Abstract
Objective:
We sought to determine the impact of positron emission tomography/computed tomography (PET/CT) on the management of presumed resectable pancreatic cancer and to assess the cost of this new staging procedure.
Summary Background Data:
PET using 18F-fluorodeoxyglucose (FDG) is increasingly used for the staging of pancreatic cancer, but anatomic information is limited. Integrated PET/CT enables optimal anatomic delineation of PET findings and identification of FDG-negative lesions on computed tomography (CT) images and might improve preoperative staging.
Material and Methods:
Patients with suspected pancreatic cancer who had a PET/CT between June 2001 to April 2004 were entered into a prospective database. Routine staging included abdominal CT, chest x-ray, and CA 19-9 measurement. FDG-PET/CT was conducted according to a standardized protocol, and findings were confirmed by histology. Cost benefit analysis was performed based on charged cost of PET/CT and pancreatic resection and included the time frame of staging and surgery.
Results:
Fifty-nine patients with a median age of 61 years (range, 40–80 years) were included in this analysis. Fifty-one patients had lesions in the head and 8 in the tail of the pancreas. The positive and negative predictive values for pancreatic cancer were 91% and 64%, respectively. PET/CT detected additional distant metastases in 5 and synchronous rectal cancer in 2 patients. PET/CT findings changed the management in 16% of patients with pancreatic cancer deemed resectable after routine staging (P = 0.031) and was cost saving.
Conclusions:
PET/CT represents an important staging procedure prior to pancreatic resection for cancer, since it significantly improvespatient selection and is cost-effective.
Positron emission tomography (PET) is increasingly used for the staging of pancreatic cancer. Integrated PET/computed tomography offers the advantages of precise anatomic delineation of FDG-positive findings and additional detection of FDG-negative lesions. In this study, PET/computed tomography impacted on the oncologic management in 16% of patients with resectable pancreatic cancer and was cost-effective.
Adenocarcinoma of the pancreas accounts for approximately 40,000 deaths each year in the United States as well as in Europe.1,2 Although surgery still offers the only option for cure, many patients develop early recurrence within 6–12 months of surgery. The dismal prognosis is related to the aggressive biology of this tumor entity and the presence of undetected extrapancreatic tumor spread at the time of surgery. Accurate staging, particularly identification of distant metastases, appears of paramount importance to properly select patients who are the most likely to benefit from surgery.
Current standard staging includes contrast-enhanced helical computed tomography (ceCT) of the abdomen and chest x-ray to detect infiltration of adjacent structures such as the superior mesenteric artery (SMA) and distant metastases.2 Endoscopic ultrasonography (EUS) is increasingly used to detect vascular encasement and to obtain histologic confirmation of cancer by ultrasound-guided fine-needle aspiration cytology (FNA), if necessary.3 In most current practice, exploratory laparotomy is performed for definitive surgical treatment, if the tumor is judged to be resectable by these means. Despite extensive preoperative staging, previously undetected metastases are found during laparotomy or laparoscopy in up to 30% of patients with pancreatic cancer.4–6
Positron emission tomography (PET) using 18F-fluorodeoxyglucose (FDG) is a noninvasive imaging technique that can be used to scan the entire body in one session. PET has been shown to be the most accurate examination for the detection of local recurrences and distant metastases in patients with colorectal cancer, with a significant impact on disease management.7–11 In patients with suspected pancreatic cancer, PET also has a high sensitivity for liver metastases and may even differentiate between malignant and benign lesions.7,9,12–14 However, the precise anatomic delineation of PET-positive findings is hampered by the limited anatomic information of PET images.15,16
To overcome this limitation, simultaneous examination by PET and computed tomography (CT) has been developed with the aim to coregister functional (PET) and anatomic information (CT) by the same scanner (PET/CT).16 This combined diagnostic test should provide better detection rates with additional information of unclear lesions that may be missed on either ceCT or PET. These theoretical advantages have recently been confirmed for colorectal and lung cancer, where PET/CT was significantly more accurate in predicting the tumor stage than PET or CT alone.17–19 The aim of this study was to evaluate the impact of PET/CT on the management of patients with suspected pancreatic cancer, who underwent a standardized conventional diagnostic work-up.
METHODS
Study Design
A prospective phase II trial on neoadjuvant chemotherapy for resectable pancreatic cancer was started in July 2001. Tumor staging in this investigation included standard abdominal ceCT (1–3 mm slices), chest x-ray, PET/CT, EUS with FNA of the primary tumor and FNA of metastatic lesions, serial serum CA 19-9 levels, and diagnostic laparoscopy. Since then, patients with focal lesions in the pancreas were routinely examined by PET/CT. The staging was performed in a stepwise manner, so that all patients had ceCT and PET/CT. When a patient appeared eligible for surgery, further staging examinations such as EUS, CA 19-9, and diagnostic laparoscopy were performed. Although patients were not eligible for neoadjuvant chemotherapy in absence of histologically proven cancer, surgery was not denied, as it is widely accepted that preoperative cytologic or histologic proof of pancreatic cancer is not a prerequisite for surgery. Therefore, surgery was performed in selected cases without preoperative histologic confirmation of cancer.
All laboratory, radiologic, and histologic data were entered into a prospective database and, after definition of inclusion criteria and study end points, data of eligible patients were analyzed in May 2004. Each patient with a focal lesion in the pancreas or with clinical suspicion of pancreatic cancer was eligible for this analysis. Follow-up was performed by personal contact with the attending or general physician. Data analysis was done in accordance with the guidelines of the local ethics committee, and written informedconsent for PET/CT scanning was available in each patient.
The main objective of this study was to evaluate the impact of PET/CT on the management of patients with pancreatic cancer and related cost of this new staging procedure. Secondary study objectives were the ability of PET/CT to differentiate benign and malignant pancreatic lesions and to detect distant metastases.
PET/CT Imaging Protocol
All patients had fasted for 4 to 6 hours and received an injection of 350–450 MBq FDG approximately 60 minutes prior to the PET/CT examination. In addition, patientsreceived oral contrast to improve delineation of abdominalstructures on CT.20 Intravenous contrast medium was notgiven because all patients underwent an additional ceCT scan.
A combined in-line PET/CT scanner (GEMS Discovery LS, Waukesha, WI) was used for all examinations. This device consists of a 4-slice Light Speed Plus CT scanner and an Advanced NXi PET scanner. A helical nonenhanced CT is performed followed by a FDG-PET during one imaging session with the patient being automatically moved from the CT to the PET gantry by shifting the table by 60 cm.
The CT scan was acquired from the top of the head to the pelvic floor using a standardized low-dose protocol of 140 kV, 80 mA, and a tube-rotation time of 0.5 second per rotation in the normal expiration phase to avoid mismatch of CT and PET data in the upper abdomen.21 Immediately after CT, PET was performed covering the same axial fields of view of the body. PET emission data were obtained using an acquisition time of 4 minutes per table position, and the section thickness of CT and PET studies were adapted to each other (5-mm contiguous slices). Because the CT data were used for transmission correction, additional transmission scanning was not necessary and imaging of the entire examination was done in less than 30 minutes.22
Data Analysis
The attenuation corrected PET images, the CT images and the integrated PET/CT images were viewed simultaneously by at least 2 nuclear medicine physicians and radiologists using eNtegra software (GE Medical Systems). Image interpretation was based on the identification of regions with increased FDG uptake on the PET images, and the anatomic delineation of all FDG-positive lesions on the integrated PET/CT images. Furthermore, all CT images were viewed separately to identify additional lesions without FDG uptake using soft tissue, lung and bone window leveling.
Findings on PET/CT were compared with results of standard staging and validated by intraoperative findings and histology of the resected specimen or biopsies. For patients, who were diagnosed to have a benign pancreatic lesion by PET/CT, and did not undergo resection, long-term outcome was assessed to confirm the diagnosis made by PET/CT.
Cost Benefit Analysis
We performed a cost benefit study to assess direct cost and benefits as a consequence of the additional application of PET/CT to the standard staging procedure for patients with suspected pancreatic cancer.23 For patients deemed resectable after standard staging and who were finally excluded from resection because of metastasis diagnosed by PET/CT, we assumed the saved cost were accruable to PET/CT. Cost analysis included all direct cost of standard staging with PET/CT and cost of surgery from a societal perspective. Cost data were derived from our accounting department and reflect real cost for the year 2004, including physician fees. Cost for pancreatic surgery were based on average charged costs of 10 consecutive pancreatic resections at our hospital. Cost for cytologic confirmation of suspected metastases including cost for cytologic work-up were added to the cost of PET/CT. We did not include any cost after hospital discharge such as palliative or adjuvant chemotherapy due to limited follow-up at the time of analysis. For sensitivity analysis, we also calculated mean daily charged cost for the postoperative period on the regular ward (cost for ICU excluded) and subtracted these cost from total cost according to the tested scenario. The time frame included the staging procedure and surgery. No discounting of cost was done due to a time frame within one year. Cost are expressed in U.S. dollars (exchange rate May 2004; 1CHF = $0.77).
Statistical Analysis
All values are presented as median (range). Statistical parameters, such as sensitivity, specificity, positive (PPV) and negative predictive values (NPV) were calculated by standard 2-by-2 tables. The McNemar test was used to test whether dependent categoric data of 2 groups differed. Statistical analysis was performed using SPSS (version 11.5). P values <0.05 were considered significant.
RESULTS
From July 2001 to April 2004, a PET/CT was performed in 59 patients with suspected pancreatic cancer. Patient characteristics are given in Table 1. A pancreatic mass was detected by ceCT in 50 patients, by magnetic resonance imaging in 1, by ERCP in 2, and by abdominal or endoscopic ultrasound, only, in 3 and 3 patients, respectively. The median time interval between ceCT and PET/CT was 10 days. Final histologic diagnosis was established in 52 patients (88%), while a definitive histologic diagnosis was not available in 7 patients (12%). The median follow-up of these 7 patients was 15 months (range, 6–18 months) with repeated EUS-guided FNA and serial CT or MRI of the pancreatic mass confirming the benign nature of the lesion in each case. Therefore, these patients were judged to have benign diseases and were not operated.
TABLE 1. Patient Characteristics
Can PET/CT Differentiate Benign From Malignant Pancreatic Lesions?
The most important step in the work-up is to decide whether the pancreatic lesion is benign or malignant, because the majority of benign lesions do not require surgery. Because PET/CT was performed as a low-dose CT without intravenous contrast, the CT portion could not be used for the determination of local resectability and dignity of the lesion, respectively. Therefore, only the PET portion of PET/CT was used for this analysis.
PET/CT had a high PPV (91%) but low NPV (64%) for cancer (Table 2). False-positive results were caused by an inflammatory pseudotumor, 1 pancreatic tuberculosis, 1 chronic pancreatitis, and 1 focal high-grade dysplasia, which was suspicious for malignancy by brush cytology. For this reason, the patient was treated in the neoadjuvant protocol, but no tumor was found in the resected specimen. Only the patient with pancreatic tuberculosis had a slightly elevated CRP of 16 mg/L (normal <5 mg/L). False-negative results in patients with histologically proven cancer occurred in 5 patients (Table 2). Three of these patients had elevated blood glucose levels (151 mg/dL, 156 mg/dL, and 184 mg/dL), and 3 had small tumors (T1 or T2).
TABLE 2. Ability of PET/CT to Differentiate Benign and Malignant Pancreatic Lesions (n = 59)
Is PET/CT Superior to ceCT for Diagnosing Pancreatic Cancer?
The diagnostic accuracy of PET/CT for the assessment of pancreatic cancer was compared with ceCT that represents the current standard imaging technique. Only the PET portion was used for this particular analysis, since a nonenhanced CT does not allow the differentiation of benign and malignant lesions. Sensitivity and specificity of ceCT were 93% and 21%, respectively. Although ceCT and PET/CT were equally sensitive for pancreatic cancer (P = 0.69, McNemar test), PET/CT has an increased specifity. However, the difference was not significant (P = 0.07, McNemar test).
Does PET/CT Detect Additional Distant Metastases in Patients With Pancreatic Cancer?
After establishing the diagnosis of pancreatic cancer and confirming local resectability of the tumor, the main objective of staging of pancreatic cancer is the identification of patients with distant metastases because they are currently thought not to be candidates for surgery. Therefore, PET/CT was evaluated for its ability to detect distant metastases in patients with pancreatic cancer.
Forty-six patients had histologically proven pancreatic malignancy. Sixteen patients had distant metastases detected by one of the applied staging procedures (including diagnostic laparoscopy), which were all histologically proven. Five patients were locally unresectable caused by the infiltration of mesenteric vessels. Finally, 25 patients underwent pancreas resection for malignancy.
Regional lymph node metastases (pN+) were found in 14 of the 25 patients (56%) with histologically proven adenocarcinoma who underwent pancreatic resection. PET/CT detected regional lymph node metastases only in 3 of these patients, of which 2 were FDG-positive.
Sixteen patients had distant metastases (see above), and standard staging detected 9 of them. Two hepatic lesions were misdiagnosed as liver metastases by routine staging, 1 in a patient with chronic pancreatitis, and 1 in a patient with pancreatic adenoma. In addition, 5 patients were only diagnosed by PET/CT (see below), and 2 small (<5 mm) liver metastasis were only found during diagnostic laparoscopy. Consequently, sensitivity and specificity of standard staging for distant metastases were 56% (9/16) and 95% (41/43), respectively.
PET/CT diagnosed distant metastases in 13 patients, of which 5 were only identified by PET/CT findings. Twelve of these patients had FDG-positive metastases. Metastases, which were only detected by PET/CT, were in the liver and retroperitoneal lymph nodes, the abdominal wall (Fig. 1), cervical lymph nodes (Fig. 2), and the lungs (n = 2). Lung metastases were FDG-negative in one patient, whereas the other patient had both FDG-positive and -negative metastases. Three patients were missed by PET/CT. One patient with a single liver metastasis was detected by ceCT, while 2 were only detected by diagnostic laparoscopy. No false positive results were obtained from PET/CT for distant metastases. Therefore, sensitivity and specificity were 81% (13/16) and 100% (43/43), respectively.
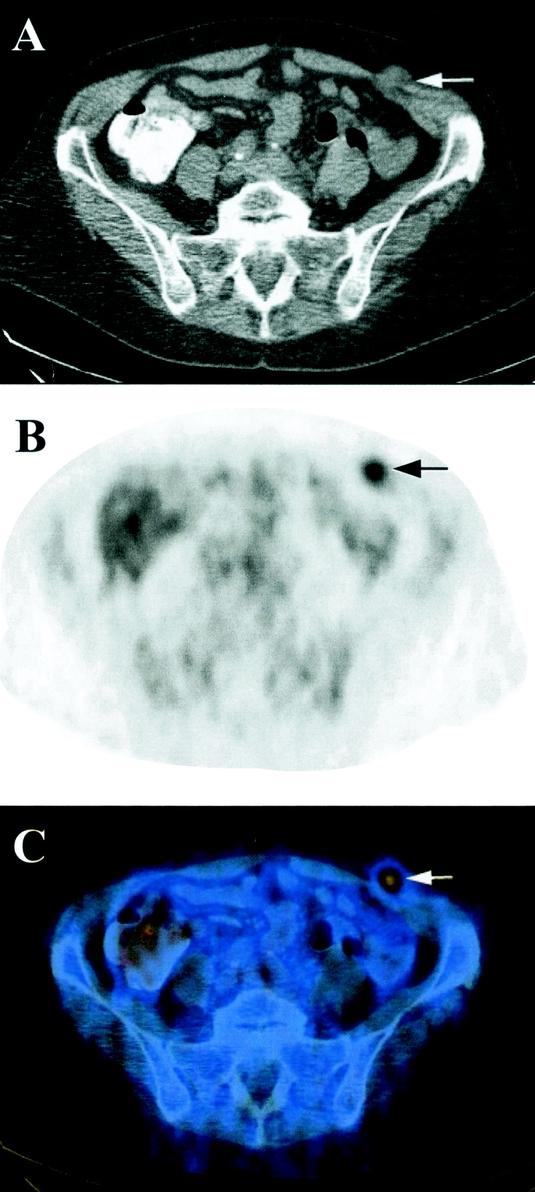
FIGURE 1. Transverse reconstruction of CT (A), PET (B), and PET/CT (C) of a 63-year-old female patient with cancer of the pancreatic tail. The metastasis in the abdominal wall (arrow) was missed on ceCT and only diagnosed because of its FDG uptake on PET/CT.
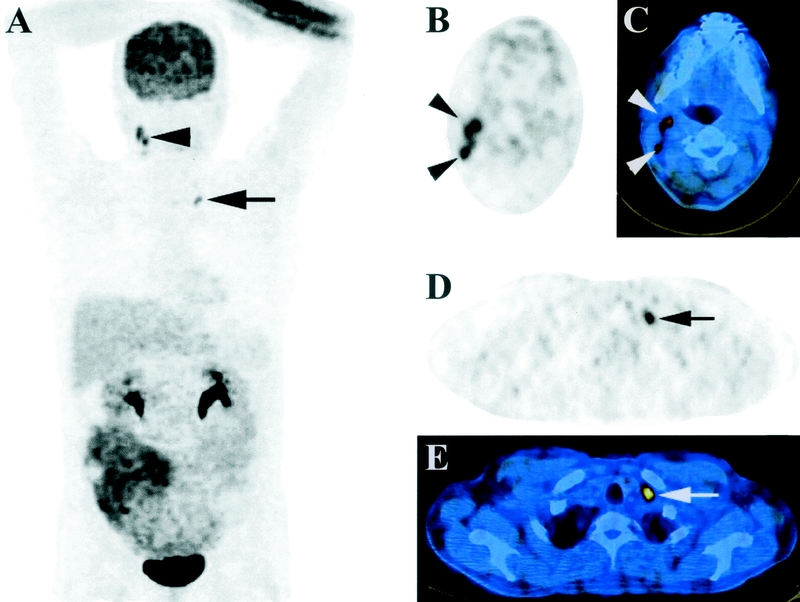
FIGURE 2. Coronal maximum intensity projection (MIP) image (A) and transverse PET, and PET/CT images at the levels of the neck (B, C) and clavicle (D, E) of a 36-year-old male patient. Pathologic FDG-uptake is found behind the left clavicle (arrows) and in the right neck (jugular chain, arrowheads). These lymph nodes were not palpable, but easily identified on transverse PET (B, D) and delineated on PET/CT (C, E) images.
PET/CT improved the detection of distant metastases compared with standard staging alone (81% versus 56%; P = 0.22, McNemar test). Standard staging followed by PET/CT further improved the detection of metastases to 88% (P = 0.06, McNemar test).
Does PET/CT Impact on the Management of Patients With Pancreatic Cancer?
The oncological management of patients with pancreatic cancer is mainly influenced by the local resectability and the detection of distant metastases. Additional malignant findings might lead to changes of the oncological management. Therefore, we evaluated the impact of PET/CT on the oncological management of patients with pancreatic cancer. A number of benign lesions (n = 17) were found by PET/CT (Table 3). Although these findings did not directly influence the treatment of pancreatic cancer, they sometimes required further diagnostic evaluation including biopsies.
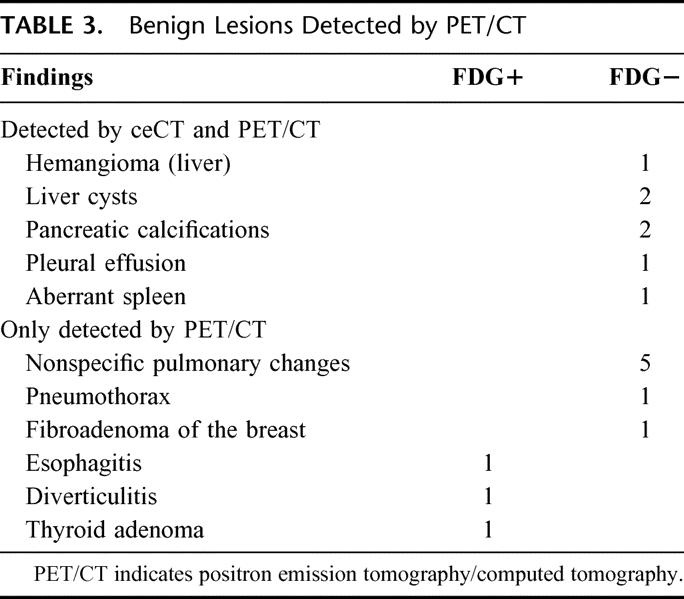
TABLE 3. Benign Lesions Detected by PET/CT
In addition to those 5 aforementioned patients in whom only PET/CT detected distant metastases, using PET/CT we diagnosed simultaneous cancer of the rectosigmoid (at 8 cm and 18 cm) in 2 patients (Fig. 3). One recto-sigmoid resection was performed with curative intention in a patient with nonmetastatic pancreatic cancer (18 cm). A palliative resection for synchronous rectal cancer (8 cm) was performed in a patient with additional metastasis of the abdominal wall. Both patients did not have symptoms from the rectal tumors, and both tumors were not detected on physical examination. Consequently, the oncological management was changed in 6/37 (16%) patients who were judged to have resectable pancreatic cancer after conventional staging due to additional findings on PET/CT.
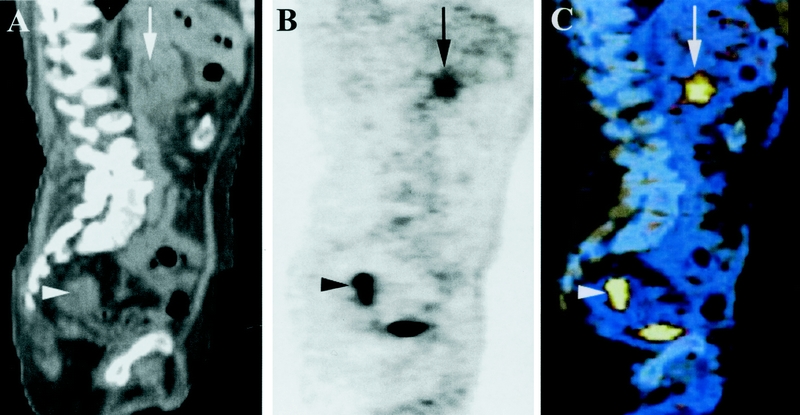
FIGURE 3. Sagittal CT (A), PET (B), and coregistered PET/CT (C) images of a 76-year-old male patient. The images show FDG-positive cancer in the head of the pancreas (arrow). In addition, FDG uptake was detected in the upper rectum (arrowhead), which referred to synchronous rectal cancer.
PET/CT findings impacted on the oncological management of patients with pancreatic cancer (15/46) significantly more often than standard staging (9/46; P = 0.03, McNemar test). In addition, PET/CT always enabled minimally invasive histologic confirmation due to exact anatomic delineation of the lesion.
Is PET/CT Cost-Effective?
The median length of stay on the intensive care unit (ICU) was 1 day (range, 1–3 days), and the median length of hospital stay including 1 preoperative day was 15 days (range, 10–40 days). The cost analysis of pancreatic resections for cancer at our hospital revealed mean costs of $37,700 per case, whereby each postoperative day on the floor accounted for $1,200. Costs of PET/CT amounted to $1,925 ($425 for FDG, $1,500 for PET/CT scanning; Table 4).
TABLE 4. Direct Costs Per Procedure and Total Cost in This Study (PET/CT, n = 59; Saved Surgery, n = 5)
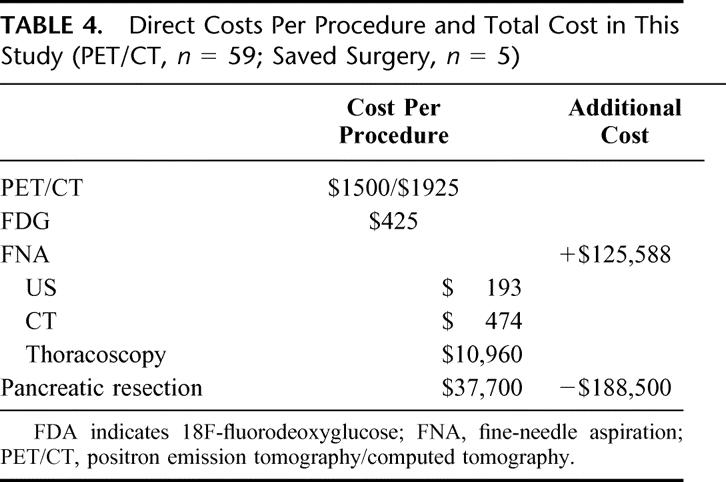
Metastases detected by PET/CT were cytologically confirmed by ultrasound (US)-guided biopsy (n = 3), CT-guided biopsy (n = 1), and thoracoscopic wedge resection (n = 1). US-guided FNA amounted for $193, CT-guided FNA for $474, and thoracoscopic wedge resection for $10,960 (overall costs). Total cost for these 5 interventions including cytologic processing amounted for $12,010.
On the basis of our series of 59 patients with suspected pancreatic cancer, 5 patients were excluded from pancreatic surgery because of metastasis diagnosed by PET/CT. Therefore, $188,500 could be saved by avoiding 5 pancreatic resections. Total cost of PET/CT for all 59 patients amounted to $125,588 (including cost for biopsies). The amount of $62,912 was finally saved by the additional use of PET/CT, which accounts for $1,066 per patient.
Sensitivity Analysis
PET/CT could be even more cost effective if it was restricted to patients who were deemed resectable after routine staging. Thirty-seven patients were judged to be surgical candidates after routine staging, but 5 patients had to be excluded because of metastasis found by PET/CT. Whereas the cost for 37 PET/CT examinations amounted to $83,238 (including cost for biopsies), $188,500 was saved by excluding5 patients with metastatic disease from surgery. By limiting PET/CT imaging to patients with resectable disease after routine staging, $105,262 could have been saved ($2844 per patient).
Because the length of hospital stay heavily influences on cost of surgery, we tested the sensitivity of our results assuming a shorter hospital stay. According to this assumption, a Whipple procedure with a 12-day or 10-day hospital stay would account for $34,100 or $30,200 per patient, respectively. However, PET/CT would still have saved $44,912 or $25,412, respectively, for the overall patient group by excluding patients with metastatic disease from surgery. These assumptions would result in cost savings of $761 and $430 per patient, respectively.
We also tested whether the type of FNA influences our analysis. If CT-guided FNA would have been applied instead of US-guided FNA, the cost for PET/CT would have increased to $126,905. Finally, we tested whether PET/CT would still be cost-saving if all metastases were confirmed by surgical interventions (eg, thoracoscopic resection). Assuming that all detected metastases were located intrathoracically, PET/CT cost (including FNA) would have amounted to $168,375 and only $20,125 could be saved ($2,853 and $341 per patient, respectively).
DISCUSSION
Surgery remains the only curative treatment of locally resectable and nonmetastatic pancreatic cancer. Although perioperative mortality has markedly decreased during the past 2 decades, complication rates remain high24 and the long-term outcome is dismal with 5-year survival rates of 10–25%.25–27 Novel staging tools are needed to improve preoperative patient selection to offer surgery only to those patients who are likely to benefit from it. Cost of novel staging tools also must be evaluated because costs have become increasingly important in many health care systems during recent years. In this study, we demonstrate that PET/CT significantly improves patient selection by changing the oncological management of patients with pancreatic cancer in 16% of the cases. Furthermore, PET/CT reduced perioperative costs by preventing unnecessary surgery. PET/CT also enabled targeted biopsies due to the exact anatomic delineation of FDG-positive lesions.
As for other adenocarcinoma, eg, colorectal and lung cancer, PET has been shown to identify otherwise undetected distant metastases in patients with pancreatic cancer.11,13,17,28 This represents a crucial aspect of staging in patients with pancreatic cancer as the occurrence of distant metastases is associated with a deleterious outcome. The main limitation of PET scanning is the poor anatomic delineation of FDG-positive lesions.15,16 In contrast, integrated PET/CT provides exact anatomic delineation of FDG positive lesions, which dramatically improves image interpretation. Physiological FDG uptake in the gastrointestinal tract, the liver and in the kidneys can easily be differentiated from malignant lesions in the retroperitoneum.15,16 Furthermore, FDG-negative lesions can be detected by the simultaneous CT scan. Supporting these putative advantages, 3 studies have convincingly shown superiority of PET/CT over PET alone for the staging of colorectal18,19 and lung17 cancer.
The main objective of this analysis was to evaluate the impact of PET/CT on the oncologic management of patients with pancreatic cancer. PET/CT might impact on the management of patients with suspected pancreatic cancer in 2 ways; ie, unnecessary surgery and further staging examinations could be avoided by reliably excluding cancer and by detecting additional metastases. Therefore, we evaluated both the ability of PET/CT to differentiate benign from malignant pancreatic lesions and to detect distant metastases.
Although no data are currently available on the use of PET/CT in the work-up of patients with pancreatic cancer, a few authors have reported on PET scanning. The sensitivity and specificity of PET/CT for the diagnosis of pancreatic cancer in the current study was similar to PET alone and consistent with previous figures available in the literature.7 However, PET and PET/CT are not used for screening asymptomatic patients, but for assessing malignancy in patients with suspected pancreatic cancer. For this reason, the PPV and NPV are much more relevant, since they describe the probabilities of FDG-positive lesions to be malignant, and of FDG-negative lesions not to be malignant, respectively. The results of our study indicate that FDG-positive pancreatic lesions have a very high probability (91%) for malignancy and were always an indication for surgery. However, because of the low NPV (64%), cancer could not be excluded based on PET/CT findings alone. In accordance with the literature, the NPV also was higher in our study if patients with elevated glucose levels were excluded (data not shown). In our series, neither the size of the pancreatic lesion nor elevated CRP values explained false PET findings.
PET and ceCT were equally effective in detecting pancreatic cancer. PET had a higher specificity than ceCT (69% versus 21%). Nevertheless, the specificity of PET is limited regarding the exclusion of pancreatic cancer, and PET should always be combined with a simultaneous CT with intravenous contrast.
PET/CT findings significantly changed the overall management of patients with pancreatic cancer compared with standard staging in the present study. Although both cases of simultaneous rectal cancer as well the metastasis in the abdominal wall were also visible on ceCT, they were missed at the time ceCT was performed, and only the FDG uptake guided to their detection. In addition, PET/CT detected FDG-negative distant metastases on the CT portion that were missed on PET. By this, PET/CT in addition to standard staging found more metastases than standard staging. However, statistical significance cannot be demonstrated when the 2 groups differ by less than 6 events regardless of the sample size. The observed difference in sensitivity (81% versus 56%) would probably be significant in a larger series of patients with the same proportion of additional metastases (16%) detected by PET/CT.
The routine use of PET is believed to not be cost-effective and has not been accepted as standard staging examination in many centers and health care systems despite its proven influence on patient selection.7 In our series of 59 patients with suspected pancreatic cancer, PET/CT was cost saving by excluding patients from resection because of metastasis. Our cost and treatment data were derived from Swiss cost structures and Swiss treatment patterns. Because costs for PET/CT are not available from other centers, a cost comparison is not possible. However, our cost for a Whipple procedure of $37,700 are well comparable with cost data derived from the United States published during recent years ($38,000),29–31 and the hospital stay in our series was within the range reported from other centers in Europe32,33 and the United States34,35 (12–20 days). Neither shortening the length of hospital stay nor the use of CT guided FNA and surgical assessment of metastasis (by a thoracoscopic or laparoscopic approach) reversed the cost-effectiveness of PET/CT.
The main limitation of our cost study is a short time frame that considers only the staging process and the perioperative period due to a limited follow-up at the time of analysis. Patients with metastatic pancreatic cancer detected by PET/CT may generate further cost because they need various palliative treatments, including chemotherapy, repeated biliary stenting, or palliative surgery.2 However, patients undergoing pancreas resection are increasingly offered adjuvant treatments,36,37 which would at least equalize these cost. Furthermore, improved patient selection by PET/CT may increase survival after surgery, which might reduce overall cost. Because PET/CT is the combination of PET and CT, the detection rate of the PET and CT portions of PET/CT are the same as for either examination alone. The major advantages of PET/CT are the simultaneous availability of both functional and anatomic information that facilitates an optimal fusion of both imaging techniques. Only by this improved imaging fusion, FDG-positive findings, eg, lymph node metastases, can be exactly identified.
As reported from the literature and also demonstrated in this study, FDG uptake is not specific for malignancy,28 and FDG-positive lesions always require histologic confirmation before a patient is denied surgery. Local resectability is still best assessed by ceCT with thin slices, particularly the determination of vascular infiltration of the SMA and celiac axis. In this study, only a low-dose CT without intravenous contrast medium was used for image fusion of PET/CT. Regular ceCT using intravenous contrast with arterial and porto-venous phases can be performed by the PET/CT scanner. Whether PET/CT with intravenous contrast (cePET/CT) may replace ceCT as an “all-in-one” staging procedure for pancreatic cancer, needs to be evaluated in future studies, but yields promises. Such “all-in-one” procedures could further increase specificity and improve cost-effectiveness of PET/CT. On the basis of our clinical and economic evaluation of PET/CT in the preoperative staging process of patients with pancreatic cancer, PET/CT proves to be beneficial and might advance to a standard staging examination for pancreatic cancer.
Footnotes
Drs. Heinrich and Goerres contributed equally to this work.
Reprints: Pierre-Alain Clavien, MD, PhD, FACS, FRCS, Department of Visceral and Transplantation Surgery, University of Zürich, Rämistrasse 100, 8091 Zürich, Switzerland. E-mail: clavien@chir.unizh.ch.
REFERENCES
- 1.Ferlay J, Bray F, Pisani P GLOBOCAN 2000: cancer incidence, mortality and prevalence worldwide. In: IARC, ed. Cancer Base No. 5. Lyon: IARC; 2001. [Google Scholar]
- 2.Li D, Xie K, Wolff R, et al. Pancreatic cancer. Lancet. 2004;363:1049–1057. [DOI] [PubMed] [Google Scholar]
- 3.Raut C, Grau A, Staerkel G, et al. Diagnostic accuracy of endoscopic ultrasound-guided fine-needle aspiration in patients with presumed pancreatic cancer. J Gastrointest Surg. 2003;7:118–128. [DOI] [PubMed] [Google Scholar]
- 4.John T, Greig J, Carter D, et al. Carcinoma of the pancreatic head and periampullary region. Ann Surg. 1995;221:156–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conlon K, Dougherty E, Klimstra D, et al. The value of minimal access surgery in the staging of patients with potentially resectable peripancreatic malignancy. Ann Surg. 1996;223:134–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fernandez-del Castillo C, Rattner D, Warshaw A. Further experience with laparoscopy and peritoneal cytology in the staging of pancreatic cancer. Br J Surg. 1995;82:1127–1129. [DOI] [PubMed] [Google Scholar]
- 7.Annovazzi A, Peeters M, Maenhout A, et al. 18-fluorodeoxyglucose positron emission tomography in nonendocrine neoplastic disorders of the gastrointestinal tract. Gastroenterology. 2003;125:1235–1245. [DOI] [PubMed] [Google Scholar]
- 8.Fong Y, Saldinger P, Akhurst T, et al. Utility of 18F-FDG positron emission tomography scanning on selection of patients for resection of hepatic colorectal metastases. Am J Surg. 1999;178:282–287. [DOI] [PubMed] [Google Scholar]
- 9.Staib L, Schirrmeister H, Reske S, et al. Is 18F-fluorodeoxyglucose positron emission tomography in recurrent colorectal cancer a contribution to surgical decision making? Am J Surg. 2000;180:1–5. [DOI] [PubMed] [Google Scholar]
- 10.Desai D, Zervos E, Arnold M, et al. Positron emission tomography affects surgical management in recurrent colorectal cancer patients. Ann Surg Oncol. 2002;10:59–64. [DOI] [PubMed] [Google Scholar]
- 11.Ruers T, Langenhoff B, Neeleman N, et al. Value of positron emission tomography with F18-Fluorodeoxyglucose in patients with colorectal liver metastases: a prospective study. J Clin Oncol. 2002;20:388–395. [DOI] [PubMed] [Google Scholar]
- 12.Nakamoto Y, Higashi T, Sakahara H, et al. Delayed 18F-Fluoro-2-Deoxy-D-Glucose positron emission tomography scan for differentiation between malignant and benign lesions in the pancreas. Cancer. 2000;89:2547–2554. [DOI] [PubMed] [Google Scholar]
- 13.Diederichs C, Staib L, Vogel J, et al. Values and limitations of 18F-Fluorodeoxyglucose-positron-emission tomography with preoperative evaluation of patients with pancreatic masses. Pancreas. 2000;20:109–116. [DOI] [PubMed] [Google Scholar]
- 14.Delbeke D, Rose D, Chapman W, et al. Optimal interpretation of FDG PET in the diagnosis, staging and management of pancreatic carcinoma. J Nucl Med. 1999;40:1784–1791. [PubMed] [Google Scholar]
- 15.Hosten N, Lemke AJ, Wiedenmann B, et al. Combined imaging techniques for pancreatic cancer. Lancet. 2000;356:909–910. [DOI] [PubMed] [Google Scholar]
- 16.Beyer T, Townsend D, Brun T, et al. A combined PET/CT scanner for clinical oncology. J Nucl Med. 2000;41:1369–1379. [PubMed] [Google Scholar]
- 17.Lardinois D, Weder W, Hany TF, et al. Staging of non-small-cell lung cancer with integrated positron-emission tomography and computed tomography. N Engl J Med. 2003;348:2500–2507. [DOI] [PubMed] [Google Scholar]
- 18.Cohade C, Osman M, Leal J, Wahl RL. Direct comparison of (18)F-FDG PET and PET/CT in patients with colorectal carcinoma. J Nucl Med. 2003;44:1797–1803. [PubMed] [Google Scholar]
- 19.Selzner M, Hany TF, Wildbrett P, et al. Does the novel PET/CT imaging modality impact on the treatment of patients with metastatic colorectal cancer to the liver? Ann Surg. 2004;240:1027–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dizendorf EV, Treyer V, Von Schulthess GK, Hany TF. Application of oral contrast media in coregistered positron emission tomography-CT. AJR Am J Roentgenol. 2002;179:477–481. [DOI] [PubMed] [Google Scholar]
- 21.Goerres GW, Burger C, Kamel E, et al. Respiration-induced attenuation artifact at PET/CT: technical considerations. Radiology. 2003;226:906–910. [DOI] [PubMed] [Google Scholar]
- 22.Burger C, Goerres G, Schoenes S, et al. PET attenuation coefficients from CT images: experimental evaluation of the transformation of CT into PET 511-keV attenuation coefficients. Eur J Nucl Med Mol Imaging. 2002;29:922–927. [DOI] [PubMed] [Google Scholar]
- 23.Gold M, Siegel J, Russell L, et al. Cost Effectiveness in Health and Medicine. New York: Oxford University Press, 1996. [Google Scholar]
- 24.Schafer M, Mullhaupt B, Clavien PA. Evidence-based pancreatic head resection for pancreatic cancer and chronic pancreatitis. Ann Surg. 2002;236:137–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yeo CJ, Cameron JL, Lillemoe KD, et al. Pancreaticoduodenectomy with or without distal gastrectomy and extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma, part 2: randomized controlled trial evaluating survival, morbidity, and mortality. Ann Surg. 2002;236:355–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Richter A, Niedergethmann M, Sturm JW, et al. Long-term results of partial pancreaticoduodenectomy for ductal adenocarcinoma of the pancreatic head: 25-year experience. World J Surg. 2003;27:324–329. [DOI] [PubMed] [Google Scholar]
- 27.van Geenen RC, van Gulik TM, Offerhaus GJ, et al. Survival after pancreaticoduodenectomy for periampullary adenocarcinoma: an update. Eur J Surg Oncol. 2001;27:549–557. [DOI] [PubMed] [Google Scholar]
- 28.Frohlich A, Diederichs CG, Staib L, et al. Detection of liver metastases from pancreatic cancer using FDG PET. J Nucl Med. 1999;40:250–255. [PubMed] [Google Scholar]
- 29.Rosemurgy AS, Bloomston M, Serafini FM, et al. Frequency with which surgeons undertake pancreaticoduodenectomy determines length of stay, hospital charges, and in-hospital mortality. J Gastrointest Surg. 2001;5:21–26. [DOI] [PubMed] [Google Scholar]
- 30.Gordon TA, Burleyson GP, Tielsch JM, et al. The effects of regionalization on cost and outcome for one general high-risk surgical procedure. Ann Surg. 1995;221:43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Porter GA, Pisters PW, Mansyur C, et al. Cost and utilization impact of a clinical pathway for patients undergoing pancreaticoduodenectomy. Ann Surg Oncol. 2000;7:484–489. [DOI] [PubMed] [Google Scholar]
- 32.Wagner M, Redaelli C, Lietz M, et al. Curative resection is the single most important factor determining outcome in patients with pancreatic adenocarcinoma. Br J Surg. 2004;91:586–594. [DOI] [PubMed] [Google Scholar]
- 33.Russell RC, Theis BA. Pancreatoduodenectomy in the treatment of chronic pancreatitis. World J Surg. 2003;27:1203–1210. [DOI] [PubMed] [Google Scholar]
- 34.Sosa JA, Bowman HM, Gordon TA, et al. Importance of hospital volume in the overall management of pancreatic cancer. Ann Surg. 1998;228:429–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Strasberg SM, Drebin JA, Soper NJ. Evolution and current status of the Whipple procedure: an update for gastroenterologists. Gastroenterology. 1997;113:983–994. [DOI] [PubMed] [Google Scholar]
- 36.Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350:1200–1210. [DOI] [PubMed] [Google Scholar]
- 37.GITSG. Pancreatic cancer. Adjuvant combined radiation and chemotherapy following curative resection. Arch Surg 1985;120:899–903. [DOI] [PubMed] [Google Scholar]


