Abstract
Objective:
To evaluate the role of postchemotherapy adjunctive surgery in patients with liver metastases from germ cell cancer (GCT).
Patients and Methods:
Forty-three male patients with nonseminoma were treated in different multicenter treatment protocols between 1990 and 1999, and they underwent hepatic surgery. The results of postchemotherapy surgical resection, histologic findings found during postchemotherapy surgery, and prognostic factors for survival were assessed.
Results:
Thirty-five of 43 patients (81%) were initially diagnosed with liver metastases and advanced GCT, and 8 patients (19%) presented with metachronous liver metastases after a median interval of 16 months (range, 6–103 months). Twelve patients (28%) had isolated liver metastases after completion of chemotherapy, while 31 patients (72%) had additional residual extrahepatic tumor masses. Liver surgery included tumor excision or segmentectomy in 32 patients (74%) and hepatectomy (right/left) or resection of multiple segments in 11 patients (26%). Histologic analysis of postchemotherapy resected residua yielded necrosis in 67%, teratoma in 12%, and viable cancer in 21%. Additional resections at other sites have been performed in 31 patients revealing necrosis in 61% (n = 19), teratoma in 29% (n = 9), and vital carcinoma in 10% (n = 3). In 39% of patients, histologic findings differed among liver and other resection sites. Refractoriness to chemotherapy was associated with a shorter survival after surgery, and a trend was seen in patients with elevation of AFP.
Conclusion:
The high rate of viable cancer and teratoma found in liver specimens, differing histologic results at residual tumor locations, and the high survival rate achieved support a multidisciplinary approach including resection of liver masses since no accurate selection of patients can narrow the use of surgery.
To evaluate the role of postchemotherapy adjunctive surgery in patients with liver metastases from germ cell cancer, 43 male patients treated in different multicenter protocols have been analyzed. The high rate of viable cancer and teratoma found in liver specimens, differing histologic results at residual tumor locations, and the high survival rate achieved support a multidisciplinary approach including resection of liver.
With the introduction of cisplatin-based combination chemotherapy, approximately 70% to 80% of patients presenting with metastatic germ cell tumors (GCT) will achieve long-term survival.1 In contrast, the 5-year overall survival rate for patients with liver involvement is approximately 49%.2 Therefore, patients are classified as “poor prognosis” according to the IGCCCG classification regardless of the other extent of metastases or concentration of serum tumor markers.2 For patients with normalized serum tumor markers, resection of residua after chemotherapy is an important part of the management of metastatic nonseminomatous GCT.3 It serves to assess response, remove chemotherapy-resistant disease, and potentially direct additional chemotherapy. There are few published reports focusing on the benefit of postchemotherapy surgery in patients with liver metastases.4,5 We here report the experience of postchemotherapy surgery in consecutive patients with metastatic GCT treated within study protocols of the German Testicular Cancer Study Group (GTCSG). The histologic findings at hepatic surgery, the morbidity associated with liver surgery, prognostic factors for survival, and the role of surgery in patients with additional extrahepatic sites are reported.
PATIENTS AND METHODS
Patients
A total of 117 patients with liver metastases from GCT were treated with chemotherapy on prospective clinical trials within different multicenter treatment protocols of the GTCSG between 1990 and 1999 at the cancer centers of Tuebingen, Charite Campus Berlin, Essen, Hannover, and Stuttgart. Forty-three of these patients underwent surgical resection for residual liver disease after completion of chemotherapy and are the subjects of this series. All patients signed informed consent for a clinical trial approved by the Tuebingen institutional review board. Treatment regimens and results of the clinical trials have been published previously,6,7 with the exception of an ongoing trial for poor-risk patients.8 Seventy-four (63%) of the 117 patients did not undergo postchemotherapy hepatic surgery and are excluded. Main reasons have been the achievement of a complete remission (n = 15), unfavorable response (n = 14), more than 3 sites with residuals masses (n = 27), as well as patient refusal (n = 12). In the remaining patients who had attained serologic complete remission but with persistent minor radiographic abnormalities, individual investigators have chosen to observe such patients without surgery (n = 6) (Fig. 1).
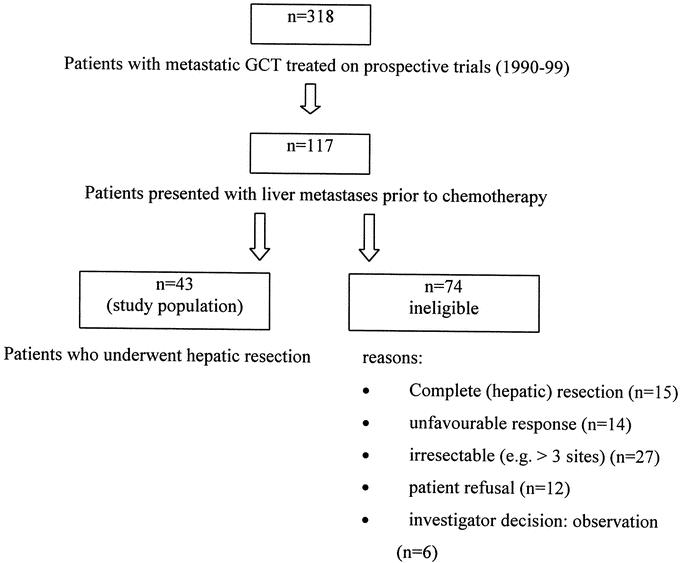
FIGURE 1. Patients.
For the data collection, a standardized questionnaire was sent to each center and completed by one of the local investigators. The following data were considered: patient characteristics, such as the demographics including age, location, and histology of the primary GCT; the extent of the disease; serum tumor marker concentrations of human β-chorionic gonadotropin (β-HCG), alpha-fetoprotein (AFP), and lactate dehydrogenase (LDH); and details on diagnostic methods, initial, and subsequent treatment including chemotherapy, surgery, evaluation of responses to treatment, and follow-up data. The completed questionnaires were checked for plausibility and data consistency at Tuebingen University Medical Center.
Response Assessment
Complete remission was defined as a complete disappearance of all evidence of disease. A partial response with marker normalization (PRm-) was defined as radiologic response >50% in tumor size and normalization of the tumor markers. PR without marker normalization (PRm+) was defined as a response >50% in size but no complete normalization of serum tumor markers. Progressive disease was defined as either residual lesions increasing in size or occurrence of new lesions or increase in serum tumor marker concentrations.
Surgery and Classification of Histologic Findings
Surgery was carried out within 3 months after the end of chemotherapy. Various surgical interventions were performed within a 3- to 6-week interval, except for 5 patients with one-stage operations. All histopathologic slides were diagnosed and classified by each center's Pathology department. Resected specimens were categorized according to the following criteria: necrosis referred to findings of necrotic debris only in the resected specimen and differentiated teratoma referred to the finding of mature teratoma in the absence of either malignant transformation or GCT such as embryonal carcinoma, yolk sac carcinoma, choriocarcinoma, or seminoma. Any of the latter histologic findings were considered as viable cancer as well as teratoma with malignant transformation. In case of a complete resection of all residual masses, patients were categorized depending on the histologic results as no evidence of disease (NED) after resection of either viable cancer, differentiated teratoma, or necrosis (pCR) in case of complete resection of all metastatic sites. During the first 2 years, follow-up examinations were performed every third month. The interval was thereafter prolonged to 6 months in the third year and to annually from the fourth year on.
Statistical Analysis
For all patients, the current status of May 2003 was gained. The univariate analysis on prognostic factors for overall survival consisted of the following categorial variables: age, evidence of extrahepatic metastases, AFP-, HCG- and LDH-elevation at presence of liver metastases, number of residual liver metastases (single versus multiple), line of chemotherapy (induction versus salvage), sensitivity to platin-based chemotherapy (remission with marker normalization versus none), simultaneous versus sequential resections, extent of secondary liver resection (“limited” excision or segmentectomy versus “extensive,” left or right hepatectomy, or multiple segmentectomies) and histology of liver metastases (necrosis, teratoma, viable cancer). All data were entered in a personal computer at the Tuebingen University Medical Center, Germany. All statistical analyses were performed with the use of the SPSS system (SPSS for windows 10.0 software, SPSS Inc., Chicago, IL). The histologic features of the liver metastases were compared with those resected at other metastatic sites using the Student t test. The Kaplan-Meier method was used to calculate survival data.9 The overall survival calculation used death due to any reason as the endpoint. A 2-sided log rank test was used. Exact 95% confidence intervals around the observed response rate were calculated from the binominal distribution. The level of significance was set to 0.05.
RESULTS
Disease Presentation
Thirty-five of the included 43 patients (81%) were initially diagnosed with “poor prognosis” GCT according to the IGCCCG classification including synchronous liver metastases. Eight patients (19%) had metachronous liver metastases diagnosed after a median time interval of 16 months (range, 6–103 months) after initial diagnosis of GCT and completion of induction chemotherapy. Two of them presented with isolated liver metastases and 6 patients with liver metastases and extrahepatic tumor sites.
Thirty-five patients (81%) had gonadal primaries. In all patients, histology of the primary tumor revealed nonseminomatous GCT. Sites of metastases included retroperitoneal lymph node (LN) metastases in 88% (n = 38), lung metastases in 72% (n = 31), mediastinal LN involvement in 21% (n = 9), bone metastases in 7% (n = 3), and metastases to the central nervous system in 5% (n = 2).
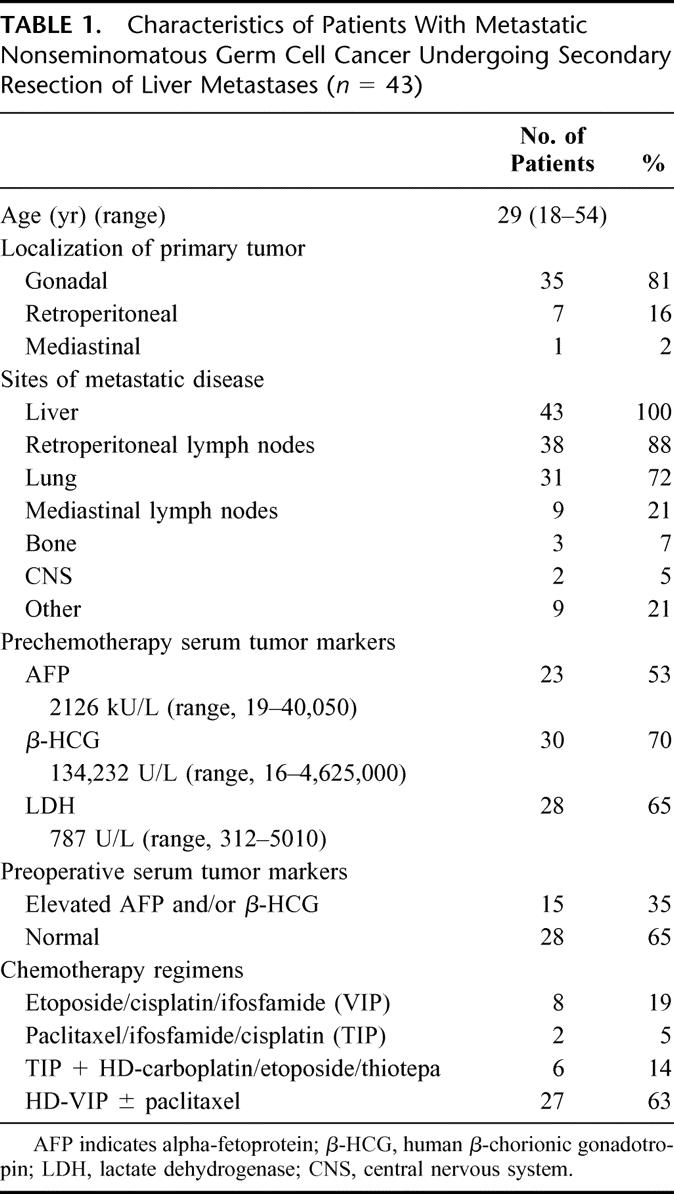
At the time of diagnosis of liver metastases, 15 patients (35%) had a single liver lesion and 28 patients (65%) had disseminated lesions in both liver lobes. Elevated levels of the serum tumor markers AFP, β-HCG, and LDH were found in 56% (n = 24), 72% (n = 31), and 63% (n = 27), respectively.
Details of the patient characteristics at primary diagnosis are summarized in Table 1.
TABLE 1. Characteristics of Patients With Metastatic Nonseminomatous Germ Cell Cancer Undergoing Secondary Resection of Liver Metastases (n = 43)
Chemotherapy Prior to Liver Surgery
Thirty-five of the patients (81%) have received cisplatin-based combinations as induction and 8 patients (19%) as salvage treatment. Three patients (7%) had received additional radiation therapy at other locations outside the liver: 2 of them for brain metastases and a single patient for bone involvement. Thirty-three of the patients (74%) had received dose-intensified chemotherapy plus autologous stem cell transplantation according to the HD-VIP or paclitaxel-HD-VIP7,8 protocol (n = 27) and 6 patients (20%) to the TIP/HD-CET regimen.6
Twenty-eight patients (65%) achieved a marker-negative partial response. Fifteen patients (35%) attained a partial response without marker normalization. The postchemotherapy tumor assessment revealed extrahepatic residual disease in 31 patients (72%), eg, in the retroperitoneum (n = 29, 90%), lungs (n = 7, 16%), and in spleen, pancreas, and kidneys (n = 1 each, 2%).
Secondary Resection of Liver Metastases
The type of liver surgery, different procedures of the resection of extrahepatic residual tumor masses, and the results of the histologic examination are outlined in Table 2.
TABLE 2. Surgical Procedures and Histologic Results in Patients With Liver Metastases (n = 43) and Extrahepatic Residual Tumor Masses (n = 31)
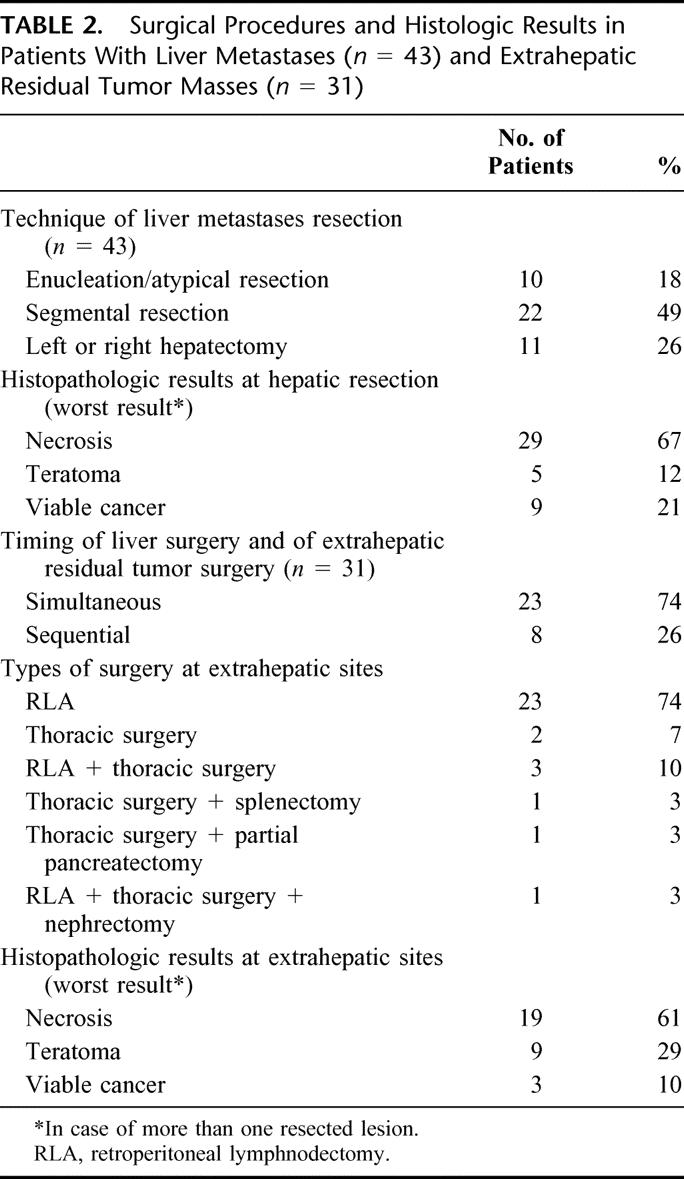
In total, 81 resections were performed in 43 patients. Twelve patients (28%) underwent only hepatic resections of residual tumor masses. Twenty-five patients (58%) had simultaneous resections of hepatic and extrahepatic lesions and in 6 patients (14%) 15 sequential surgical procedures including hepatic and at least one extrahepatic tumor resection were performed.
In 32 patients (74%), the extent of liver resection has been restricted to either excision/atypical resection (n = 10) or segmental resection (n = 22). Eleven patients (26%) underwent an extended liver surgery either with left- or right-sided hepatectomy or multiple segmental resection. In total, 4 patients (9%) experienced major perioperative complications. One patient required reoperation because of hepatic bleeding after left hepatectomy (2%). None of the patients died of surgery-related complications.
Hepatic and Extrahepatic Resection Specimens
Resections of liver metastases revealed necrosis in 29 patients (67%), differentiated teratoma in 5 patients (12%), and viable cancer in 9 patients (21%). Surgical exploration of the extrahepatic residual tumor masses yielded necrosis in 19 patients (61%), differentiated teratoma in 9 patients (29%), and viable cancer in 3 patients (10%). Table 3 compares the histologic findings at hepatic and extrahepatic residual tumor sites. In patients with different histologic findings, the worst case of histology was taken into account.
TABLE 3. Comparison of Histologic Findings at Resection of Liver Metastases and of Extrahepatic Residua (n = 31 Patients)
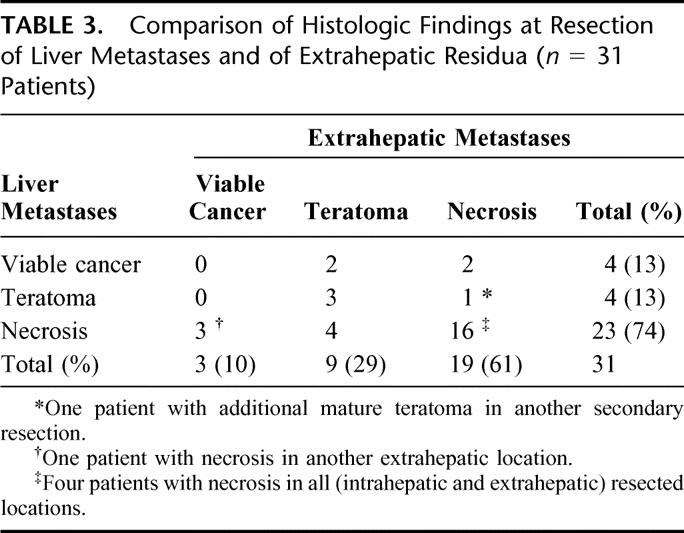
Twelve of the 31 patients (39%) who underwent liver surgery and one or more resections at other sites showed dissimilar histologic findings in hepatic and extrahepatic tumor masses. Five of these 12 patients (42%) demonstrated less favorable pathologic features (mature teratoma or viable cancer) at liver resection and necrosis at the other sites, while in 7 patients (56%) histopathologic results revealed necrosis in the liver and teratoma or viable cancer at the extrahepatic sites. In addition, 2 patients showed dissimilar histologic findings within 2 extrahepatic locations (6%).
Outcome
The remission status after postchemotherapy surgery was pCR in 27 patients (63%), NED teratoma in 3 patients (7%), and NED viable cancer in 7 patients (16%). Six patients (14%) had progressive disease after chemotherapy and incomplete secondary resections. Extensive secondary surgery rendered 10 patients disease-free considering hepatic and extrahepatic tumor locations (to a total rate of 86%). After a median follow-up time of 23 months (range, 8–216 months) in all patients and of 37 months (range, 16–216 months) in surviving patients, 34 patients (79%) were alive. Twenty-seven of them (79%) had no evidence of disease and 7 patients (21%) were alive with persistent disease. Two of 7 patients (29%) had progressive disease both at intrahepatic and extrahepatic sites; 5 patients experienced an extrahepatic relapse (71%). Within the follow-up period, 9 patients died of disease (21%): 2 of them due to progression of liver metastases and 7 patients due to systemic progression. The calculated 5-years survival is 70.9% (95% confidence interval, 54.6–87.2).
Prognostic Factors for Survival
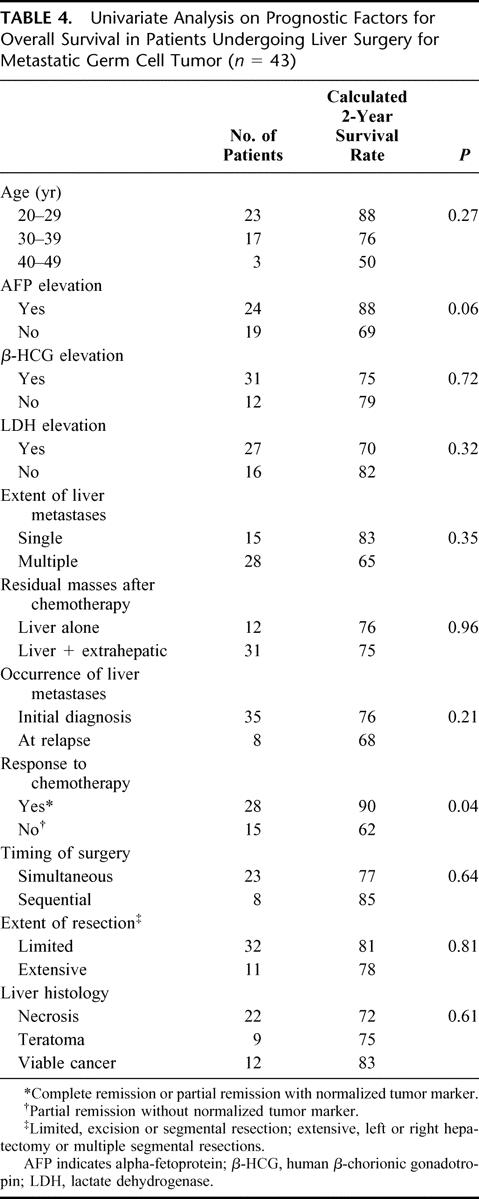
Negative factors for survival were the elevation of serum tumor marker AFP at diagnosis of liver metastases and refractoriness to chemotherapy. Neither age, nor synchronous nor metachronous appearance of liver metastases, nor extent of hepatic surgery, nor the histologic results of resected hepatic residual tumors were of significant prognostic importance. The results of the univariate analysis are listed in Table 4.
TABLE 4. Univariate Analysis on Prognostic Factors for Overall Survival in Patients Undergoing Liver Surgery for Metastatic Germ Cell Tumor (n = 43)
DISCUSSION
The initial presence of liver metastases represents an independent “poor prognosis” criterion for patients with metastatic nonseminomatous gonadal and extragonadal GCT.2,10 Postchemotherapy surgical removal of residual tumor masses in patients with metastatic GCT is an established adjunctive treatment. Particularly in patients with nonseminomatous GCT, resection is recommended since accurate noninvasive methods to predict necrotic tissue after chemotherapy are lacking.11,12 After completion of induction chemotherapy, 15% to 20% of residual tumor masses histologically contain viable cancer, 45% to 50% necrotic tissue, and 30% to 40% mature teratoma.13,14 In case of differentiated teratoma, often non-GCT components with the propensity to transform into sarcomas or other solid cancer types may be present. Therefore, the majority of investigators advise the excision of all tumor residua if technically feasible because there is a high chance of different histologic results being present in patients with more than one metastatic site. Dissimilar histologic results at different anatomic localizations have been reported in 25% to 47% of patients.3,15–21
The therapeutic options for patients with liver metastases are less well described because they usually represent a group of patients presenting with multiple tumor sites, often technically difficult to resect; however, complete resection of residual tumor masses contributes to a long-term cure. Hepatic surgery in patients with GCT has to date only been specifically addressed in 2 publications. One series comprised of 57 male patients revealing necrosis in 16%, residual teratoma in 51%, and residual GCT in 33% of the total of 60 hepatic resections.4 Sixty-eight concomitant procedures have been performed, including mainly retroperitoneal lymphadenectomy and thoracic procedures, but also cholecystectomy, nephrectomy, and inferior vena cava resections. Extrahepatic metastases were present in 51 of 57 patients (89%). Sixty-nine percent of patients were alive after 2 years, 63% of whom had no evidence of disease. One of the patients with necrosis and 6 patients with mature teratoma died of progressive GCT; 7 patients with viable residual liver tumors remained with NED, demonstrating the value of this aggressive surgical approach.
Another retrospective investigation reviewed 37 patients, including 4 females with GCT.5 Sixty-two percent of those patients were alive with no evidence of disease after a median follow-up of 66 months (range, 31–134 months). Univariate analysis identified the presence of pure embryonal carcinoma in the primary tumor, liver metastases >30 mm in greatest dimension at the time of surgery, and the presence of viable cancer residua as negative prognostic factors for survival. The authors proposed to select patients for liver surgery according to the size of the residual liver masses. Patients with residual tumor masses that measure ≤10 mm in greatest dimension should be considered for close follow-up because they had a high probability of necroses. Male patients with masses >30 mm represented a high-risk group who were not likely to benefit from liver surgery because of the high rate of complications. However, these conclusions were based on a small sample size of and the 5-year survival rate for patients with resected of liver metastases >30 mm was 47% in this investigation, which appears to be in the range of survival rates usually observed in patients fulfilling “poor prognosis” criteria. In addition, the extent of the surgical procedure largely depends on the location of the metastases in the liver, and not inevitable on the size of the lesion. In the series reported here, the rate of severe complications was 9%, although 33% of patients underwent extended resection procedures. There were no perioperative deaths seen both in the Indianapolis series4 and in the current report. Rivoire et al5 reported an overall mortality rate of 3% and the rate of severe complications was 27%. Three patients in his series required reoperation (8%). Concomitant cytoreductive procedures were performed in 73% of patients.5 Thus, morbidity might be depending on extent of liver surgery; however, the majority of patients had undergone a limited liver surgery procedure. Therefore, secondary liver surgery in advanced GCT patients based on the available literature is a feasible approach in experienced centers, which may render a substantial number of patients long-term disease-free. Currently, the overall mortality risk for major hepatic resections is <5% in specialized centers.
After chemotherapy and secondary resection of liver metastases, 86% of the patients in the current series showed no evidence of disease and calculated 5-year overall survival rate is 70.9% (95% confidence interval, 54.6–87.2) (Fig. 2). This appears to be in the highest range of data reported in large evaluations for the whole group of “poor prognosis” patients.2 Thus, the existence of liver metastases may not deteriorate the outcome compared with other “poor prognosis” patients without evidence of hepatic metastases. Survival probability for patients harboring vital carcinoma was 72.9% (95% confidence interval, 40.6–99.8) at 5 years.
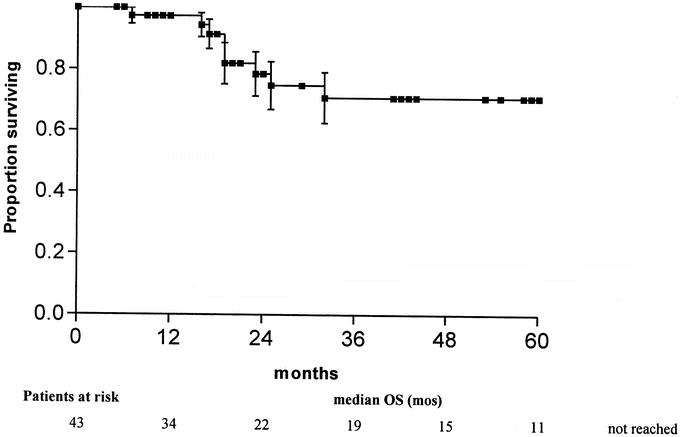
FIGURE 2. Overall survival of 43 patients with metastatic GCT who underwent postchemotherapy hepatic surgery.
In the present investigation, the histologic examination revealed corresponding rates of necrosis in hepatic and extrahepatic residual tumors (67% and 61%). In 61% of the patients, the histologic specimens of hepatic and extrahepatic residuals were identical. In slightly more than half of the patients with differing histologic findings, the less favorable features were found in the extrahepatic location while necrosis occurred in the liver. Obviously, there seems to be no inferior chemotherapy response in liver metastases from GCT compared with other sites.
Univariate analysis identified the elevation of AFP serum levels at the time of diagnosis and refractoriness to chemotherapy as negative prognostic factors for overall survival. Liver metastases at first diagnosis or at relapse, extent of liver involvement, time and extent of hepatic surgery, and even the histology of resected liver metastases were not influencing survival in patients with hepatic metastases from testicular GCT. However, it must be kept in mind that this analysis might be biased by the selection of patients allocated to hepatic surgery. Resection was only considered in patients with at least some response to chemotherapy and not in patients with overt progression.
CONCLUSION
Viable cancer or teratoma was found in one third of postchemotherapy liver specimens. In 40% of patients, differing histologic results at intrahepatic and extrahepatic residual tumor locations were present. Aggressive surgery (enabled by the young age of patients with metastatic germ cell tumors and the lack of comorbidities), including resection of all residual lesions at any location, produces a high survival rate in this selected group of patients. Compared with the results of liver surgery in other metastatic malignancies, postchemotherapy liver resections in germ cell tumors are highly curative. Despite the high chance of cure, 60% to 70% of patients will undergo an unnecessary approach. Neither any imaging procedures nor any prognostic model has been able to reliably predict residual tumor mass histology.13,22 Therefore, the consensus is to remove all residual masses if technically feasible. This is also based on the observation that viable cancer as well as differentiated teratoma are found in liver specimens and that different histologic results at residual tumor locations are a frequent finding.14,15,21 In patients with residual masses at multiples sites including the liver, it might be an acceptable procedure to spare patients from further surgery if the histology of the primarily resected mass is only necrosis.23,24 Alternatively, patients with different residua and marker-negative disease may be offered an ultrasonography-guided biopsy of the liver metastases, and in those patients with a negative biopsy for undifferentiated tumor or teratoma surveillance will be an acceptable therapeutic decision.
Footnotes
Reprints: Jörg Thomas Hartmann, MD, Department of Hematology/Oncology/Immunology, UKT Medical Center II, Eberhard-Karls-University Tuebingen, Otfried-Mueller-Str. 10 72076, Tuebingen, Germany. E-mail: joerg.hartmann@med.uni-tuebingen.de.
REFERENCES
- 1.Bosl GJ, Motzer RJ. Testicular germ-cell cancer. N Engl J Med. 1997;337:242–253. [DOI] [PubMed] [Google Scholar]
- 2.International Germ Cell Consensus Classification. A prognostic factor-based staging system for metastatic germ cell cancers: International Germ Cell Cancer Collaborative Group. J Clin Oncol. 1997;15:594–603. [DOI] [PubMed] [Google Scholar]
- 3.Toner GC, Panicek DM, Heelan RT, et al. Adjunctive surgery after chemotherapy for nonseminomatous germ cell tumors: recommendations for patient selection. J Clin Oncol. 1990;8:1683–1694. [DOI] [PubMed] [Google Scholar]
- 4.Hahn TL, Jacobson L, Einhorn LH, et al. Hepatic resection of metastatic testicular carcinoma: a further update. Ann Surg Oncol. 1999;6:640–644. [DOI] [PubMed] [Google Scholar]
- 5.Rivoire M, Elias D, De Cian F, et al. Multimodality treatment of patients with liver metastases from germ cell tumors: the role of surgery. Cancer. 2001;92:578–587. [DOI] [PubMed] [Google Scholar]
- 6.Rick O, Bokemeyer C, Beyer J, et al. Salvage treatment with paclitaxel, ifosfamide, and cisplatin plus high-dose carboplatin, etoposide, and thiotepa followed by autologous stem-cell rescue in patients with relapsed or refractory germ cell cancer. J Clin Oncol. 2001;19:81–88. [DOI] [PubMed] [Google Scholar]
- 7.Schmoll HJ, Kollmannsberger C, Metzner B, et al. Long-term results of first-line sequential high dose VIP chemotherapy plus autologous stem cell support for patients with advanced metastatic germ cell cancer: an extended phase II study of the German Testicular Cancer Study Group. J Clin Oncol. 2003;23:4083–4091. [DOI] [PubMed] [Google Scholar]
- 8.Hartmann JT, Schleucher N, Metzner B, et al. Phase I/II study of sequential high dose VIP plus paclitaxel supported by PBSC in patients with ‘poor prognosis’ germ cell tumor (GCT) [Abstract 691]. Proc Am Soc Clin Oncol. 2001;20:173a. [Google Scholar]
- 9.Kaplan EL, Maier P. Non-parametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 10.Hartmann JT, Nichols CR, Droz JP, et al. Prognostic variables for response and outcome in patients with extragonadal germ-cell tumors. Ann Oncol. 2002;13:1017–1028. [DOI] [PubMed] [Google Scholar]
- 11.Geller NL, Bosl GJ, Chan EY. Prognostic factors for relapse after complete response in patients with metastatic germ cell tumors. Cancer. 1989;63:440–445. [DOI] [PubMed] [Google Scholar]
- 12.Tait D, Peckham MJ, Hendry WF, et al. Post-chemotherapy surgery in advanced non-seminomatous germ-cell testicular tumours: the significance of histology with particular reference to differentiated (mature) teratoma. Br J Cancer. 1984;50:601–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steyerberg EW, Keizer HJ, Fossa SD, et al. Prediction of residual retroperitoneal mass histology after chemotherapy for metastatic nonseminomatous germ cell tumor: multivariate analysis of individual patient data from six study groups. J Clin Oncol. 1995;13:1177–1187. [DOI] [PubMed] [Google Scholar]
- 14.Hartmann JT, Schmoll HJ, Kuczyk MA, et al. Postchemotherapy resections of residual masses from metastatic non-seminomatous testicular germ cell tumors. Ann Oncol. 1997;8:531–538. [DOI] [PubMed] [Google Scholar]
- 15.Hartmann JT, Candelaria M, Kuczyk MA, et al. Comparison of histological results from the resection of residual masses at different sites after chemotherapy for metastatic non-seminomatous germ cell tumours. Eur J Cancer. 1997;33:843–847. [DOI] [PubMed] [Google Scholar]
- 16.Tiffany P, Morse MJ, Bosl G, et al. Sequential excision of residual thoracic and retroperitoneal masses after chemotherapy for stage III germ cell tumors. Cancer. 1986;57:978–983. [DOI] [PubMed] [Google Scholar]
- 17.Mandelbaum I, Yaw PB, Einhorn LH, et al. The importance of one-stage median sternotomy and retroperitoneal node dissection in disseminated testicular cancer. Ann Thorac Surg. 1983;36:524–528. [DOI] [PubMed] [Google Scholar]
- 18.Gerl A, Clemm C, Schmeller N, et al. Sequential resection of residual abdominal and thoracic masses after chemotherapy for metastatic non-seminomatous germ cell tumours. Br J Cancer. 1994;70:960–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brenner PC, Herr HW, Morse MJ, et al. Simultaneous retroperitoneal, thoracic, and cervical resection of postchemotherapy residual masses in patients with metastatic nonseminomatous germ cell tumors of the testis. J Clin Oncol. 1996;14:1765–1769. [DOI] [PubMed] [Google Scholar]
- 20.Qvist HL, Fossa SD, Ous S, et al. Post-chemotherapy tumor residuals in patients with advanced nonseminomatous testicular cancer.Is it necessary to resect all residual masses? J Urol. 1991;145:300–302. [DOI] [PubMed] [Google Scholar]
- 21.Aprikian AG, Herr HW, Bajorin DF, et al. Resection of postchemotherapy residual masses and limited retroperitoneal lymphadenectomy in patients with metastatic testicular nonseminomatous germ cell tumors. Cancer. 1994;74:1329–1334. [DOI] [PubMed] [Google Scholar]
- 22.Oldenburg J, Alfsen GC, Lien HH, et al. Postchemotherapy retroperitoneal surgery remains necessary in patients with nonseminomatous testicular cancer and minimal residual tumor masses. J Clin Oncol. 2003;21:3310–3317. [DOI] [PubMed] [Google Scholar]
- 23.Herr HW. Does necrosis on frozen-section analysis of a mass after chemotherapy justify a limited retroperitoneal resection in patients with advanced testis cancer? Br J Urol. 1997;80:653–657. [DOI] [PubMed] [Google Scholar]
- 24.Schmoll HJ, Souchon R, Krege S, et al. European consensus on diagnosis and treatment of germ cell cancer: a report of the European Germ Cell Cancer Consensus Group (EGCCCG). Ann Oncol. 2004;15:1377–1399. [DOI] [PubMed] [Google Scholar]


