Abstract
Objectives:
To evaluate the prognostic impact of anatomic versus nonanatomic resection on the patients’ survival after resection of a single hepatocellular carcinoma (HCC).
Summary of Background Data:
Anatomic resection is a reasonable treatment option for HCC; however, its clinical significance remains to be confirmed.
Methods:
Curative hepatic resection was performed for a single HCC in 210 patients; the patients were classified into the anatomic resection (n = 156) and nonanatomic resection (n = 54) groups. In 84 patients assigned to the anatomic resection group, segmentectomy or subsegmentectomy was performed. We evaluated the outcome of anatomic resection, including segmentectomy and subsegmentectomy, in comparison with that of nonanatomic resection, by the multivariate analysis taking into consideration 14 other clinical factors.
Results:
Both the 5-year overall survival and disease-free survival rates in the anatomic resection group were significantly better than those in the nonanatomic resection group (66% versus 35%, P = 0.01, and 34% versus 16%, P = 0.006, respectively). In the segmentectomy and subsegmentectomy group, the 5-year overall and disease-free survival rates were 67% and 28%, respectively, both of which were also higher than the corresponding rates in the nonanatomic resection group (P = 0.007 and P = 0.001, respectively). The results of multivariate analysis revealed that anatomic resection was a significantly favorable factor for overall and disease-free survivals: the hazard ratios were 0.57 (95% confidence interval, 0.32–0.99, P= 0.04), and 0.65 (0.43–0.96, P = 0.03).
Conclusion:
Anatomic resection for a single HCC yields more favorable results rather than nonanatomic resection.
We evaluated the clinical significance of anatomic versus nonanatomic resection for hepatocellular carcinoma. Anatomic resection was an independently favorable factor for both overall and disease-free survivals in cases with a single hepatocellular carcinoma.
Hepatocellular carcinoma (HCC) is one of the most common cancers worldwide. Hepatic resection is now established as the first-line therapeutic option for HCC.1,2 However, a high incidence of postoperative recurrence with a 3-year recurrence rate of up to 60%3–5 remains a serious problem.
One of the major forms of recurrence of HCC is intrahepatic metastasis via vascular invasion. HCC has a high propensity to invade the portal and hepatic veins.6 Indeed, vascular invasion and intrahepatic metastasis are among the risk factors that most strongly influence the postoperative prognosis.7–12 Therefore, eradication of intrahepatic metastasis is the most crucial consideration for improving the surgical outcome in HCC.
Anatomic resection, which was originally introduced as segmentectomy and subsegmentectomy by Makuuchi et al,13 is systematic removal of a hepatic segment confined by tumor-bearing portal tributaries. Because of the high likelihood of the cancer cells from HCC spreading through the portal venous system, anatomic resection is theoretically effective for eradication of the intrahepatic metastases of HCC.13,14 The potential superiority of anatomic resection for HCC has been indicated.5,14–20 However, the supporting evidence is insufficient.
For the last 8 years, we have consecutively performed anatomic resection as first-line treatment of HCC. The purpose of this study was to evaluate the outcome of anatomic resection for a single HCC in terms of the long-term results.
PATIENTS AND METHODS
Study Population
Between 1994 and 2001, 315 patients underwent initial and curative hepatic resection for HCC. Among the 315 patients, 105 were excluded from this analysis for the following reasons: multiple tumors (86 patients), secondary malignancy diagnosed within 5 years prior to the hepatic resection (13 patients), and spontaneous rupture of the HCC (6 patients). To clearly evaluate the effects of anatomic resection on the eradication of intrahepatic metastasis, we also excluded cases with multiple HCC, not only those with intrahepatic metastases but also those with multicentric tumors. The remaining 210 patients were divided into two groups: the anatomic (n = 156) and nonanatomic (n = 54) resection groups. Anatomic resection includes segmentectomy and subsegmentectomy (Makuuchi's procedure, n = 84),13 sectoriectomy, hemihepatectomy, and trisectoriectomy, based on Couinaud's classification.21 The operative procedures conducted in the anatomic resection group are shown in Table 1. Nonanatomic resection consisted of limited resection22 and enucleation.
TABLE 1. Operative Procedures of Anatomic Resection
Surgical Techniques
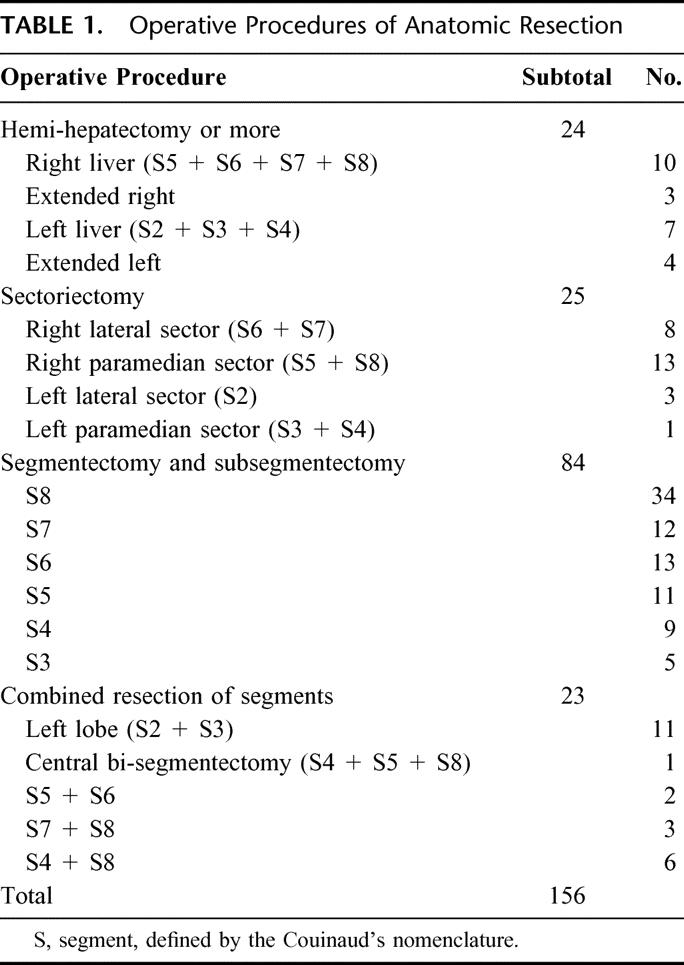
Indications for hepatic resection and the types of operative procedures were determined based on our criteria (Makuuchi's criteria), ie, the presence or absence of ascites, the serum total bilirubin level, and the indocyanine green retention rate at 15 minutes (ICG R15).23 Operation allocation depended on Makuuchi's criteria, not on time period. We divided the liver along the demarcation line appearing after occlusion of the portal vein and hepatic artery in hemihepatectomy and sectoriectomy, or after injection of dye into the portal vein confining the tumor-bearing area under intraoperative ultrasound guidance in the case of anatomic segmentectomy and subsegmentectomy (Fig. 1A). 13 In nonanatomic resection, we divided the liver along a line so as to secure a surgical margin of at least 5 mm, if possible. When it was impossible, liver parenchymal transection was performed not to expose the tumor surface. We divided the liver parenchyma by the clamp-crushing method or an ultrasonic dissector.24 In anatomic resection, we took into account the following key points: accurate decision of the resection area, accurate approach to the portal triad to be resected with the help of ultrasonography, and full exposure of the landmark vessels on the cutting surface, such as the right and middle hepatic veins, in the cases of anatomic resection of the whole segment 8 (Fig. 1B).
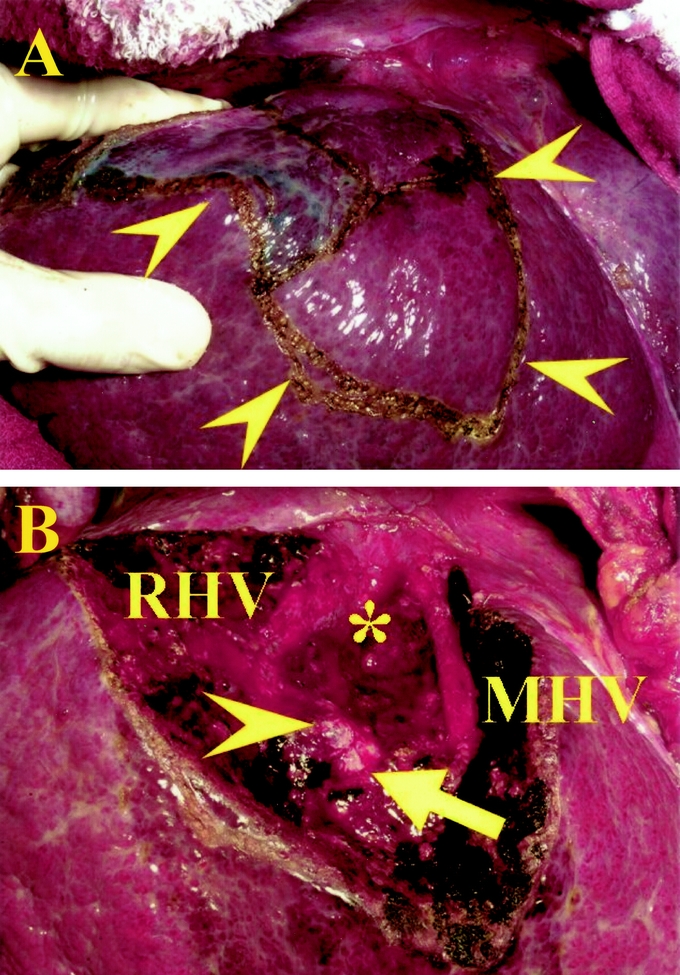
FIGURE 1. The views before (A) and after (B) anatomic resection of segment 8. A, The surface of segment 8 is marked, according to the stained area after blue dye injection (arrowheads). B, The landmark veins were exposed longitudinally in the cutting surface: the inferior vena cava (*), the right hepatic vein (RHV), and the middle hepatic vein (MHV, arrowheads). The stumps of the ventral (arrow) and dorsal (arrowhead) branches of Glisson's triad in segment 8 are seen.
The size of the tumors and length of the surgical margin were measured before fixation of the specimens. The background liver status, level of tumor cell differentiation, and cancer spread were assessed by microscopic examination of the specimens. Cancer spread was defined by the presence of microscopic vascular invasion and/or intrahepatic metastasis, as had been done in our previous report.12
Follow-up
After discharge, all the patients were examined for recurrence by ultrasonography every 2 months and by dynamic computed tomography every 4 months, as previously described.12 The median follow-up period after the surgery was 3.3 years (range, 0.2–7.9 years). When recurrence was suspected, we performed hepatic angiography with injection of iodized oil (Lipiodol; Guerbet Laboratories, Aulnay Sous Bois, France) followed by Lipiodol computed tomography. Recurrence was defined as the appearance of a new lesion with radiologic features typical of HCC, as confirmed by two or more imaging modalities. In this study, disease-free survival was defined as an interval between the operation and the date of the diagnosis of the first recurrence or the last follow-up.
Statistical Analysis
The survival curves of the anatomic and nonanatomic resection groups were generated by the Kaplan-Meier method and compared by the log-rank test. To investigate the prognostic significance of the operative procedure (anatomic versus nonanatomic resection), we performed the multivariate regression analysis with the Cox proportional hazard model, using a variable-selection method by the backward-elimination procedure. P < 0.15 was set as the cutoff for the elimination.
In the multivariate analysis, we chose 14 factors as potential confounders, considering their clinical significance and the results of previous reports.4,5,7–11,19,25 Because any factors that are of potential importance can be incorporated into the multivariate analysis whether or not they are statistically significant,26 we entered some nonsignificant factors in the univariate analysis into the model of the multivariate analysis in the present study. The 14 factors were as follows: age (older versus younger than 65 years), sex, preoperative serum total bilirubin level (more versus less than 1 mg/dL), ICG R15 (more versus less than 20%), Child-Pugh class (A versus B), background liver status (cirrhosis versus noncirrhosis) as assessed histologically, tumor size (larger versus smaller than 30 mm), cancer spread (present or absent), tumor cell differentiation (well versus moderate or poor), serum α-fetoprotein level (more versus less than 100 ng/mL), plasma des-γ-carboxy prothrombin (positive versus negative), history of red blood cell transfusion (yes versus no), surgical margin (greater versus smaller than 5 mm), and tumor exposure (yes versus no).
The Mann-Whitney U test and the χ2 test were used for the continuous and the categorical data, respectively. Calculations were performed with the help of the Stat View 5.0 computer software (SAS Institute Inc., Cary, NC).
RESULTS
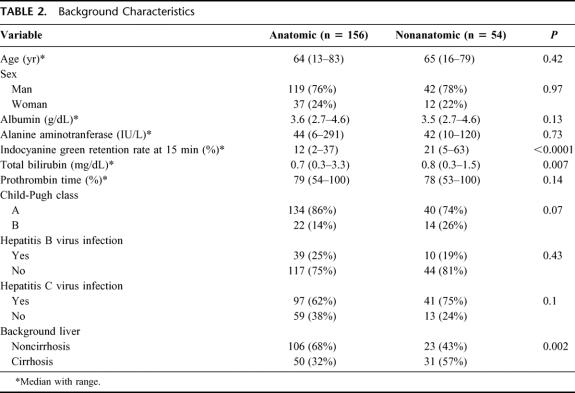
The background characteristics in the anatomic and nonanatomic resection groups are shown in Table 2. The values of ICG R15 and total bilirubin in the anatomic resection group were significantly lower than those in the nonanatomic resection. All patients enrolling into this study had no ascites before surgery. The nonanatomic resection group included more patients with cirrhosis than the anatomic resection group.
TABLE 2. Background Characteristics
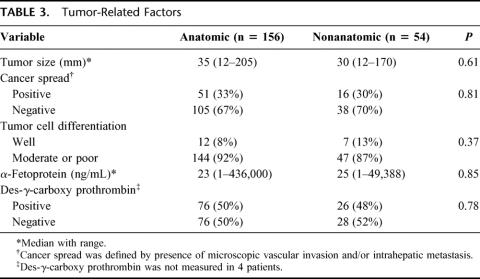
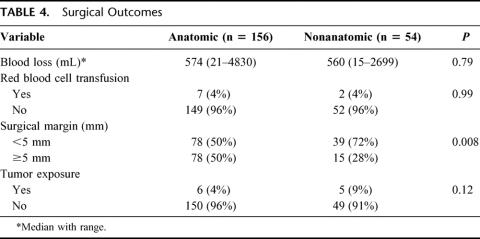
In all the 210 cases, all of the recognizable tumors were resected without iatrogenic rupture of the tumors. As for the cancer-related factors, there were no significant differences between the 2 groups (Table 3). Table 4 shows the surgical results in the two groups. Intraoperative blood loss and incidence of red cell transfusion were similar between the two groups. The length of the surgical margin in the nonanatomic resection group was smaller than that in the anatomic resection group (2 mm; range, 0–11 mm; versus 4 mm, range, 0–55 mm; P = 0.0002).
TABLE 3. Tumor-Related Factors
TABLE 4. Surgical Outcomes
No hospital deaths occurred. The overall and disease-free survival curves of the anatomic and nonanatomic resection groups are illustrated in Figures 2A and B, respectively. The postoperative results were significantly better in the anatomic resection group than in the nonanatomic resection group, in term of both the overall and the disease-free survival rates (P = 0.01 and 0.006, respectively). The overall 1-, 3-, and 5- year survival rates were 95%, 84%, and 66% in the anatomic resection group, and 93%, 66%, and 35% in the nonanatomic resection group, respectively; on the other hand,the 1-, 3-, and 5- year disease-free survival rates were 76%, 50%, and 34% in the anatomic resection group, and 69%, 20%, and 16% in the nonanatomic resection group, respectively.
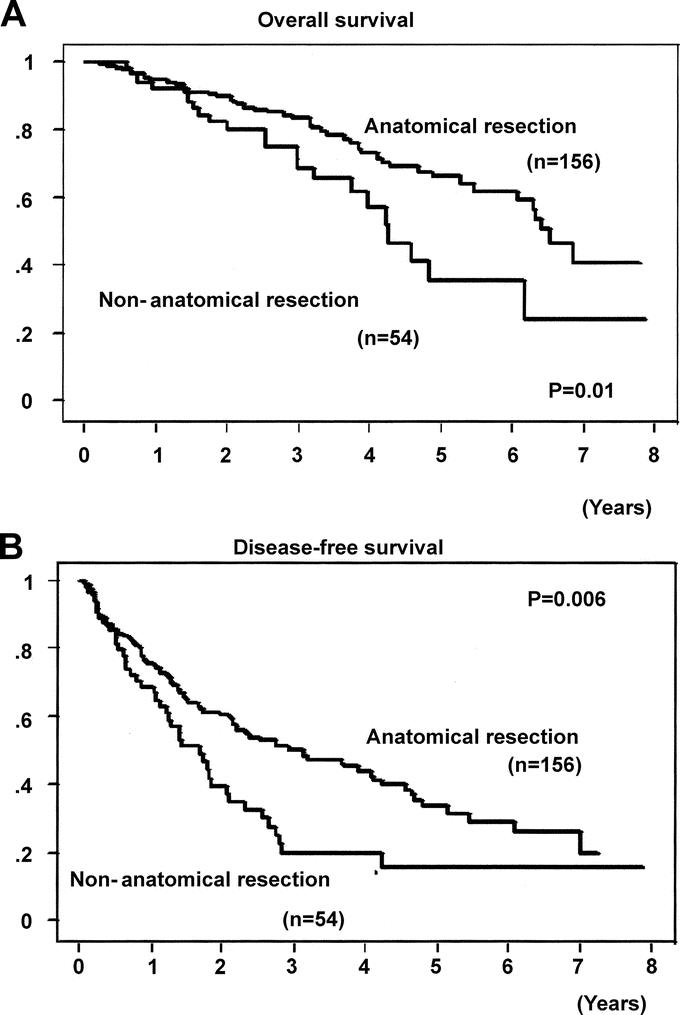
FIGURE 2. Overall (A) and disease-free (B) survival curves after anatomic and nonanatomic resections for a single hepatocellular carcinoma.
The segmentectomy and subsegmentectomy group (n = 84) also had significantly better overall and disease-free survivals than the nonanatomic resection group (P = 0.007 and P = 0.001, respectively, Fig. 3). In the segmentectomy and subsegmentectomy group, the overall 1-, 3-, and 5- year survival rates were 97, 87%, and 67%, respectively, while the 1-, 3-, and 5- year disease-free survival rates were 82%, 55%, and 28%, respectively.
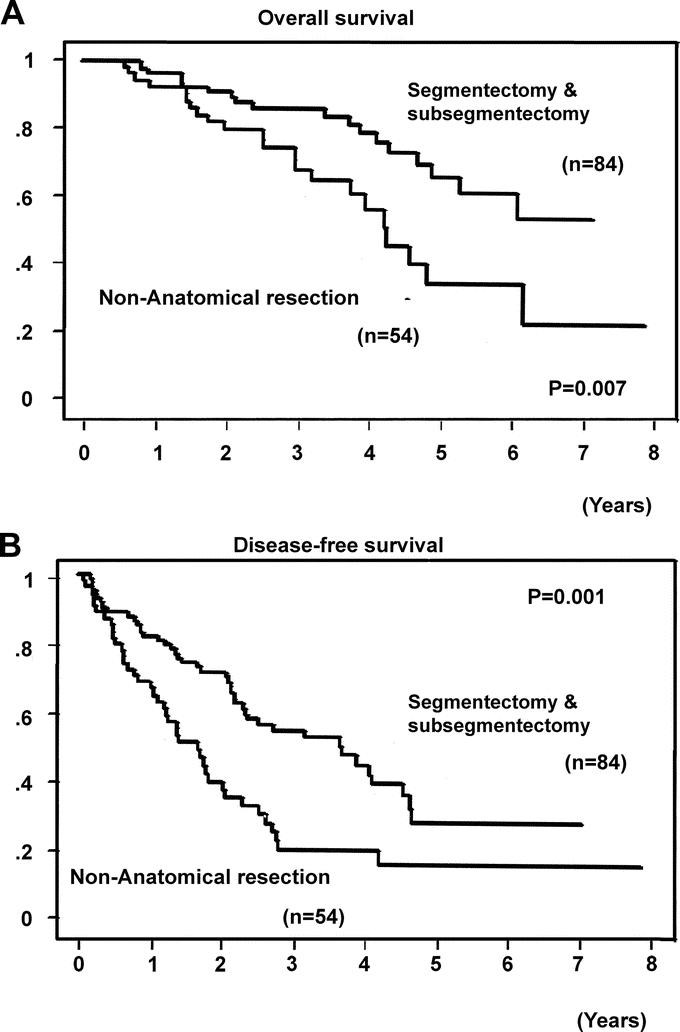
FIGURE 3. Overall (A) and disease-free (B) survival curves after segmentectomy and subsegmentectomy, and after nonanatomic resection, for a single hepatocellular carcinoma.
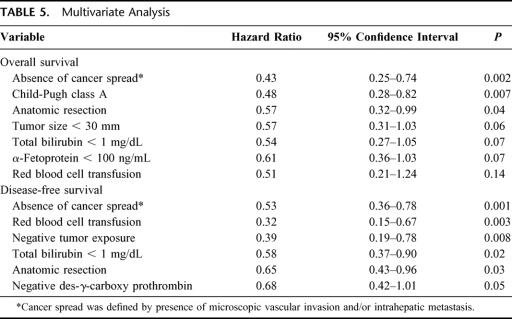
Multivariate analysis identified three factors (absence of cancer spread, Child-Pugh class A, and anatomic resection) as significantly influencing the overall survival rate, and five factors (absence of cancer spread, red blood cell transfusion, negative tumor exposure, total bilirubin < 1 mg/dL, and anatomic resection) as significantly influencing the disease-free survival rate (Table 5). Anatomic resection was confirmed to be an independent favorable factor for overall and disease-free survival: the hazard ratios with 95% confidence intervals and the P values were 0.57, 0.32–0.99, P = 0.04, and 0.65, 0.43–0.96, P = 0.03, respectively. Absence of cancer spread was also a significantly favorable factor for overall and disease-free survival (0.43, 0.25–0.74, P = 0.002; and 0.53, 0.36–0.78, P = 0.001, respectively).
TABLE 5. Multivariate Analysis
DISCUSSION
This retrospective study shows that anatomic resection contributes to better overall and disease-free survival after initial surgery in cases with a single HCC. It is suggested that anatomic resection would be superior to nonanatomic resection as an operative procedure for HCC.
In treatment of HCC, eradication of intrahepatic metastasis occurring via vascular invasion is one of the most important considerations, as suggested by the previous reports.7–12 The results of the multivariate analysis in this study, which showed that the presence of cancer spread (vascular invasion and/or intrahepatic metastasis) was an independently unfavorable factor in relation to the postsurgical outcome, also supports this suggestion. However, the most suitable operative procedure for efficient eradication of intrahepatic metastasis has not yet been established. To maximally preserve noncancerous, functional liver parenchyma, some investigators have recommended the use of nonanatomic, or limited, resection.22 However, the nonanatomic approach would be disadvantageous when considered from the standpoint of eradication of intrahepatic metastasis. The results of ablation therapy for HCC, excluding cases with cancer seeding and/or tumors located in the dead angle in extracorporeal ultrasound examination, may be the same as the results of a nonanatomic approach for this cancer.
On the other hand, to remove minute cancerous foci with a main HCC, wide hepatic resection, such as right or left hemihepatectomy, has been recommended.27 However, the policy of wide resection would restrict not only the indications of surgical therapy for HCC, but also the chance of a repeat resection, which would be the most effective for a recurrence.28
Anatomic resection, taking into consideration both preservation, to the maximal extent possible, of liver functional parenchyma and eradication of intrahepatic metastasis, would be a theoretically reasonable procedure. To our knowledge, only four papers have reported the effectiveness of anatomic resection in terms of the long-term results: 3 based on univariate analyses18–20 and one in terms of the disease-free survival.5 The multivariate analyses in the present study showed that three factors (absence of cancer spread, Child-Pugh class A, and anatomic resection) significantly influenced the overall survival, and five factors (absence of cancer spread, red blood cell transfusion, negative tumor exposure, total bilirubin < 1 mg/dL, and anatomic resection) significantly influenced the disease-free survival rate. Among them, only two of the factors, namely, anatomic resection and absence of cancer spread, significantly influenced both the disease-free survival and the overall survival according to the results of the multivariate analyses. These results strongly support the clinical efficacy of anatomic resection for HCC.
There may be skepticism that the different background characteristics between the two groups (Table 1), especially in terms of liver function, might bias the results of this study. To minimize and adjust for the effects of the differences, we adopted the Cox proportional hazard model with 14 other potential confounders. The hazard ratios of anatomic resection for both overall and disease-free survival in the multivariate analyses are small enough to support its clinical importance (0.57; 95% confidence interval, 0.32–0.99; and 0.65; 95% confidence interval, 0.43–0.96, respectively). Because interpretation of our surgical outcomes is probably affected by potential sources of selection bias due to Makuuchi's criteria, it is difficult to defend the main conclusion of this study without randomization.
If confounders in a multivariate analysis are limited only to the significant factors in univariate analysis, some factors, which are accidentally nonsignificant despite their potential importance, may be excluded. Therefore, according to the Bradburn et al recommendation,26 we chose 14 factors as confounders, after weighing their clinical importance, whether or not they were significant in the univariate analysis. Indeed, this method was also adopted in a previous study.29 The present study indicated that anatomic resection would be suitable option of choice for HCC.
Liver transplantation is the most reasonable surgical approach for HCC associated with the injured liver. The long-term results of liver transplantation are satisfactory in patients with HCC who satisfy the Milan's criteria.30 However, the application of liver transplantation for malignant diseases is limited, owing to severe graft shortage.31 There has been a report suggesting that HCC developing in a well-compensated cirrhotic liver might be treated with hepatic resection.32
The segmentectomy and subsegmentectomy group also showed better long-term results than the nonanatomic resection group, although this was not statistically confirmed by the results of the multivariate analyses, perhaps due to the small number of patients in the study. The results, indeed, suggest that segmentectomy and subsegmentectomy would be as effective for HCC as hemihepatectomy or sectoriectomy. However, further investigation is needed to evaluate the validity of this suggestion.
CONCLUSION
Compared with nonanatomic resection, anatomic resection is effective to improve the rates of overall and disease-free survival after initial and curative resection in cases of a single HCC.
Footnotes
Supported by a Grant-in-Aid for scientific research from the Ministry of Education, Science, Sports and Culture (Grant No.12470252) and by a grant from the Kanae Foundation for Life & Socio-medical Science.
Reprints: Masatoshi Makuuchi, MD, PhD, Division of Hepato-Biliary-Pancreatic Surgery, Department of Surgery, Graduate School of Medicine, University of Tokyo, 7-3-1, Hongo, Bunkyo-ku, Tokyo 113-8655, Japan. E-mail: makuuchi-tky@umin.ac.jp.
REFERENCES
- 1.Fan ST, Lo CM, Liu CL, et al. Hepatectomy for hepatocellular carcinoma: toward zero hospital deaths. Ann Surg. 1999;229:322–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Torzilli G, Makuuchi M, Inoue K, et al. No-mortality liver resection for hepatocellular carcinoma in cirrhotic and noncirrhotic patients: is there a way? A prospective analysis of our approach. Arch Surg. 1999;134:984–992. [DOI] [PubMed] [Google Scholar]
- 3.Belghiti J, Panis Y, Farges O, et al. Intrahepatic recurrence of hepatocellular carcinoma complicating cirrhosis. Ann Surg. 1990;214:114–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fan ST, Ng IOL, Poon RTP, et al. Hepatectomy for hepatocellular carcinoma: the surgeon's role in long-term survival. Arch Surg. 1999;134:1124–1130. [DOI] [PubMed] [Google Scholar]
- 5.Imamura H, Matsuyama Y, Miyagawa Y, et al. Prognostic significance of anatomical resection and des-γ-carboxy prothrombin in patients with hepatocellular carcinoma. Br J Surg. 1999;86:1032–1038. [DOI] [PubMed] [Google Scholar]
- 6.Nakashima T, Kojiro M. Pathologic characteristics of hepatocellular carcinoma. Semin Liver Dis. 1986;6:259–266. [DOI] [PubMed] [Google Scholar]
- 7.Yamanaka N, Okamoto E, Toyosawa A, et al. Prognostic factors after hepatectomy for hepatocellular carcinomas: a univariate and multivariate analysis. Cancer. 1990;65:1104–1110. [DOI] [PubMed] [Google Scholar]
- 8.Shirabe K, Kanematsu T, Matsumata T, et al. Factors linked to early recurrence of small hepatocellular carcinoma after hepatectomy: univariate and multivariate analyses. Hepatology. 1991;14:802–805. [DOI] [PubMed] [Google Scholar]
- 9.Izumi R, Shimizu K, Ii T, et al. Prognostic factors of hepatocellular carcinoma in patients undergoing hepatic resection. Gastroenterology. 1994;106:720–727. [DOI] [PubMed] [Google Scholar]
- 10.Vauthey JN, Klimstra D, Franceschi D, et al. Factors affecting long-term outcome after hepatic resection for hepatocellular carcinoma. Am J Surg. 1995;169:28–35. [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto J, Kosuge T, Takayama T, et al. Recurrence of hepatocellular carcinoma after surgery. Br J Surg. 1996;83:1219–1222. [PubMed] [Google Scholar]
- 12.Takayama T, Makuuchi M, Hirohashi S, et al. Early hepatocellular carcinoma as an entity with a high cure rate of surgical cure. Hepatology. 1998;28:1141–1246. [DOI] [PubMed] [Google Scholar]
- 13.Makuuchi M, Hasegawa H, Yamazaki S. Ultrasonically guided subsegmentectomy. Surg Gynecol Obstet. 1986;161:346–350. [PubMed] [Google Scholar]
- 14.Casting D, Garden J, Bismuth H. Segmental liver resection using ultrasound-guided selective portal venous occlusion. Ann Surg. 1989;210:20–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lui WY, Chau GY, Loong CC, et al. Hepatic segmentectomy for curative resection of primary hepatocelluar carcinoma. Arch Surg. 1995;130:1090–1097. [DOI] [PubMed] [Google Scholar]
- 16.Emond J. Anatomical hepatectomy for resection or transplantation. Am J Surg. 1996;172:29–34. [DOI] [PubMed] [Google Scholar]
- 17.Billingsley KG, Jarnagin WR, Fong Y, et al. Segment-oriented hepatic resection in the management of malignant neoplasms of the liver. J Am Coll Surg. 1998;187:471–481. [DOI] [PubMed] [Google Scholar]
- 18.Kosuge T, Makuuchi M, Takayama T, et al. Long-term results after resection of hepatocellular carcinoma: experience of 480 cases. Hepatogastroenterology. 1993;40:328–332. [PubMed] [Google Scholar]
- 19.Fuster J, García-Valdecasas JC, Grande L, et al. Hepatocellular carcinoma and cirrhosis: results of surgical treatment in a European series. Ann Surg. 1996;223:297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Regimbeau JM, Kianmanesh R, Farges O, et al. Extent of liver resection influences the outcome in patients with cirrhosis and small hepatocellular carcinoma. Surgery. 2002;131:311–317. [DOI] [PubMed] [Google Scholar]
- 21.Couinaud C. Lobes et segments hepatiques [in French]. Presse Med. 1954;62:709–712. [PubMed] [Google Scholar]
- 22.Kanematsu T, Takenaka K, Matsumata T, et al. Limited hepatic resection effective for selected cirrhotic patients with primary liver cancer. Ann Surg. 1984;199:51–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Makuuchi M, Kosuge T, Takayama T, et al. Surgery for small liver cancers. Semin Surg Oncol. 1993;9:298–304. [DOI] [PubMed] [Google Scholar]
- 24.Takayama T, Makuuchi M, Kubota K, et al. Randomized comparison of ultrasonic versus clamp transection of the liver. Arch Surg. 2001;136:922–926. [DOI] [PubMed] [Google Scholar]
- 25.Lee CS, Sheu JC, Wang M, et al. Long-term outcome after surgery for asymptomatic small hepatocellular carcinoma. Br J Surg. 1996;83:330–333. [DOI] [PubMed] [Google Scholar]
- 26.Bradburn MJ, Clark TG, Love SB, et al. Survival analysis: III. Multivariate data analysis and assessing its adequacy and fit. Br J Cancer. 2003;89:605–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ozawa K, Takayasu T, Kumada K, et al. Experience with 225 hepatic resections for hepatocellular carcinoma over 4-year period. Am J Surg. 1991;161:677–682. [DOI] [PubMed] [Google Scholar]
- 28.Minagawa M, Makuuchi M, Takayama T, et al. Selection criteria for repeat hepatectomy in patients with recurrent hepatocellular carcinoma. Ann Surg. 2003;238:703–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clark TG, Stewart ME, Altman DG, et al. A prognostic model for ovarian cancer. Br J Cancer. 2001;85:944–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. [DOI] [PubMed] [Google Scholar]
- 31.Bismuth H, Chiche L, Adam R, et al. Liver resection versus transplantation for hepatocellular carcinoma in cirrhotic patients. Ann Surg. 1993;213:145–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamamoto J, Iwatsuki S, Kosuge T, et al. Should hepatomas be treated with hepatic resection or transplantation? Cancer 1999;86:1151–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]