastases. J Surg Res. 2001;95:8–12. doi: 10.1006/jsre.2000.5988. [DOI] [PubMed] [Google Scholar]
- 94. Yamakado K Nakatsuka A Ohmori S et al. Radiofrequency ablation combined with chemoembolization in hepatocellular carcinoma: treatment response based on tumor size and morphology. J Vasc Interv Radiol. 2002;13:1225–1232. doi: 10.1016/s1051-0443(07)61969-1. [DOI] [PubMed] [Google Scholar]
- 95. Yamakado K Nakatsuka A Akeboshi M et al. Percutaneous radiofrequency ablation of liver neoplasms adjacent to the gastrointestinal tract after balloon catheter interposition. J Vasc Interv Radiol. 2003;14:1183–1186. doi: 10.1097/01.rvi.0000086530.86489.05. [DOI] [PubMed] [Google Scholar]
- 96. Yamasaki T Kurokawa F Shirahashi H et al. Percutaneous radiofrequency ablation therapy for patients with hepatocellular carcinoma during occlusion of hepatic blood flow: comparison with standard percutaneous radiofrequency ablation therapy. Cancer. 2002;95:2353–2360. doi: 10.1002/cncr.10966. [DOI] [PubMed] [Google Scholar]
- 97. Zago M Bisagni P Bona S et al. Laparoscopic radiofrequency ablation of liver tumours: what are its indications? Surg Endosc 2002. 16 184 11961636 [Google Scholar]
- 98. Zheng RQ Kudo M Minami Y et al. Stage IV hepatocellular carcinoma with portal venous tumor thrombus: complete response after combined therapy of repeated arterial chemoembolization and radiofrequency ablation. J Gastroenterol. 2003;38:406–409. doi: 10.1007/s005350300073. [DOI] [PubMed] [Google Scholar]
- 99. Bleicher RJ Allegra DP Nora DT et al. Radiofrequency ablation in 447 complex unresectable liver tumors: lessons learned. Ann Surg Oncol. 2003;10:52–58. doi: 10.1245/aso.2003.03.018. [DOI] [PubMed] [Google Scholar]
- 100. Chan RP Asch M Kachura J et al. Radiofrequency ablation of malignant hepatic neoplasms. Can Assoc Radiol J. 2002;53:272–278. [PubMed] [Google Scholar]
- 101. Hori T Nagata K Hasuike S et al. Risk factors for the local recurrence of hepatocellular carcinoma after a single session of percutaneous radiofrequency ablation. J Gastroenterol. 2003;38:977–981. doi: 10.1007/s00535-003-1181-0. [DOI] [PubMed] [Google Scholar]
- 102. Ossip MG Kachura JR Ho CS et al. Radiofrequency ablation of liver tumors: local progression-free survival and factors for failure of effectiveness. J Vasc Interv Radiol. 2004;15:208. [Google Scholar]
- 103. Poon RT Ng KK Lam CM et al. Learning curve for radiofrequency ablation of liver tumors: prospective analysis of initial 100 patients in a tertiary institution. Ann Surg. 2004;239:441–449. doi: 10.1097/01.sla.0000118565.21298.0a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Goldberg SN Gazelle GS Compton CC et al. Treatment of intrahepatic malignancy with radiofrequency ablation: radiologic-pathologic correlation. Cancer. 2000;88:2452–2463. [PubMed] [Google Scholar]
- 105. Harrison LE Koneru B Baramipour P et al. Locoregional recurrences are frequent after radiofrequency ablation for hepatocellular carcinoma. J Am Coll Surg. 2003;197:759–764. doi: 10.1016/S1072-7515(03)00750-6. [DOI] [PubMed] [Google Scholar]
- 106. Rossi S Di Stasi M Buscarini E et al. Percutaneous RF interstitial thermal ablation in the treatment of hepatic cancer. AJR Am J Roentgenol. 1996;167:759–768. doi: 10.2214/ajr.167.3.8751696. [DOI] [PubMed] [Google Scholar]
- 107. Bilchik AJ Rose DM Allegra DP et al. Radiofrequency ablation: a minimally invasive technique with multiple applications. Cancer J Sci Am. 1999;5:356–361. [PubMed] [Google Scholar]
- 108. Solbiati L Goldberg SN Ierace T et al. Hepatic metastases: percutaneous radio-frequency ablation with cooled-tip electrodes. Radiology. 1997;205:367–373. doi: 10.1148/radiology.205.2.9356616. [DOI] [PubMed] [Google Scholar]
- 109. Solbiati L Livraghi T Goldberg SN et al. Percutaneous radio-frequency ablation of hepatic metastases from colorectal cancer: long-term results in 117 patients. Radiology. 2001;221:159–166. doi: 10.1148/radiol.2211001624. [DOI] [PubMed] [Google Scholar]
- 110. Rossi S Rosa L Mongiovetti M et al. Radiofrequency thermal ablation (RFTA) of nonresectable hepatocellular carcinoma (HCC) nodules in cirrhosis. J Hepatol 2001. 34 11 11211886 [Google Scholar]
- 111. Bilchik AJ Wood TF Allegra DP Radiofrequency ablation of unresectable hepatic malignancies: lessons learned. Oncologist. 2001;6:24–33. doi: 10.1634/theoncologist.6-1-24. [DOI] [PubMed] [Google Scholar]
- 112. Kettenbach J Kostler W Rucklinger E et al. Percutaneous saline-enhanced radiofrequency ablation of unresectable hepatic tumors: initial experience in 26 patients. AJR Am J Roentgenol. 2003;180:1537–1545. doi: 10.2214/ajr.180.6.1801537. [DOI] [PubMed] [Google Scholar]
- 113. Livraghi T Goldberg SN Lazzaroni S et al. Hepatocellular carcinoma: radio frequency ablation of medium and large lesions. Radiology. 2000;214:761–768. doi: 10.1148/radiology.214.3.r00mr02761. [DOI] [PubMed] [Google Scholar]
- 114. Wood TF Rose DM Chung M et al. Radiofrequency ablation of 231 unresectable hepatic tumors: indications, limitations, and complications. Ann Surg Oncol. 2000;7:593–600. doi: 10.1007/BF02725339. [DOI] [PubMed] [Google Scholar]
- 115. Dodd GD Frank MS Aribandi M et al. Computer analysis of the size of the thermal injury created by overlapping ablations. AJR Am J Roentgenol. 2001;177:777–782. doi: 10.2214/ajr.177.4.1770777. [DOI] [PubMed] [Google Scholar]
- 116. Mulier S Ni Y Miao Y et al. Size and geometry of hepatic radiofrequency lesions. Eur J Surg Oncol. 2003;29:867–878. doi: 10.1016/j.ejso.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 117. Nanko M Shimada H Yamaoka H et al. Micrometastatic colorectal cancer lesions in the liver. Surg Today. 1998;28:707–713. doi: 10.1007/BF02484616. [DOI] [PubMed] [Google Scholar]
- 118. Shirabe Takemaya K Gion T et al. Analysis of prognostic factors in hepatic resection for metastatic colorectal carcinoma with special reference to the surgical margin. Br J Surg. 1997;84:1077–1080. [PubMed] [Google Scholar]
- 119. Lai ECS You KT Ng IOL et al. The pathological basis of resection margin for hepatocellular carcinoma. World J Surg. 1993;17:786–791. doi: 10.1007/BF01659097. [DOI] [PubMed] [Google Scholar]
- 120. Maeda T Takenaka K Adachi E et al. Small hepatocellular carcinoma of single nodular type: a specific reference to its surrounding cancerous area undetected radiologically and macroscopically. J Surg Oncol. 1995;60:75–79. doi: 10.1002/jso.2930600202. [DOI] [PubMed] [Google Scholar]
- 121. Gillams AR Lees WR The importance of large vessel proximity in thermal ablation of liver tumours. Radiology. 1999;213:123. [Google Scholar]
- 122. Jiao LR Hansen PD Havlik R et al. Clinical short term results of radiofrequency ablation in primary and secondary liver tumors. Am J Surg. 1999;177:303–306. doi: 10.1016/s0002-9610(99)00043-4. [DOI] [PubMed] [Google Scholar]
- 123. Scudamore CH Shung IL Patteron EJ et al. Radiofrequency ablation followed by resection of malignant liver tumors. Am J Surg. 1999;177:411–417. doi: 10.1016/s0002-9610(99)00068-9. [DOI] [PubMed] [Google Scholar]
- 124. Lu DS Raman SS Vodopich DJ et al. Effect of vessel size on creation of hepatic radiofrequency lesions in pigs: assessment of the ‘heat sink’ effect. AJR Am J Roentgenol. 2002;178:47–51. doi: 10.2214/ajr.178.1.1780047. [DOI] [PubMed] [Google Scholar]
- 125. Chinn SB Lee FTJr Kennedy GD et al. Effect of vascular occlusion on radiofrequency ablation of the liver: results in a porcine model. AJR Am J Roentgenol. 2001;176:789–795. doi: 10.2214/ajr.176.3.1760789. [DOI] [PubMed] [Google Scholar]
- 126. Rossi S Garbagnati F De Francesco I et al. Relationship between the shape and size of radiofrequency induced thermal lesions and hepatic vascularization. Tumori. 1999;85:137–141. [PubMed] [Google Scholar]
- 127. Scott DJ Fleming JB Watumull LM et al. The effect of hepatic inflow occlusion on laparoscopic radiofrequency ablation using simulated tumors. Surg Endosc. 2002;16:1286–1291. doi: 10.1007/s004640080167. [DOI] [PubMed] [Google Scholar]
- 128. Tungjitkusolmun S Staelin ST Haemmerich D et al. Three-dimensional finite-element analyses for radio-frequency hepatic tumor ablation. IEEE Trans Biomed Eng. 2002;49:3–9. doi: 10.1109/10.972834. [DOI] [PubMed] [Google Scholar]
- 129. Mulier S Mulier P Ni Y et al. Complications of radiofrequency coagulation of liver tumours. Br J Surg. 2002;89:1206–1222. doi: 10.1046/j.1365-2168.2002.02168.x. [DOI] [PubMed] [Google Scholar]
- 130. Curley SA Radiofrequency ablation of malignant liver tumors. Ann Surg Oncol. 2003;10:338–347. doi: 10.1245/aso.2003.07.017. [DOI] [PubMed] [Google Scholar]
- 131. Siperstein AE Rogers SJ Hansen PD et al. Laparoscopic thermal ablation of hepatic neuroendocrine tumor metastases. Surgery. 1997;122:1147–1155. doi: 10.1016/s0039-6060(97)90221-x. [DOI] [PubMed] [Google Scholar]
- 132. Horigome H Nomura T Nakao H et al. Half-deployed method: percutaneous radiofrequency ablation therapy using clustered electrodes for malignant liver tumors proximal to large vessels. AJR Am J Roentgenol. 2001;177:948. doi: 10.2214/ajr.177.4.1770948. [DOI] [PubMed] [Google Scholar]
- 133. Poon RT Ng KK Lam CM et al. Radiofrequency ablation for subcapsular hepatocellular carcinoma. Ann Surg Oncol. 2004;11:281–289. doi: 10.1245/aso.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 134. McGahan JP Browning PD Brock JM et al. Hepatic ablation using radiofrequency electrocautery: preliminary investigation. Invest Radiol. 1990;25:267–270. doi: 10.1097/00004424-199003000-00011. [DOI] [PubMed] [Google Scholar]
- 135. Rossi S Fornari F Pathies C et al. Thermal lesions induced by 480 KHz localized current field in guinea pig and pig liver. Tumori. 1990;76:54–57. doi: 10.1177/030089169007600114. [DOI] [PubMed] [Google Scholar]
- 136.Buscarini L, Fornari F, Rossi S. Interstitial radiofrequency hyperthermia in the treatment of small hepatocellular carcinoma: percutaneous US guidance of electrode needle. In: Anderegg et al., eds. Ultraschall Diagnostik 91. Berlin: Springer, 1992:218–222. [Google Scholar]
- 137. Miao Y Ni Y Mulier P et al. Radiofrequency liver ablation with saline infusion through a screw tip cannulated electrode. Radiology. 1996;201:422. doi: 10.1006/jsre.1997.5133. [DOI] [PubMed] [Google Scholar]
- 138. Tanabe KK Curley SA Dodd GD et al. Radiofrequency ablation: the experts weigh in. Cancer. 2004;100:641–650. doi: 10.1002/cncr.11919. [DOI] [PubMed] [Google Scholar]
- 139. Choy PY Koea J McCall J et al. The role of radiofrequency ablation in the treatment of primary and metastatic tumours of the liver: initial lessons learned. N Z Med J. 2002;115:U128. [PubMed] [Google Scholar]
- 140. Suh S Won J Lee D et al. Radio-frequency ablation of liver metastasis: comparison of percutaneous ablation and intraoperative ablation. J Vasc Interv Radiol. 2004;15:219. [Google Scholar]
- 141. Silas AM Kruskal JB Kane RA Intraoperative ultrasound. Radiol Clin North Am. 2001;39:429–448. doi: 10.1016/s0033-8389(05)70290-6. [DOI] [PubMed] [Google Scholar]
- 142. Rose SC Hassanein TI Bouvet M et al. Delivery of radiofrequency ablation probes to the targeted liver malignancy: using all the players on the field. J Vasc Interv Radiol. 2002;13:1060–1061. doi: 10.1016/s1051-0443(07)61875-2. [DOI] [PubMed] [Google Scholar]
- 143. Mahvi DP Lee FT Radiofrequency ablation of hepatic malignancies: is heat better than cold? Ann Surg. 1999;230:9–11. doi: 10.1097/00000658-199907000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Poon RT Fan ST Tsang FH et al. Locoregional therapies for hepatocellular carcinoma: a critical review from the surgeon's perspective. Ann Surg. 2002;235:466–486. doi: 10.1097/00000658-200204000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Siperstein A Garland A Engle K et al. Laparoscopic radiofrequency ablation of primary and metastatic liver tumors: technical considerations. Surg Endosc. 2000;14:400–405. doi: 10.1007/s004640000067. [DOI] [PubMed] [Google Scholar]
- 146. Smith MK Mutter D Forbes LE et al. The physiologic effect of the pneumoperitoneum on radiofrequency ablation. Surg Endosc. 2004;18:35–38. doi: 10.1007/s00464-001-8235-2. [DOI] [PubMed] [Google Scholar]
- 147. Kudo M Local ablation therapy for hepatocellular carcinoma: current status and future perspectives. J Gastroenterol. 2004;39:205–214. doi: 10.1007/s00535-003-1280-y. [DOI] [PubMed] [Google Scholar]
- 148.Kojiro M. The evolution of pathologic features of hepatocellular carcinoma. In: Tabor E, ed. Viruses and Liver Cancer. Amsterdam: Elsevier, 2002:113–122. [Google Scholar]
- 149. Ambiru S Miyazaki M Isono T et al. Hepatic resection for colorectal metastases: analysis of prognostic factors. Dis Colon Rectum. 1999;42:632–639. doi: 10.1007/BF02234142. [DOI] [PubMed] [Google Scholar]
- 150. Scott DJ Young WN Watumull LM et al. Accuracy and effectiveness of laparoscopic versus open hepatic radiofrequency ablation. Surg Endosc. 2000;14:162. doi: 10.1007/s004640080066. [DOI] [PubMed] [Google Scholar]
- 151. Antoch G Kuehl H Vogt FM et al. Value of CT volume imaging for optimal placement of radiofrequency ablation probes in liver lesions. J Vasc Interv Radiol. 2002;13:1155–1161. doi: 10.1016/s1051-0443(07)61958-7. [DOI] [PubMed] [Google Scholar]
- 152. Rose SC Hassanein TI Easter DW et al. Value of three-dimensional US for optimizing guidance for ablating focal liver tumors. J Vasc Interv Radiol. 2001;12:507–515. doi: 10.1016/s1051-0443(07)61892-2. [DOI] [PubMed] [Google Scholar]
- 153. Denys AL de Baere T Kuoch V et al. Radio-frequency tissue ablation of the liver: in vivo and ex vivo experiments with four different systems. Eur Radiol. 2003;13:2346–2352. doi: 10.1007/s00330-003-1970-0. [DOI] [PubMed] [Google Scholar]
- 154.Heaney CJ, Charboneau JW, Callstrom MR, et al. Superimposed image analysis of pre- and immediate post-RFA contrast-enhanced CT predicts local control of HCC. Radiological Society of North America, 89th Scientific Assembly and Annual Meeting Program, November 30-December 5, 2003, McCormick Palace, Chicago, 670.
- 155. Solbiati L Goldberg SN Livraghi T et al. Radiofrequency thermal ablation: increased treatment effect with saline pre-treatment. Radiology. 2000;217:607. [Google Scholar]
- 156. Zhang Z Wu M Chen H et al. Percutaneous radiofrequency ablation combined with transcatheter arterial chemoembolization for hepatocellular carcinoma. Zhonghua Wai Ke Za Zhi. 2002;40:826–829. [PubMed] [Google Scholar]
- 157. Goldberg SN Hahn PF Tanabe KK et al. Percutaneous radiofrequency tissue ablation: does perfusion mediated tissue cooling limit coagulation necrosis? J Vasc Interv Radiol. 1998;9:101–111. doi: 10.1016/s1051-0443(98)70491-9. [DOI] [PubMed] [Google Scholar]
- 158. Patterson EJ Scudamore CH Owen DA et al. Radiofrequency ablation of porcine liver in vivo: effects of blood flow and treatment time on lesion size. Ann Surg. 1998;227:559–565. doi: 10.1097/00000658-199804000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Nicoli N Casaril A Marchiori L et al. Intraoperative and percutaneous radiofrequency thermal ablation in the treatment of hepatocellular carcinoma. Chir Ital. 2000;52:29–40. [PubMed] [Google Scholar]
- 160. Lees WR Gillams AR Schumillian C et al. Hypotensive anaesthesia improves the effectiveness of radiofrequency ablation in the liver. Radiology 2000. 217 228 229 11012449 [Google Scholar]
- 161. Asch MR Kachura JR Gallinger S Safety and effect of percutaneous temporary portal vein occlusion on the size of thermal lesion in porcine livers. J Vasc Interv Radiol 2002. 13 61 11788696 [Google Scholar]
- 162. Nakashima Y Nakashima O Hsia CC et al. Vascularization of small hepatocellular carcinomas: correlation with differentiation. Liver. 1999;19:12–18. doi: 10.1111/j.1478-3231.1999.tb00003.x. [DOI] [PubMed] [Google Scholar]
- 163.Solbiati L, Ierace T, Goldberg SN, et al.Radiofrequency thermal ablation of liver metastases. In: Bartolozzi C, Lencioni R, eds. Liver Malignancies: Diagnostic and Interventional Radiology. New York: Springer, 1999:339–353. [Google Scholar]
- 164. Helton WS Minimizing complications with radiofrequency ablation for liver cancer: the importance of properly controlled clinical trials and standardized reporting. Ann Surg. 2004;239:459–463. doi: 10.1097/01.sla.0000120150.95389.ba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Fong Y A promising technique for liver cancer? Cancer J. 1999;5:339–340. [PubMed] [Google Scholar]
- 166. Gazelle GS Lester JS Goldberg SN et al. RF ablation vs hepatic resection in patients with suspected crc liver metastases: a decision-analytic model to assess cost-effectiveness. Radiology. 2002;225:488. [Google Scholar]
- 167. Gillams AR Lees WR Ledermann JR et al. Image guided ablation of colorectal liver metastases: time for a randomized, contrlled trial versus hepatic resection. Radiology. 1999;213:212–213. [Google Scholar]
- 168. Livraghi T Gazelle GS Percutaneous radiofrequency ablation of liver metastases in potential candidates for resection: the ‘test-of-time’ approach [Reply]. Cancer. 2003;98:2304–2305. doi: 10.1002/cncr.11426. [DOI] [PubMed] [Google Scholar]
- 169. Oshowo A Gillams A Harrison E et al. Comparison of resection and radiofrequency ablation for treatment of solitary colorectal liver metastases. Br J Surg. 2003;90:1240–1243. doi: 10.1002/bjs.4264. [DOI] [PubMed] [Google Scholar]
- 170. Birth M Hildebrand P Dahmen G et al. Present state of radio frequency ablation of liver tumors in Germany. Chirurg. 2004;75:417–423. doi: 10.1007/s00104-003-0801-9. [DOI] [PubMed] [Google Scholar]
- 171. Abdalla EK Vauthey JN Ellis LM et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg. 2004;239:818–825. doi: 10.1097/01.sla.0000128305.90650.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Bilchik AJ Faries M Radiofrequency ablation of hepatic malignancies: inexpensive and minimally invasive but should it replace resection? Ann Surg Oncol. 2003;10:1002–1004. doi: 10.1245/aso.2003.09.915. [DOI] [PubMed] [Google Scholar]
- 173. Elias D Radiofrequency: storm looming over hepatic surgery. Ann Chir. 2000;125:815–817. doi: 10.1016/s0003-3944(00)00013-4. [DOI] [PubMed] [Google Scholar]
- 174. Figueras J Llado L Percutaneous radiofrequency ablation of liver metastases in potential candidates for resection. The ‘test-of-time’ approach. Cancer. 2003;98:2303–2305. doi: 10.1002/cncr.11782. [DOI] [PubMed] [Google Scholar]
- 175.Full list of references is available from the corresponding author upon request.