Abstract
Objective:
The purpose of this study was to analyze our experience with saline-linked cautery in hepatic surgery.
Summary Background Data:
Safe and efficient hepatic parenchymal transection is predicated on the ability to simultaneously address 2 tasks: parenchymal dissection and hemostasis. To date, no single instrument has been designed that addresses both of these tasks. Saline-linked cautery is now widely used in liver surgery and is reported to decrease blood loss during liver transection, but data on its exact benefits are lacking.
Methods:
From a single institution, prospective liver surgery database, we identified 32 consecutive patients with noncirrhotic livers who underwent resection for primary or metastatic disease using a 2-surgeon technique with saline-linked cautery and ultrasonic dissection (SLC+UD) from December 2002 to January 2004. From the same database, we identified a contemporary and matched set of 32 patients who underwent liver resection with similar indications using ultrasonic dissection alone (UD alone). Operative and anesthetic variables were retrospectively analyzed to identify differences between the 2 groups.
Results:
The 2 groups were equivalent in terms of age, gender, tumor histology, tumor number, and tumor size. The UD+SLC group had a decreased duration of inflow occlusion (20 minutes versus 30 minutes, P = 0.01), blood loss (150 mL versus 250 mL, P = 0.034), and operative time (187 minutes versus 211 minutes, P = 0.027). Postoperative liver function and complication rates were similar in each group.
Conclusions:
The 2-surgeon technique for liver parenchymal transection using SLC and UD in noncirrhotic livers is safe and may provide advantages over other techniques.
The authors compare their experience with a novel technique for liver parenchymal transection using saline-linked cautery and ultrasonic dissection (n = 32) to a matched set of patients who underwent liver resection using ultrasonic dissection alone (n = 32). Analysis of operative variables reveals that patients undergoing liver resection using the new technique had shorter inflow occlusion time (20 minutes versus 30 minutes, P = 0.01), less blood loss (150 mL versus 250 mL, P = 0.034), and shorter operative time (187 minutes versus 211 minutes, P = 0.027), without an increase in morbidity.
Over the past 40 years, the safety of liver resection has dramatically improved. This point has recently been emphasized in several reports from high-volume centers, which have documented mortality rates of less than 5%, even for major liver resection.1–3 The enhanced safety of liver surgery has been attributed to a number of factors, most notably the development of anesthetic and surgical techniques that reduce intraoperative blood loss. These techniques include low central venous pressure anesthesia, the use of vascular inflow occlusion, and the development and use of more effective instruments for parenchymal transection.
Efficient and safe liver parenchymal transection is dependent on the ability to simultaneously address 2 tasks: parenchymal division and hemostasis. Because no single instrument has been developed that is adequate for both of these tasks, most hepatic parenchymal transections are performed using a combination of instruments and techniques. In our practice, we have used an ultrasonic dissector for transection and monofilament suture ligatures and titanium clips to accomplish hemostasis. With this technique, our intraoperative blood loss was acceptable, and morbidity and mortality rates were low. In 2001, we began using a saline-linked cautery device to coagulate and divide small parenchymal vessels and for liver surface hemostasis. The device delivers energy in the radiofrequency range via a continuous stream of saline dripped from the device tip. The system coagulates small vessels and liver parenchyma without char production, which is the limitation of standard electrocautery.
When we combined saline-linked cautery (SLC) with ultrasonic dissection (UD) during liver transection, we noted shorter operative times and a reduction in the duration of hepatoduodenal ligament occlusion. In this paper, we report a statistical analysis of multiple preoperative and perioperative variables related to 64 matched liver resections, comparing our experience before and after the addition of SLC to UD for liver transection.
PATIENTS AND METHODS
Following institutional review board approval, we searched a single institution prospectively collected hepatobiliary surgical database for noncirrhotic patients who underwent liver resection for primary hepatobiliary malignancy or metastatic disease. After excluding cases with cirrhosis, severe steatosis, and cases with associated procedures, such as combined liver and colon resection, we identified 32 consecutive liver resections performed from December 2002 to January 2004, in which UD and SLC (UD+SLC) were used for liver transection. We then identified a group of 32 patients with similar demographic and pathologic features who underwent liver resection where UD was used alone (UD alone). All patients in the UD alone group underwent liver resection between September 2001 and December 2003. The 2 hepatobiliary surgeons (J.-N.V., E.K.A.) who performed all procedures introduced the combined technique into their practice sequentially. In total, both surgeons performed 145 liver resections during the study period (September 2001 to January 2004).
Postoperative morbidity was measured in 2 ways. First, we tested all patients for the development of abnormal liver function. Peak total bilirubin, alanine aminotransferase, aspartate aminotransferase, and prothrombin time through patient discharge were recorded. Second, patients were monitored for the development of postoperative fluid collections and/or biliary fistulas. For the purpose of this study, we defined biliary fistula as bilious drainage lasting more than 7 postoperative days.4
At the conclusion of each liver resection, the attending surgeon and anesthesiologist jointly completed a hepatobiliary surgery data sheet that included a number of operative and anesthetic variables. The attending anesthesiologist determined intraoperative blood loss by subtracting the amount of irrigant fluid instilled during the procedure, which was recorded on an ongoing basis during the operation, from thetotal amount of fluid contained in the suction canister at the conclusion of the procedure. This calculation included the fluid wrung from all laparotomy pads used during the procedure and aspirated into the same canister. All data points recorded on the hepatobiliary surgery data sheet were then entered into the prospective hepatobiliary surgery database.
For statistical analysis, demographic, pathologic, and perioperative details were extracted from this database, and differences in operative and anesthetic variables between the 2 patient groups were compared using the χ2 test and the Mann-Whitney U test. Peak liver function test values were compared between the groups using the Student t test. We performed all statistical analyses with the STATISTICA software program (version 6.1, StatSoft, Inc., Tulsa, OK).
Operative Technique
Following laparotomy and exploration for intra-abdominal metastasis, the liver was mobilized in the standard fashion. Intraoperative sonography was used to confirm the extent of disease and to plan the parenchymal transection plane. Glisson capsule was scored with electrocautery, and 4-0 polypropylene stay sutures were placed along the plane of intended transection. The primary surgeon dissected the hepatic parenchyma from the patient's left side using the Cavitron Ultrasonic Surgical Aspirator System (CUSA, Valleylab, Boulder, CO). The secondary surgeon operated the SLC device (Dissecting Sealer DS 3.0, TissueLink Medical, Inc., Dover, NH) from the patient's right side (Fig. 1). Inflow occlusion was used only when bleeding from the transection plane obscured visualization of the intraparenchymal anatomy.
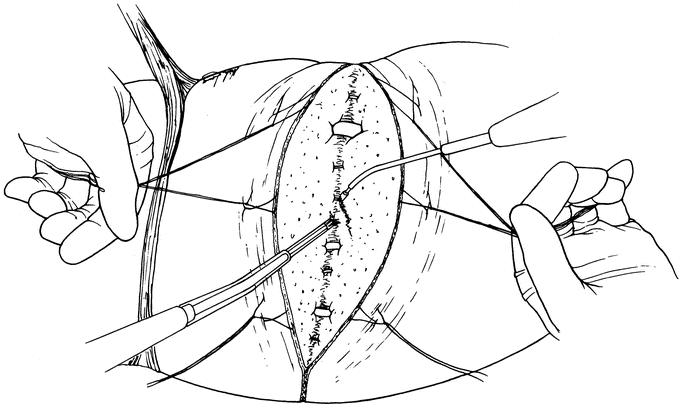
FIGURE 1. Two-surgeon technique for hepatic parenchymal transection. Using the ultrasonic dissection device, the primary surgeon directs the dissection from the patient's left side. Simultaneously, the secondary surgeon operates the saline-linked cautery device from the patient's right side. Traction on 4-0 polypropylene stay sutures is used to expose the deepening transection plane.
We did not use the SLC device to divide liver parenchyma. The liver parenchyma was dissected, and the intraparenchymal vascular anatomy was defined using the UD device so that a decision on hemostatic technique could be made based on vessel size. The SLC device was used to coagulate and divide dissected vessels 3 mm or smaller. Vessels from 3 mm to 5 mm were controlled with titanium clips and divided sharply. The few larger vessels and portal triads that were encountered were ultrasonically dissected, controlled with 3-0 silk ties in continuity, and divided sharply (Fig. 2). Traction on the stay sutures was used to separate and expose the deepening transection plane. We selectively placed closed suction drains at the conclusion of each procedure.
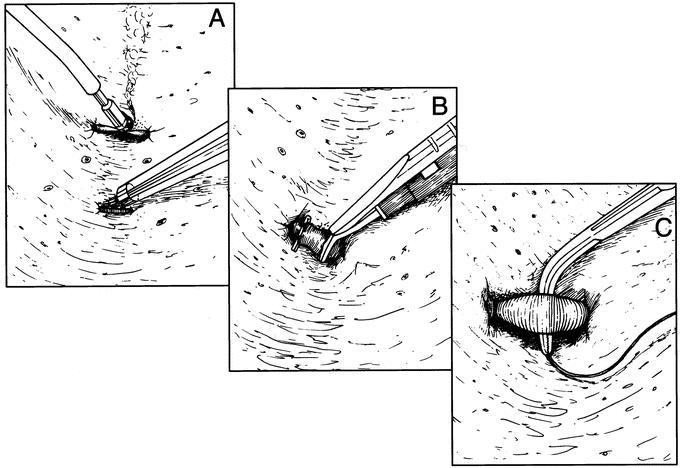
FIGURE 2. The technique for vascular control during parenchymal transection is based on vessel size. A, Vessels less than 3 mm are coagulated and divided using saline-linked cautery. B, Vessels from 3 to 5 mm are controlled with titanium clips and sharply divided. C, Vascular structures larger than 5 mm are controlled with 3-0 silk ties and sharply divided.
RESULTS
Table 1 demonstrates that the distribution of demographic and pathologic variables was equivalent between the 2 patient groups. The distribution of tumor number, tumor size, and extent of resection was also equal between the 2 groups (Table 2). There were no procedures in which gross positive margins were seen. Final pathologic analysis identified 3 patients in the UD+SLC group and 2 patients in the UD alone group with microscopic positive margins (P = 0.64, χ2 test). We observed no difference in the number of complications between the 2 study groups (Table 2). There were no perioperative deaths in either group, and no patients required reexploration. Three patients in the UD+SLC group and 2 patients in the UD alone group required percutaneous drainage for fluid collection and/or biliary fistula. The difference in the incidence of percutaneous drainage was not statistically significant (P = 0.64, χ2 test). In each case, the collection or fistula resolved following a short course of percutaneous drainage and, when indicated, antibiotic therapy.
TABLE 1. Demographic and Pathologic Variables
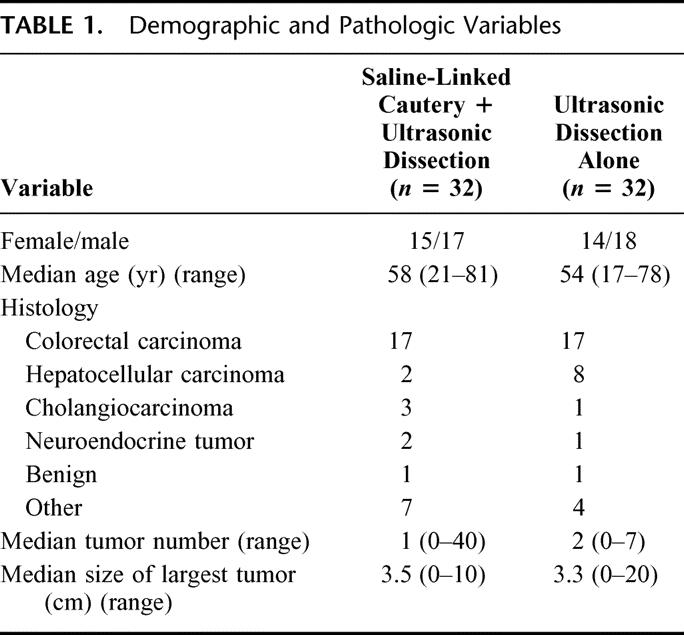
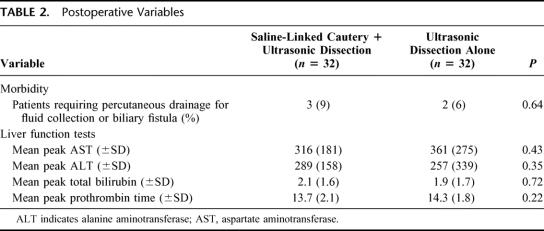
TABLE 2. Postoperative Variables
Results of our statistical analysis of operative and anesthetic variables are shown in Table 3. This analysis revealed that inflow occlusion time was shorter in the group of patients undergoing liver resection using the 2-surgeon technique. The percentage of cases in each group where an interval of inflow occlusion was used was similar; however, median total inflow occlusion time was reduced from 30 minutes to 20 minutes (P = 0.01). In the UD+SLC group we saw a concomitant reduction in operative blood loss. Patients in this group had a median blood loss of 150 mL versus 250 mL in the UD alone group (P = 0.034). Finally, median operative time was significantly lower when SLC was used (187 minutes versus 211 minutes, P = 0.027). The shorter total operative time was independent of the extent of the resection (Fig. 3).
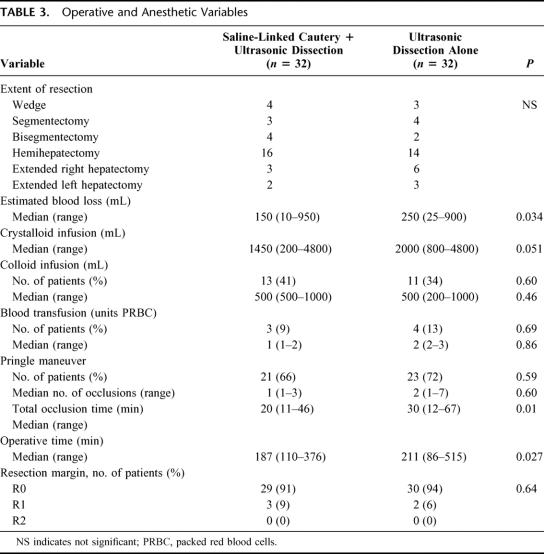
TABLE 3. Operative and Anesthetic Variables
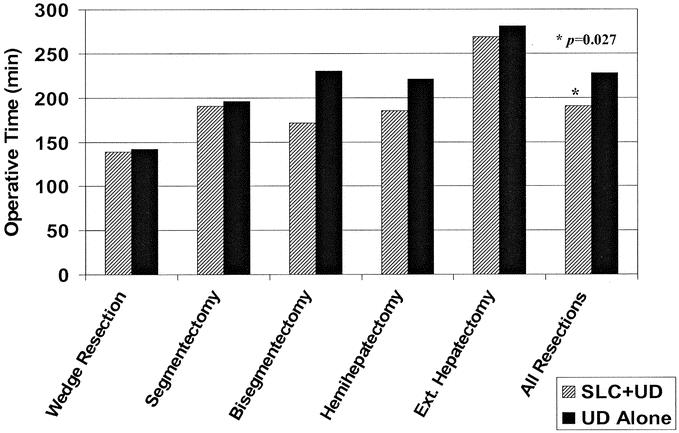
FIGURE 3. Comparison of operative time based on parenchymal transection technique and extent of resection. SLC+UD, saline-linked cautery and ultrasonic dissection; UD alone, ultrasonic dissection alone.
DISCUSSION
The task of parenchymal division in liver surgery has traditionally been accomplished with a crushing technique.5,6 Although simple and rapid, the use of finger fracture and crush-clamp techniques can be associated with significant peritransectional blood loss. This has led to the development of more precise instruments for parenchymal transection, including high-velocity water jet and ultrasonic dissection.7,8 In 1996, Fan et al compared parenchymal transection using a crush-clamp technique to an ultrasonic dissection technique and found that parenchymal transection using the ultrasonic dissection technique was associated with a reduction in blood loss and transfusion requirements.9 Although UD does not directly address hemostasis, when compared with crushing techniques, it may enhance visualization of intraparenchymal vessels that cross the transection plane. This may allow for vascular control prior to division with a reduction in blood loss during transection.
Hemostasis during parenchymal transection has traditionally been addressed with suture ligature, surgical clips, and topical agents. Standard electrocautery has not been effective in controlling bleeding from the transected liver; however, a number of advanced electrocautery devices have been introduced for this purpose. In the early 1990s, argon beam coagulation was introduced as an advance over standard electrocautery, biologic glue, and sutures for liver surface hemostasis.10 In 1997, Rau et al introduced the technique of transection with high-frequency current supported by a saline jet and reported shortened transection times.11 Later, standard bipolar cautery was applied to liver transection, and the value of intraparenchymal vessel precoagulation in reducing peritransectional bleeding was appreciated.12 Taking the precoagulation concept to the extreme, Weber et al have reported the technique of heat coagulative necrosis, where multiple linear cautery probes are inserted into the liver parenchyma along the transection plane prior to division.13 Finally, an early experience with a dedicated bipolar vessel-sealing device has been described.14,15
Despite its widespread implementation in liver surgery, structured analyses of experience with SLC are lacking. Review of the literature reveals only one report in which the use of SLC in liver surgery is compared with traditional techniques. In January 2004, Sakamoto et al retrospectively compared their experience with 16 liver resections in which SLC was used in combination with a bipolar vessel-sealing device and a matched set of 16 patients undergoing liver resections in which a crush-clamp technique was used.15 They found that fewer patients in the SLC group required inflow occlusion and that blood loss was reduced. Differences in total operative time were not reported, but liver transection time was prolonged in the SLC group.
Another group has recently published a small-volume experience where SLC was used for hemostasis in liver resection.16 In 8 of the 9 reported cases, only 2 of which were major hepatectomies, transection was accomplished using a crushing technique and the CUSA device. Unlike our technique, this group appears to have used SLC following transection to achieve hemostasis at the cut surface of the liver. Anecdotally, the authors report a reduction in estimated blood loss and minimal need for hepatic inflow occlusion; however, a comparison to other transection techniques is lacking.
Our data show that, in 2 well-matched patient groups undergoing liver resection, the addition of SLC to UD for parenchymal transection results in a reduction in operative time. We account for the shorter operative times seen in the UD+SLC group in several phases of the operation. Before the introduction of SLC, we separated the parenchymal dissection and hemostasis tasks but used time-consuming suture ligation to control active bleeding. Using SLC, we are able to coagulate small vessels prior to division and find that the need for suture control of intraparenchymal vessels is minimized and that there is a marked reduction in time taken to pass instruments.
Limiting the time required for hemostasis during and after liver transection may also reduce the length of the operation. We have observed that our technique results in less bleeding during transection and bleeding from the remnant liver surface at the conclusion of transection is usually insignificant. This observation is supported by our data, which show that patients who were operated on using UD with SLC had significantly less operative blood loss. Although the analysis of our data shows a difference in estimated blood loss between the 2 study groups, this did not translate into a difference in the number of units transfused. This is likely because the absolute blood loss in each group was low and the number of patients who required transfusion was less than 15%.
Finally, our technique may also shorten operative time by allowing 2 surgeons to simultaneously participate in the parenchymal transection. The primary surgeon directs the dissection using the UD device, and the secondary surgeon is actively involved by using the SLC device for hemostasis. In addition to maximizing the efficiency of liver transection, the 2-surgeon technique also creates an ideal environment for training residents and fellows in hepatic surgery. While retaining control of the dissection, the attending surgeon can help the trainee to correctly delineate the vascular and biliary anatomy within the transection plane. Operative time is not compromised, and the goal of low intraoperative blood loss is maintained.
Our data also show that, in the UD+SLC group, there was less dependence on inflow occlusion during transection. When inflow occlusion was used, its duration was 30% shorter than in patients who underwent transection without SLC. Although we recognize that a controlled period of ischemic preconditioning just prior to liver transection may be beneficial,17,18 it is clear that prolonged inflow occlusion during parenchymal transection can be detrimental.19 We find that our technique leads to a rapid transection of the liver parenchyma and a reduction in prolonged ischemic times.
There are several limitations to this study that warrant comment. First, the study design was a nonrandomized comparison between 2 groups of patients undergoing liver resection. Patients with cirrhosis and those with severe steatosis, in whom SLC may not be as effective, were excluded from the analysis. Despite this potential limitation, both groups were contemporary and well matched for other demographic, pathologic, and operative variables. Second, other factors not captured by our analysis may have contributed to the decreased operative time. In our recent practice, for example, we have tended to use a midline incision for left liver resections, which may be technically easier and faster to close. Third, this study compared SLC to manual hemostatic techniques, not to another thermocoagulation device. Some surgeons use standard bipolar cautery to coagulate small intraparenchymal vessels in a fashion similar to our SLC technique. We find that SLC is an advance over standard bipolar cautery because small vessels are not only coagulated by SLC but can also be divided using the energy produced by the device, reducing the passage of instruments during parenchymal transection. In addition, other surgeons have reported the ability of SLC to produce necrosis at the transected liver surface up to 20 mm in depth, which may give SLC an advantage over other electrocautery systems in terms of margin control.20
To date, no single instrument has been developed for liver surgery that adequately addresses the tasks of parenchymal division and hemostasis. Our data suggest that a targeted approach, which separates the tasks of parenchymal division and hemostasis, has several advantages. In addition, we find that our technique balances the demands of preserving normal hepatic parenchyma and maintaining adequate oncologic margins. While the potential benefits of the 2-surgeon technique on oncologic outcome await confirmation in future studies, we introduce the technique of hepatic parenchymal transection using SLC and UD as a safe approach with advantages over other surgical techniques.
Footnotes
Reprints: Jean-Nicolas Vauthey, MD, Department of Surgical Oncology, University of Texas M.D. Anderson Cancer Center, 1515 Holcombe Boulevard, Unit 444, Houston, TX 77030. E-mail: jvauthey@mdanderson.org.
REFERENCES
- 1.Belghiti J, Hiramatsu K, Benoist S, et al. Seven hundred forty-seven hepatectomies in the 1990s: an update to evaluate the actual risk of liver resection. J Am Coll Surg. 2000;191:38–46. [DOI] [PubMed] [Google Scholar]
- 2.Vauthey JN, Pawlik TM, Abdalla EK, et al. Is extended hepatectomy for hepatobiliary malignancy justified? Ann Surg. 2004;239:722–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fan ST, Lo CM, Liu CL, et al. Hepatectomy for hepatocellular carcinoma toward zero hospital deaths. Ann Surg. 1999;229:322–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fong Y, Brennan MF, Brown K, et al. Drainage is unnecessary after elective liver resection. Am J Surg. 1996;171:158–162. [DOI] [PubMed] [Google Scholar]
- 5.Lin TY. A simplified technique for hepatic resection: the crush method. Ann Surg. 1974;180:285–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bismuth H. Surgical anatomy and anatomical surgery of the liver. World J Surg. 1982;6:3–9. [DOI] [PubMed] [Google Scholar]
- 7.Hodgson WB, DelGuercio LM. Preliminary experience in liver surgery using the ultrasonic scalpel. Surgery. 1984;95:230–234. [PubMed] [Google Scholar]
- 8.Baer HU, Gilg M, Maddern GJ, et al. High velocity water jet dissection in liver surgery. Helv Chir Acta. 1992;59:437–442. [PubMed] [Google Scholar]
- 9.Fan ST, Lai ECS, Lo CM, et al. Hepatectomy with an ultrasonic dissector for hepatocellular carcinoma. Br J Surg. 1996;83:117–120. [DOI] [PubMed] [Google Scholar]
- 10.Postema RR, Plaisier PW, ten Kate FJW, et al. Haemostatis after partial hepatectomy using argon beam coagulation. Br J Surg. 1993;80:1563–1565. [DOI] [PubMed] [Google Scholar]
- 11.Rau HG, Buttler ER, Baretton G, et al. Jet-cutting supported by high frequency current: new technique for hepatic surgery. World J Surg. 1997;21:254–259. [DOI] [PubMed] [Google Scholar]
- 12.Yamamoto Y, Ikai I, Kume M, et al. New simple technique for hepatic parenchymal resection using a Cavitron Ultrasonic Surgical Aspirator and bipolar cautery equipped with a channel for water dripping. World J Surg. 1999;23:1032–1037. [DOI] [PubMed] [Google Scholar]
- 13.Weber JC, Navarra G, Jiao LR, et al. New technique for liver resection using heat coagulative necrosis. Ann Surg. 2002;236:560–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strasberg SM, Drebin JA, Linehan D. Use of a bipolar vessel-sealing device for parenchymal transection during liver surgery. J Gastrointest Surg. 2002;6:569–574. [DOI] [PubMed] [Google Scholar]
- 15.Sakamoto Y, Yamamoto J, Kokudo N, et al. Bloodless liver resection using the monopolar floating ball plus ligasure diathermy: preliminary results of 16 liver resections. World J Surg. 2004;28:166–172. [DOI] [PubMed] [Google Scholar]
- 16.Di Carlo I, Barbagallo F, Toro A, et al. Hepatic resections using a water-cooled, high-density, monopolar device: a new technology for safer surgery. J Gastrointest Surg. 2004;8:596–600. [DOI] [PubMed] [Google Scholar]
- 17.Imamura H, Takayama T, Sugawara Y, et al. Pringle's manoeuvre in living donors. Lancet. 2002;360:2049–2050. [DOI] [PubMed] [Google Scholar]
- 18.Clavien P, Yadav S, Sindram D, et al. Protective effects of ischemic preconditioning for liver resection performed under inflow occlusion in humans. Ann Surg. 2000;232:155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wei AC, Poon R, Fan ST, et al. Risk factors for perioperative morbidity and mortality after extended hepatectomy for hepatocellular carcinoma. Br J Surg. 2003;90:33–41. [DOI] [PubMed] [Google Scholar]
- 20.Topp SA, McClurken M, Lipson D, et al. Saline-linked surface radiofrequency ablation: factors affecting steam popping and depth of injury in the pig liver. Ann Surg. 2004;239:518–527. [DOI] [PMC free article] [PubMed] [Google Scholar]