Abstract
Objective:
We sought to examine the expression and functional role of osteonectin in primary and metastatic pancreatic ductal adenocarcinoma (PDAC).
Background:
The glycoprotein osteonectin plays a vital role in cell–matrix interactions and is involved in various biologic processes. Overexpression of osteonectin is present in malignant tumors and correlates with disease progression and poor prognosis.
Methods:
Expression of osteonectin was analyzed by quantitative polymerase chain reaction and immunohistochemistry in pancreatic tissues and by enzyme-linked immunosorbent assay in the serum of patients and donors. Recombinant osteonectin and specific antisense oligonucleotides were used to examine the effects of osteonectin on induction of target genes, and on proliferation and invasiveness of pancreatic cancer cells.
Results:
There was a 31-fold increase in osteonectin mRNA levels in PDAC and a 16-fold increase in chronic pancreatitis as compared with the normal pancreas (P < 0.01). By immunohistochemistry, faint immunoreactivity was detected in the normal pancreas. In contrast, strong staining of the cancer cells was observed in addition to extensive osteonectin immunoreactivity in surrounding fibroblasts and in the extracellular matrix. In metastatic tissues, strong immunoreactivity was observed in fibroblasts and in extracellular matrix surrounding metastatic cancer cells, whereas the signal was absent in most tumor cells. In vitro studies showed that osteonectin was able to inhibit cancer cell growth while promoting invasiveness of pancreatic tumor cells.
Conclusion:
Osteonectin is markedly overexpressed in pancreatic cancer and has the potential to increase the invasiveness of pancreatic cancer cells.
The glycoprotein osteonectin plays a critical role in malignant tumors and correlates with disease progression and poor prognosis. In the present study we revealed an influence on pancreatic cancer growth and invasion and an overexpression of osteonectin in human pancreatic cancer tissues.
Pancreatic ductal adenocarcinoma (PDAC) is the fourth-leading cause of cancer-related death in Western countries.1 It is a devastating disease with an overall 5-year survival rate of less than 1% and a median survival time after diagnosis of approximately 5 to 6 months. At time of diagnosis, between 75% and 85% of patients with PDAC have unresectable tumors, and conventional therapies virtually are ineffective.2 Furthermore, patients typically have metastatic lesions at the time of diagnosis, and most patients undergoing potentially curative tumor resection will die within the first 2 postoperative years as the result of local recurrence or distant metastasis.2 An improved understanding of the molecular characteristics of PDAC is the only means of identifying new markers for early diagnosis and potential targets for therapeutic intervention.2–4 Therefore, in recent decades many laboratories have focused on understanding the molecular alterations that occur in PDAC.
Osteonectin, also called “secreted protein acidic and rich in cysteine” (SPARC), is a 32- to 43-kDa calcium-binding glycoprotein that belongs to a group of matrix-associated factors mediating cell–matrix interactions.5–7 This glycoprotein can be divided into 3 distinct modules. Module I (aa 3–51) is highly acidic, binds calcium ions with low affinity, interacts with hydroxyapatite, and has been implicated in the mineralization of cartilage and bone.5,8,9 Module II (aa 52–132) is a cysteine-rich structure that is homologous to a repeated domain in follistatin, a protein that binds to activin and inhibin, members of the transforming growth factor (TGF)-β superfamily. Module III (aa 133–285) constitutes the extracellular calcium-binding (EC) module,10 which has been shown to bind to endothelial cells and to inhibit their proliferation.11 The EC module binds to several collagen types, including the basement membrane collagen type IV, in a calcium-dependent manner. The affinity of osteonectin for collagens is increased by cleavage of a single peptide bond located in the α-helical region in the EC module.12 The specificity of this cleavage has been demonstrated in vitro with a number of matrix metalloproteinases (MMP).13 Interestingly, osteonectin has also been shown to increase the production14 and activity of MMPs.15
Osteonectin is involved in various biologic processes, including tissue remodeling, wound repair, morphogenesis, angiogenesis, and cellular differentiation, proliferation, and migration.16–18 Osteoblasts, endothelial cells, megakaryocytes, vascular smooth muscle cells, chondrocytes, steroidogenic cells, and placental trophoblasts have been described to produce osteonectin.19–21 It inhibits the proliferation of a variety of cells, primarily through modulation of cell adhesion, growth factor activity, and cell cycle progression from G1 to S phase.22 Osteonectin overexpression has been reported in a variety of human malignancies,23 including breast,15,24 colon,25 esophageal,26 prostate,27 hepatocellular,28 and bladder carcinomas,29 as well as in melanomas,30 astrocytomas,31 and meningiomas.32 Several in vitro and in vivo studies have linked osteonectin expression with tumor invasion; deregulated expression of osteonectin has been found to correlate with disease progression and/or poor prognosis.24–26,29,30,32–35 Furthermore, studies have shown that inhibition of osteonectin expression by antisense-RNA blocks tumor formation by human melanoma cells in vivo.30
Up to now, there is only limited information available regarding the potential role of osteonectin in pancreatic diseases.36,37 Therefore, in the present study we examined (1) the expression and localization of osteonectin in both primary and metastatic human pancreatic cancers as well as in chronic pancreatitis; (2) the possible biologic effects of osteonectin on human pancreatic cancer cells; and (3) the mechanisms of action of osteonectin and the possible pathways involved in the activation of osteonectin in human pancreatic cancer.
MATERIALS AND METHODS
Patients and Tissue Collection
Human pancreatic cancer tissue samples were obtained from 51 patients (25 women, 26 men, median age 65 years [range, 28–82 years]) and chronic pancreatitis (CP) tissue samples from 37 patients (11 women, 26 men, median age 54 years [range, 25–73 years]) who underwent pancreatic resection at the University Hospitals of Berne (Switzerland) and Heidelberg (Germany). Normal human pancreatic tissue samples were obtained through an organ donor program from 24 previously healthy individuals (10 women, 14 men, median age 45 years [range, 20–74 years]). Freshly removed tissue samples were immediately fixed in paraformaldehyde solution for 12 to 24 hours and paraffin-embedded for immunohistochemical analysis. Concomitantly, tissue samples for RNA extraction were immediately snap frozen in liquid nitrogen in the operating room and maintained at −80°C until use. Serum samples were collected from 34 normal, 32 CP, and 72 pancreatic cancer patients. The Human Subject Committees of the University Hospitals of Berne (Switzerland) and Heidelberg (Germany) approved the studies, and written informed consent was obtained from all patients.
Real-Time Light Cycler Quantitative Polymerase Chain Reaction (QRT-PCR)
All reagents and equipment for mRNA/cDNA preparation were purchased from Roche Applied Science (Mannheim, Germany). mRNA was prepared by automated isolation using MagNA Pure LC instrument and isolation kits I (for cells) and II (for tissue samples). cDNA was prepared using the 1st Strand cDNA Synthesis Kit for RT-PCR according to the manufacturer's instructions. Real-time PCR was performed with the Light Cycler Fast Start DNA SYBR Green Kit as described previously.4,38 The number of specific (osteonectin, MMP-2, p21, vascular endothelial growth factor [VEGF]) transcripts was normalized to housekeeping genes (cyclophilin-B [CPB] and hypoxanthine guanine phosphoribosyltransferase [HPRT]). All primers were obtained from Search-LC (Heidelberg, Germany).
Immunohistochemistry
Immunohistochemical analysis was performed using the streptavidin–peroxidase technique and DAKO EnVision+ Systems (Dako Cytomation GmbH, Hamburg, Germany) according to the manufacturer's protocol. Briefly, consecutive 3- to 5-μm thick paraffin-embedded tissue sections were deparaffinized and dehydrated. Slides were incubated for 10 minutes in 0.3% hydrogen peroxide to block endogenous peroxidase activity. Then, the sections were incubated for 30 minutes at room temperature with 10% normal goat serum prior to overnight incubation at 4°C with mouse monoclonal antiosteonectin antibodies (Hematological Technologies, Inc., Essex Junction, VT) diluted in phosphate-buffered saline (4 μg/mL). Secondary goat antimouse peroxidase-labeled polymer was used. Slides were counterstained with Mayer's hematoxylin. To ensure specificity of the primary antibodies, anti-osteonectin antibody was incubated with human recombinant osteonectin at 4°C overnight. Then, consecutive tissue sections were incubated with the absorbed antibody or with equal amounts of mouse IgG1, κ-chain. In these cases, no specific immunoreactivity was detected.
Cell Culture
Aspc-1, BxPc-3, Capan-1, Colo-357, MIA PaCa-2, Panc-1, SU 8686, and T3M4 cells were grown in RPMI media. Cells were maintained at 37°C in humidified air with 5% CO2. Medium was supplemented with penicillin G (100 U/ml), streptomycin (100 μg/mL) and 10% fetal bovine serum. Cells were split into 96-well plates for MTT cell growth assays, into 24-well plates for Matrigel invasion assays, and into 6-well plates for fluorescence-activated cell sorting (FACS) and QRT-PCR analysis. Duplicate cultures were treated with recombinant osteonectin or with antisense nucleotides as described before.39
Osteonectin Enzyme-Linked Immunosorbent Assay (ELISA)
Serum osteonectin levels were measured using an osteonectin ELISA kit (Hematological Technologies, Inc.) according to the manufacturer's instructions.
Matrigel Invasion Assays
Invasion assays were performed using a BD Biocoat Matrigel Invasion Chamber (BD Biosciences, Heidelberg, Germany) according to the manufacturer's protocol. Briefly, Matrigel was rehydated using bicarbonate-based culture medium (DMEM serum-free) and incubated in 37°C, 5% CO2 atmosphere for 2 hours. Pancreatic cancer cells were seeded at 5 × 104 cells/mL and incubated overnight at 37°C in a humidified atmosphere containing 5% CO2. Then, cells were treated with exogenous osteonectin (Hematological Technologies, Inc.) and further incubated for 24 hours. Afterward, noninvading cells were removed from the upper surface of the membrane by swiping with a cotton-tipped swab, and membrane was fixed with Carnoy's fixative (75% methanol with 25% acetone). Subsequently, the cells were stained with 1.5% toluidine blue and counted to calculate the invasion percentage and index.
Induction of TGF-β1
Human pancreatic cancer cells were seeded in 24-well plates in 10% fetal bovine serum growth media and allowed to attach for 12 hours. For TGF-β induction experiments, cells were serum-starved overnight and incubated thereafter in serum-free medium in the absence or presence of 200 pM TGF-β1 (R&D Systems Inc., Minneapolis, MN).4 After incubation for the indicated time periods, RNA was extracted and QRT-PCR was conducted as described.
MTT Cell Growth Assays
Cell proliferation was studied using 3-(4,5-methylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) assays. Briefly, cells were seeded at a density of 5 × 103 cells/well in 96-well plates, grown overnight, and exposed to different concentrations of exogenous osteonectin for 48 hours. Then, MTT was added (5 μg/well) for 4 hours. Formazan products were solubilized with acidic isopropanol and the optical density was measured at 570 nm.
FACS Analysis
Both adherent and floating cells were collected for analysis. Cell cycle analysis was performed using a hypotonic fluorochrome solution (propidium iodide 50 μg/mL, sodium citrate 0.1%, Triton X-100 0.1%).40 Flow cytometry was performed using FACScan (Beckton Dickinson, New Jersey, NJ).
Statistics
Patient data are expressed as median and range. Experimental results are expressed as mean ± SEM of at least 3 independent experiments unless indicated otherwise. For statistical analysis, Student t test or the Mann-Whitney U test were used. P < 0.05 was taken as the level of significance.
RESULTS
Expression and Localization of Osteonectin in Pancreatic Tissues
To accurately compare the in vivo expression profile of osteonectin in the normal and diseased pancreas, QRT-PCR was conducted using RNA isolated from normal (n = 24), CP (n = 37), and PDAC (n = 51) tissue sections. This analysis revealed a 31-fold increase in osteonectin mRNA levels in PDAC and a 16-fold increase in CP as compared with the normal pancreas (P < 0.01). Moreover, 82% (42/51) of PDAC and 70% (26/37) of CP samples exhibited osteonectin mRNA levels exceeding the highest levels observed in normal tissues (Fig. 1A).
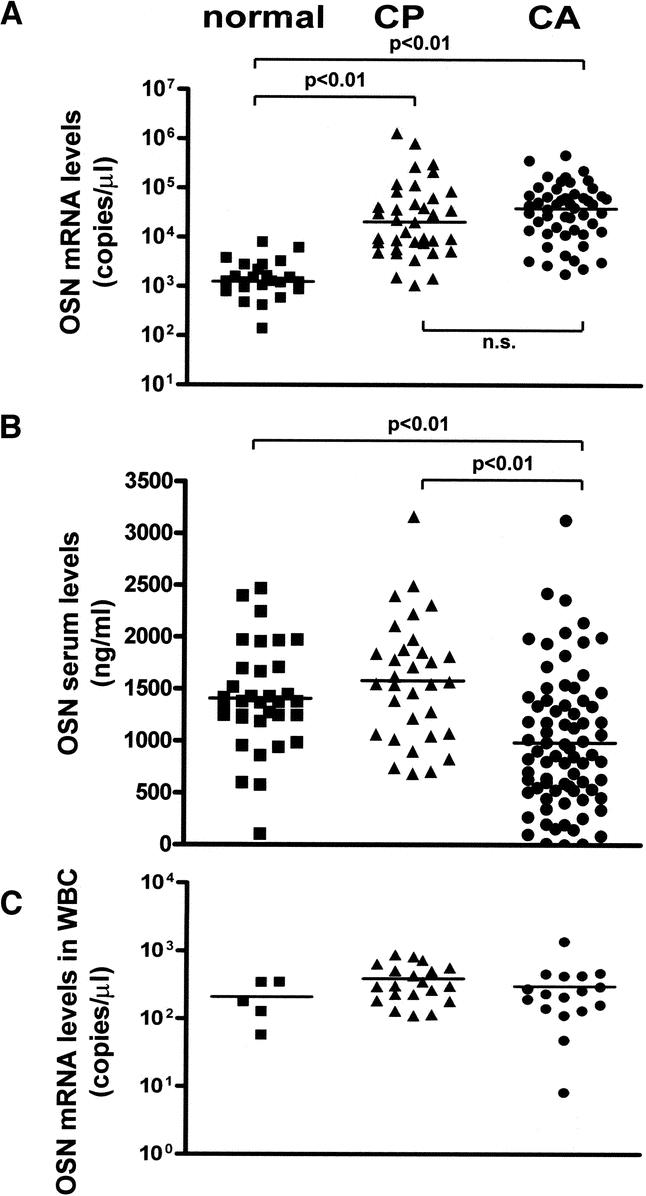
FIGURE 1. Osteonectin mRNA expression and serum levels. A, Osteonectin mRNA levels in 24 normal pancreatic tissues, 37 CP tissues, and 51 PDAC tissues. B, Osteonectin protein levels in the serum of 14 healthy controls, 19 CP patients, and 45 PDAC patients. C, Osteonectin mRNA levels in peripheral white blood cells from 5 healthy controls, 20 CP patients, and 17 PDAC patients. Horizontal lines represent the median (ns indicates not significant).
Given the high expression of osteonectin in diseased tissues, we evaluated the potential diagnostic and prognostic value of osteonectin as specific marker for CP or pancreatic cancer. To this end, ELISA was used to measure osteonectin levels in serum samples obtained from 34 healthy controls, 32 patients with CP, and 72 PDAC patients. Interestingly, there were significantly lower osteonectin serum levels in pancreatic cancer patients compared with both healthy controls and CP patients (P < 0.01) (Fig. 1B). There was, however, no significant correlation between osteonectin mRNA levels in tissues and osteonectin serum levels for individual PDAC patients (data not shown). Because osteonectin serum levels might derive not only from diseased pancreatic tissues but also from white blood cells (WBC), we further examined osteonectin mRNA levels in these cells of 5 healthy controls, 20 CP patients, and 17 PDAC patients by QRT-PCR. There was no significant difference of osteonectin mRNA levels in WBC between healthy controls, CP and PDAC patients (Fig. 1C). Therefore, it is likely that the osteonectin serum levels in healthy controls and CP/PDAC patients are determined by osteonectin expression levels in WBC rather than by osteonectin expression in the diseased pancreas.
To identify the cellular source and localization of osteonectin in pancreatic tissues, immunohistochemistry was carried out next using 10 normal, 15 CP, 30 primary, and 15 metastatic PDAC tissue samples. Faint osteonectin immunoreactivity was detected in the acinar cells, islets, and ECM of normal pancreatic tissues. No staining was detected in ductal cells (Fig. 2A). In contrast, in CP tissue samples, strong osteonectin immunoreactivity was observed in the tubular complexes and degenerating acinar cells as well as in the extracellular matrix, nerves and infiltrating inflammatory cells (Fig. 2B–D).
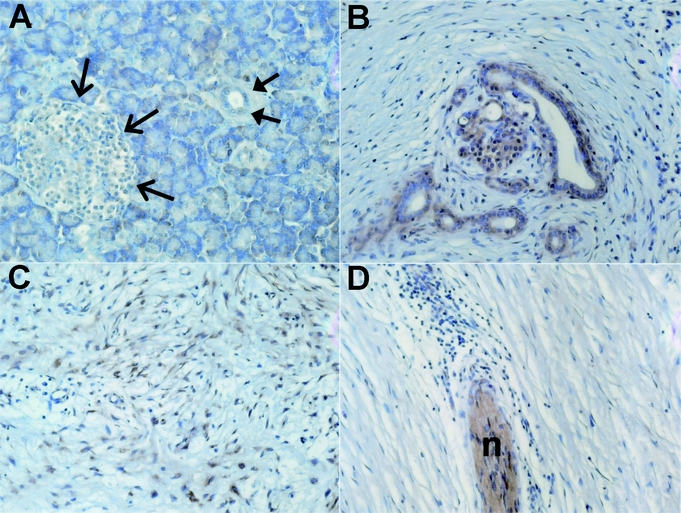
FIGURE 2. Osteonectin expression and localization in the normal pancreas and chronic pancreatitis (CP). A, Osteonectin localization in the normal pancreas; open arrows depict an islet, closed arrows depict a normal duct. B–D, Osteonectin in chronic pancreatitis tissues; with B showing tubular complexes, C the extracellular matrix, and D nerve (n) with surrounding inflammatory cells.
In all primary PDAC samples, extensive cytoplasmic osteonectin immunostaining was present in the cancer cells (Fig. 3A–D). In several PDAC samples, strong scattered nuclear staining of the cancer cells also was observed (Fig. 3D, arrows). Furthermore, osteonectin immunoreactivity was observed in the fibroblasts and in the ECM surrounding the cancer cells in most of the PDAC samples (Fig. 3). In addition, weak-to-moderate osteonectin immunoreactivity was observed in endothelial cells, the muscular layer of the blood vessels, nerves, and infiltrating inflammatory cells in some PDAC samples. There was no correlation between inflammatory cell infiltrates and osteonectin expression levels neither in the tumor cells nor in the ECM. In metastatic PDAC samples, strong osteonectin immunoreactivity was observed in the fibroblasts and in the extracellular matrix surrounding the metastatic cancer cells (Fig. 3E–G). In contrast, weak to absent osteonectin immunoreactivity was observed in the metastatic cancer cells (Fig. 3F and G).
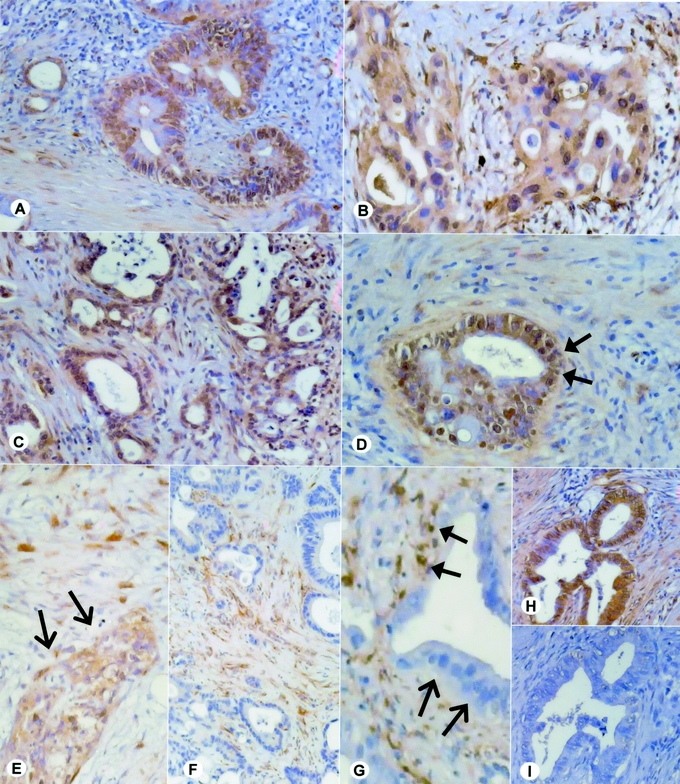
FIGURE 3. Osteonectin expression and localization in primary and metastatic pancreatic cancer (PDAC). A–D, Osteonectin localization in primary PDAC; the arrows in D depict the occasionally observed nuclear staining pattern. E–G, Osteonectin localization in metastatic PDACs. E depicts osteonectin expression by the metastatic tumor cells, F and G depict osteonectin expression in the tissue surrounding (closed arrows) the tumor cells, which are devoid of osteonectin immunoreactivity (open arrows). H and I, To ensure specificity of the primary antibodies, the osteonectin antibody was incubated with human recombinant osteonectin. Consecutive tissue sections were incubated with the untreated (H) and the adsorbed (I) antibody.
To ensure specificity of the observed staining pattern, antiosteonectin antibodies were incubated with human recombinant osteonectin and consecutive tissue sections were incubated with preabsorbed antibody. Preincubation with osteonectin completely abolished the immunoreactivity (Fig. 3H and I).
The Functional Role of Osteonectin in Pancreatic Cancer Cells
To analyze the functional role of osteonectin in pancreatic cancer, first the expression level of osteonectin mRNA was examined in 8 pancreatic cancer cell lines as well as in pancreatic stellate cells by QRT-PCR. Pancreatic stellate cells expressed exceedingly high osteonectin mRNA levels, whereas expression was lower and differed significantly among the pancreatic cancer cell lines (Fig. 4A). The levels of osteonectin mRNA were high in SU 8686 cells and Panc-1 pancreatic cancer cells, moderate to low in Colo-357, MIA PaCa-2 and T3M4 cells, and undetectable in Aspc-1, BxPc-3 and Capan-1 pancreatic cancer cells (Fig. 4A). No correlation was observed between osteonectin expression levels and differentiation or basal growth characteristics of the analyzed cell lines.
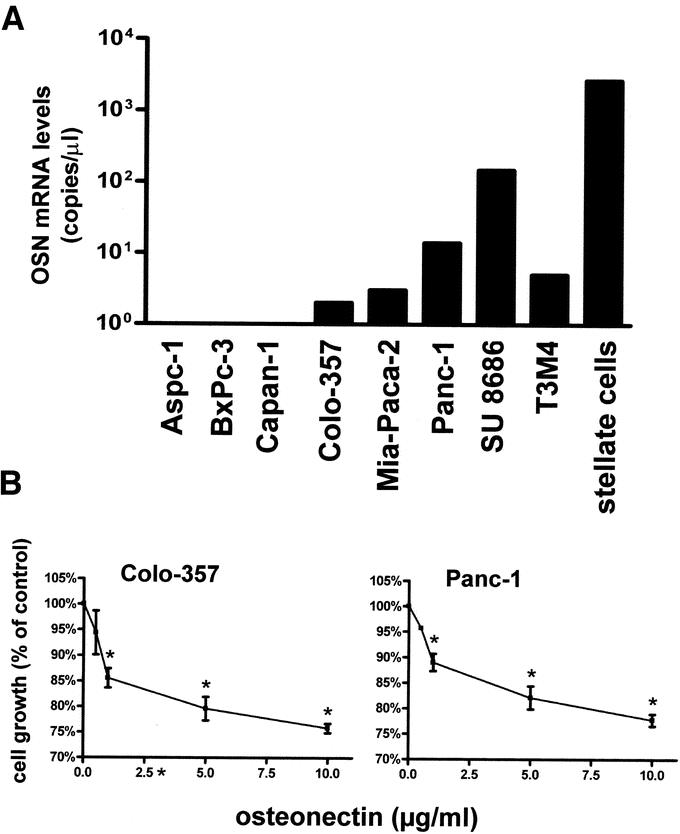
FIGURE 4. Osteonectin expression levels and effects on growth in pancreatic cancer cells. A, Osteonectin mRNA levels in cultured pancreatic cancer cell lines and pancreatic stellate cells. B, The indicated pancreatic cancer cells were treated with exogenous human recombinant osteonectin for 48 hours. Data are presented as mean ± SEM of 3 independent experiments. *P < 0.05.
Effect of Osteonectin on the Growth of Pancreatic Cancer Cells In Vitro
To examine the effects of osteonectin on pancreatic cancer cell growth, MTT assays were conducted next. Colo-357, which exhibited low endogenous osteonectin levels and Panc-1 cells, with high endogenous osteonectin levels, were used for the subsequent experiments. Cells were treated with exogenous human recombinant osteonectin for 48 hours. Osteonectin caused dose-dependent growth inhibition in both Colo-357 and Panc-1 cells. Thus, Colo-357 pancreatic cancer cells displayed maximal 24% growth inhibition at 10 μg/mL osteonectin and Panc-1 cells 23% growth inhibition at the same concentration of exogenous osteonectin (Fig. 4B). To analyze the mechanisms of this growth inhibition, FACS analysis was carried out next. Colo-357 cells and Panc-1 cells were synchronized by serum starvation overnight, released into the cell cycle, and treated with exogenous osteonectin (5 μg/mL) for 24–72 hours before cell cycle analysis. This analysis demonstrated that exogenous osteonectin caused transient G1/S phase accumulation in Colo-357 cells, most evident at 48 hours; apoptotic changes or toxicity were not observed (Fig. 5). In contrast, no changes in cell cycle distribution could be observed in Panc-1 cells (data not shown).
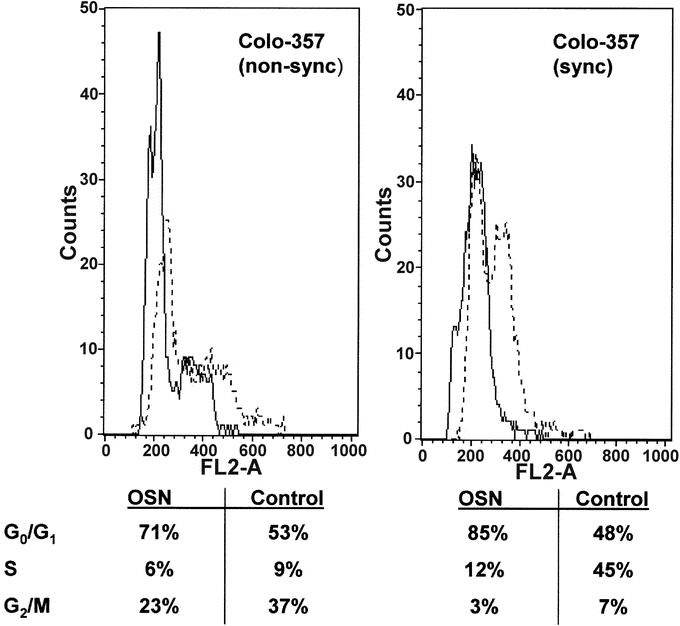
FIGURE 5. Effect of osteonectin on cell cycle distribution. FACS analysis in Colo-357 cells that were nonsynchronized (left panel) or synchronized by serum starvation overnight (right panel). Cells were treated with exogenous osteonectin (5 μg/mL; solid line) for 48 hours before cell cycle analysis or left untreated (dotted line).
To further analyze the effects of osteonectin on pancreatic cancer cells, specific antisense oligonucleotides were used. In Panc-1 cells, the high endogenous osteonectin mRNA expression was reduced by approximately 25–35% at 24–72 hours after the transfection of 10 μM osteonectin antisense oligonucleotides (data not shown).
Effect of Osteonectin on the Invasiveness of Pancreatic Cancer Cells In Vitro
To examine the possible proinvasive role of osteonectin in pancreatic cancer cells, invasion assays were conducted next. Colo-357 cells were treated with osteonectin (5 μg/mL) for 24 hours. Osteonectin significantly increased (14-fold) the invasive capacity of Colo-357 cells as compared with the control (Fig. 6A). Downregulation of osteonectin expression in Panc-1 cells by antisense oligonucleotides led to reduced invasive capacity of Panc-1 (−45% compared with control cells; Fig. 6B, C).
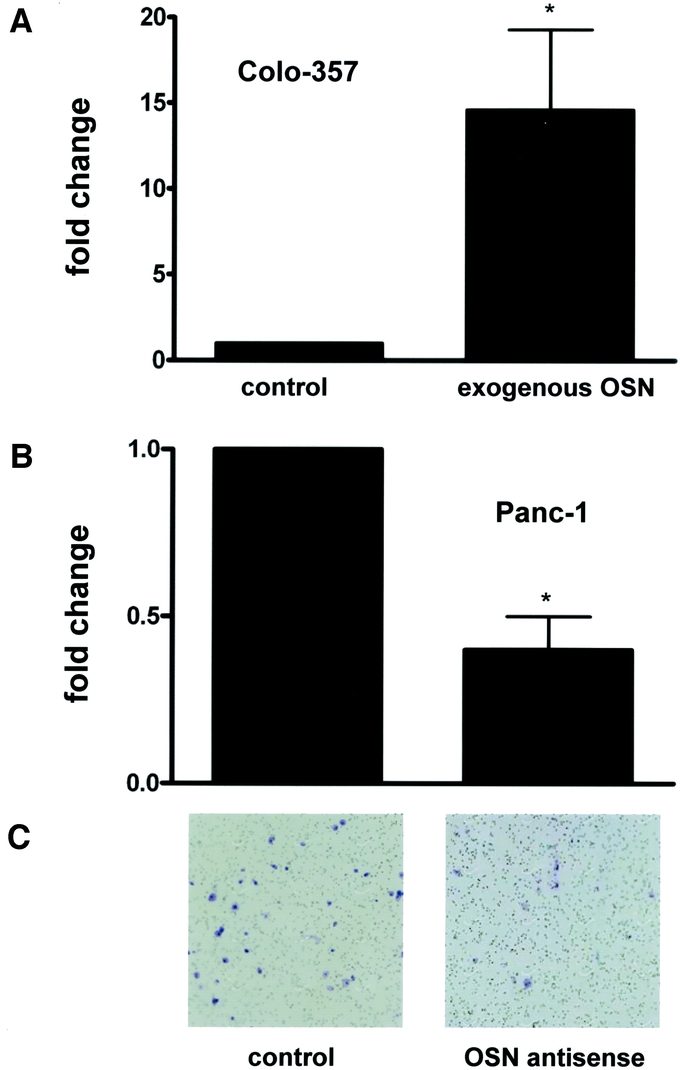
FIGURE 6. Effects of osteonectin on pancreatic cancer cell invasion. A, Colo-357 cells were incubated with exogenous osteonectin (5μg/ml) for 24 hours. B, Panc-1 cells were transfected with specific osteonectin antisense oligonucleotides for 24 hours. Data are presented as mean ± SEM of 3 independent experiments. C, Exemplary picture of control and osteonectin antisense-treated Panc-1 cells. *P < 0.05.
Regulation of Proinvasive and Growth Inhibitory Genes by Osteonectin in Pancreatic Cancer Cells
To gain insight into the molecular basis of both increased tumor invasiveness and the antiproliferative role of osteonectin in pancreatic cancer cells, the correlation between expression of osteonectin, 2 invasion-related genes (MMP-2 and VEGF), and a cell cycle-associated gene (p21) were examined. Colo-357 cells were exposed to exogenous osteonectin (5 μg/mL) for the indicated time periods and subsequently analyzed by QRT-PCR analysis (Fig. 7). In Panc-1 cells, expression of osteonectin was downregulated by osteonectin antisense oligonucleotides before QRT-PCR analysis (Fig. 7). Expression of MMP-2 mRNA dramatically increased (up to 10-fold) in Colo-357 cells 24 hours after the application of exogenous osteonectin. In Panc-1 cells, downregulation of osteonectin expression by antisense treatment resulted in reduction of MMP-2 mRNA levels (−27% after 72 hours, as compared with untreated cells; Fig. 7). Furthermore, expression of p21 doubled in Colo-357 cells 24 hours after the application of exogenous osteonectin. In Panc-1 cells, osteonectin antisense treatment resulted in a 21% reduction of p21 mRNA levels after 48 hours as compared with untreated cells (Fig. 7). Interestingly, expression of VEGF was downregulated in Colo-357 cells by exogenous osteonectin (−56% after 24 hours), and in Panc-1 cells, downregulation of osteonectin expression resulted in an increase (+58% after 24 hours) of VEGF mRNA levels (Fig. 7).
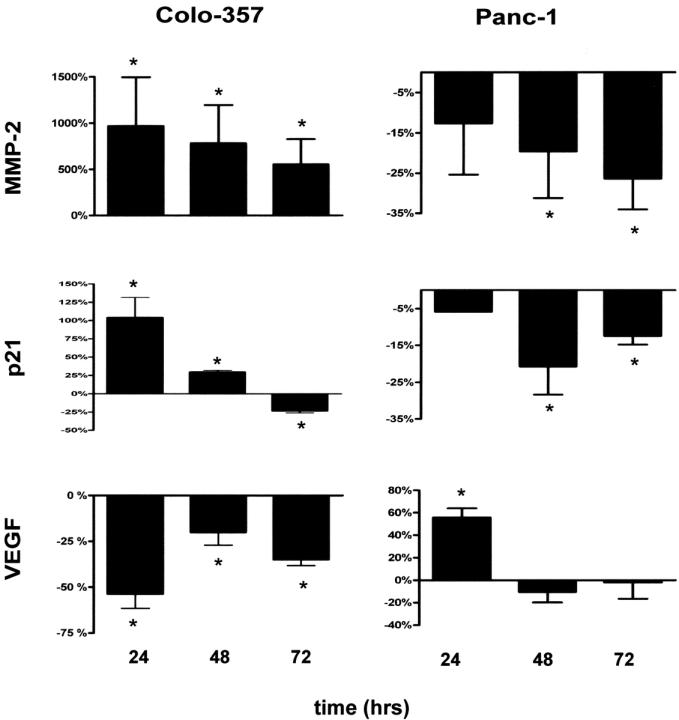
FIGURE 7. Effects of osteonectin on the expression of MMP-2, p21, and VEGF. Colo-357 cells were incubated with exogenous osteonectin (5 μg/mL) for the indicated time (left panel). Panc-1 pancreatic cancer cells were transfected with specific osteonectin antisense oligonucleotides for the indicated time (right panel). Data are presented as mean ± SEM of 3 independent experiments. *P < 0.05.
Effects of TGF-β on Expression of Osteonectin in Pancreatic Cancer Cells
To analyze possible mechanisms of increased osteonectin expression in pancreatic cancer cells, we examined the effects of TGF-β1 on mRNA expression of osteonectin in Colo-357 cells and in Panc-1 cells, which are known to respond to TGF-β1. Exogenous TGF-β1 resulted in transient reduction (approximately −55% after 30 minutes until 6 hours) of osteonectin expression in Panc-1 cells, whereas no effect was observed on osteonectin expression in Colo-357 cells (data not shown).
DISCUSSION
In the present study we show that in primary PDAC, osteonectin was expressed in tumor cells and the surrounding ECM components including fibroblasts and endothelial cells, which indicates that osteonectin may promote tumor cell infiltration into adjacent pancreatic tissues by affecting tumor–ECM interactions. The high expression of osteonectin in primary pancreatic cancers is in contrast to a recent study showing that osteonectin was rarely expressed in the cancers themselves, possibly due to aberrant methylation.37 However, in agreement with the study mentioned, in metastatic PDAC, expression of osteonectin was present predominantly in the stroma surrounding and adjacent to the metastatic tumor cells, whereas its expression in the metastatic tumor cells was below the level of detection in a significant proportion of the samples examined. These findings indicate that osteonectin might have an essential role in tumor progression at the site of interface between metastatic tumor cells and the surrounding host cells. Whether the enhanced expression of osteonectin in the ECM of pancreatic tumors acts to promote tumor cell invasion or blocks tumor growth and spread as it was suggested by a recent study in osteonectin-null mice,36 has to be analyzed in the future. In addition, further studies are also necessary to elucidate the exact role of osteonectin in CP, a potentially premalignant disease. It could be hypothesized, however, that osteonectin has some function in the carcinogenic process.
Osteonectin Influences Invasion and Metastasis
Osteonectin is associated with morphogenesis and tissue remodeling and has been shown to regulate several biologic processes related to angiogenesis, tumor cell migration, and invasion.41–44 Recently, it was demonstrated that increased osteonectin expression promotes the invasive capacity of glioblastoma cells in vitro,45 and in vivo osteonectin was associated with the invasiveness of meningiomas and gliomas.32 Furthermore, suppression of osteonectin expression by human melanoma cells results in a significant reduction in the in vitro adhesive and invasive capacities of tumor cells and was associated with a completely blocked tumor formation by human melanoma cells in nude mice.46 In PDAC, exogenous human recombinant osteonectin treatment significantly increased the invasive capacity of the non-osteonectin-producing Colo-357 cell line, whereas downregulation of osteonectin expression resulted in reduced invasiveness of Panc-1 cells, which overexpress osteonectin. These results suggest that osteonectin plays an important role in the invasive and metastatic phenotype of PDAC through autocrine and/or paracrine mechanisms.
Osteonectin and Its Interaction With Metalloproteinases
Expression of osteonectin was shown to significantly correlate with MMP-2 expression in esophageal carcinoma patients47 and to modulate the expression of MMP-2 in fibroblasts and human monocytes.48 In addition, osteonectin increases the production and activity of MMP-2 in invasive human breast and prostate cancer cell lines.14,15,27 Furthermore, the invasive capacity of melanoma cells correlates positively with increased levels of MMP-2, and reduction of osteonectin in these cells is accompanied by a decrease in MMP-2.46 We examined this potential correlation between osteonectin and MMP-2 as a possible mechanism for the promotion of tumor cell invasion in PDAC. By using induction experiments, it could be shown that exogenous osteonectin significantly increased the expression of MMP-2 in Colo-357 cells, whereas downregulation of osteonectin in Panc-1 cells resulted in a reduction of MMP-2 expression. These results show for the first time that osteonectin directly induces the expression of MMP-2 in PDAC. Interestingly, in vivo and in vitro overexpression of MMP-2 has previously been demonstrated in PDAC in comparison with the normal pancreas.49–51 Furthermore, it has been shown that MMP-2 activation in pancreatic cancer plays a significant role in tumor invasion, metastasis, and early recurrence after pancreatic resection and that it is associated with the development of the characteristic desmoplastic reaction in pancreatic cancer.49–51 In this context, it can be hypothesized that osteonectin promotes tumor invasion in PDAC through direct induction of MMP-2, which in turn degrades the ECM surrounding the tumor cells and tumor vasculature. Additionally, these results suggest that osteonectin and MMP-2 might participate in an autocrine and/or paracrine positive feedback loop in PDAC. Thus, osteonectin stimulates the expression and activity of MMP-2 and in turn, MMP-2 proteolytically cleaves osteonectin, which increases the affinity of osteonectin for collagen and presumably its localization to the basement membrane.
Osteonectin and Cell Cycle Regulation
The role of osteonectin in cell cycle regulation is controversial. It was shown that osteonectin stimulates cell proliferation during periodontal ligament repair,52 whereas in melanoma cells no effect of osteonectin on cell cycle regulation was observed.46 In addition, exogenous osteonectin inhibits cell proliferation and induces apoptosis in ovarian cancer cells.53 Our results show that osteonectin is associated with suppression of pancreatic cancer cell growth in vitro. Exogenous osteonectin inhibits the proliferation of several pancreatic cancer cells, however, without induction of apoptosis. Our results are consistent with a recent report showing essentially the same antiproliferative effects of osteonectin in pancreatic cancer cells.37 Additionally, we could show for the first time that the antiproliferative effect of osteonectin in PDAC is associated with G1 accumulation. These results are supported by previous reports demonstrating that osteonectin arrests cells in the mid-G1 phase of the cell cycle54 and that overexpression of osteonectin increases the percentage of cells in G0-G1 for osteonectin transfected glioma cells.55 The exact mechanisms by which osteonectin inhibits cellular proliferation are still poorly understood. However, our results show that osteonectin induces the expression of p21, which is known to be able to induce a G1 cell cycle block. These results together suggest that although osteonectin promotes tumor invasion, it also decreases tumor cell proliferation. In line with this observation, recent reports indicate that osteonectin promotes glioma invasion by mechanisms that both promote tumor cell migration and delay cell proliferation, and that the extent to which it effects both phenotypes depends on the amount of osteonectin secreted by the tumor cells.55
Osteonectin and Its Interaction With VEGF and TGF-β
Osteonectin is intimately involved in the regulation of angiogenesis through its interaction with VEGF. VEGF is expressed and secreted by many tumors and contributes to tumor expansion associated with angiogenesis. Our study shows that expression of osteonectin and VEGF are closely regulated and that osteonectin overexpression directly inhibits VEGF expression in pancreatic cancer cells. The exact impact of VEGF downregulation by osteonectin is not clear, however, it was shown that osteonectin in its intact form inhibits VEGF-induced proliferation of HMEC but stimulates increased permeability of the endothelial cell barrier for extravasation of tumor cells.56 Nevertheless, the functional significance of osteonectin interaction with VEGF in PDAC requires further investigations.
The biology of osteonectin is further complicated by the fact that osteonectin and another growth factor, TGF-β1, mediate overlapping activities, as well as induce each other's expression.57 Thus, osteonectin induces TGF-β1 expression in mesangial and epithelial cells58–60 and, conversely, TGF-β1 stimulates osteonectin expression in a number of cell types, including fibroblasts, keratinocytes, smooth muscle cells, and endothelial cells.61–64 Our study shows that osteonectin induces the expression of TGF-β1 by pancreatic cancer cells (data not shown), whereas TGF-β1 expression reduces the expression of osteonectin in tumor cells. It is likely that osteonectin and TGF-β1 participate in a feedback loop that influences their production. However, further experiments are needed to address the mechanisms of induction and to investigate whether there is a specific interaction between osteonectin and TGF-β in PDAC.
In conclusion, we report that (1) osteonectin is significantly overexpressed both in CP and PDAC as compared with the normal pancreas; (2) osteonectin promotes the invasiveness of pancreatic cancer cells possibly in part by the induction of MMP-2 expression; (3) osteonectin inhibits the growth of pancreatic cancer cells through cell cycle arrest, which is at least in part mediated by the induction of p21. Our results collectively suggest that osteonectin contributes to the invasion and metastasis of pancreatic cancer cells, implying that osteonectin is a candidate therapeutic target for the design of treatment strategies specifically directed towards the inhibition of this pathway in PDAC.
Footnotes
Drs. Guweidhi and Kleeff contributed equally to this work.
Reprints: Helmut Friess, MD, Department of General Surgery, University of Heidelberg, Im Neuenheimer Feld 110, 69120 Heidelberg, Germany. E-mail: helmut_friess@med.uni-heidelberg.de.
REFERENCES
- 1.Jemal A, Tiwari RC, Murray T, et al. Cancer statistics, 2004. CA Cancer J Clin. 2004;54:8–29. [DOI] [PubMed] [Google Scholar]
- 2.Li D, Xie K, Wolff R, et al. Pancreatic cancer. Lancet. 2004;363:1049–1057. [DOI] [PubMed] [Google Scholar]
- 3.Friess H, Ding J, Kleeff J, et al. Microarray-based identification of differentially expressed growth- and metastasis-associated genes in pancreatic cancer. Cell Mol Life Sci. 2003;60:1180–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guo J, Kleeff J, Li J, et al. Expression and functional significance of CDC25B in human pancreatic ductal adenocarcinoma. Oncogene. 2004;23:71–81. [DOI] [PubMed] [Google Scholar]
- 5.Lane TF, Sage EH. The biology of SPARC, a protein that modulates cell-matrix interactions. FASEB J. 1994;8:163–173. [PubMed] [Google Scholar]
- 6.Mason IJ, Taylor A, Williams JG, et al. Evidence from molecular cloning that SPARC, a major product of mouse embryo parietal endoderm, is related to an endothelial cell ‘culture shock’ glycoprotein of Mr 43,000. Embo J. 1986;5:1465–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwarzbauer JE, Spencer CS. The Caenorhabditis elegans homologue of the extracellular calcium binding protein SPARC/osteonectin affects nematode body morphology and mobility. Mol Biol Cell. 1993;4:941–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maurer P, Mayer U, Bruch M, et al. High-affinity and low-affinity calcium binding and stability of the multidomain extracellular 40-kDa basement membrane glycoprotein (BM-40/SPARC/osteonectin). Eur J Biochem. 1992;205:233–240. [DOI] [PubMed] [Google Scholar]
- 9.Romberg RW, Werness PG, Lollar P, et al. Isolation and characterization of native adult osteonectin. J Biol Chem. 1985;260:2728–2736. [PubMed] [Google Scholar]
- 10.Hohenester E, Maurer P, Hohenadl C, et al. Structure of a novel extracellular Ca(2+)-binding module in BM-40. Nat Struct Biol. 1996;3:67–73. [DOI] [PubMed] [Google Scholar]
- 11.Kupprion C, Motamed K, Sage EH. SPARC (BM-40, osteonectin) inhibits the mitogenic effect of vascular endothelial growth factor on microvascular endothelial cells. J Biol Chem. 1998;273:29635–29640. [DOI] [PubMed] [Google Scholar]
- 12.Sasaki T, Hohenester E, Gohring W, et al. Crystal structure and mapping by site-directed mutagenesis of the collagen-binding epitope of an activated form of BM-40/SPARC/osteonectin. EMBO J. 1998;17:1625–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sasaki T, Gohring W, Mann K, et al. Limited cleavage of extracellular matrix protein BM-40 by matrix metalloproteinases increases its affinity for collagens. J Biol Chem. 1997;272:9237–9243. [DOI] [PubMed] [Google Scholar]
- 14.Tremble PM, Lane TF, Sage EH, et al. SPARC, a secreted protein associated with morphogenesis and tissue remodeling, induces expression of metalloproteinases in fibroblasts through a novel extracellular matrix-dependent pathway. J Cell Biol. 1993;121:1433–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gilles C, Bassuk JA, Pulyaeva H, et al. SPARC/osteonectin induces matrix metalloproteinase 2 activation in human breast cancer cell lines. Cancer Res. 1998;58:5529–5536. [PubMed] [Google Scholar]
- 16.Bradshaw AD, Sage EH. SPARC, a matricellular protein that functions in cellular differentiation and tissue response to injury. J Clin Invest. 2001;107:1049–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brekken RA, Sage EH. SPARC, a matricellular protein: at the crossroads of cell-matrix communication. Matrix Biol. 2001;19:816–827. [DOI] [PubMed] [Google Scholar]
- 18.Reed MJ, Sage EH. SPARC and the extracellular matrix: implications for cancer and wound repair. Curr Top Microbiol Immunol. 1996;213:81–94. [DOI] [PubMed] [Google Scholar]
- 19.Kelm RJ Jr., Hair GA, Mann KG, et al. Characterization of human osteoblast and megakaryocyte-derived osteonectin (SPARC). Blood. 1992;80:3112–3119. [PubMed] [Google Scholar]
- 20.Porter PL, Sage EH, Lane TF, et al. Distribution of SPARC in normal and neoplastic human tissue. J Histochem Cytochem. 1995;43:791–800. [DOI] [PubMed] [Google Scholar]
- 21.Whitson SW, Harrison W, Dunlap MK, et al. Fetal bovine bone cells synthesize bone-specific matrix proteins. J Cell Biol. 1984;99:607–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brekken RA, Sage EH. SPARC, a matricellular protein: at the crossroads of cell-matrix. Matrix Biol. 2000;19:569–580. [DOI] [PubMed] [Google Scholar]
- 23.Framson PE, Sage EH. SPARC and tumor growth: where the seed meets the soil? J Cell Biochem. 2004;92:679–690. [DOI] [PubMed] [Google Scholar]
- 24.Bellahcene A, Castronovo V. Increased expression of osteonectin and osteopontin, two bone matrix proteins, in human breast cancer. Am J Pathol. 1995;146:95–100. [PMC free article] [PubMed] [Google Scholar]
- 25.Porte H, Chastre E, Prevot S, et al. Neoplastic progression of human colorectal cancer is associated with overexpression of the stromelysin-3 and BM-40/SPARC genes. Int J Cancer. 1995;64:70–75. [DOI] [PubMed] [Google Scholar]
- 26.Porte H, Triboulet JP, Kotelevets L, et al. Overexpression of stromelysin-3, BM-40/SPARC, and MET genes in human esophageal carcinoma: implications for prognosis. Clin Cancer Res. 1998;4:1375–1382. [PubMed] [Google Scholar]
- 27.Jacob K, Webber M, Benayahu D, et al. Osteonectin promotes prostate cancer cell migration and invasion: a possible mechanism for metastasis to bone. Cancer Res. 1999;59:4453–4457. [PubMed] [Google Scholar]
- 28.Le Bail B, Faouzi S, Boussarie L, et al. Osteonectin/SPARC is overexpressed in human hepatocellular carcinoma. J Pathol. 1999;189:46–52. [DOI] [PubMed] [Google Scholar]
- 29.Yamanaka M, Kanda K, Li NC, et al. Analysis of the gene expression of SPARC and its prognostic value for bladder cancer. J Urol. 2001;166:2495–2499. [PubMed] [Google Scholar]
- 30.Ledda F, Bravo AI, Adris S, et al. The expression of the secreted protein acidic and rich in cysteine (SPARC) is associated with the neoplastic progression of human melanoma. J Invest Dermatol. 1997;108:210–214. [DOI] [PubMed] [Google Scholar]
- 31.Rempel SA, Golembieski WA, Ge S, et al. SPARC: a signal of astrocytic neoplastic transformation and reactive response in human primary and xenograft gliomas. J Neuropathol Exp Neurol. 1998;57:1112–1121. [DOI] [PubMed] [Google Scholar]
- 32.Rempel SA, Ge S, Gutierrez JA. SPARC: a potential diagnostic marker of invasive meningiomas. Clin Cancer Res. 1999;5:237–241. [PubMed] [Google Scholar]
- 33.Massi D, Franchi A, Borgognoni L, et al. Osteonectin expression correlates with clinical outcome in thin cutaneous malignant melanomas. Hum Pathol. 1999;30:339–344. [DOI] [PubMed] [Google Scholar]
- 34.Thomas R, True LD, Bassuk JA, et al. Differential expression of osteonectin/SPARC during human prostate cancer progression. Clin Cancer Res. 2000;6:1140–1149. [PubMed] [Google Scholar]
- 35.Wewer UM, Albrechtsen R, Fisher LW, et al. Osteonectin/SPARC/BM-40 in human decidua and carcinoma, tissues characterized by de novo formation of basement membrane. Am J Pathol. 1988;132:345–355. [PMC free article] [PubMed] [Google Scholar]
- 36.Puolakkainen PA, Brekken RA, Muneer S, et al. Enhanced growth of pancreatic tumors in SPARC-null mice is associated with decreased deposition of extracellular matrix and reduced tumor cell apoptosis. Mol Cancer Res. 2004;2:215–224. [PubMed] [Google Scholar]
- 37.Sato N, Fukushima N, Maehara N, et al. SPARC/osteonectin is a frequent target for aberrant methylation in pancreatic adenocarcinoma and a mediator of tumor-stromal interactions. Oncogene. 2003;22:5021–5030. [DOI] [PubMed] [Google Scholar]
- 38.Li J, Kleeff J, Guweidhi A, et al. RUNX3 expression in primary and metastatic pancreatic cancer. J Clin Pathol. 2004;57:294–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Adwan H, Bauerle TJ, Berger MR. Downregulation of osteopontin and bone sialoprotein II is related to reduced colony formation and metastasis formation of MDA-MB-231 human breast cancer cells. Cancer Gene Ther. 2004;11:109–120. [DOI] [PubMed] [Google Scholar]
- 40.Nicoletti I, Migliorati G, Pagliacci MC, et al. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods. 1991;139:271–279. [DOI] [PubMed] [Google Scholar]
- 41.Briggs J, Chamboredon S, Castellazzi M, et al. Transcriptional upregulation of SPARC, in response to c-Jun overexpression, contributes to increased motility and invasion of MCF7 breast cancer cells. Oncogene. 2002;21:7077–7091. [DOI] [PubMed] [Google Scholar]
- 42.Murphy-Ullrich JE, Lane TF, Pallero MA, et al. SPARC mediates focal adhesion disassembly in endothelial cells through a follistatin-like region and the Ca(2+)-binding EF-hand. J Cell Biochem. 1995;57:341–350. [DOI] [PubMed] [Google Scholar]
- 43.Sage EH. Terms of attachment: SPARC and tumorigenesis. Nat Med. 1997;3:144–146. [DOI] [PubMed] [Google Scholar]
- 44.Young BA, Wang P, Goldblum SE. The counteradhesive protein SPARC regulates an endothelial paracellular pathway through protein tyrosine phosphorylation. Biochem Biophys Res Commun. 1998;251:320–327. [DOI] [PubMed] [Google Scholar]
- 45.Golembieski WA, Ge S, Nelson K, et al. Increased SPARC expression promotes U87 glioblastoma invasion in vitro. Int J Dev Neurosci. 1999;17:463–472. [DOI] [PubMed] [Google Scholar]
- 46.Ledda MF, Adris S, Bravo AI, et al. Suppression of SPARC expression by antisense RNA abrogates the tumorigenicity of human melanoma cells. Nat Med. 1997;3:171–176. [DOI] [PubMed] [Google Scholar]
- 47.Yamashita K, Upadhay S, Mimori K, et al. Clinical significance of secreted protein acidic and rich in cystein in esophageal carcinoma and its relation to carcinoma progression. Cancer. 2003;97:2412–2419. [DOI] [PubMed] [Google Scholar]
- 48.Shankavaram UT, DeWitt DL, Funk SE, et al. Regulation of human monocyte matrix metalloproteinases by SPARC. J Cell Physiol. 1997;173:327–334. [DOI] [PubMed] [Google Scholar]
- 49.Ellenrieder V, Alber B, Lacher U, et al. Role of MT-MMPs and MMP-2 in pancreatic cancer progression. Int J Cancer. 2000;85:14–20. [DOI] [PubMed] [Google Scholar]
- 50.Gress TM, Muller-Pillasch F, Lerch MM, et al. Balance of expression of genes coding for extracellular matrix proteins and extracellular matrix degrading proteases in chronic pancreatitis. Z Gastroenterol. 1994;32:221–225. [PubMed] [Google Scholar]
- 51.Gress TM, Muller-Pillasch F, Lerch MM, et al. Expression and in-situ localization of genes coding for extracellular matrix proteins and extracellular matrix degrading proteases in pancreatic cancer. Int J Cancer. 1995;62:407–413. [DOI] [PubMed] [Google Scholar]
- 52.Fujita T, Shiba H, Sakata M, et al. SPARC stimulates the synthesis of OPG/OCIF, MMP-2 and DNA in human periodontal ligament cells. J Oral Pathol Med. 2002;31:345–352. [DOI] [PubMed] [Google Scholar]
- 53.Yiu GK, Chan WY, Ng SW, et al. SPARC (secreted protein acidic and rich in cysteine) induces apoptosis in ovarian cancer cells. Am J Pathol. 2001;159:609–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Funk SE, Sage EH. The Ca2(+)-binding glycoprotein SPARC modulates cell cycle progression in bovine aortic endothelial cells. Proc Natl Acad Sci USA. 1991;88:2648–2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rempel SA, Golembieski WA, Fisher JL, et al. SPARC modulates cell growth, attachment and migration of U87 glioma cells on brain extracellular matrix proteins. J Neurooncol. 2001;53:149–160. [DOI] [PubMed] [Google Scholar]
- 56.Kato Y, Lewalle JM, Baba Y, et al. Induction of SPARC by VEGF in human vascular endothelial cells. Biochem Biophys Res Commun. 2001;287:422–426. [DOI] [PubMed] [Google Scholar]
- 57.Francki A, McClure TD, Brekken RA, et al. SPARC regulates TGF-beta1-dependent signaling in primary glomerular mesangial cells. J Cell Biochem. 2004;91:915–925. [DOI] [PubMed] [Google Scholar]
- 58.Bassuk JA, Pichler R, Rothmier JD, et al. Induction of TGF-beta1 by the matricellular protein SPARC in a rat model of glomerulonephritis. Kidney Int. 2000;57:117–128. [DOI] [PubMed] [Google Scholar]
- 59.Francki A, Bradshaw AD, Bassuk JA, et al. SPARC regulates the expression of collagen type I and transforming growth factor-beta1 in mesangial cells. J Biol Chem. 1999;274:32145–32152. [DOI] [PubMed] [Google Scholar]
- 60.Schiemann BJ, Neil JR, Schiemann WP. SPARC inhibits epithelial cell proliferation in part through stimulation of the transforming growth factor-beta-signaling system. Mol Biol Cell. 2003;14:3977–3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ford R, Wang G, Jannati P, et al. Modulation of SPARC expression during butyrate-induced terminal differentiation of cultured human keratinocytes: regulation via a TGF-beta-dependent pathway. Exp Cell Res. 1993;206:261–275. [DOI] [PubMed] [Google Scholar]
- 62.Reed MJ, Vernon RB, Abrass IB, et al. TGF-beta 1 induces the expression of type I collagen and SPARC, and enhances contraction of collagen gels, by fibroblasts from young and aged donors. J Cell Physiol. 1994;158:169–179. [DOI] [PubMed] [Google Scholar]
- 63.Shiba H, Fujita T, Doi N, et al. Differential effects of various growth factors and cytokines on the syntheses of DNA, type I collagen, laminin, fibronectin, osteonectin/secreted protein, acidic and rich in cysteine (SPARC), and alkaline phosphatase by human pulp cells in culture. J Cell Physiol. 1998;174:194–205. [DOI] [PubMed] [Google Scholar]
- 64.Wrana JL, Overall CM, Sodek J. Regulation of the expression of a secreted acidic protein rich in cysteine (SPARC) in human fibroblasts by transforming growth factor beta. Comparison of transcriptional and post-transcriptional control with fibronectin and type I collagen. Eur J Biochem. 1991;197:519–528. [DOI] [PubMed] [Google Scholar]


