Abstract
Objective:
To examine treatment trends in invasive lobular carcinoma (ILC) over the last 15 years and, in particular, to compare rates of recurrence and disease-free survival associated with breast conservation therapy compared with mastectomy.
Summary Background Data:
The biologic characteristics of ILC make it difficult to estimate the extent of the disease by either clinical examination or mammography, and can also make it difficult to detect axillary lymph node metastases. Because of this, there has been a bias toward treating ILC with aggressive therapy.
Methods:
Patients with ILC were selected from the National Cancer Data Base (1989–2001) using an extensive set of inclusion and exclusion criteria. A total of 21,596 patients were selected, including 8108 who received breast conservation therapy and 13,488 who received mastectomy. Analysis included demographic characteristics, trends in usage of sentinel lymph node biopsy, rates of local and distant recurrence, and 5-year disease-free survival rates.
Results:
The use of breast conversation therapy increased almost threefold during the study period. From 1998 to 2001, the use of sentinel node biopsy increased more than twofold in the breast conservation group (an average of 23% in 1998 versus 57% in 2001), compared with limited usage in the mastectomy group (an average of 10% in 1998 versus 23% in 2001). Local recurrence rates were very low and disease-free survival rates were correspondingly high in both treatment groups for all diagnosis years and across all pathologic tumor size/lymph node status designations.
Conclusions:
Less invasive treatment options are becoming widely used for invasive lobular carcinoma, yielding outcomes equivalent to those seen with more aggressive treatment.
Because of the infiltrative growth pattern and frequent discontinuity seen in invasive lobular carcinoma, there has been a bias toward treating patients with more aggressive surgery, including mastectomy and standard axillary lymph node dissection. Date from the National Cancer Data Base indicate a growing trend toward less invasive treatment, with good treatment outcomes obtained using breast conserving surgery and sentinel lymph node biopsy.
Over the past 20 years, the trend in breast cancer management has been toward less invasive treatment strategies. This trend has been fueled in large part by the growing use of screening mammography, which has led to a significant decrease in the average size of tumors when they are first discovered. Several large-scale clinical trials have demonstrated that breast conservation therapy (BCT) consisting of segmental mastectomy and radiation treatment is as effective as standard mastectomy for the surgical treatment of these small tumors.1,2 The routine use of axillary lymph node dissection (ALND) has also been called into question because small tumors are less likely to be associated with axillary disease and because other factors are playing a larger role in determining optimal adjuvant treatment strategies. Numerous studies have now shown that sentinel lymph node biopsy (SLNB) provides an accurate assessment of the disease status of the axilla while avoiding the significant morbidity associated with ALND.3,4
The studies validating the use of BCT and SLNB for the management of early-stage invasive breast cancer were not designed to look at histology as an independent variable affecting outcome parameters. Thus, even when different histologic tumor types have been included and categorized, the overall outcomes of the studies have been heavily weighted by the most common histologic type, invasive ductal carcinoma (IDC).
The second most common type of invasive breast cancer is invasive lobular carcinoma (ILC), accounting for 8% to 14% of all invasive breast cancers.5 ILC is biologically quite distinct from IDC. Rather than showing discrete tumor foci, ILC is pathologically characterized by innocuous looking round or spindle-shaped cells that show a single-file growth pattern, or are dispersed beyond the mammographic or gross lesion in a seemingly random manner.5 Clinically, ILC may be apparent only as a poorly defined thickening of the breast, rather than presenting as a dominant mass. This makes the extent of the disease difficult to estimate on clinical examination, and the unusual growth pattern can also make ILC hard to visualize by mammography. Although ultrasonography (US) has been shown to be more sensitive than mammography in detecting ILC,6 it may also significantly underestimate the size of ILC lesions.7 Magnetic resonance imaging (MRI) is more accurate than either mammography or US in defining the extent of the disease but is less widely available.8 Because of the infiltrative growth pattern and frequent discontinuities, there is a higher incidence of resection margin involvement than for IDC and a higher rate of intrasurgical conversion to mastectomy.9 Finally, ILC has a higher incidence of bilaterality, multifocality, and multicentricity than IDC.10
The same biologic characteristics that make ILC difficult to detect clinically or mammographically can make it harder to detect axillary lymph node metastases.11 Nodes may remain nonpalpable even when extensively involved, and the bland-looking metastatic tumor cells may mimic histiocytes or other benign cell types. Keratin immunohistochemistry is helpful in distinguishing cancer cells from the background but has only recently been used.
Because of these characteristics, there has been a bias toward treating ILC with aggressive surgery, with most small series indicating a 3- to 6-fold excess in patients receiving mastectomy compared with BCT.12–14 Nonetheless, a number of retrospective studies have demonstrated that, for patients with ILC who successfully undergo BCT, outcomes are equivalent to those obtained in patients with IDC treated withBCT15–22 or in ILC patients treated with mastectomy.12–14,23–25 With 2 exceptions, these studies have been small, limiting the usefulness of the findings. One exception is a large-scale study by Sastre-Garau et al from the Institute Curie in Paris.21 They reported on a series of 11,036 patients with nonmetastatic breast cancer seen during the 1981 to 1991 period. Of these, 7341 were treated with BCT, including 480 patients with ILC, 154 with ILC/IDC, and 6797 with non-ILC (including 91% IDC and 9% other histologic types). They reported slightly lower 5-year local recurrence rates in ILC compared with non-ILC (9% versus 14%, respectively). A second large study by Winchester et al25 used data from the National Cancer Data Base (NCDB) to examine presentation, treatment, and outcomes in patients with ILC. Outcome assessments were restricted to patients diagnosed between 1985 and 1988. Overall survival rates at 5 years were similar between patients with ILC and IDC and between ILC patients receiving BCT and those receiving mastectomy.
The current study was designed to capitalize on the large amount of additional data that have accrued to the NCDB since the publication of the Winchester study. We extracted data covering the 12-year period from 1989 to 2001 to examine management trends in ILC, including the growing use of SLNB, and to compare rates of local recurrence, distance recurrence, and disease-free survival (DFS) as a function of surgical treatment (BCT versus mastectomy). Because of the much larger data set at our disposal, we were able to narrowly define our study sample to provide a more definitive look at these issues.
METHODS
NCDB
Data from the NCDB were used to analyze trends in ILC management from 1989 to 2001. The NCDB is a nationwide oncology database founded in 1989 as a joint project of the American Cancer Society and the Commission on Cancer of the American College of Surgeons. It holds information on about 70% of all newly diagnosed cases of cancer in the United States from approximately 1600 hospitals in 50 states (over 900,000 cases per year), and includes demographic, clinical, and health system data elements needed to assess the quality of care.
Patient Selection
Because of the large size of the NCDB, it is possible to select relatively homogeneous patient samples to use in answering specific research questions. For this study, we established the following patient selection criteria:
Diagnosis of pure ILC (ie, no lobular carcinoma in situ [LCIS], no mixed ILC/IDC or ILC/LCIS). This is in keeping with the coding of the database, although we recognize that a significant percentage of “pure ILC” will contain at least a small LCIS component.
Early-stage disease, defined pathologically as T1/node negative, T1/node positive, T2/node negative, or T2/node positive. These designators were used in place of AJCC/UICC stages to avoid problems in interpretation that might arise due to revisions of the staging system in 1997 and 2001.
Diagnosis years restricted. For analysis of trends in surgical management of the primary tumor, patients were selected from diagnosis years grouped as 1989–1990, 1994–1995, and 2000–2001. For analysis of trends in detection of axillary lymph node metastasis, patients were selected from single diagnosis years 1998, 1999, 2000, and 2001. (Data on the use of SLNB only started to be reported from the cancer registries in 1998, but the technique has quickly come into widespread use since that time.)
One or more axillary lymph nodes removed and examined in all patients to ensure proper pathologic classification of axillary lymph node status.
-
Locoregional treatment type specified as either:
BCT: defined as removal of the primary tumor, assessment of the axilla by either SLNB or standard ALND or both, and postoperative radiation therapy (XRT). For this group, the categories of partial mastectomy, lumpectomy, and re-excision were combined.
Mastectomy: defined as removal of the whole breast, assessment of the axilla by either SLNB or ALND or both, with or without XRT. For this group, the categories of total simple mastectomy and modified radical mastectomy were combined.
In addition to the patients who did not meet the above inclusion criteria, the following patients were also specifically excluded:
Patients in the following treatment categories.
No surgery performed.
Subcutaneous mastectomy.
Mastectomy not otherwise specified.
Surgery not otherwise specified.
Unknown if surgery performed.
Patients with known bilateral breast cancer or contralateral breast surgery.
Patients who had no axillary lymph nodes removed or for whom information was not available about whether nodes were removed.
Patients for whom pathologic information about axillary lymph node status was not available.
Patients with positive supraclavicular or internal mammary lymph nodes.
BCT patients who did not receive XRT or whose XRT treatment status was unknown.
Patients who received neoadjuvant chemotherapy.
Using these inclusion and exclusion criteria, 21,596 patients were selected for the study sample, including 8108 in the BCT group and 13,488 in the mastectomy group.
Analysis
The 2 locoregional treatment groups (BCT and mastectomy) were analyzed qualitatively by age, race, diagnosis year group, pathologic tumor size, pathologic nodal status, estrogen receptor status, type of postoperative adjuvant therapy, and geographic area. Trends in the usage of SLNB for subsets of patients defined by pathologic tumor size and lymph node status (T1/node negative, T1/node positive, T2/node negative, T2/node positive) were compared graphically for BCT and mastectomy. The number of patients who received SLNB was calculated as the number receiving SLNB alone plus the number receiving a combination of SLNB and standard ALND. Rates of local and distant recurrence for the 2 earlier time periods (1989–1990 and 1994–1995) were assessed qualitatively for associations with treatment type (BCT or mastectomy) and pathologic T/N designation. Recurrence rates (local plus distant) for the 2 earlier time periods were analyzed quantitatively using Cox regression analysis to assess the independent contributions of surgical treatment type, pathologic T/N designation, age, and geographic region. Five-year DFS rates were compared for BCT versus mastectomy using Kaplan-Meier analysis.
RESULTS
Patient and Tumor Characteristics
Patient and tumor characteristics for the 21,596 patients in the study sample are shown in Table 1. ILC patients who received BCT tended to be younger than those receiving mastectomy, with a shift of approximately 10% of patients from the 70 years or older category to the 51 to 69 years category. There was no major difference in ethnicity between the 2 treatment groups. Over the 3 time periods analyzed, the percentage of total ILC patients receiving BCT increased almost 3-fold, from 17.9% in 1989 to 1990 to 51.5% in 2000 to 2001. This change was marked by a parallel decrease in the percentage of total ILC patients receiving mastectomy from 82.1% to 48.5%.
TABLE 1. Patient and Treatment Characteristics for Patients With Invasive Lobular Carcinoma
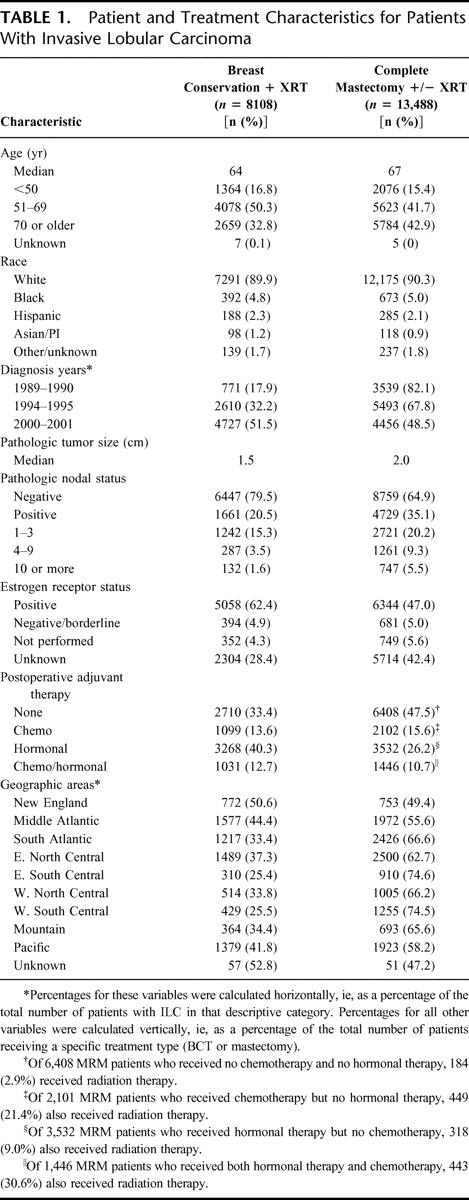
There were notable differences between treatment groups in characteristics associated with disease severity. The median pathologic tumor size in patients who received mastectomy was 33.0% larger than in BCT patients. Mastectomy patients were also more likely than BCT patients to be node positive (35.1% versus 20.5%, respectively), and less likely than BCT patients to be ER-positive (47.0% versus 62.4%, respectively). (It should be noted that hormone receptor data were unavailable for 28.4% of BCT patients and 42.4% of mastectomy patients. This reflects the fact that this information was not reported from cancer registries until 1998, and then only voluntarily.)
Nearly half of the patients who received mastectomy received no postoperative adjuvant therapy, compared with one third of the patients who received BCT. There was no difference between the 2 treatment groups in the percentage of patients receiving adjuvant chemotherapy or chemotherapy in combination with hormonal therapy, but mastectomy patients were substantially less likely than BCT patients to have received hormonal therapy alone (26.2% versus 40.3%, respectively).
When the number of patients receiving BCT was considered as a percentage of the total number of ILC patients treated by either BCT or mastectomy in each geographic area, there was a bicoastal trend toward higher usage of BCT. Thus, the highest percentages of BCT use were found in the New England, the middle Atlantic, and the Pacific regions (50.6%, 44.4%, and 41.8% of ILC patients, respectively), while the lowest percentages were found in the east south central and the west south central regions (25.4% and 25.5%, respectively).
Axillary Lymph Node Assessment
Figure 1 shows trends in the use of SLNB for the assessment of axillary lymph nodes in patients with ILC. The data points indicate the percentage of patients receiving SLNB alone or SLNB in combination with standard ALND, as a function of the total number of patients in that treatment group who had one or more axillary lymph nodes surgically removed and examined. For BCT patients (Fig. 1A), there was a substantial increase in the use of SLNB from 1998 to 2001, regardless of T/N designation. Thus, 23% of BCT patients (averaged across all T/N designations) received SLNB in 1998, compared with 57% in 2001. For mastectomy patients, on the other hand, slightly less than 10% (averaged across all T/N designations) received SLNB in 1998, and that amount increased to only 23% in 2001 (Fig. 1B).
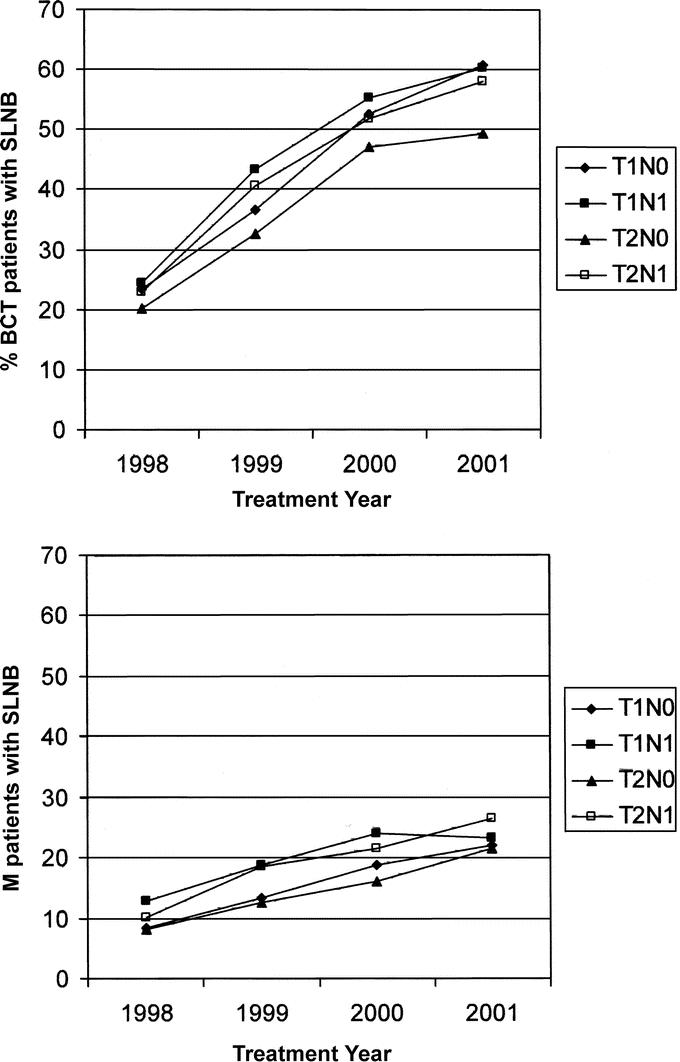
FIGURE 1. Percentage of patients with invasive lobular carcinoma who received a sentinel lymph node biopsy (either alone or in combination with standard axillary lymph node dissection) as a function of the total number of patients who had at least one axillary lymph node surgically removed and assessed. A, Patients treated with breast conservation therapy (BCT). B, Patients treated with mastectomy (M).
Of the pathologically node-negative BCT patients who received SLNB, approximately half had SLNB without a follow-up ALND. In T1N0 patients, this ranged from 38% of patients in 1998 to 60% of patients in 2001. Similarly in T2N0 patients who received SLNB, the percentage receiving SLNB alone ranged from 41% in 1999 to 56% in 2001. In node-positive BCT patients, on the other hand, the majority of patients (75% to 90%) in all diagnosis years who received SLNB went on to receive a regular ALND.
Locoregional Recurrence and Distant Recurrence as a Function of Pathologic T/N Designation and Treatment Type
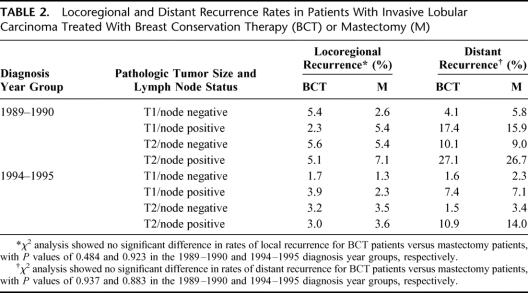
Rates of locoregional and distant recurrence as a function of diagnosis year group, pathologic T/N designation, and treatment type are shown in Table 2. In both diagnosis year groups, there were no significant differences between BCT patients and mastectomy patients in rates of locoregional or distant recurrence. Across all T/N designations, rates of locoregional recurrence were uniformly low, ranging from 1.3% to 7.1%. Rates were modestly increased in the 1989 to 1990 diagnosis year group compared with the 1994 to 1995 diagnosis year group, but there were no apparent differences as a function of treatment type (BCT versus mastectomy). Distant recurrence rates were low for all T1/node-negative tumors (1.6%–5.8%), regardless of diagnosis year group or treatment type. For T1/node-positive, T2/node-negative, and T2/node-positive tumors, distant recurrence rates were 2- to 3-fold higher in the 1989 to 1990 group compared with the 1994 to 1995 group but were essentially the same for the 2 treatment types.
TABLE 2. Locoregional and Distant Recurrence Rates in Patients With Invasive Lobular Carcinoma Treated With Breast Conservation Therapy (BCT) or Mastectomy (M)
In the Cox regression analysis, pathologic T/N designation was the most significant factor in predicting total recurrence rate (locoregional plus distant) in both diagnosis year groups (P < 0.001).
Disease-Free Survival as a Function of Surgical Treatment
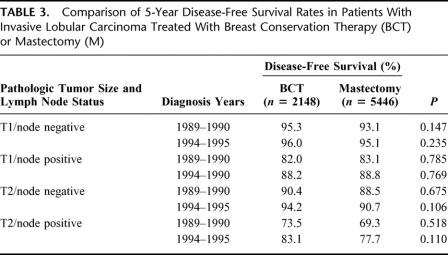
Of the 21,596 patients in the original study group, the 9183 patients from the 2000 to 2001 diagnosis year group (4727 BCT and 4456 mastectomy) were excluded because of insufficient follow-up time for 5-year DFS analysis. An additional 4819 patients were excluded if they were in the category “unknown if recurred,” if no 5-year follow-up was available, or if there were conflicting data in multiple data fields. This resulted in a cohort of 7594 patients (2148 BCT and 5446 mastectomy) that was used to compare 5-year DFS rates (Table 3). The Kaplan-Meier analysis showed no significant differences in DFS rates between treatment groups for any T/N designation in any diagnosis year group.
TABLE 3. Comparison of 5-Year Disease-Free Survival Rates in Patients With Invasive Lobular Carcinoma Treated With Breast Conservation Therapy (BCT) or Mastectomy (M)
DISCUSSION
Use of BCT for the Treatment of ILC
ILC is the second most common type of invasive breast cancer. It has been the subject of increasing interest because of reports that the incidence of ILC has increased in postmenopausal women over the last 15 years, possibly in response to the growing use of estrogen-progestin hormone replacement therapy in this age group.26,27
This study used the extensive data set available in the NCDB to examine trends in the management of ILC during this same 15-year period. We were especially interested in looking at developments in minimally invasive treatment strategies for this group. We found that the percentage of ILC patients using BCT had increased almost 3-fold over the study period and that patient outcomes (locoregional and distant recurrence, 5-year disease-free survival) were similar in BCT patients compared with mastectomy patients, stage for stage.
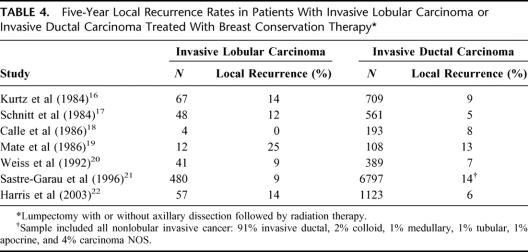
Two types of studies have been used previously to look at the effectiveness of BCT as a treatment option for ILC. In one type of study, outcomes in ILC treated with BCT were compared with outcomes in IDC treated with BCT. The 7 studies shown in Table 4 compared 5-year local recurrence rates in patients treated with BCT.16–22 Five of the 7 studies showed slightly increased local recurrence rates in ILC compared with IDC, but the small numbers of patients with ILC limit the clinical importance of these findings. These studies also do not directly address the question of whether BCT is equivalent to mastectomy for the management of ILC.
TABLE 4. Five-Year Local Recurrence Rates in Patients With Invasive Lobular Carcinoma or Invasive Ductal Carcinoma Treated With Breast Conservation Therapy
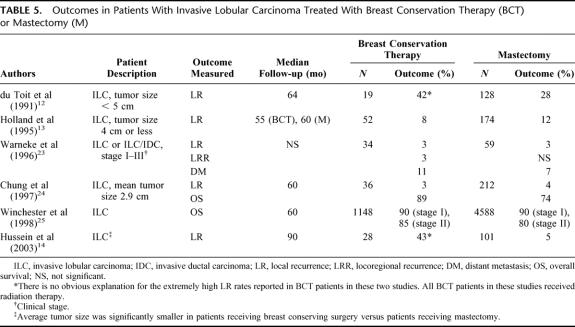
In the other type of study, outcomes were compared in ILC treated with BCT versus ILC treated with mastectomy. The 6 studies shown in Table 5 looked at a variety of outcomes at follow-up times ranging from 55 months to 90 months.12–14,23–25 In 2 studies,12,14 patients receiving BCT showed extremely high rates of local recurrence (42%–43%) compared with rates of 3% to 8% shown in other studies in this series13,23,24 or the 2% to 4% shown in the 1994 to 1995 diagnosis year group in the current report. It is not clear what may have contributed to the high rates seen in these 2 studies, but very low sample sizes may have been a factor. With the exception of the NCDB study from Winchester et al,25 all of these studies are based on small sample sizes from local populations.
TABLE 5. Outcomes in Patients With Invasive Lobular Carcinoma Treated With Breast Conservation Therapy (BCT) or Mastectomy (M)
The study from Winchester et al25 used NCDB data from 5736 patients (1148 ILC and 4588 IDC) from 1984 to 1988 and 1990 to 1993. The treatment outcomes for the 1984 to 1988 treatment group were analyzed by American Joint Committee on Cancer (AJCC) stage, with pathologic stage augmented by clinical stage when necessary. They reported 5-year overall survival rates of 90% for stage I patients, regardless of treatment type, and 85% and 80% for stage II patients receiving BCT and mastectomy, respectively.
A potential problem with this study was the use of clinical staging to augment pathologic staging, since both mammographic and ultrasound estimates of tumor size for ILC frequently underestimate pathologic size.5,7 Thus, it is possible that some outcomes reported in the Winchester study might actually represent a higher-stage disease than the one that was used for the analysis. In the current report, we sought to avoid this problem and a related problem concerning revisions in the AJCC staging system during the study period by reporting outcomes based on pathologic tumor size and lymph node status (eg, T1/node negative) rather than formal AJCC stage.
Geographic Variation in the Use of BCT for the Treatment of ILC
There was a trend toward increased use of BCT on the east and west coasts, with decreased use in the center of the country, especially in the south central regions. This is consistent with similar trends that have been previously reported in histologically mixed populations of breast cancer patients (Fig. 2). Osteen et al28 demonstrated this bicoastal trend, based on data from 41,680 patients in the NCDB who were originally treated between 1985 and 1988. They hypothesized that the nonuniform distribution might stem, in part, from the fact that BCT was still a relatively new technique, with results from the initial randomized trial published just a short time before the 1988 cohort was treated. However, a later study by Albain et al29 using patients who had been randomized into 2 Southwest Oncology Group intergroup trials between 1989 and 1995 showed a strikingly similar distribution. (Note that the bars shown in Fig. 2 for the Albain et al study29 represent node-negative patients only.) For the histologically selected subsample of breast cancer patients presented in this study, it is encouraging that, although the bicoastal trend is still apparent, the percentage of patients receiving BCT has increased in all geographic regions, especially in the south central regions.
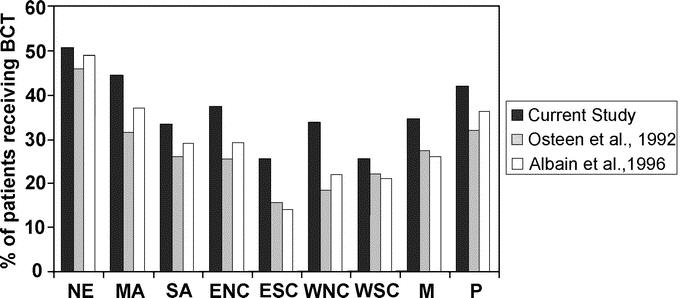
FIGURE 2. Percentage of patients receiving breast conservation therapy as a function of geographic region. (Percentage was calculated as the proportion of the total number of patients receiving either breast conservation or mastectomy.) NE, New England; MA, mid-Atlantic; SA, south Atlantic; ENC, east north central; ESC, east south central; WNC, west north central; WSC, west south central; M, mountain; P, Pacific. Data from the study by Osteen et al28 represent histologically mixed AJCC stage I and II patients. Data from the study by Albain et al29 represent a node-negative subsample of histologically mixed AJCC stage I and II patients.
ILC and Axillary Lymph Node Assessment
Although SLNB is rapidly becoming a treatment standard for patients with early-stage breast cancer, there are certain patient groups for whom its use has been debated. For example, the use of SLNB in patients who have received neoadjuvant chemotherapy has come under scrutiny because of concern that chemotherapy might interfere with the structure or function of the lymphatic system. In the case of ILC, some have questioned whether, because of the ill-defined features of the primary tumor, there might be difficulty in recognizing nodal metastases.
Grube et al11 addressed this question in a study that examined the results of SLNB in 105 patients with ILC. They were able to identify the sentinel node in 97% of cases with an accuracy of 100% and a false-negative rate of 0%. In a more recent study, Classe et al30 compared the results of SLNB in 208 patients with IDC with 35 patients with ILC. They found that the predictive value from SLNB was the same in both histologic types. They also reported that the rate of micrometastasis as diagnosed by immunohistochemical staining techniques may be overestimated in patients with ILC, but this remains to be confirmed in a larger study. Overall, these studies suggest that SLNB is not only accurate in ILC but may be much more useful than standard ALND, due to the difficulty of detecting axillary lymph node metastases in this patient group with standard staining procedures.
In the current study, the use of SLNB in patients with ILC who were treated with BCT increased from 23% in 1998 to 57% in 2001, mirroring the growing use of this technique seen in IDC. For mastectomy patients, on the other hand, slightly less than 10% received SLNB in 1998, and that value increased to only 23% in 2001. This is consistent with results reported by Edge et al31 using data from 3003 stage I and II cancer patients from the National Comprehensive Cancer Network Breast Cancer Outcomes Project who were treated between July 1997 and December 2000. In that study, of those patients in whom the axillary lymph nodes were examined, 53% of BCT patients received SLNB alone or in combination with ALND, compared with only 18% of mastectomy patients.
The limited use of SLNB in mastectomy patients is troubling, since the advantages of SLNB over ALND in terms of postsurgical morbidity certainly extend to mastectomy patients. A possible contributory factor is the fear that an ALND performed as a second surgery in a mastectomy patient who had a positive sentinel node might be problematic if the patient has undergone an immediate reconstruction. This could result in surgical difficulties and might also affect the subsequent use of radiation therapy. Sabel et al32 addressed this issue in a retrospective study of 51 patients who underwent SLNB concomitantly with mastectomy. Their patient data, in combination with survey results from 25 surgical oncologists at other institutions, led them to conclude that SLNB in conjunction with a mastectomy is a safe option for selected patients. Alternatively, Brady et al33 suggest that the ideal approach for patients desiring reconstruction may be to perform SLNB as a separate procedure before the mastectomy, using the results to guide the decision about whether to proceed with an immediate reconstruction.
Using the NCDB
The current study used the large and diverse NCDB to assess changing treatment standards and outcomes in patients with ILC. There are limitations to using large databases, including hidden biases that are difficult to detect, to control, or to correct. For example, in comparing outcomes between BCT and mastectomy, it is likely that patients with more severe disease would be preferentially partitioned into the mastectomy treatment group. Thus, in the T2 tumor size category, which includes tumors ranging from 2 to 5 cm in size, one might expect that patients with smaller tumors would be more likely to receive BCT, while those with larger tumors would either be recommended for neoadjuvant chemotherapy or would receive a mastectomy directly.
It is also possible that patients in certain demographic categories might be preferentially partitioned into a particular treatment group, regardless of tumor stage. Diab et al34 reported that treatment approaches in the elderly are, on average, quite different from those used in their younger counterparts. In their study sample, the majority of women 56 to 85 years of age (64%–88%) received modified radical mastectomies; older women are also less likely to receive additional local therapy or adjuvant systemic therapy, with the exception of tamoxifen.
Nonetheless, the advantages of using a large database far outweigh the limitations. The extremely large sample sizes make it possible to use extensive inclusion and exclusion criteria to tailor a very clean study. The NCDB draws from a diverse patient population and can address treatment and outcome differences based on ethnicity, age, or geographic location. Finally, the NCDB allows the analysis of trends over long periods of time, which is especially critical for the assessment of subtle differences in treatment efficacy.
CONCLUSION
Because of the infiltrative growth pattern and frequent discontinuity seen in ILC, there has been a bias toward treating patients with more aggressive surgery, including mastectomy and standard axillary lymph node dissection. Indeed, if clear surgical margins can be obtained, many patients with ILC can be effectively treated with BCT, as demonstrated in the current analysis. SLNB, especially when used in conjunction with immunohistochemical staining, is also an appropriate technique in patients with ILC, sparing them the morbidities commonly associated with a full axillary dissection.
Footnotes
The Commission on Cancer Breast Disease Site Team: Paul L. Baron, MD, FACS, Charleston Surgical Associates Charleston, SC; James L. Connolly, MD, Beth Israel Deaconess Medical Center Boston, MA; Rosemary B. Duda, MD, FACS, Beth Israel Deaconess Medical Center Boston, MA; Stephen B. Edge, MD, FACS, Roswell Park Cancer Institute Buffalo, NY; James A. Edney, MD, FACS, University of Nebraska Medical Center Omaha, NE; Suzanne Klimberg, MD, University of Arkansas Little Rock, AK; Robert R. Kuske, MD, Scottsdale Radiology Oncology Scottsdale, AZ; A. Marilyn Leitch, MD, FACS, University of Texas Southwestern Medical Center Dallas, TX; Joseph Lipscomb, PhD, Emory University Atlanta, GA; Lisa Ann Newman, MD, FACS, University of Michigan Ann Arbor, MI; Geoffrey L. Robb, MD, FACS, University of Texas MD Anderson Cancer Center Houston, TX; Edward Allen Sickles, MD, FACR, UCSF Medical Center San Francisco, CA; George Sledge, MD, University of Indiana Indianapolis, IN; Andrew Stewart, MA, The American College of Surgeons Commission on Cancer Chicago, IL; David P. Winchester, MD, FACS, Evanston Hospital Evanston, IL.
Reprints: S. Eva Singletary, MD, FACS, Department of Surgical Oncology, University of Texas M.D. Anderson Cancer Center, 1515 Holcombe Boulevard, Box 444, Houston TX 77030-4095. E-mail: esinglet@mdanderson.org.
REFERENCES
- 1.Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347:1233–1241. [DOI] [PubMed] [Google Scholar]
- 2.Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347:1227–1232. [DOI] [PubMed] [Google Scholar]
- 3.Krag D, Weaver D, Ashikaga T, et al. The sentinel node in breast cancer: a multicenter validation study. N Engl J Med. 1998;339:941–6. [DOI] [PubMed] [Google Scholar]
- 4.Veronesi U, Paganelli G, Viale G, et al. Sentinel lymph node biopsy and axillary dissection in breast cancer: results in a large series. J Natl Cancer Inst. 1999;91:368–373. [DOI] [PubMed] [Google Scholar]
- 5.Arpino G, Bardou VJ, Clark GM, et al. Infiltrating lobular carcinoma of the breast: tumor characteristics and clinical outcome. Breast Cancer Res. 2004;6:R149–R156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Selinko VL, Middleton LP, Dempsey PJ. Role of sonography in diagnosing and staging invasive lobular carcinoma. J Clin Ultrasound. 2004;32:323–332. [DOI] [PubMed] [Google Scholar]
- 7.Pritt B, Ashikaga T, Oppenheimer RG, et al. Influence of breast cancer histology on the relationship between ultrasound and pathology tumor size measurements. Mod Pathol. 2004;17:905–910. [DOI] [PubMed] [Google Scholar]
- 8.Weinstein SP, Orel SG, Heller R, et al. MR imaging of the breast in patients with invasive lobular carcinoma. AJR Am J Roentgenol. 2001;176:399–406. [DOI] [PubMed] [Google Scholar]
- 9.Yeatman TJ, Cantor AB, Smith TJ, et al. Tumor biology of infiltrating lobular carcinoma: implications for management. Ann Surg. 1995;222:549–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ashikari R, Huvos AG, Urban JA, et al. Infiltrating lobular carcinoma of the breast. Cancer. 1973;31:110–116. [DOI] [PubMed] [Google Scholar]
- 11.Grube BJ, Hansen NM, Ye X, et al. Tumor characteristics predictive of sentinel node metastases in 105 consecutive patients with invasive lobular carcinoma. Am J Surg. 2002;184:372–376. [DOI] [PubMed] [Google Scholar]
- 12.du Toit RS, Locker AP, Ellis IO, et al. An evaluation of differences in prognosis, recurrence patterns and receptor status between invasive lobular and other invasive carcinomas of the breast. Eur J Surg Oncol. 1991;17:251–257. [PubMed] [Google Scholar]
- 13.Holland P, Shah A, Howell A, et al. Lobular carcinoma of the breast can be managed by breast-conserving therapy. Br J Surg. 1991;82:1364–1366. [DOI] [PubMed] [Google Scholar]
- 14.Hussein M, Lioe TF, Finnegan J, et al. Surgical treatment for invasive lobular carcinoma of the breast. Breast. 2003;12:23–35. [DOI] [PubMed] [Google Scholar]
- 15.Bouvet M, Ollila DW, Hunt KK, et al. Role of conservation therapy for invasive lobular carcinoma of the breast. Ann Surg Oncol. 1997;4:650–654. [DOI] [PubMed] [Google Scholar]
- 16.Kurtz JM, Jacquemier J, Torhorst J, et al. Conservation therapy for breast cancers other than infiltrating ductal carcinoma. Cancer. 1989;63:1630–635. [DOI] [PubMed] [Google Scholar]
- 17.Schnitt SJ, Connolly JL, Harris JR, et al. Pathologic predictors of early local recurrence in Stage I and II breast cancer treated by primary radiation therapy. Cancer. 1984;53:1049–1057. [DOI] [PubMed] [Google Scholar]
- 18.Calle R, Vilcoq JR, Zafrani B, et al. Local control and survival of breast cancer treated by limited surgery followed by irradiation. Int J Radiat Oncol Biol Phys. 1986;12:873–878. [DOI] [PubMed] [Google Scholar]
- 19.Mate TP, Carter D, Fischer DB, et al. A clinical and histopathologic analysis of the results of conservation surgery and radiation therapy in stage I and II breast carcinoma. Cancer. 1986;58:1995–2002. [DOI] [PubMed] [Google Scholar]
- 20.Weiss MC, Fowble B, Solin LJ, et al. Outcome of conservative therapy for invasive breast cancer by histologic subtype. Int J Radiat Oncol Biol Phys. 1992;23:941–947. [DOI] [PubMed] [Google Scholar]
- 21.Sastre-Garau X, Jouve M, Asselain B, et al. Infiltrating lobular carcinoma of the breast. Cancer. 1996;77:113–120. [DOI] [PubMed] [Google Scholar]
- 22.Harris EE, Hwang W, Santiago R, et al. Long-term outcomes for breast conservation therapy in invasive lobular carcinoma of the breast. Int J Radiat Oncol Biol Phys. 2003;57(suppl 2):359–360. [Google Scholar]
- 23.Warneke J, Berger R, Johnson C, et al. Lumpectomy and radiation treatment for invasive lobular carcinoma of the breast. Am J Surg. 1996;172:496–500. [DOI] [PubMed] [Google Scholar]
- 24.Chung MA, Cole B, Wanebo JH, et al. Optimal surgical treatment of invasive lobular carcinoma of the breast. Ann Surg Oncol. 1997;4:545–550. [DOI] [PubMed] [Google Scholar]
- 25.Winchester DJ, Chang HR, Graves TA, et al. A comparative analysis of lobular and ductal carcinoma of the breast: presentation, treatment, and outcomes. J Am Coll Surg. 1998;186:416–422. [DOI] [PubMed] [Google Scholar]
- 26.Coates RJ, Marchbanks PA, Norman SA, et al. Relation of regimens of combined hormone replacement therapy to lobular, ductal, and other histologic types of breast carcinoma. Cancer. 2002;95:2455–2464. [DOI] [PubMed] [Google Scholar]
- 27.Li CI, Malone KE, Porter PL, et al. Relationship between long durations and different regimens of hormone therapy and risk of breast cancer. JAMA. 2003;289:3254–3263. [DOI] [PubMed] [Google Scholar]
- 28.Osteen RT, Steele GD, Menck HR, et al. Regional differences in surgical management of breast cancer. CA Cancer J Clin. 1992;42:39–43. [DOI] [PubMed] [Google Scholar]
- 29.Albain KS, Green SR, Lichter AS, et al. Influence of patient characteristics, socioeconomic factors, geography, and systemic risk on the use of breast-sparing treatment in women enrolled in adjuvant breast cancer studies: an analysis of two intergroup trials. J Clin Oncol. 1996;14:3009–3017. [DOI] [PubMed] [Google Scholar]
- 30.Classe JM, Loussouarn D, Campion L, et al. Validation of axillary sentinel lymph node detection in the staging of early lobular invasive breast carcinoma: a prospective study. Cancer. 2004;100:935–941. [DOI] [PubMed] [Google Scholar]
- 31.Edge SB, Niland JC, Bookman MA, et al. Emergence of sentinel node biopsy in breast cancer as standard-of-care in academic comprehensive cancer centers. J Natl Cancer Inst. 2003;95:1514–1521. [DOI] [PubMed] [Google Scholar]
- 32.Sabel MS, Degnim A, Wilkins EG, et al. Mastectomy and concomitant sentinel lymph node biopsy for invasive breast cancer. Am J Surg. 2004;187:673–678. [DOI] [PubMed] [Google Scholar]
- 33.Brady B, Fant J, Jones R, et al. Sentinel lymph node biopsy followed by delayed mastectomy and reconstruction. Am J Surg. 2003;185:114–117. [DOI] [PubMed] [Google Scholar]
- 34.Diab SG, Elledge RM, Clark GM. Tumor characteristics and clinical outcome of elderly women with breast cancer. J Natl Cancer Inst. 2000;92:550–556. [DOI] [PubMed] [Google Scholar]