Abstract
Objective:
To measure sexual function and quality of life (QOL) after rectal cancer treatment.
Summary Background Data:
Previous studies on sexual function after rectal cancer treatment have focused on males and have not used validated instruments.
Methods:
Patients undergoing curative rectal cancer surgery from 1980 to 2003 were administered a questionnaire, including the Female Sexual Function Index (FSFI) or International Index of Erectile Function (IIEF), and the EORTC QLQ-C30/CR-38. Multiple logistic regression was used to test associations of clinical factors with outcomes.
Results:
Eighty-one women (81.0%) and 99 men (80.5%) returned the questionnaire; 32% of women and 50% of men are sexually active, compared with 61% and 91% preoperatively (P < 0.04); 29% of women and 45% of men reported that “surgery made their sexual lives worse.” Mean (SD) FSFI and IIEF scores were 17.5 (11.9) and 29.3 (22.8). Specific sexual problems in women were libido 41%, arousal 29%, lubrication 56%, orgasm 35%, and dyspareunia 46%, and in men libido 47%, impotence 32%, partial impotence 52%, orgasm 41%, and ejaculation 43%. Both genders reported a negative body image. Patients seldom remembered discussing sexual risks preoperatively and seldom were treated for dysfunction. Current age (P < 0.001), surgical procedure (P = 0.003), and preoperative sexual activity (P = 0.001) were independently associated with current sexual activity. Gender (male, P = 0.014), surgical procedure (P = 0.005), and radiation therapy (P = 0.0001) were independently associated with the outcome “surgery made sexual life worse.” Global QOL scores were high.
Conclusions:
Sexual problems after surgery for rectal cancer are common, multifactorial, inadequately discussed, and untreated. Therefore, sexual dysfunction should be discussed with rectal cancer patients, and efforts to prevent and treat it should be increased.
Validated questionnaires measuring sexual function and quality of life (QOL) were returned by 180 patients curatively treated for rectal cancer. Male and female sexual dysfunction was common and multifactorial, but overall QOL was preserved. Male gender, abdominoperineal resection, and radiation therapy were independently associated with an increased rate of sexual dysfunction.
Surgery for rectal cancer may result in physiologic and psychologic changes that alter a patient's sexual functioning and quality of life (QOL). While a number of studies have examined rates of sexual dysfunction and QOL scores after surgery for rectal cancer, the primary focus has been on the male sexual issues of erectile dysfunction and retrograde ejaculation.1–4 Other factors that may contribute to sexual dysfunction such as the patient's psychologic response to the cancer, scar or ostomy, and the reaction of the sexual partner have not been examined. Moreover, female sexual dysfunction after surgery for rectal cancer has been relatively ignored,5 due in part to the reluctance of women with rectal cancer to respond to questions about their sexuality.6–10 Where sexual issues in women have been studied, the focus has usually been on dyspareunia. In addition, validated instruments for measuring sexual functioning have seldom been used in studies of sexual changes after rectal cancer surgery.11
The goals of the current study were: 1) to assess the frequency of female and male sexual dysfunction, using validated instruments, 2) to determine factors associated with posttreatment sexual problems, and 3) to qualitatively examine contributing factors to and manifestations of these problems.
METHODS
Study Population
For this study, all patients with rectal cancer who had undergone surgery with curative intent at the Mount Sinai Hospital from 1980 to 2003 were identified from a database of all colorectal cancer operations. Living rectal cancer patients were included if they were younger than 86 years at the time of the survey, English-speaking, and free of recurrent disease.
A total of 512 patients with rectal cancer operations were identified. Of these, 200 were deceased, 11 had palliative surgery, 22 were alive with recurrence, 26 were >85 years old, 7 did not speak English, 19 were lost to follow-up, 2 had dementia, and 2 did not wish to be contacted. Therefore, 223 patients (100 women and 123 men) were mailed the questionnaire packet.
This study was conducted with the approval of the Mount Sinai Hospital Research Ethics Board.
Questionnaires
Survey Procedures
Self-administered questionnaires were implemented using multiple wave mailings after the survey methods of Dillman.12 Females were sent the Female Sexual Function Index, the European Organization for Research and Treatment of Cancer (EORTC) QLQ-C30 and -CR38 questionnaires, and the female version of a questionnaire developed by our group. Males were sent the International Index of Erectile Function, the EORTC QLQ-C30 and -CR38 questionnaires, and the male version of our questionnaire.
Female Sexual Function Index
The Female Sexual Function Index (FSFI) is a 19-item, validated questionnaire that was designed to evaluate female sexual dysfunction, especially female sexual arousal disorder.13 The scale is divided into 6 domains: desire (D), arousal (A), lubrication (L), orgasm (O), satisfaction (S), and pain (P). Each item is scored with a 5- or 6-point Likert scale. The maximum score for each domain is 6 and the maximum total score is 36. For the present study, a total score more than 1 standard deviation below the mean of a normal population (as reported by Rosen et al13) was considered “abnormal” (total score <25.2).
International Index of Erectile Function
The International Index of Erectile Function (IIEF) is a 15-item, validated instrument designed to measure sexual functioning in males.14 The index is comprised of 5 domains, erectile function (EF), orgasmic function (OF), sexual desire (SD), intercourse satisfaction (IS) and overall satisfaction (OS). Each item of the IIEF is scored on a 5- or 6-point Likert scale. Scores for each domain are variable, and the total score range is 5 to 75. For the present study, a total score more than 1 standard deviation below the mean of a normal group (as reported by Rosen et al14) was considered “abnormal” (total score less than 42.9).
EORTC QLQ-C30 and EORTC QLQ-CR38
The EORTC QLQ-C30 and EORTC QLQ-CR38 are validated instruments designed to measure QOL in colorectal cancer patients.15,16 The C30 “core” cancer QOL questionnaire has 15 domains, and the colorectal cancer-specific CR-38 questionnaire has 12 domains. Calculated domain scores range from 0 to 100. For functional scales, including the Global Health Status/QOL domain (QL2) and the Sexual Functioning (SX) and Sexual Enjoyment domains (SE), higher scores reflect better functioning. For symptom scales, including the Female and Male Sexual Problem domains (FSX and MSX), a higher score reflects more symptoms and worse functioning. Although scores for all domains were obtained, only the domains relevant to the current study (QL2, SX, SE, FSX, and MSX) were analyzed, as suggested by the EORTC Study Group on Quality of Life.16
Additional Questionnaire
An additional questionnaire was designed to measure a patient's medical and sexual history. It also contains items designed to determine whether the ostomy or bowel function, urinary function, body image, and psychologic and/or physical effects contribute to a patient's reported sexual dysfunction. Separate female and male versions of the questionnaire (female, 33 items; male, 30 items) were developed. The questionnaire was tested for face validity by a panel of colorectal surgeons and nurses, and it was pretested in a small group of rectal cancer patients.
Imputing Scores
For each questionnaire item, some respondents may have intentionally or accidentally given no response. For the FSFI and IIEF, subjects who failed to respond were excluded from the analyses of total scores and affected domain scores. For the EORTC, the mean was imputed for missing items when at least 50% of domain items were completed, according to EORTC scoring guidelines.17
Surgical Technique
Since the 1980s, “nerve-preserving” surgery has been the standard of care in the treatment of rectal cancer at our institution. Surgical technique combines total mesorectal excision with identification and preservation of the hypogastric and splanchnic nerves, along with the pelvic autonomic nerve plexus. These nerves are only sacrificed if there is direct tumor extension. Denonvillier's fascia is left on the prostate, unless the tumor is anterior and at this level. The inferior mesenteric artery is usually divided at its origin.
Statistical Analysis
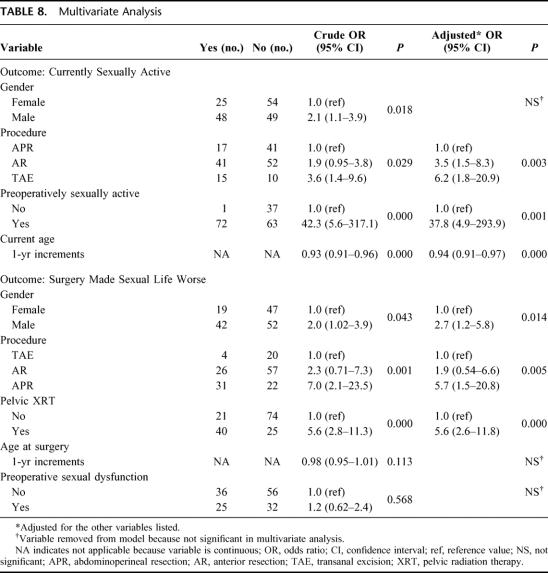
Results were reported descriptively, and Student t tests and univariate χ2 tests were performed where appropriate. Multiple logistic regression was used to determine clinical factors predicting 2 dichotomous outcomes: 1) currently sexually active and 2) “surgery made sexual life worse.” The models were created using a modified backward, stepwise methodology where candidate variables were identified a priori from a literature review. The candidate variables for the 2 models are listed in Table 8. SPSS for Windows version 12.0 (SPSS, Inc., Chicago, IL) was used to perform statistical tests.
TABLE 8. Multivariate Analysis
RESULTS
Response Rate and Study Population
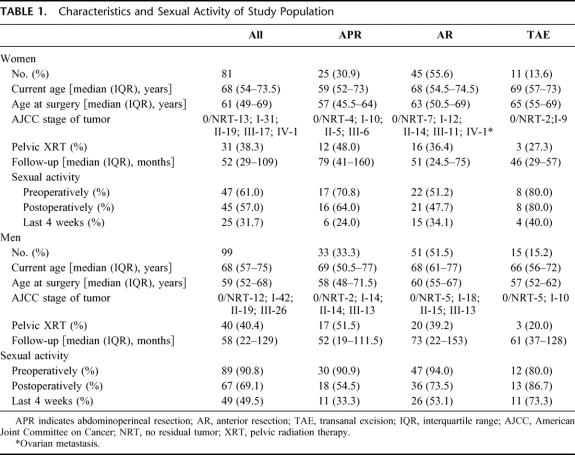
Eighty-one of 100 women (81.0%) and 99 of 123 men (80.5%) completed and returned the questionnaire packet. Of these 180 subjects, 98 patients left one or more question blank. Characteristics of the study population are shown in Table 1, along with a summary of patients’ levels of sexual activity before surgery, after surgery, and during “the last 4 weeks.”
TABLE 1. Characteristics and Sexual Activity of Study Population
Female Sexual Function and Quality of Life
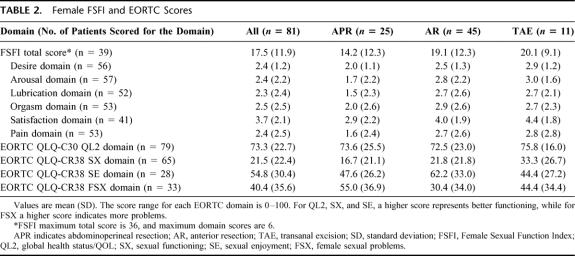
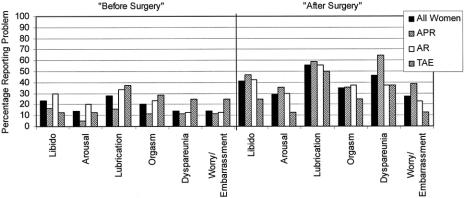
Responses of female study subjects to the questionnaires are summarized in Tables 2 and 3 and Figure 1. The mean FSFI total and domain scores are lower than those previously reported for patients with female sexual arousal disorder, except for the S domain (Table 2).13 The abdominoperineal resection (APR) patients had the lowest mean scores in all domains, particularly for pain (P). Twenty-four (61.5%) of the 39 patients who answered all FSFI items had a total score more than one standard deviation below the mean of a normal population.
TABLE 2. Female FSFI and EORTC Scores
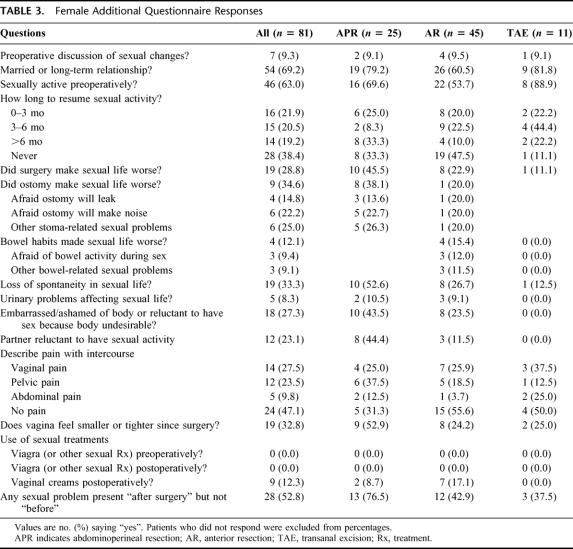
TABLE 3. Female Additional Questionnaire Responses
FIGURE 1. Specific female sexual problems before and after surgery.
Table 3 summarizes women's responses to items of the additional questionnaire. Of note, relatively fewer women in the anterior resection (AR) group were in a relationship, a factor that probably influenced the likelihood of postoperative sexual activity for that group. Figure 1 depicts the percentages of women reporting various sexual symptoms “before surgery” and “after surgery.” Twenty-seven women (49.1%) reported dyspareunia “after surgery,” compared with 14.0% “before surgery.”
Women Currently Sexually Active
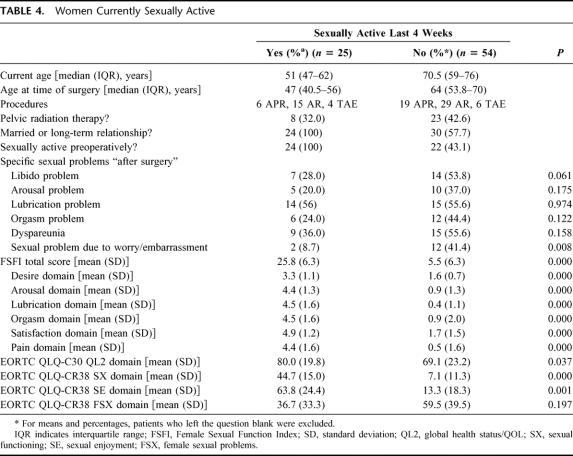
Twenty-five of the 81 women stated they are currently sexually active. These women were compared with those not sexually active in Table 4. Sexually active women were younger and more likely to have been sexually active before surgery, and more likely to be in a relationship. The FSFI and EORTC sexual domain scores for this group were significantly better than for those not sexually active (P < 0.001). However, more than half of both groups reported new sexual problems “after surgery.”
TABLE 4. Women Currently Sexually Active
Women for Whom “Surgery Made Sexual Life Worse”
There were 19 women (28.8%) who stated that “surgery made their sexual lives worse.” The median age at surgery was 50 years. Ten patients had an APR, 8 had an AR, and 1 had a transanal excision (TAE). Fourteen had pelvic radiation therapy (73.7%). Twenty-six percent were sexually active within the last 4 weeks, while 89.5% were sexually active before surgery.
These women reported multiple contributing factors. The ostomy caused a negative change in sexual life for 8 of 10 APR patients (80%). Three of 10 stated they were afraid their appliance may leak, 5 were afraid the ostomy will make noise, and 5 had other reasons (“embarrassed, less spontaneous;” “ex-husband could not deal with it;” “image;” “I feel damaged”). Four of 7 patients without an ostomy felt that bowel changes made their sexual life worse. Ten of 19 were ashamed of their body (0 “before surgery”). Eleven believed that their partner was reluctant to have sex with them after surgery, 7 believed their partner found them less attractive, and 7 believed their partner was afraid of hurting them. Seventeen women (94.4%) reported a loss of spontaneity.
Sixteen patients in this group (94.1%) reported specific sexual problems “after surgery” but not “before surgery.” Excluding those who reported having the same problem “before surgery,” percentages with each problem were as follows: libido problems, 72.7%; arousal problems, 66.7%; lubrication problems, 78.6%; orgasm problems, 75.0%; and dyspareunia 100%. With respect to the character of pain with intercourse, 7 patients reported vaginal pain with intercourse, and 8 reported pelvic pain with intercourse. Thirteen of 16 said their vagina “feels smaller or tighter” since surgery.
Male Sexual Function and Quality of Life
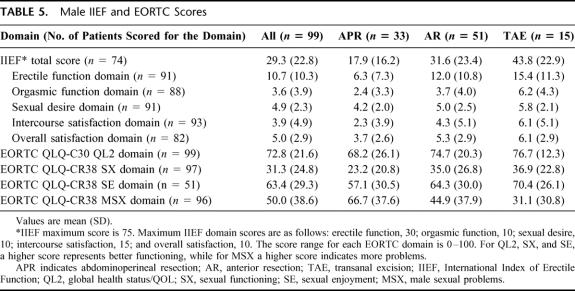
Responses of male subjects to the questionnaires are summarized in Tables 5 and 6 and Figure 2. The mean IIEF total and domain scores were low (Table 6) and were similar to the mean scores of patients with erectile dysfunction (ED) reported by Rosen et al.14 Fifty-one (68.9%) of the 74 patients who answered all IIEF items had an abnormal total score.
TABLE 5. Male IIEF and EORTC Scores
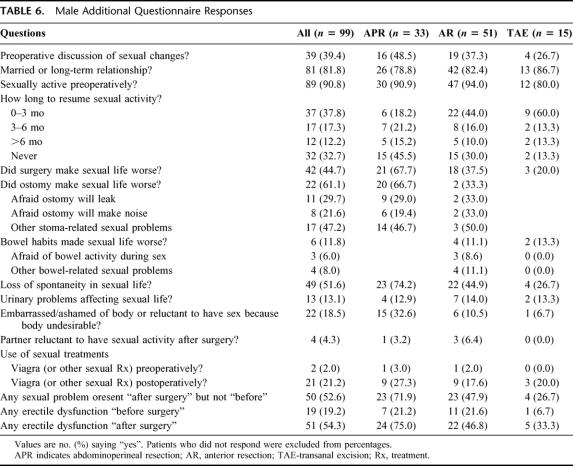
TABLE 6. Male Additional Questionnaire Responses
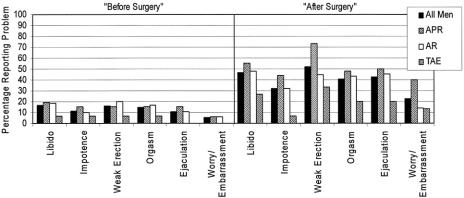
FIGURE 2. Specific male sexual problems before and after surgery.
Some degree of ED was reported by 19.2% of men “before surgery” and 54.3% of men “after surgery,” while complete impotence was reported by 11.2% “before surgery” and 31.9% “after surgery.” Percentages were higher in the APR group. Abnormal ejaculation was reported by 10.4% of men “before surgery” and 42.5% “after surgery.”
Men Currently Sexually Active
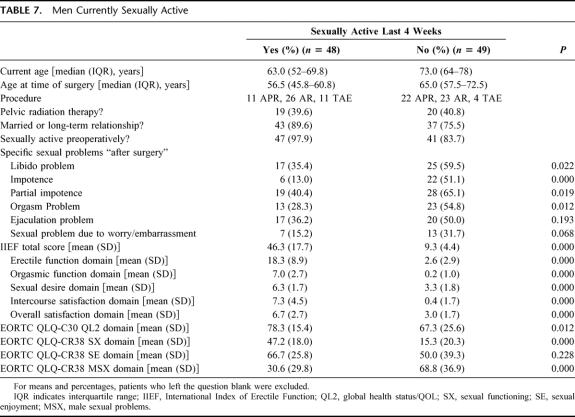
The subset of men who were sexually active within the last 4 weeks includes 48 individuals. Data for this group are compared with those not sexually active in Table 7. Sexually active patients were younger and more likely to be in a relationship than those not sexually active. These men reported significantly fewer sexual problems and had better sexual scores than the men not currently sexually active. Nevertheless, 22 patients who were currently sexually active (46.8%) reported new sexual problems “after surgery.”
TABLE 7. Men Currently Sexually Active
Men for Whom “Surgery Made Sexual Life Worse”
Forty-two men stated their sexual life was worse due to surgery. Their median age at the time of surgery was 59 years. Twenty-one had an APR, 18 had an AR, and 3 had a TAE. Twenty-five (59.5%) had XRT, including all 3 of the TAE patients; 100% were sexually active preoperatively, while 40.5% were currently sexually active.
The ostomy caused a negative change in sexual life for 85.7% of the APR patients in this group. Six patients were afraid that the appliance would leak, 3 were afraid the ostomy would make noise, and 9 had other reasons (“appearance,” “odor,” “body image,” “smell,” “movement during sex,” “feel less manly,” “impotence”). Four (22.2%) of the patients without an ostomy felt that bowel problems affected their sexual lives. A loss of spontaneity was reported by 95.2%. In terms of body image, 32.5% were ashamed of their body (0 “before surgery”).
Thirty-nine of the men (92.9%) reported one or more specific new sexual problem “after surgery.” If men who reported having each problem “before surgery” are excluded, the percentages of men with each problem were as follows: low libido, 77.4%; impotence, 55.3%; partial impotence, 88.2%; orgasm problems, 62.5%; ejaculation problems, 63.9%; and worry or embarrassment, 27.8%. Considering all 42 patients in this group, 90.5% had ED: 6 patients preoperatively and 38 postoperatively.
Factors Affecting Current Sexual Activity and “Surgery Made Sexual Life Worse”
Multivariate analyses of factors predictive of “current sexual activity” and “surgery made sexual life worse” were performed (Table 8). Men and women were pooled together. APR, preoperative sexually inactivity, and older age were significant, independent predictors of current sexual inactivity. Patients receiving pelvic radiation therapy, having an APR, or of male gender were significantly more likely to state that “surgery made their sexual life worse,” while age and preoperative sexual dysfunction were not predictive in multivariate analysis.
DISCUSSION
This study shows that when highly sensitive instruments are used, a high rate of sexual dysfunction is detected in men after rectal cancer excision, despite the routine use of nerve-preserving techniques. We found that 43% of sexually active men and 69% of men overall had IIEF scores that were considered abnormal. In addition, this study shows that sexual dysfunction is an issue for women. Thirty-nine percent of sexually active women and 62% of all women respondents had FSFI scores that were considered abnormal. This is despite the fact that nerve-sparing surgery was used routinely at our institution.
Widely varying rates of sexual dysfunction after rectal surgery have been reported in the literature, and comparisons between studies are difficult due to differing exclusion criteria and outcome measures.2,18 There are several reasons why rates of sexual dysfunction reported here appear high compared with other recent studies. First, this study did not exclude patients based on age (except over 85 years) or preexisting sexual inactivity or dysfunction, exclusions that bias results toward better function.2,4,5 Second, questionnaires that considered the effects of physiologic, psychologic, and social factors on sexual function were used, unlike other reports, which have tended to focus on self-reported physiologic function only, particularly impotence and retrograde ejaculation. The one previous study that used the IIEF to measure male sexual function after nerve-preserving rectal cancer surgery reported mean postoperative IIEF scores of EF 13.5, IS 5.4, OF 4.4, SD 4.8, and OS 4.5, only slightly better than the mean scores in this study, even though the patients in this study are older and a greater percentage had APR. In this study, the proportion of individuals who were sexually active is also lower than that reported by Havenga et al, who found that 70% of men and 60% of women were sexually active.5 This may be due to our strict definition of sexual activity “in the last 4 weeks” as well as liberal inclusion criteria.
As mentioned, this study is unique in terms of its inclusiveness and the detail with which sexual issues were explored. To facilitate comparisons with other published reports, data are presented for all patients, patients currently sexually active, and those for whom “surgery made sexual life worse.” The third analysis was chosen because it separates patients with dysfunction they attribute to rectal cancer treatment from those with preexisting dysfunction only or dysfunction they attribute to other causes. Forty-five percent of men and 29% of women felt that rectal cancer treatment made their sexual lives worse. Because these percentages represent patients’ subjective measure of the effects of rectal cancer treatment, they will be useful numbers for preoperative counseling.
Another finding was the high rate of pretreatment sexual dysfunction. Thirty-five percent of men and 47% of women reported that they had sexual problems before treatment. Sexual problems are likely very common in the North American population. This is illustrated by a recent study of erectile dysfunction in Canadian men that revealed a prevalence of 32.2% among men 45 years of age or older.19 Our data are concordant with smaller studies focusing on male sexual function after rectal cancer treatment20,21 and reinforce the importance of preoperative assessment of sexual function in the clinical and research settings.
This study also shows that sexual dysfunction is multifactorial. Multiple physiologic problems and effects of the ostomy (particularly in men), body image, and partner were reported. These data confirm that additional physical and psychologic factors influence postoperative sexuality. In addition, this study shows that most patients do not get treatment of sexual dysfunction, despite a randomized trial showing efficacy of sildenafil (Viagra) in 79% of male patients with ED after rectal excision for cancer or inflammatory bowel disease.22
The use of validated male and female sexual function questionnaires is unique in studying patients following rectal surgery. The mean scores in this study are poor compared with normal controls (Rosen et al: means 30.5 for FSFI13 and 60.8 for IIEF14) and similar to the scores of patients with diagnosed sexual dysfunction reported by Rosen et al.13,14 However, the subjects in the Rosen et al studies were younger and presumably healthier than the patients in this study.13,14 The decision to designate a “normal versus abnormal” cutoff of 1 standard deviation below the mean scores of normal subjects was admittedly arbitrary. However, it was then possible to determine the proportion of patients with sexual dysfunction, as measured by validated instruments. One disadvantage of the FSFI and IIEF is that scores are greatly influenced by current sexual inactivity. In the case of no sexual activity in “the last 4 weeks,” a patient receives a score of 0 for multiple items, even if sexual function was good prior to that time frame.
Not surprisingly, patients with an APR, older age, or preoperative sexual inactivity were less likely to be sexually active. These data are in agreement with other studies.1,5 Patients who had an APR or pelvic radiation therapy and males were more likely to state that “surgery made their sexual life worse,” even after adjusting for age and the other factors. The effects of radiotherapy for rectal cancer on sexual function are poorly understood;23 however, as many as 62% of patients receiving radiotherapy for prostate cancer experience new ED.24,25 In terms of females, these data are consistent with the hypothesis that surgery- and radiation-induced scarring alters sexual function. It is interesting to note that 18 of the 19 women who said their “vagina feels smaller or tighter” had an APR, XRT, or both. Future trials examining rectal radiation therapy should include measures of sexual function for males and females.
The high frequency of sexual problems also warrants an increased effort to discuss these issues before treatment. Only 9% of women and 39% of men remember discussing sexual effects of treatment preoperatively. Previous studies have also shown a failure to document possible sexual effects of surgery in the informed consent process.26 These findings highlight the need for discussing and documenting sexual dysfunction as a surgical risk. Perhaps written information should be provided to facilitate this conversation, and for patients to share with partners. As well, meticulous surgical technique is necessary to minimize injury to autonomic nerves in females and males.
On the other hand, mean EORTC global health/QOL scores were reassuring, approximating population norms.27 QOL domains of the EORTC-QLQ-C30 and CR-38 have been shown to discriminate between clinically different groups of colorectal cancer patients, such as those with and without recurrence,7 and those undergoing different treatments.28 Most recently, Engel et al published a prospective QOL study in rectal cancer patients using serial administrations of these EORTC questionnaires.29 While we found that the mean Global QOL domain (QL2) scores of our nonsexually active patients were significantly worse than those who were sexually active, these “worst” scores approximated the mean scores reported by Engel et al.29 That is, despite sexual problems or inactivity, overall QOL is preserved. Furthermore, we believe that the older age of the “nonsexually active” group may explain the difference in mean global QOL scores.
This is one of several studies to show a discrepancy between overall QOL and sexual dysfunction. Camilleri-Brennan and Steele, as well as others, have published several papers showing the phenomenon of excellent overall QOL despite high rates of sexual dysfunction.3,30,31 Cancer cure appears to be of overriding importance to most patients. Nevertheless, we believe that potential sexual dysfunction should be a routine part of the informed consent process for rectal cancer surgery and for radiation therapy for rectal cancer. Patients’ personal feelings about the importance of sexual function should be considered in the choice of surgical procedure. Unfortunately, combining less radical surgery with XRT may not spare sexual function.
Methodologic limitations of this study include the potential for sampling and selection bias, and recall bias. The study population is a “convenience cohort” of living rectal cancer patients who are free of recurrence and were treated in an urban, tertiary care hospital. Therefore, results may not be generalizable to all patients undergoing rectal cancer surgery. Procedure subgroups were not randomly assigned and differ in tumor characteristics as well as treatment. Also, while the strength of this study is the high response rate, some questions were left blank, particularly by older women who are not currently sexually active. Most importantly, this study is retrospective; therefore, comparisons of preoperative and postoperative function are inherently inaccurate. Future studies should measure sexual function preoperatively and postoperatively, using validated instruments such as the IIEF and FSFI, so that the current problem of noncomparability across studies is improved.
CONCLUSION
On the basis of this study, several conclusions may be drawn. First, a significant percentage of rectal cancer patients have preexisting sexual dysfunction. Second, postoperative sexual dysfunction is common in both men and women, although it does not appear to have a negative impact on global QOL. Third, those patients who feel that rectal cancer treatment negatively affected their sexual lives cite multiple physiologic and psychologic components to the problem. Finally, rectal cancer patients do not typically remember a preoperative discussion of potential sexual problems and seldom get treatment of sexual dysfunction. Future studies should use validated instruments administered preoperatively and postoperatively to characterize sexual dysfunction following treatment of rectal cancer.
ACKNOWLEDGMENTS
The authors thank Karen West of the EORTC Quality of Life Group, Jules Mitchel of Target Health, and the Christelle Berne of the MAPI Research Institute for granting permission to use the EORTC, FSFI, and IIEF questionnaires, respectively. The authors also thank Anat Ravid and Laura Santoro for their important work maintaining our colorectal cancer database.
Footnotes
Reprints: Robin S. McLeod, MD, Department of Surgery, Mount Sinai Hospital, 600 University Avenue, Room 451, Toronto, Ontario M5G1X5, Canada. E-mail: rmcleod@mtsinai.on.ca.
REFERENCES
- 1.Sprangers MA, Taal BG, Aaronson NK, et al. Quality of life in colorectal cancer: stoma vs. nonstoma patients. Dis Colon Rectum. 1995;38:361–369. [DOI] [PubMed] [Google Scholar]
- 2.Havenga K, Maas CP, DeRuiter MC, et al. Avoiding long-term disturbance to bladder and sexual function in pelvic surgery, particularly with rectal cancer. Semin Surg Oncol. 2000;18:235–243. [DOI] [PubMed] [Google Scholar]
- 3.Camilleri-Brennan J, Steele RJ. Quality of life after treatment for rectal cancer. Br J Surg. 1998;85:1036–1043. [DOI] [PubMed] [Google Scholar]
- 4.Masui H, Ike H, Yamaguchi S, et al. Male sexual function after autonomic nerve-preserving operation for rectal cancer. Dis Colon Rectum. 1996;39:1140–1145. [DOI] [PubMed] [Google Scholar]
- 5.Havenga K, Enker WE, McDermott K, et al. Male and female sexual and urinary function after total mesorectal excision with autonomic nerve preservation for carcinoma of the rectum. J Am Coll Surg. 1996;182:495–502. [PubMed] [Google Scholar]
- 6.Sailer MD, Sebastian E, Fuchs K-H. How useful is the EORTC QLQ-CR38 in the pre- and post-operative evaluation of patients with rectal cancer? QoL Newsletter 2000;25:12–13. [Google Scholar]
- 7.Camilleri-Brennan J, Steele RJ. The impact of recurrent rectal cancer on quality of life. Eur J Surg Oncol. 2001;27:349–353. [DOI] [PubMed] [Google Scholar]
- 8.Camilleri-Brennan J, Steele RJ. Objective assessment of quality of life following panproctocolectomy and ileostomy for ulcerative colitis. Ann R Coll Surg Engl. 2001;83:321–324. [PMC free article] [PubMed] [Google Scholar]
- 9.Allal AS, Bieri S, Pelloni A, et al. Sphincter-sparing surgery after preoperative radiotherapy for low rectal cancers: feasibility, oncologic results and quality of life outcomes. Br J Cancer. 2000;82:1131–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Camilleri-Brennan J, Steele RJ. Prospective analysis of quality of life after reversal of a defunctioning loop ileostomy. Colorectal Dis. 2002;4:167–171. [DOI] [PubMed] [Google Scholar]
- 11.Kim NK, Aahn TW, Park JK, et al. Assessment of sexual and voiding function after total mesorectal excision with pelvic autonomic nerve preservation in males with rectal cancer. Dis Colon Rectum. 2002;45:1178–1185. [DOI] [PubMed] [Google Scholar]
- 12.Dillman DA. Mail and Internet Surveys: The Tailored Design Method, 2nd ed. New York: John Wiley & Sons, 2000:464. [Google Scholar]
- 13.Rosen R, Brown C, Heiman J, et al. The Female Sexual Function Index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther. 2000;26:191–208. [DOI] [PubMed] [Google Scholar]
- 14.Rosen RC, Riley A, Wagner G, et al. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49:822–830. [DOI] [PubMed] [Google Scholar]
- 15.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. [DOI] [PubMed] [Google Scholar]
- 16.Sprangers MA, te Velde A, Aaronson NK. The construction and testing of the EORTC colorectal cancer-specific quality of life questionnaire module (QLQ-CR38): European Organization for Research and Treatment of Cancer Study Group on Quality of Life. Eur J Cancer. 1999;35:238–247. [DOI] [PubMed] [Google Scholar]
- 17.Fayers PM, Bjordal K, Groenvold M, et al. The EORTC QLQ-C30 Scoring Manual, 3rd ed. Brussels: European Organisation for Research and Treatment of Cancer, 2001. [Google Scholar]
- 18.Lindsey I, Guy RJ, Warren BF, et al. Anatomy of Denonvilliers’ fascia and pelvic nerves, impotence, and implications for the colorectal surgeon. Br J Surg. 2000;87:1288–1299. [DOI] [PubMed] [Google Scholar]
- 19.Canadian Male Sexual Health Council. Prevalence of Erectile Dysfunction. Canadian Male Sexual Health Council, 2004.
- 20.Nesbakken A, Nygaard K, Bull-Njaa T, et al. Bladder and sexual dysfunction after mesorectal excision for rectal cancer. Br J Surg. 2000;87:206–210. [DOI] [PubMed] [Google Scholar]
- 21.Leveckis J, Boucher NR, Parys BT, et al. Bladder and erectile dysfunction before and after rectal surgery for cancer. Br J Urol. 1995;76:752–756. [DOI] [PubMed] [Google Scholar]
- 22.Lindsey I, George B, Kettlewell M, et al. Randomized, double-blind, placebo-controlled trial of sildenafil (Viagra) for erectile dysfunction after rectal excision for cancer and inflammatory bowel disease. Dis Colon Rectum. 2002;45:727–732. [DOI] [PubMed] [Google Scholar]
- 23.Temple LK, Wong WD, Minsky B. The impact of radiation on functional outcomes in patients with rectal cancer and sphincter preservation. Semin Radiat Oncol. 2003;13:469–477. [DOI] [PubMed] [Google Scholar]
- 24.Little DJ, Kuban DA, Levy LB, et al. Quality-of-life questionnaire results 2 and 3 years after radiotherapy for prostate cancer in a randomized dose-escalation study. Urology. 2003;62:707–713. [DOI] [PubMed] [Google Scholar]
- 25.Beard CJ, Lamb C, Buswell L, et al. Radiation-associated morbidity in patients undergoing small-field external beam irradiation for prostate cancer. Int J Radiat Oncol Biol Phys. 1998;41:257–262. [DOI] [PubMed] [Google Scholar]
- 26.Chorost MI, Weber TK, Lee RJ, et al. Sexual dysfunction, informed consent and multimodality therapy for rectal cancer. Am J Surg. 2000;179:271–274. [DOI] [PubMed] [Google Scholar]
- 27.Fayers PM, Curran D, et al. EORTC QLQ-C30 Reference Values. Brussels: EORTC, 1998. [Google Scholar]
- 28.Sailer M, Fuchs KH, Fein M, et al. Randomized clinical trial comparing quality of life after straight and pouch coloanal reconstruction. Br J Surg. 2002;89:1108–1117. [DOI] [PubMed] [Google Scholar]
- 29.Engel J, Kerr J, Schlesinger-Raab A, et al. Quality of life in rectal cancer patients: a four-year prospective study. Ann Surg. 2003;238:203–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Camilleri-Brennan J, Steele RJ. Prospective analysis of quality of life and survival following mesorectal excision for rectal cancer. Br J Surg. 2001;88:1617–1622. [DOI] [PubMed] [Google Scholar]
- 31.Chatwin NA, Ribordy M, Givel JC. Clinical outcomes and quality of life after low anterior resection for rectal cancer. Eur J Surg. 2002;168:297–301. [DOI] [PubMed] [Google Scholar]