Abstract
Objective:
To analyze results of 70 patients undergoing left hepatic trisectionectomy and to clarify its current role.
Summary Background Data:
Left hepatic trisectionectomy remains a complicated hepatectomy, and few reports have described the long-term results of the procedure.
Methods:
Short-term and long-term outcomes of 70 consecutive patients who underwent left hepatic trisectionectomy from January 1993 to February 2004 were analyzed.
Results:
Of the 70 patients, 36 had colorectal liver metastasis, 24 had cholangiocarcinoma, 4 had hepatocellular carcinoma, and the remaining 6 had other tumors. Overall morbidity, 30-day and 90-day mortality rates were 46%, 7%, and 9%, respectively. Multivariate analysis disclosed that preoperative jaundice and intraoperative blood transfusion were positive independent predictors for postoperative morbidity; however, there were no independent predictors for postoperative mortality. Postoperative morbidity (87% versus 35%, P < 0.001) and mortality (20% versus 5%, P = 0.108) were observed more frequently in patients with preoperative obstructive jaundice than in those without jaundice. Each survival according to tumor type was acceptable compared with reported survivals. Survival for patients with colorectal liver metastasis undergoing left hepatic trisectionectomy with concomitant partial resection of the remnant liver was similar to those without this concomitant procedure. This concomitant procedure was not associated with postoperative morbidity and mortality.
Conclusions:
Left hepatic trisectionectomy remains a challenging procedure. Preoperative obstructive jaundice considerably increases perioperative risk. Concomitant partial resection of the remaining liver appears to be safe and offers the potential for cure in patients with colorectal metastasis affecting all liver segments.
Seventy consecutive patients undergoing left hepatic trisectionectomy have been analyzed. Left hepatic trisectionectomy remains a challenging procedure; however, concomitant partial resection of the remaining liver appears to be safe and offers the potential for cure in patients with colorectal metastasis affecting all liver segments.
Left hepatic trisectionectomy (resection of hepatic segments 2, 3, 4, 5, and 8 ± 1) was first described in detail by Starzl as left hepatic trisegmentectomy in 19821 and has recently been renamed.2 This procedure has enabled the resection of advanced tumors with curative intent. Despite advances in surgical technique and perioperative management,3–7 it remains the most challenging of the major anatomic hepatectomies with higher complication rates than other hepatic resection,8,9 and worldwide experience remains small. Although several authors have focused on this complicated hepatectomy,3,4,10–13 few reports have described the results of large series14 and long-term follow-up has rarely been considered.13 In this study, we analyze perioperative features and long-term outcome of 70 consecutive patients undergoing left hepatic trisectionectomy in our hepatobiliary unit.
This is the largest series reported to date, and we consider predictive factors for morbidity and mortality, follow-up data, and present an appraisal of the current role of this difficult procedure.
MATERIALS AND METHODS
From January 1993 to February 2004, 756 hepatectomies (right-sided hepatectomy, n = 362; left-sided hepatectomy, n = 126; central hepatectomy, n = 3; sectionectomy or less, n = 265) have been performed in the HPB and Transplant Unit at St. James's University Hospital in Leeds, UK. Of the 756 patients, 70 (9%) underwent left hepatic trisectionectomy and were enrolled in this study; 69 of the 70 patients were operated on by the senior author (J.P.A.L.). There were 41 male and 29 female patients with a mean age of 57 years, ranging from 30 to 76 years. One of the 70 patients underwent the left hepatic trisectionectomy using the ante-situm technique15 and the remaining 69 patients underwent resection in standard fashion.
Inevitably, operative techniques have developed with increasing experience. Currently, our technique is as follows, and this is largely as described by Blumgart et al.3 After mobilization of both the right and left liver, the left portal vein and the left hepatic artery are divided separately at the base of the umbilical fissure. In cases planned for caudate lobe (segment 1) resection, the left portal vein and the left hepatic artery are divided at their origins to interrupt the blood supply to the caudate lobe. The right anterior sectional portal and arterial branches are divided separately by opening the right Glissonian sheath, if possible, or the entire right anterior sectional portal pedicle can be isolated and divided at this stage, staying outside the Glissonian sheath. When the vessels are difficult to identify extrahepatically, they are divided during liver parenchymal transection. The lesser omentum is divided (ligating any accessory left hepatic arterial branches). If the caudate lobe is to be excised, it needs to be mobilized from the inferior vena cava, with ligation or suture of its short hepatic veins. This maneuver can usually be accomplished from the left side, but if there is significant involvement of the caudate lobe with tumor, then it may be necessary to approach the caudate veins from the right. In this case, it is important to preserve any major inferior or middle right hepatic veins which may be draining segment 6. The hepatocaval confluence is most safely approached from the left side: the middle and left hepatic veins can be isolated and slung together for subsequent division by passing behind these structures anterior to the inferior vena cava to emerge between the middle and right hepatic veins. If the right anterior sectional portal vein and hepatic artery have been divided, then the middle and left hepatic veins should be divided immediately, and our usual practice is to use a surgical stapling device. However, in the past or in cases of very difficult access, we have used a 3/0 monofilament polypropylene suture after division between vascular clamps. This division creates 2 distinct advantages: a clear line of demarcation appears at the junction of segments 6 and 7 with 5 and 8; and the extended left liver to be removed becomes considerably more mobile. If the right anterior sectional portal vein and hepatic artery have not been divided, then division of the left and middle hepatic veins must wait until that point in the parenchymal transection, or the extended left liver will become congested resulting in more difficult access and increased blood loss. In some cases, it is necessary to divide and reconstruct the major portal and hepatic arterial structures. This is best done after parenchymal transection to gain access for the reconstruction phase. In cases of perihilar cholangiocarcinoma, as extrahepatic bile duct excision is required, the common bile duct is divided in the head of the pancreas. All other points of biliary division are done during parenchymal transection to prevent bile duct injury. Liver parenchymal transection is done in our center using the CUSA (Valleylab, Inc., Boulder, CO) under low central venous pressure anesthesia (<5 cm H2O), and lifting the right posterior section ventrally to minimize the venous bleeding by reducing the “central venous pressure” within the liver remnant.16 An intermittent Pringle maneuver is resorted to when the bleeding is considered excessive,17,18 and rarely we have used total vascular isolation.4 Residual vascular and biliary division is done at appropriate stages of the hepatic transection. In cases of perihilar cholangiocarcinoma, it is our usual practice to complete all aspects of the parenchymal transection before division of the segment 6 and 7 hepatic ducts, lifting the extended left liver, dropping the right posterior section back to divide the ducts as far away from the tumor as possible. In cases of metastasis, it is our practice to retain the hepatic duct confluence, dividing the segment 1, 2/3, 4, and 5/8 ducts individually to avoid biliary injury. In cases with bile duct resection, reconstruction between the right posterior sectional bile duct or the segment 6 and 7 bile ducts and the jejunum is performed by Roux-en Y hepaticojejunostomy.
Preoperative clinical data, operation and pathology reports, postoperative complications, and long-term survival were reviewed. Continuous variables were expressed as mean± SD and compared using the unpaired t test for univariate analyses. Categorical variables were compared by the χ2 test or the Fisher exact test, where appropriate. Multivariate analyses were done using the logistic regression model (forward stepwise method) to determine independent predictors of outcome. Long-term survival was calculated using the Kaplan-Meier method and compared by the log-rank test. A P value less than 0.05 was considered to indicate statistical significance.
RESULTS
Of the 70 patients, 31 had primary liver tumor (intrahepatic cholangiocarcinoma, n = 11; perihilar cholangiocarcinoma, n = 13; hepatocellular carcinoma, n = 4; primary carcinoid tumor, n = 2; sarcoma, n = 1), while 39 had liver metastases (colorectal cancer, n = 36; esophageal cancer, n= 1; duodenal carcinoid, n = 1; small bowel carcinoid, n = 1) (Table 1). Fifteen patients had preoperative obstructive jaundice and biliary drainage was performed in 11 patients whose serum total bilirubin level was in excess of 300 μmol/L (normal range, 3–19 μmol/L) at presentation (endoscopic retrograde drainage, n = 6; percutaneous transhepatic drainage, n = 5). The latest serum total bilirubin level before surgery was 29.2 ± 54.8 μmol/L, ranging from 2 to 286 μmol/L in all patients. No patient underwent preoperative portal vein embolization.
TABLE 1. Diseases and Combined Procedures of the 70 Patients Undergoing Left Hepatic Trisectionectomy
Concomitant caudate lobe resection, extrahepatic bile duct resection and lymphadenectomy were performed in 40 (57%), 29 (41%), and 25 (36%) of the 70 patients, respectively (Table 1). Most of patients with intrahepatic or perihilar cholangiocarcinoma underwent those concomitant resections. Sixteen (44%) of the 36 patients with liver metastases from colorectal cancer also underwent concomitant partial resection of the remnant right posterior hepatic section for multiple metastases (Fig. 1). Combined portal vein resection and reconstruction was carried out in 9 patients with cholangiocarcinoma and 1 with liver metastasis from colorectal cancer. Other organs were involved by primary or secondary tumors and resected in 6 patients (partial gastrectomy, n = 1; pancreatoduodenectomy with total gastrectomy, n = 1; partial resection of the small bowel, n = 1; colectomy, n = 2; partial duodenectomy and colectomy, n = 1). Tumor thrombus extending into the right atrium was removed in another patient and 1 patient underwent right oophorectomy for an ovarian cyst. The average operation time was 347 ± 145 minutes (range, 90–665 minutes) and the median amount of intraoperative blood transfusion was 2 units (range, 0–20 units) with 40 patients (57%) requiring transfusion.

FIGURE 1. Magnetic resonance imaging of a 58-year-old man with multiple liver metastases from a sigmoid colon cancer. Tumors are demonstrated in almost all hepatic segments. This patient underwent left hepatic trisectionectomy and partial resections of the remnant liver, and is alive without recurrence 10 months after the surgery. The number in the figure indicates the segment where the tumor exists.
Of the 70 patients, 32 (46%) had postoperative complications (Table 2). Transient liver failure was the most common complication (n = 12, 17%), which was followed by intra-abdominal bleeding (n = 6, 9%) and bile leak (n = 6, 9%). Relaparotomy was performed in 10 patients because of intra-abdominal bleeding (n = 5), bile leak (n = 2), portal vein thrombosis (n = 1), bowel obstruction (n = 1), or wound dehiscence (n = 1). Six patients (9%) died following postoperative complications (Tables 2, 3). Five patients died within 30 days of surgery (7%) and the sixth died at 40 days. No other deaths occurred within 90 days of surgery, so we have classed all 6 deaths as hospital mortality (9%) for the purposes of comparisons. Four of those 6 patients had preoperative obstructive jaundice due to cholangiocarcinoma and the remaining 2 patients had liver metastases from colorectal cancer. All of the 6 patients who died had required intraoperative blood transfusion. Five patients had more than 2 postoperative complications. The overall postoperative hospital stay was 17 ± 12 days (range, 5–59 days).
TABLE 2. Postoperative Complications of the 70 Patients Undergoing Left Hepatic Trisectionectomy

TABLE 3. Clinical Data of 6 Nonsurvivors After Left Hepatic Trisectionectomy
Six preoperative and 10 intraoperative variables were analyzed for postoperative morbidity. By univariate analyses, diagnosis of cholangiocarcinoma, preoperative jaundice, solitary tumor, longer operation time, a larger amount of intraoperative blood transfusion, concomitant caudate lobe resection, extrahepatic bile duct resection, lymphadenectomy, and portal vein resection were statistically significantly associated with postoperative morbidity (Table 4). Multivariate analysis by a logistic regression model was performed using those 9variables and concomitant partial resection of the right posterior section. As the result, preoperative jaundice and intraoperative blood transfusion were the positive independent predictors of postoperative morbidity (Table 5). Thirteen preoperative and intraoperative variables and 6 postoperative variables were analyzed for postoperative mortality. By univariate analysis, solitary tumor, postoperative liver failure, renal failure, sepsis, portal vein thrombosis, and upper gastrointestinal bleeding were associated with mortality (Table 6). However, multivariate analysis using those 6 variables failed to disclose any independent predictors.
TABLE 4. Univariate Analysis for the 70 Patients According to Morbidity
TABLE 5. Results of Logistic Regression Analysis for Postoperative Morbidity

TABLE 6. Univariate Analysis for the 70 Patients According to Mortality
After the left hepatic trisectionectomy, 9 patients underwent a total of 13 reoperations for radiologic recurrent disease after 4 to 43 months of survival (Table 7). Of the 9 patients, 5 had liver metastases from colorectal cancer, 3 had intrahepatic cholangiocarcinoma, and the remaining 1 had primary carcinoid of the liver. This latter patient is reported in a recent series from our center.19 Partial resection of the remnant liver was performed in 4 patients and liver transplantation in 2 patients. Wedge resection of the lung was done in 3 patients. One patient underwent resection of periportal lymphadenopathy and resection of peritoneal deposits, which were found at laparotomy for raised CEA. Histologic examination revealed that those deposits were adenocarcinoma, although the lymph nodes were cancer negative. Currently, this patient remains disease free after a further 9 months of follow-up. Four of the 9 patients underwent 2 reoperations. As results, 7 of the 9 patients were alive with follow-up period ranging from 14 to 67 months after the left hepatic trisectionectomy (2–41 months from last surgery) and 2 of those patients have survived more than 5 years.
TABLE 7. Procedures Performed and Outcome of 9 Patients Who Underwent Reoperation for Recurrence
Survival curves according to diseases are shown in Figure 2A. Two of 4 patients with hepatocellular carcinoma survived more than 5 years. The 1-, 3-, and 5-year survival rates for patients with intrahepatic cholangiocarcinoma were 73%, 11%, and 11%, respectively. As 10 of 13 patients with hilar cholangiocarcinoma underwent surgery after January 2002, there were only 3 2-year survivors and the predicted 2-year survival rate is 59%. The 1-, 3-, and 5-year survival rates for patients with colorectal metastasis were 85%, 48%, and 40%, respectively, and the median survival for these patients was 32 months. The patients with liver metastases from colorectal cancer were divided into 2 groups according to those with or without concomitant partial resection of the right posterior section (Fig. 2B). Survivals for the patients of the 2 groups were similar to each other.
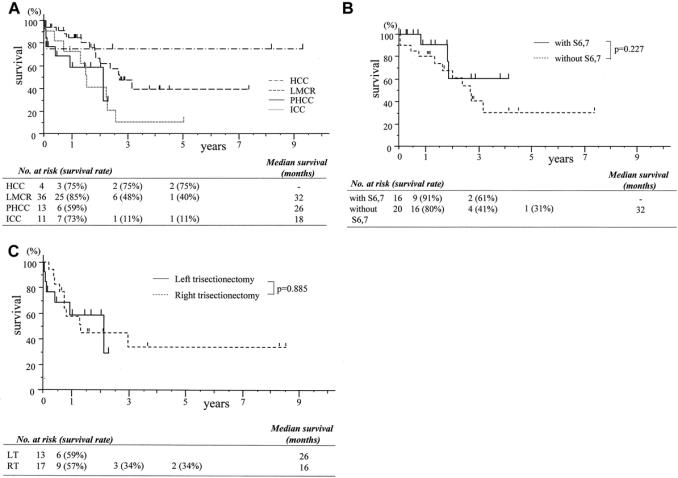
FIGURE 2. A, Survival curves according to tumor type. These survivals include postoperative mortality. HCC, hepatocellular carcinoma; PHCC, perihilar cholangiocarcinoma; ICC, intrahepatic cholangiocarcinoma; LMCR, liver metastasis from colorectal cancer. B, Survival curves of patients with liver metastasis from colorectal cancer. These survivals include postoperative mortality. There is no significant difference between survivals for patients who underwent left hepatic trisectionectomy with and without partial resection of the remnant liver. with S6,7, left hepatic trisectionectomy with partial resection of the remnant liver; without S6,7, left hepatic trisectionectomy without partial resection of the remnant liver. C, Survival curves of patients who underwent hepatic trisectionectomy for perihilar cholangiocarcinoma between January 1993 and February 2004 in our center. LT, left hepatic trisectionectomy; RT, right hepatic trisectionectomy.
DISCUSSION
Although several surgeons have reported on this complicated procedure, most of them have experienced less than 20 patients.4,10–12 Only one report, from the Memorial Sloan-Kettering Cancer Center, has described the results of a large series: 51 patients.14 However, this report failed to disclose any independent predictors for postoperative morbidity and mortality. In contrast, multivariate analysis in our series disclosed that preoperative obstructive jaundice and intraoperative blood transfusion were independent predictors of postoperative morbidity (Table 5). The difference might result from the number of the patients and distribution of diseases. In our series, 24 (34%) of the 70 patients had biliary cancer and 15 (21%) had preoperative obstructive jaundice (Table 1), whereas 5 (10%) of the 51 patients had biliary cancer and 5 (10%) had obstructive jaundice in the Memorial Sloan-Kettering Cancer Center series. Concomitant caudate lobe resection (57% versus 35%), extrahepatic bile duct resection (41% versus 12%), partial resection of the remnant liver (26% versus 8%), and portal vein resection (14% versus 6%) were performed on more patients in our series. Despite this, postoperative morbidity (46% versus 53%) and mortality rates (9% versus 8%) were similar.
Several authors have stressed that hyperbilirubinemia and cholangitis are strongly associated with increased in-hospital mortality,9,20 and preoperative obstructive jaundice was one of the independent predictors of postoperative complications in our series (Table 5). The wide confidence interval is mainly due to the large relative risk and partly due to the small number of jaundiced patients. Actually, postoperative morbidity (87% versus 35%, P < 0.001) and mortality (20% versus 5%, P = 0.108) were observed more frequently in patients with preoperative obstructive jaundice than in those without jaundice (Tables 4, 6). From our results, unfortunately left hepatic trisectionectomy may not be considered safe for patients with obstructive jaundice. However, the long-term outcome of the patients with cholangiocarcinoma was acceptable compared with reported outcomes, despite the advanced stage of patients in our series who had required left trisectionectomy.21–26 Although Neuhaus et al21 stressed superiority of the right hepatic trisectionectomy for hilar cholangiocarcinoma, the outcome was similar between the right and left hepatic trisectionectomies carried out in our center during this study period (Fig. 2C). Vauthey et al13 also found similar outcomes for these 2 extended procedures. Therefore, this procedure should be also indicated for selected patients with obstructive jaundice, but probably using some additional procedures in an attempt to prevent postoperative complications. Although portal vein embolization and bile replacement have not been carried out in our series, some authors have described the possibility of these procedures for preventing liver failure and sepsis.5,6,27,28 To reduce perioperative risk, it is possible that these potentially helpful procedures should be adopted.
Intraoperative blood loss could not be analyzed because it is not routinely accurately calculated in our hospital. However, the amount of blood transfusion, which we found to be an independent predictor for postoperative morbidity, inevitably correlates with the amount of intraoperative blood loss, and this reflects operative difficulty. A large amount of blood loss and subsequent homologous blood transfusion in hepatectomy can increase the incidence of postoperative complications.29–31 Furthermore, homologous blood transfusion carries with it the potential risk of an immunosuppressive effect and may adversely affect the prognosis of patients with gastrointestinal carcinomas.31–33 Extended hepatic resection is associated with a higher risk to require blood transfusion. In our series, 40 (57%) of the 70 patients received homologous blood transfusion with a median amount of 2 units. Some workers have suggested that autologous blood donation may be an option, although its benefit in hepatectomy remains controversial.31,34,35
In our series, reoperation has been performed aggressively even in patients with recurrent tumors after extensive hepatic resection. As a result, a total of 13 reoperations were carried out in 9 (13%) patients (Table 7). Partial resection of the remnant liver was done 5 times in 4 patients. Liver transplantation was performed in 2 patients with intrahepatic cholangiocarcinoma, 1 of whom has survived more than 5 years without recurrence. Although liver transplantation for recurrent tumor is controversial, especially for cholangiocarcinoma,21,22,36–38 this patient could not have survived without liver transplantation. Our data suggest that further surgery should be considered whenever patients have recurrent tumor after left trisectionectomy to offer potential long-term survival.
The survival for patients with hepatocellular carcinoma, intrahepatic or perihilar cholangiocarcinoma, or liver metastasis from colorectal cancer is acceptable in comparison with reported survivals (Fig. 2A).11,12,21–26,39–42 Recently, Vauthey et al13 have found that the need to perform radiofrequency ablation for additional disease in the remaining unresected segment of liver after right or left trisectionectomy was associated with a poor outcome. In contrast, in our study, survival for patients with liver metastasis from colorectal cancer who underwent left hepatic trisectionectomy with concomitant partial resection of the right posterior section was similar to those without the concomitant procedure (Fig. 2B). Furthermore, concomitant partial resection of the remnant liver was not associated with an increase in postoperative complications or mortality (Tables 4, 6). Our data suggest that combined left hepatic trisectionectomy and partial resection of the remnant liver can be a useful surgical option in patients with multiple colorectal metastases affecting all segments of the liver.
Collectively, left hepatic trisectionectomy remains a challenging procedure. Preoperative obstructive jaundice considerably increases perioperative risk. Concomitant partial resection of the remaining liver appears to be safe and offers the potential for cure in patients with colorectal metastasis affecting all liver segments.
Footnotes
Reprints: J. Peter A. Lodge, MD, FRCS, HPB and Transplant Unit, St. James's University Hospital, Beckett Street, Leeds LS9 7TF, UK. E-mail: PeterLodge@aol.com.
REFERENCES
- 1.Starzl TE, Iwatsuki S, Shaw BW Jr, et al. Left hepatic trisegmentectomy. Surg Gynecol Obstet. 1982;155:21–27. [PMC free article] [PubMed] [Google Scholar]
- 2.The terminology committee of the IHPBA: the Brisbane 2000 terminology of hepatic anatomy and resections. HPB. 2000;2:333–339. [Google Scholar]
- 3.Blumgart LH, Baer HU, Czerniak A, et al. Extended left hepatectomy: technical aspects of an evolving procedure. Br J Surg. 1993;80:903–906. [DOI] [PubMed] [Google Scholar]
- 4.Huguet C, Stipa F, Gavelli A. Extended left hepatectomy with vascular exclusion. J Am Coll Surg. 1994;178:288–292. [PubMed] [Google Scholar]
- 5.Makuuchi M, Thai BL, Takayasu K, et al. Preoperative portal embolization to increase safety of major hepatectomy for hilar bile duct carcinoma: a preliminary report. Surgery. 1990;107:521–527. [PubMed] [Google Scholar]
- 6.Nagino M, Nimura Y, Kamiya J, et al. Right and left trisegment portal vein embolization before hepatic trisegmentectomy for hilar bile duct carcinoma. Surgery. 1995;117:677–681. [DOI] [PubMed] [Google Scholar]
- 7.Makuuchi M, Hasegawa H, Yamazaki S. Intraoperative ultrasonic examination for hepatectomy. Ultrasound Med Biol. 1983;2(suppl):493–497. [PubMed] [Google Scholar]
- 8.Belghiti J, Hiramatsu K, Benoist S, et al. Seven hundred forty-seven hepatectomies in the 1900s: an update to evaluate the actual risk of liver resection. J Am Coll Surg. 2000;191:38–46. [DOI] [PubMed] [Google Scholar]
- 9.Melendez J, Ferri E, Zwillman M, et al. Extended hepatic resection: a 6-year retrospective study of risk factors for perioperative mortality. J Am Coll Surg. 2001;192:47–53. [DOI] [PubMed] [Google Scholar]
- 10.Hasegawa H, Yamasaki S, Makuuchi M, et al. Poor prognoses following left hepatic trisegmentectomies for cancer. Jpn J Clin Oncol. 1989;19:271–275. [PubMed] [Google Scholar]
- 11.Iwatsuki S, Starzl TE. Personal experience with 411 hepatic resections. Ann Surg. 1988;208:421–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poon RTP, Fan ST, Lo CM, et al. Extended hepatic resection for hepatocellular carcinoma in patients with cirrhosis: is it justified? Ann Surg. 2002;236:602–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vauthey JN, Pawlik TM, Abdalla EK, et al. Is extended hepatectomy for hepatobiliary malignancy justified? Ann Surg. 2004;239:722–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Povoski SP, Fong Y, Blumgart LH. Extended left hepatectomy. World J Surg. 1999;23:1289–1293. [DOI] [PubMed] [Google Scholar]
- 15.Lodge JPA. Ex vivo and in situ hypothermic hepatic resection. In: Blumgart LH, Fong Y, eds. Surgery of the Liver and Biliary Tract, 3rd ed. London: Saunders, 2000:1773–1784. [Google Scholar]
- 16.Johnson M, Manner R, Wu AV. Correlation between blood loss and inferior vena caval pressure during liver resection. Br J Surg. 1998;85:188–190. [DOI] [PubMed] [Google Scholar]
- 17.Pringle JH. Notes on the arrest of hepatic hemorrhage due to trauma. Ann Surg. 1908;48:541–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Man K, Fan ST, Ng IO, et al. Prospective evaluation of Pringle maneuver in hepatectomy for liver tumors by a randomized study. Ann Surg. 1997;226:704–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fenwick SW, Wyatt JI, Toogood GJ, et al. Hepatic resection and transplantation for primary carcinoid tumors of the liver. Ann Surg. 2004;239:210–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanai M, Nimura Y, Kamiya J, et al. Preoperative intrahepatic segmental cholangitis in patients with advanced carcinoma involving the hepatic hilus. Surgery. 1996;119:498–504. [DOI] [PubMed] [Google Scholar]
- 21.Neuhaus P, Jonas S, Bechstein WO, et al. Extended resection for hilar cholangiocarcinoma. Ann Surg. 1999;230:808–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iwatsuki S, Todo S, Marsh W, et al. Treatment of hilar cholangiocarcinoma (Klastkin tumors) with hepatic resection or transplantation. J Am Coll Surg. 1998;187:358–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jarnagin WR, Fong Y, DeMatteo RP, et al. Staging, respectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg. 2001;234:507–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nimura Y, Hayakawa N, Kamiya J, et al. Hepatic segmentectomy with caudate lobe resection for bile duct carcinoma of the hepatic hilus. World J Surg. 1990;14:535–544. [DOI] [PubMed] [Google Scholar]
- 25.Madariaga JR, Iwatsuki S, Todo S, et al. Liver resection for hilar and peripheral cholangiocarcinoma: a study of 62 cases. Ann Surg. 1998;227:70–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.El Rassi ZE, Partensky C, Scoazec JY, et al. Peripheral cholangiocarcinoma: presentation, diagnosis, pathology and management. Eur J Surg Oncol. 1999;25:375–380. [DOI] [PubMed] [Google Scholar]
- 27.Hemming AW, Reed AI, Howard RJ, et al. Preoperative portal vein embolization for extended hepatectomy. Ann Surg. 2003;237:686–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kamiya S, Nagino M, Kanazawa H, et al. The value of bile replacement during external biliary drainage. Ann Surg. 2004;239:510–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gozzetti G, Mazziotti A, Grazi GL, et al. Liver resection without blood transfusion. Br J Surg. 1995;82:1105–1110. [DOI] [PubMed] [Google Scholar]
- 30.Shimada M, Takenaka K, Fujiwara Y, et al. Risk factors linked to postoperative morbidity in patients with hepatocellular carcinoma. Br J Surg. 1998;85:195–198. [DOI] [PubMed] [Google Scholar]
- 31.Shinozuka N, Koyama I, Arai T, et al. Autologous blood transfusion in patients with hepatocellular carcinoma undergoing hepatectomy. Am J Surg. 2000;179:42–45. [DOI] [PubMed] [Google Scholar]
- 32.Foster RS Jr, Costanza MC, Foster JC, et al. Adverse relationship between blood transfusions and survival after colectomy for colon cancer. Cancer. 1985;55:1195–1201. [DOI] [PubMed] [Google Scholar]
- 33.Heiss MM, Mempel W, Delanoff C, et al. Blood transfusion-moderated tumor recurrence: first result of a randomized study of autologous versus allogeneic blood transfusion in colorectal cancer surgery. J Clin Oncol. 1994;12:1859–1867. [DOI] [PubMed] [Google Scholar]
- 34.Chan AC, Blumgart LH, Wuest DL, et al. Use of preoperative autologous blood donation in liver resection for colorectal metastasis. Am J Surg. 1998;175:461–465. [DOI] [PubMed] [Google Scholar]
- 35.Itamoto T, Katayama K, Nakahara H, et al. Autologous blood storage before hepatectomy for hepatocellular carcinoma with underlying liver disease. Br J Surg. 2003;90:23–28. [DOI] [PubMed] [Google Scholar]
- 36.Robles R, Figueras J, Turrion VS, et al. Spanish experience in liver transplantation for hilar and peripheral cholangiocarcinoma. Ann Surg. 2004;239:265–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meyer CG, Penn I, James L. Liver transplantation for cholangiocarcinoma: results in 207 patients. Transplantation. 2000;69:1633–1637. [DOI] [PubMed] [Google Scholar]
- 38.De Vreede I, Steer JL, Burch PA, et al. Prolonged disease-free survival after orthotopic liver transplantation plus adjuvant chemoirradiation for cholangiocarcinoma. Liver Transplant. 2000;6:309–316. [DOI] [PubMed] [Google Scholar]
- 39.Chang CH, Chau GY, Lui WY, et al. Long-term results of hepatic resection for hepatocellular carcinoma originating from the noncirrhotic liver. Arch Surg. 2004;139:320–325. [DOI] [PubMed] [Google Scholar]
- 40.Fan ST, Ng IO, Poon RT, et al. Hepatectomy for hepatocellular carcinoma: the surgeon's role in long-term survival. Arch Surg. 1999;134:1124–1130. [DOI] [PubMed] [Google Scholar]
- 41.Fong Y, Fortner J, Sun RL, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bolton JS, Fuhrman GM. Survival after resection of multiple bilobar hepatic metastases from colorectal carcinoma. Ann Surg. 2000;231:743–751. [DOI] [PMC free article] [PubMed] [Google Scholar]