Abstract
Objective:
The objective of this study was to evaluate, in an international multicenter phase III trial, the accuracy, use, and morbidity of intraoperative lymphatic mapping and sentinel node biopsy (LM/SNB) for staging the regional nodal basin of patients with early-stage melanoma.
Summary Background Data:
Since our introduction of LM/SNB in 1990, this technique has been widely adopted and has become part of the American Joint Committee on Cancer (AJCC) staging system. Eleven years ago, the authors began the international Multicenter Selective Lymphadenectomy Trial (MSLT-I) to compare 2 treatment approaches: wide excision (WE) plus LM/SNB with immediate complete lymphadenectomy (CLND) for sentinel node (SN) metastases, and WE plus postoperative observation with CLND delayed until the subsequent development of clinically evident nodal metastases.
Methods:
After each center achieved 85% accuracy of SN identification during a 30-case learning phase, patients with primary cutaneous melanoma (≥1 mm with Clark level ≥III, or any thickness with Clark level ≥IV) were randomly assigned in a 4:6 ratio to WE plus observation (WEO) with delayed CLND for nodal recurrence, or to WE plus LM/SNB with immediate CLND for SN metastasis. The accuracy of LM/SNB was determined by comparing the rates of SN identification and the incidence of SN metastases in the LM/SNB group versus the subsequent development of nodal metastases in the regional nodal basin of those patients with tumor-negative SNs. Early morbidity of LM/SNB was evaluated by comparing complication rates between the 2 treatment groups. Trial accrual was completed on March 31, 2002, after enrollment of 2001 patients.
Results:
Initial SN identification rate was 95.3% overall: 99.3% for the groin, 95.3% for the axilla, and 84.5% for the neck basins. The rate of false-negative LM/SNB during the trial phase, as measured by nodal recurrence in a tumor-negative dissected SN basin, decreased with increasing case volume at each center: 10.3% for the first 25 cases versus 5.2% after 25 cases. There were no operative mortalities. The low (10.1%) complication rate after LM/SNB increased to 37.2% with the addition of CLND; CLND also increased the severity of complications.
Conclusions:
LM/SNB is a safe, low-morbidity procedure for staging the regional nodal basin in early melanoma. Even after a 30-case learning phase and 25 additional LM/SNB cases, the accuracy of LM/SNB continues to increase with a center's experience. LM/SNB should become standard care for staging the regional lymph nodes of patients with primary cutaneous melanoma.
Elective lymphadenectomy for early-stage melanoma is controversial as a staging procedure because 80% of patients do not have nodal metastases. Interim results of a phase III international trial show that lymphatic mapping and sentinel node biopsy is highly accurate and adds little morbidity to the care of early-stage melanoma.
The single most important prognostic factor for patients with early-stage melanoma is the tumor status of regional lymph nodes draining the primary tumor.1 Before we developed intraoperative lymphatic mapping and sentinel node biopsy (LM/SNB), the only method to identify regional nodal metastases and stage the nodal basin was elective complete lymph node dissection (CLND) with pathologic examination of each excised node using hematoxylin and eosin (H&E) staining. However, this technique is labor-intensive and it samples only a small portion of each node, thereby underestimating the true frequency of nodal metastasis by as much as 14%.2
Part of the controversy about elective CLND as a staging procedure relates to its effectiveness versus its potential morbidity and cost. Because only approximately 20% of patients with an intermediate-thickness primary are expected to have metastases in the regional nodes, 80% of patients undergoing elective CLND are at risk for acute wound problems and for the chronic morbidities of lymphedema, nerve injury, and anesthetic complications without actual survival benefit.1 LM/SNB was designed to identify occult nodal metastases and stage the regional nodal basin, thereby targeting the critical subset of patients who might benefit from the removal of occult nodal metastases before they become palpable while avoiding the morbidity of CLND in the 80% of patients without regional nodal metastases.
In 1977, we described the use of cutaneous lymphoscintigraphy to identify regional lymphatic basins at risk for metastasis from truncal or head/neck primary melanomas that have ambiguous drainage patterns.3 Our lymphoscintigraphic mapping studies and subsequent investigations with antibodies to S-100 protein4 showed that early-stage regional metastasis targets one or 2 tumor-proximate nodes5 and that lymph nodes closest to the primary tumor are immune downregulated.6 These findings were the impetus for the intraoperative use of vital dyes to identify the sentinel node (SN), ie, the first lymph node within the lymphatic basin reached by lymph draining the primary lesion. A feline model demonstrated the feasibility of LM/SNB,7 and in 1985, we began our first clinical studies of LM/SNB to determine which patients with early-stage melanoma had regional nodal metastases and therefore might benefit from immediate CLND at the time of wide excision of the primary.8
In 1990, we reported our initial series of 223 patients with early-stage cutaneous melanoma who underwent LM/SNB after injection of isosulfan blue or patent blue V dye.8,9 Although cutaneous lymphoscintigraphy was used only for selected primary melanomas with potentially ambiguous drainage patterns such as those on the trunk, blue-stained SNs were identified in 194 of 237 (82%) basins. CLND was performed after all LM/SNB procedures, so that the tumor status of SNs and non-SNs could be compared by H&E staining and immunohistochemistry. SN histology accurately predicted the tumor status of the entire nodal basin; only 2 of 194 (1%) lymph node basins had metastases confined to (allegedly) non-SNs. Complete nodal staging could thus be obtained by focused examination of the SN alone. After extensive phase II trials, we abandoned routine elective CLND as a staging procedure, performing CLND only in patients with tumor-positive SNs.10–12 Studies of LM/SNB in melanoma,13–19 breast cancer,20–22 colon cancer,23 lung cancer,24 and virtually all solid neoplasms that spread to lymph nodes25 have confirmed the SN concept of an orderly progression of metastatic cells from the primary site through the lymphatics to one or 2 regional SNs.
Because LM/SNB requires technical expertise in surgery, nuclear medicine, and pathology, and because we had observed a shallow learning curve in our initial experience, we were concerned about its accuracy outside high-volume melanoma centers.9 In 1994, we initiated the first Multicenter Selective Lymphadenectomy Trial (MSLT-I) to evaluate LM/SNB for staging the regional lymph nodes in patients with early-stage primary cutaneous melanoma; the trial applied uniform entry criteria and standardized operative, pathologic, and nuclear medicine techniques (Fig. 1). MSLT-I was designed to compare primary and secondary end points associated with 2 treatments: wide excision (WE) plus LM/SNB and WE plus postoperative nodal observation (WEO). Eighteen centers in Europe, Australia, and the United States joined the trial after demonstrating an 85% rate of SN identification in a 30-case learning phase. During the learning phase, LM/SNB was followed by CLND and histopathologic examination of all non-SNs. Accrual to MSLT-I was completed in March 2002 with 2001 patients. This report, which is based on the third interim analysis of data from MSLT-I, examines the morbidity and accuracy of LM/SNB for detection of SN metastases that, if left intact, will lead to clinical recurrence of melanoma in the regional nodes. The efficacy of the procedure in regard to disease-free and melanoma-specific survival will be reported subsequently.
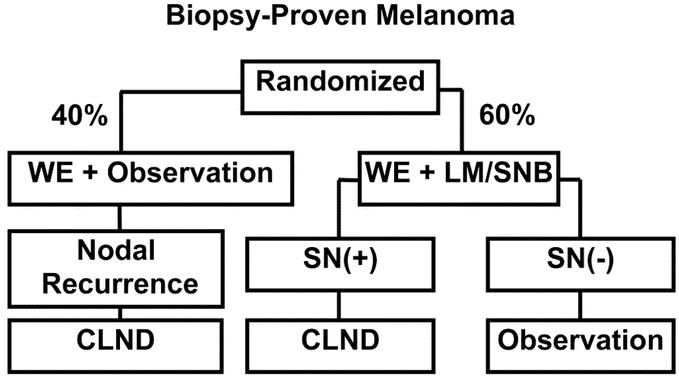
FIGURE 1. MSLT-I study design. Patients with primary cutaneous melanoma ≥1 mm or Clark level IV are assigned in a 60:40 distribution to wide excision (WE) plus lymphatic mapping and sentinel node biopsy, with immediate complete lymphadenectomy (CLND) for occult nodal metastases, or to WE plus observation, with delayed CLND or other treatment of palpable nodal metastases. All patients are followed up for disease-free and melanoma-specific survival.
PATIENTS AND METHODS
MSLT-I Patient Population
Eligible patients had invasive primary cutaneous melanoma of the head and neck, trunk, extremities, sole of the foot, palm of the hand, or a subungual site. Patients with primary melanomas on the ear were excluded. Primary melanomas were Clark level III and Breslow thickness ≥1 mm or Clark level IV/V with any Breslow thickness (Fig. 1). Patients entered the trial no more than 10 weeks after skin biopsy, and LM/SNB was performed within 12 weeks of diagnosis. The age range was 18 to 75 years. Patients were ineligible after any operative procedure that could have disrupted lymphatic drainage patterns from the primary site, including prior wide excision of the primary with a shortest margin ≥1.5 cm. They were also ineligible if they had a history of melanoma or other invasive malignancy within 5 years of the diagnosis of melanoma and/or if their life expectancy (excluding the diagnosis of melanoma) was less than 10 years. Other exclusion criteria were primary or secondary immune deficiency and pregnancy. All patients provided informed consent based on the approved protocol of each institution's review board.
MSLT-I Treatment Arms
Patients were randomly assigned in a 4:6 ratio to WEO or to WE plus LM/SNB with immediate CLND if the SN contained metastases (Fig. 1). Wide excision was performed with operative margins ≥2 cm; the technique of LM/SNB is briefly described subsequently. Patients randomized to WEO underwent CLND only if they developed clinically apparent nodal recurrence (generally in the absence of other known metastases). The larger proportion of patients undergoing LM/SNB was intended to facilitate rapid accrual to this treatment arm without disrupting the power to detect significant differences in primary and secondary end points.
Technique of Lymphatic Mapping and Sentinel Node Biopsy
The mapping technique used in the MSLT was based on the technique we first described in 1990 before the Society of Surgical Oncology8 and published in 1992,9 and which has previously been described in this journal.11 When the trial was initiated in 1994, LM/SNB was performed after a single intradermal injection of 1 to 2 mL of vital blue dye (patent blue or isosulfan blue) around the primary tumor or excisional biopsy wound. Shortly after the start of the trial, a combination of blue dye and radioisotope was used for LM/SNB; SNs were identified not only by the presence of blue staining, but also by the radioactivity measured by a handheld gamma probe.
Preoperative Lymphoscintigraphy
Preoperative dynamic cutaneous lymphoscintigraphy was required and was performed with the radiocolloid available to each multicenter site (varies by country) as previously described.11 Approximately 18.5 to 30 Mbq (0.5–0.8 mCi) of radiopharmaceutical was injected at the primary site. A scintillation camera documented the drainage pattern from the primary through the dermal lymphatics to the regional lymph nodes. The skin overlying the SN was marked by the nuclear medicine physician to assist the surgeon in locating the SN during LM/SNB.
Because of variation in the transit speed of various radiopharmaceuticals and the distance from the primary to the regional basin,26 dynamic imaging is essential to differentiate SNs from secondary non-SNs. In our experience, SNs are usually identified within 30 minutes; after 4 hours, SNs and non-SNs may be difficult to differentiate as a result of migration of the radiocolloid up the lymphatic chain to nodes beyond the SN.15,26
MSLT-I Learning Phase
During the learning phase, each participating center was required to complete at least 30 consecutive cases of LM/SNB plus immediate CLND with an 85% SN identification rate confirmed by histopathologic assessment of all nodes in the CLND specimen. Each individual surgeon documented at least 15 consecutive cases. Documentation included a detailed operative note and a description of how the SN was identified, ie, operative visualization and/or pathologic confirmation of a blue-stained node. CLND was performed in all learning cases to confirm the absence of metastases in non-SNs when the reported SN was free of tumor. No center entered the trial until all learning-phase cases were reviewed by the trial coordinating board (surgeons, nuclear medicine physician, and pathologist). After the first few years, intraoperative radiolymphoscintigraphy was introduced as an adjunct for SN identification12,15,16–19 and centers incorporated the handheld gamma probe into the mapping procedure.
MSLT-I Trial Phase
Each participating center performed LM/SNB by the same technique used during the learning phase, except for the later use of radiocolloids and gamma probe. CLND was recommended if no SN was identified during LM/SNB, and it was routinely performed if the SN contained tumor. Patients in both treatment groups were monitored postoperatively with routine clinical examination, blood tests, and chest x-rays every 3 months for the first 2 years, every 4 months during the third year, every 6 months during years 4 and 5, and then yearly until year 10. Follow-up was calculated from the date of randomization to last follow-up examination or death.
Histopathologic Examination of the Sentinel Node
SN specimens were reviewed as permanent sections; multiple sections were carefully examined by both H&E and immunohistochemical staining for S-100, HMB-45, and later MART-1 or Melan-A, as previously described.2,11 Immunohistochemistry is essential because evaluation of the SN by H&E alone misses up to 12% of positive nodes.5 When CLND was performed, non-SNs were examined by conventional H&E staining alone. Slides from all primary melanoma specimens and slides from 20% of LM/SNB (SN) and 20% of CLND (non-SN) specimens were reviewed by the pathology group at the John Wayne Cancer Institute (JWCI).
Monitoring of MSLT-I Sites
Sites were monitored periodically by JWCI's clinical trials group; monitoring frequency was once or twice a year if a site was still accruing patients and every 2 years thereafter.
Statistics
Demographic and clinical factors were tabulated in side-by-side columns for the 2 treatment groups; mean, standard deviation, and median were used for continuous variables, and frequency and percentage were used for categorical variables. t test and chi-squared test were used to compare characteristics between the 2 treatment groups. Survival distribution was estimated using Kaplan-Meier's method and compared using log-rank test. Cox proportional hazard regression model was used to compare the 2 treatment groups. While other prognostic factors, including age, gender, primary site, Breslow thickness, and ulceration, were adjusted. All analyses were performed using SAS software package and all tests were 2-sided with a significance level of 0.05.
RESULTS
By March 31, 2002, 2001 patients had entered MSLT-I. There were 797 patients randomized to the WEO arm and 1204 to the WE plus LM/SNB arm. Twenty-six (1.3%) patients dropped out after randomization. JWCI's pathology group reviewed 1897 slides from primary melanomas and confirmed the diagnosis in all patients; however, there were some changes in primary tumor stage (described in a separate report). The data presented in this report are based on JWCI pathology diagnosis. Audit of the source documents identified 2 patients who had palpable regional nodes and therefore were not eligible for the study. The remaining 1973 patients were eligible for analysis after a median follow-up of 54 months (range, 3.0 months to 10 years).
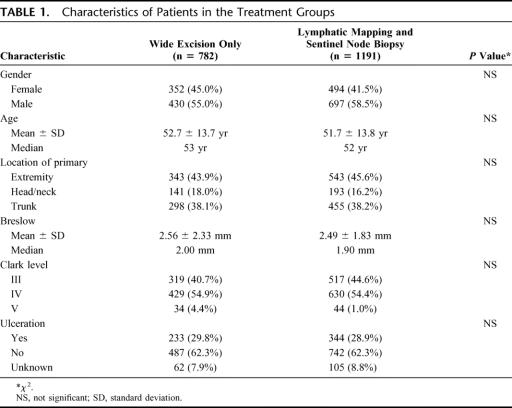
To assure an even distribution of prognostic factors, patients were randomized by the primary tumor's anatomic site (extremity vs nonextremity) and by the primary tumor's microstage as determined by Breslow thickness and by Clark level and/or ulceration for lesions thinner than 1.0 mm. The LM/SNB patients were remarkably similar to the WEO patients in regard to characteristics of the primary melanoma (Table 1).
TABLE 1. Characteristics of Patients in the Treatment Groups
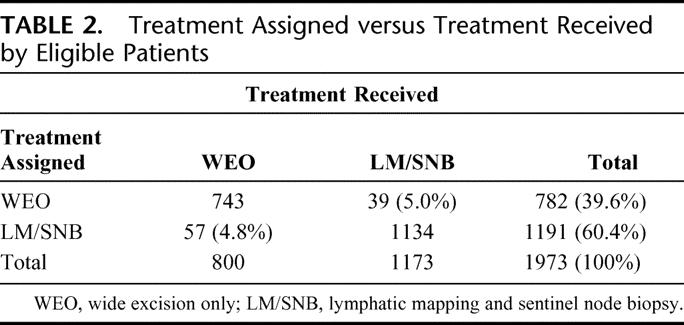
Thirty-nine (5.0%) patients assigned to WEO actually received WE plus LM/SNB; 57 (4.8%) patients assigned to WE plus LM/SNB actually received WEO. Thus, 800 patients received WEO and 1173 patients received WE plus LM/SNB (Table 2). Because the purpose of this report is to evaluate the surgical procedure in regard to morbidity and accuracy in staging the regional nodal basin, this analysis is based on treatment actually received.
TABLE 2. Treatment Assigned versus Treatment Received by Eligible Patients
Accuracy of Sentinel Node Identification as Reported by the Surgeon
The surgeon's perception of accuracy in SN identification was based on visualization of a blue-stained lymphatic channel leading to a blue-stained node or identification of the node that contained the highest level of radioactivity as measured by the gamma probe. Because some of the 1173 patients had primary melanomas that drained to more than one basin, LM/SNB was attempted in 1419 basins. The overall rate of SN identification was 95.3% (1352 of 1419). This high rate of success in part reflects the fact that most MSLT-I centers had some prior experience with LM/SNB.
Inguinal (99.3%, 417 of 420) or axillary (96.6%, 715 of 740) mapping was more successful than mapping of the cervical area (84.5%, 185 of 219) or ectopic drainage sites such as those in popliteal, epitrochlear, or parascapular areas (87.5%, 35 of 40). The overall lower incidence of successful SN identification in the neck may reflect the relatively small number of head and neck primaries (18%); perhaps even more important are the complex lymphatic anatomy and the generally small size of lymph nodes in this region. Numbers were too small to compare the success of mapping for other (ectopic) lymphatic basins. Nevertheless, the relatively low accuracy of SN identification in ectopic basins is related to the overall lack of experience in mapping these sites.
Dissected-Basin Recurrence in Patients With Tumor-Negative Sentinel Nodes
The most accurate method to determine the predictive accuracy of LM/SNB in identifying biologically significant occult nodal metastases is to assess the incidence of same-basin recurrence in patients who have tumor-negative SNs. Fifty-nine (6.3%) of the 944 patients with tumor-negative SNs developed regional nodal recurrence at a median follow- up of 54 months. Of the 59 patients, 48 (81%) had recurrence in the SN drainage basin and 11 had recurrence in a basin that was not sampled. Fifty-two of the 944 patients (5.5%) developed local/in-transit recurrence: 36 patients had local/in-transit recurrence without nodal recurrence, 8 had local/in-transit recurrence before nodal recurrence, and 8 had local/in-transit recurrence after nodal recurrence. Local or intransit recurrence that preceded nodal recurrence may have been the source of metastasis to the previously dissected lymph basin.
Relationship Between Case Load and Dissected-Basin Recurrence
Because we observed a shallow learning curve in our initial report of the technique,9 the MSLT-I was designed with a mandatory 30-case learning phase. Is 30 cases enough to optimally reduce the false-negative rate of LM/SNB? To answer this question, we determined rates of same-basin nodal recurrence at 10 centers that had entered a total of 918 patients in MSLT-I. At these 10 of the higher volume centers, the dissected-basin recurrence rate was 10.3% for the first 25 cases of the trial phase and 5.2% after 25 cases (P = 0.0136, log-rank test) (Fig. 2). This suggests that although a learning phase of 30 cases may yield a high rate of SN identification, 25 additional cases can further increase the surgeon's proficiency with the procedure.20,27
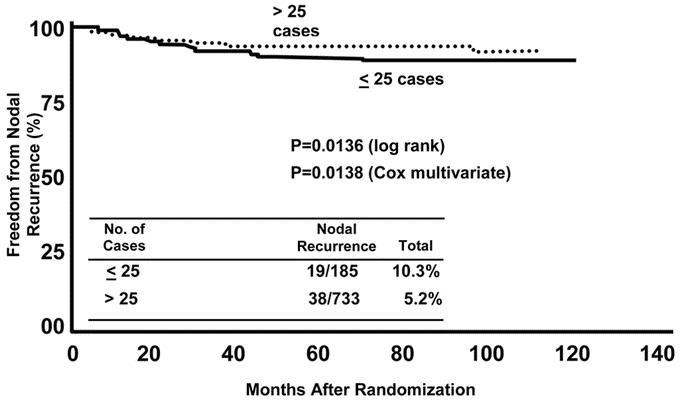
FIGURE 2. Relationship between nodal recurrence after a tumor-negative lymphatic mapping and sentinel node biopsy procedure and volume of cases (below or above 25) at 10 MSLT-I centers. Minimum duration of follow up was 36 months.
Surgical Morbidity of Wide Excision versus Wide Excision Plus Lymphatic Mapping and Sentinel Node Biopsy versus Wide Excision Plus Lymphatic Mapping and Sentinel Node Biopsy Plus Complete Lymphadenectomy
Of the 1973 eligible patients, 1969 were included in the morbidity analysis; the remaining 4 patients (2 in the LM/SNB arm and 2 in the WEO arm) were excluded as a result of absence of surgical toxicity forms.
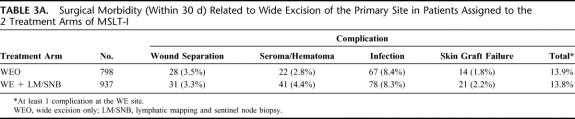
There were no operative mortalities. As shown in Table 3A, surgical complications associated with WE of the primary site were quite low and almost identical in the 2 groups. The incidence of at least one local wound complication was 13.9% (111 of 798) in the WEO arm and 13.8% (162 of 1171) in the LM/SNB arm (P = 0.9621, chi-squared test). Thus, LM/SL did not influence the incidence of surgical morbidity at the primary site. The incidence of allergic reactions to the blue dye administered at the time of LM/SNB was 0.17% (2 of 1173) and consisted of blue-colored urticaria (hives). No patient developed an anaphylactic reaction to blue dye.
TABLE 3A. Surgical Morbidity (Within 30 d) Related to Wide Excision of the Primary Site in Patients Assigned to the 2 Treatment Arms of MSLT-I
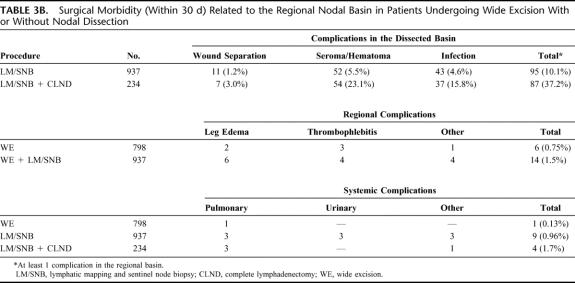
Regional and systemic complications were minimal with WE and increased only slightly with LM/SNB (Table 3B). As expected, surgical morbidity in the regional nodal basin dramatically increased when LM/SNB was followed by immediate CLND (37.2% for LM/SNB with immediate CLND vs 10.1% for LM/SNB without CLND; P < 0.0001, chi-squared test), confirming that LM/SNB is a much less morbid diagnostic procedure for staging the regional nodes than elective CLND.28
TABLE 3B. Surgical Morbidity (Within 30 d) Related to the Regional Nodal Basin in Patients Undergoing Wide Excision With or Without Nodal Dissection
Surgical Quality Control
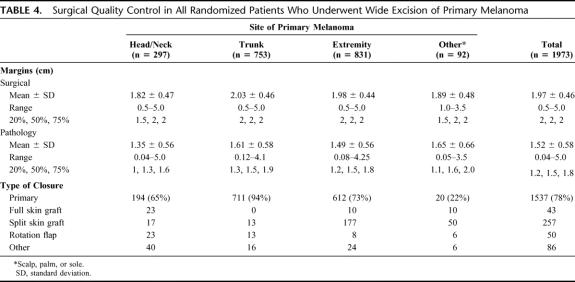
The MSLT-I protocol required WE with 2-cm margins. As shown in Table 4, the mean surgical margin of 1.97 cm was congruent with the mean pathology margin of 1.52 cm (assuming a 25% shrinkage factor). In most cases (1537 of 1973, or 77.9%), WE was followed by primary closure; less frequently, the wound was closed by skin grafts (300 of 1973, or 15.2%) or rotation flaps (50 of 1973, or 2.5%). In 29% of cases, the underlying muscle fascia was removed at the time of surgery.
TABLE 4. Surgical Quality Control in All Randomized Patients Who Underwent Wide Excision of Primary Melanoma
DISCUSSION
Interim data from MSLT-I clearly show that LM/SNB performed by an experienced nuclear medicine, surgical oncology, and pathology team is a safe, accurate, low-morbidity method of identifying patients with lymph node metastases from a primary cutaneous melanoma. The relatively infrequent occurrence of dissected-basin recurrence (6.3%) at a median follow-up of almost 6 years suggests a high rate of successful SN identification. We found an 18.1% (144 of 800) incidence of clinical nodal recurrence during nodal observation (similar to the incidence of tumor-positive SNs); this suggests that most if not all occult SN metastases will eventually become palpable nodal recurrences in the regional nodal basin and require delayed CLND or other treatment at that time.
SN identification is conceptually simple but technically challenging; its success requires a dedicated and experienced multidisciplinary team of surgeons, nuclear medicine physicians, and pathologists. In our 1999 report in this journal,11 we measured mastery of LM/SNB by the rate of SN identification. In the present report, we used long-term follow-up of MSLT data to monitor rates of dissected-basin nodal recurrence. Our findings indicate that a learning phase of 30 cases may not be adequate for mastery of LM/SNB; the nodal recurrence rate in basins with tumor-negative SNs was 10.3% for the first 25 cases of the trial phase, but dropped to 5.1% after 25 cases (Fig. 2). A recently completed review of more than 700 non-MSLT patients who underwent dual-agent (radiopharmaceutical plus blue dye) LM/SNB at JWCI during the last 10 years revealed a dissected-basin nodal recurrence rate of only 1.7% in those with tumor-negative SNs.29 This low recurrence rate likely relates to our extensive experience with LM/SNB.
The technique for histopathologic analysis of the SN is another key area of standardization that still engenders discussion and controversy. Because less than 2% of SN volume is actually sectioned and only 8 to 20 sections are examined for occult metastases, tumor foci can be missed—as has been pointed out by us30 and others.31,32 Coinjection of carbon dye to map the most likely intranodal site of tumor foci for the pathologist and molecular analysis33,34 of paraffin-embedded SN specimens may further upstage the nodal basins of patients whose SNs are tumor-free by H&E and immunohistochemical staining, and should therefore increase the accuracy of the technique. We have already demonstrated that molecular staging of histopathologically negative SNs is an independent prognostic factor in non-MSLT patients followed up for at least 8 years.34 The clinical significance of reverse transcriptase–polymerase chain reaction positivity in paraffin-embedded sections of microscopically tumor-negative SNs, and the need for routine CLND if the SN contains tumor will be examined in a second multicenter trial (MSLT-II), as shown in Figure 3.
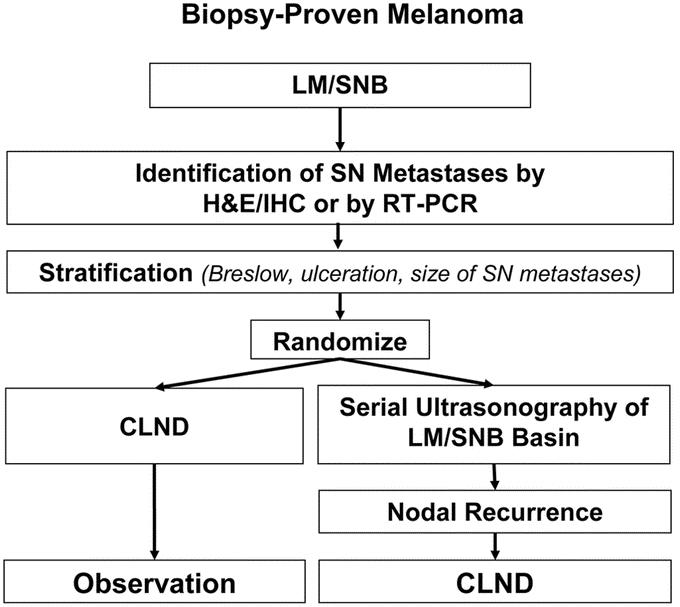
FIGURE 3. Design of MSLT-II.
Standardization is essential to maintain quality control in any multicenter trial, particularly when expertise is required from personnel in different disciplines. The nuclear medicine physician identifies the nodal basin(s) at risk, determines the number of lymphatic channels leading to separate SNs, and accurately marks the cutaneous location overlying each SN to direct the surgeon. Each surgeon should be familiar with the common lymphatic drainage patterns for different areas of the body, aberrant routes of the lymphatics, and aberrant locations of the SN.12,35 Imaging must be timed to avoid missing true SNs or incorrectly identifying non-SNs as SNs.26,35
Two criteria can be used to compare the accuracy of LM/SNB in MSLT-I: 1) the incidence of SN metastases, which appears similar when adjusting for risk factors for nodal metastases; and 2) the incidence of dissected-basin recurrence in patients whose SNs were initially reported as histopathologically negative. The latter criterion is important because it assesses the accuracy of the 3 different disciplines in correctly identifying and evaluating the SN, and it determines whether histology and immunohistochemical techniques can detect all biologically and clinically significant occult metastases. Some dissected-basin recurrences could be explained by SN micrometastases not detected by pathologists or by surgical misidentification of the true SN or failure to find additional SNs in the same basin.30 However, because the incidence of nodal basin recurrence increases with follow- up to 5 years,29 long-term data are essential to accurately judge the incidence of recurrence in reportedly tumor-negative nodal basins.
This report examining the accuracy of MSLT-I data confirms the crucial importance of a learning period for LM/SNB, as noted in our initial description of the technique.9,11,27 Experienced investigators have achieved high rates of SN identification and accurate pathologic evaluation only after ascending a learning curve to technical expertise. The results of long-term follow-up in MSLT-I indicate that the 30-case learning curve is shallow and that experience with an additional 25 cases is usually required to identify the SN with at least 95% accuracy. The MSLT results reported here suggest that routine application of LM/SNB may not be appropriate among surgical teams who treat only a few patients with melanoma each year; these patients may be best served by referral to high-volume melanoma centers.
Early complications from MSLT-I were uncommon and were not increased by the addition of LM/SNB to treatment of the primary site. Wound separation, hematoma, and infection were more common after graft repair than after primary closure; however, limiting WE margins to 2 cm reduced the incidence of graft repair to only 15%. Factors such as age above 50 years, male gender, and tobacco use have been shown to increase the incidence of local wound complications.36
Complications of CLND range from those confined to the wound such as seroma, hematoma, or infection to more chronic abnormalities of dysesthesia or lymphedema.37 As expected, the incidence of wound problems at the CLND site increased after LM/SNB, in part because of surgical trauma and tissue injury. In this study, we did not evaluate the complications of delayed CLND in the WEO arm, but we would expect a possibly higher incidence of chronic lymphedema or dysesthesia because nodal tumor burden is higher.37,38
CONCLUSION
The final results on the overall therapeutic use of LM/SNB in MSLT-I will not be available until the fifth and final analysis, which will depend on longer follow-up for more events to determine melanoma-specific survival in the 2 treatment arms. However, the use of LM/SNB in staging the regional nodal basin has been clearly demonstrated by the third interim analysis of MSLT-I data (median follow up 54 months). MSLT-I data show, for the first time in a randomized, multicenter trial, that LM/SNB can accurately identify occult nodal metastases that will lead to more advanced, palpable nodal disease if left in situ. Because almost all patients with occult SN metastases will experience disease progression and most require therapeutic CLND when nodal tumor becomes palpable, little purpose is served by not performing LM/SNB for staging and prognosis and to identify candidates for immediate therapeutic CLND.
We believe that LM/SNB should continue to be an important part of the AJCC staging system and is the standard of care for patients with early-stage melanoma. Because only 10% to 20% of patients with tumor-positive SNs have tumor-positive non-SNs identified during CLND, and because the risk of additional (non-SN) tumor-positive nodes may depend on SN tumor burden,39 it is possible that eventually the morbidity of CLND will be avoided for most patients with early, clinically occult nodal involvement. We and others have identified certain factors predictive of tumor-positive non-SNs in patients with tumor-positive SNs.39–41 However, none of these factors are 100% accurate in predicting which patients will have additional positive non-SNs. Therefore, MSLT-II will examine the indications for CLND when the SN contains tumor (Fig. 3), and it will determine whether immediate CLND provides a therapeutic advantage over care based on postoperative ultrasonographic monitoring of the nodal basin.
ACKNOWLEDGMENTS
The authors thank the members of the Data Safety Monitoring Committee: John Kirkwood, MD (Chairman); Martin Mihm, MD; John Daly, MD; Gary Smith, MD; Michael Kutner, PhD (Statistician); Marshall Urist, MD; and Norman Begin, Esq. (Patient Advocate). The authors are also grateful for the support and assistance of the MSLT coordinating group at the John Wayne Cancer Institute: Lisa van Kreuningen, Stacey Stern, Yasmine Franklin, Angelica Gurrola, Laura Janis, Amber Nichols, and Paul Linke.
Discussions
Dr. Marshall M. Urist (Birmingham, Alabama): Today Donald Morton has presented the initial results of a landmark international trial which tests 1 of the basic tenants of cancer therapy by asking the question: Does the early diagnosis and resection of regional metastatic disease result in a significant increase in the cure rate for patients with clinically localized melanoma? Randomized trials of prophylactic lymph node basin dissections for melanoma have not demonstrated a survival benefit. Similar trials in breast cancer have reached the same conclusions. However, none of these trials have been powered to detect differences of 10% or less. While we would like to have the final answer today, the follow-up is not long enough in this trial to reach conclusions about survival.
The current interim analysis reports to us the morbidity, accuracy and prognostic value of intraoperative lymphatic mapping and sentinel lymphadenectomy. The authors conclude that sentinel lymph node biopsy is a safe and accurate technique associated with low morbidity. As the number of surgical procedures increased, the surgeon's skills also improved. When the diagnosis of lymphatic metastasis was delayed until lymph nodes became palpable, there was no increase in the proportion of patients with metastases, however the number of positive nodes per patient was significantly increased.
Like any good trial, this study opens far more questions than it answers. Some of these questions might be:
False negative rates, measured as the number of infield recurrences, were low after a surgeon had performed a large number of cases. Does this mean that sentinel lymph node biopsy should be limited to high volume centers or high volume surgeons, or both?
Second, who should have a sentinel lymph node biopsy? The thickness threshold in this study was 1 millimeter, however the node positive rate remains low even for patients who have tumors as thick as 1.5 millimeters. Why is 1 millimeter the threshold?
Third, does the anatomic site make an important difference for the accuracy of this procedure? Other authors have reported that patients with head and neck primary melanomas had lymphatic drainage that was more often multidirectional. What do you do when a patient has a sentinel node in the parotid gland?
In breast cancer, there is an increasing trend both here in the United States and in Europe for performing ultrasound as part of lymphatic staging. In this case, some patients can be determined to have a positive regional lymph node by fine needle aspiration and thereby decrease the number of patients who have to undergo sentinel lymph node biopsy. Should this be incorporated into the treatment of patients with melanoma?
Finally, and perhaps most importantly, lymphatic mapping and sentinel lymphadenectomy has been criticized for 2 reasons. First, because it has not been proven to be therapeutic itself. Secondly, because there is no effective systemic adjuvant therapy to apply if a patient has a positive regional node. The question is: Should this technique be utilized while we are waiting for the results about survival from this trial?
Dr. Donald L. Morton (Santa Monica, California): Thank you, Dr. Urist, for your comments and for your participation in the Data Safety Monitoring Board for this trial.
Should sentinel node biopsy (SNB) be limited to high-volume centers? I do not believe that surgeons who treat only a few melanoma patients each year will have the experience required for a high degree of mapping accuracy. If SNB is undertaken in community hospitals, it should be limited to a few surgeons who have extensive experience with the technique.
Because its morbidity is so low, SNB is practical for any primary melanoma that has a depth of at least 1 mm. We have found that the incidence of sentinel node metastasis is about 4% for lesions that are 0.75–1.0 mm and about 2% for thinner lesions. However, certain factors increase the risk of sentinel node metastasis from a thin primary melanoma: age less than 40 years, shave biopsy with positive deep margins, significant regression or ulceration, and Clark level >IV. Any of these factors indicates consideration of SNB, since the procedure's morbidity is so low.
SNB is technically more challenging when the primary melanoma is in the head and neck area. MSLT-I interim data show a success rate of 85% for head and neck melanomas vs. 98% for melanomas in other sites. However, the data do not indicate that melanomas on the head and neck have an appreciably higher rate of recurrence when the sentinel node is tumor-negative. Longer follow-up is necessary for a definitive conclusion.
The role of ultrasonography in patients undergoing SNB will be evaluated in the second MSLT trial (MSLT-II), which randomizes in patients with tumor-positive sentinel nodes to postoperative nodal observation with ultrasonography or to immediate complete lymphadenectomy. Because only 10–12% of patients with tumor-positive sentinel nodes will also have positive nonsentinel nodes, it may be reasonable to undertake completion lymphadenectomy only if ultrasonography monitoring reveals subclinical nodal recurrence.
Finally, I believe that SNB is already justified as standard care, not only for its prognostic value but also because it provides information that can help a patient decide about adjuvant therapy and clinical trials. When we have completed the follow-up of this trial, I expect that the data will show a therapeutic benefit for SNB and immediate complete lymphadenectomy in patients with intermediate-thickness melanomas.
Dr. Gerard V. Aranha (Maywood, Illinois): Dr. Morton and colleagues, I enjoyed your paper. I wanted to ask you, though: How did you decide upon the positivity of the sentinel node? Did you use a frozen section or immunohistochemistry? If you used frozen section, did you have a large number of false negatives?
Dr. Donald L. Morton (Santa Monica, California): Sentinel node positivity was determined by staining permanent sections with hematoxylin and eosin and with antibodies to S-100, HMB-45 and later MART-1 or Melan-A. We do not use frozen sections because we have found that rapid immunohistochemistry has a 5–10% false-negative rate compared to permanent sections. In addition, immediate immunohistochemical staining of frozen sections is labor- and cost-intensive.
Dr. Daniel G. Coit (New York, New York): Thank you very much not only for introducing this technique to the surgical community but for sharing your slides with me ahead of time just to let me think about this. I have 2 questions for you.
First of all, 1 of the issues we have been struggling with recently is, what really is the threshold for a clinically relevant positive node? It has a little bit to do with Dr. Aranha's question. Did you find any difference or any threshold of melanoma in a lymph node that was not prognostically significant?
The other question I think is a matter of definition and is one of ongoing discussion. That is, what in actuality is the false negative rate of this procedure? If 20% of patients have a positive sentinel node and an additional 5% were going to fail in the nodal basin, have we not missed 20% of the positive nodes?
Dr. Donald L. Morton (Santa Monica, California): When MSLT-I was organized, we graded the sentinel node as either tumor-positive or tumor-negative. Subsequently, we have found that the amount of tumor in this node may have considerable prognostic importance. We therefore plan to review tumor-positive sentinel nodes from MSLT-I to look for a correlation between intranodal tumor burden and long-term clinical outcome.
Although the rate of false-negative results probably will never drop to zero, interim MSLT-I data show that it is only about 5% in centers with more than 25 cases. Below 25 cases, the rate is about 10%. There is no question that the false-negative rate is related to experience; at the John Wayne Cancer Institute, a recent review of almost 800 non-MSLT patients who underwent SNB showed a false-negative rate of 1.7%. Since the purpose of SNB is to determine the tumor status of the sentinel node, we believe its accuracy should be based on all patients who undergo the procedure, not just those who have tumor-positive sentinel nodes.
Dr. Marc K. Wallack (New York, New York): Dr. Morton, first of all you are to be commended on another landmark paper on work that you have done and sentinel node technology has affected how we approach both breast cancer and melanoma.
My question to you is: In my practice, I have now 3 patients that had melanomas, 0.75 millimeters depth of invasion, that I did not do sentinel node biopsies on. What I want you to consider – because all 3 now are dying of metastatic melanoma, and probably had I done a sentinel node on those patients, they may not be dying. Therefore I propose this to you: Don't we need to extend our criteria for sentinal nodes to 0.75 or T 1 lesions? And is there a way that when you work out your next trial, that essentially you include into one of the groups a 0.75 mm melanoma, as you mentioned previously, and include that in a subset with others such as the 1-millimeter depth of invasion melanoma for sentinel node biopsy?
Dr. Donald L. Morton (Santa Monica, California): Dr. Wallack, that is a very good point. When we examined our experience at the John Wayne Cancer Institute, we found that certain factors are associated with a higher incidence of positive nodes in patients with thinner melanomas. One of the most important factors is shave biopsy. A shave biopsy that leaves the base of the lesion will cause the pathologist to underestimate lesion depth. We therefore recommend SNB for a positive deep margin on shave biopsy.
Age also is an important factor; for reasons that are not yet clear, the incidence of tumor-positive sentinel nodes appears to be higher in younger patients with thinner lesions.
In either case, the morbidity of SNB in experienced hands is so low that the procedure can be justified in patients with thinner lesions.
Dr. Hiram C. Polk, Jr. (Louisville, Kentucky): If I may ask 2 points, 1 to Marc Wallack's comment about the occasional metastasis from the very thin melanoma. We have 2 out of 1000. We treated 1000 patients less than 0.75, and there have been 2 patients with metastasis. So that is a very uncommon event, at least in our experience. Would you comment in closing on the cost of the lymphoscintigraphy, the OR time, and the extra added things this puts into the equation?
Dr. Donald L. Morton (Santa Monica, California): A good question that is unfortunately impossible to answer because, as you know, there is a fairly wide gap between health care costs and insurance/Medicare reimbursements. We estimate that preoperative lymphoscintigraphy, intraoperative lymphatic mapping, SNB, and histopathologic assessment of the SNB specimen add about $2000 to the cost of treating patients with clinically localized primary melanoma.
DR. Ashok R. Shaha (New York, New York): Dr. Morton, I am still finding it very difficult to make a decision regarding the sentinel node in the parotid region, and I just wanted to get your feeling about doing the sentinel node biopsy and then coming back later on and doing a completion parotidectomy, which is obviously much more difficult. Or should we go ahead at the initial procedure, get a frozen section, and then proceed with sentinel lymph node parotidectomy and upper neck dissection, which really becomes a much bigger operation. I wanted to get your feeling about this issue.
Dr. Donald L. Morton (Santa Monica, California): We believe that biopsy of sentinel nodes in the parotid is minimally invasive, and we have experienced no difficulty in performing subsequent complete parotidectomy when a sentinel node contains tumor. Certainly lymphatic mapping of the parotid area can be tricky, and the surgeon must conduct a meticulous search for all nodes that have been identified during preoperative lymphoscintigraphy. Since we have not found frozen section analysis to be completely accurate, we believe that the tumor status of the sentinel node is best determined on permanent sections.
Footnotes
Supported by grant CA 29605 from the National Cancer Institute.
Presented at the 125th Annual Meeting of the American Surgical Association, April 14–16, 2005, Palm Beach, Florida.
Reprints: Donald L. Morton, MD, John Wayne Cancer Institute, 2200 Santa Monica Blvd., Santa Monica, CA 90404. E-mail: mortond@jwci.org.
REFERENCES
- 1.Morton DL, Wanek L, Nizze JA, et al. Improved long-term survival after lymphadenectomy of melanoma metastatic to regional nodes. Analysis of prognostic factors in 1134 patients from the John Wayne Cancer Clinic. Ann Surg. 1991;214:491–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cochran AJ, Wen DR, Morton DL. Occult tumor cells in the lymph nodes of patients with pathological stage I malignant melanoma. An immunohistological study. Am J Surg Pathol. 1988;12:612–618. [DOI] [PubMed] [Google Scholar]
- 3.Robinson DS, Sample WF, Fee HJ, et al. Regional lymphatic drainage in primary malignant melanoma of the trunk determined by colloidal gold scanning. Surg Forum. 1977;28:147–148. [PubMed] [Google Scholar]
- 4.Gaynor R, Irie R, Morton DL, et al. S100 protein is present in cultured human malignant melanomas. Nature. 1980;286:400–401. [DOI] [PubMed] [Google Scholar]
- 5.Cochran AJ, Wen DR, Herschman HR. Occult melanoma in lymph nodes detected by antiserum to S-100 protein. Int J Cancer. 1984;34:159–163. [DOI] [PubMed] [Google Scholar]
- 6.Cochran AJ, Pihl E, Wen DR, et al. Zoned immune suppression of lymph nodes draining malignant melanoma: histologic and immunohistologic studies. J Natl Cancer Inst. 1987;78:399–405. [PubMed] [Google Scholar]
- 7.Wong JH, Cagle LA, Morton DL. Lymphatic drainage of skin to a sentinel lymph node in a feline model. Ann Surg. 1991;214:637–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morton D, Cagle L, Wong J, et al. Intraoperative lymphatic mapping and selective lymphadenectomy: technical details of a new procedure for clinical stage I melanoma. Presented at the Annual Meeting of the Society of Surgical Oncology; Washington, DC; 1990.
- 9.Morton DL, Wen DR, Wong JH, et al. Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch Surg. 1992;127:392–399. [DOI] [PubMed] [Google Scholar]
- 10.Essner R, Conforti A, Kelley MC, et al. Efficacy of lymphatic mapping, sentinel lymphadenectomy, and selective complete lymph node dissection as a therapeutic procedure for early-stage melanoma. Ann Surg Oncol. 1999;6:442–449. [DOI] [PubMed] [Google Scholar]
- 11.Morton DL, Thompson JF, Essner R, et al. Validation of the accuracy of intraoperative lymphatic mapping and sentinel lymphadenectomy for early-stage melanoma. Ann Surg. 1999;230:453–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bostick P, Essner R, Sarantou T, et al. Intraoperative lymphatic mapping for early-stage melanoma of the head and neck. Am J Surg. 1997;174:536–539. [DOI] [PubMed] [Google Scholar]
- 13.Thompson JF, McCarthy WH, Bosch CM, et al. Sentinel lymph node status as an indicator of the presence of metastatic melanoma in regional lymph nodes. Melanoma Res. 1995;5:255–260. [DOI] [PubMed] [Google Scholar]
- 14.Reintgen D, Cruse CW, Wells K, et al. The orderly progression of melanoma nodal metastases. Ann Surg. 1994;220:759–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Essner R, Bostick PJ, Glass EC, et al. Standardized probe-directed sentinel node dissection in melanoma. Surgery. 2000;127:26–31. [DOI] [PubMed] [Google Scholar]
- 16.Bostick P, Essner R, Glass E, et al. Comparison of blue dye and probe-assisted intraoperative lymphatic mapping in melanoma to identify sentinel nodes in 100 lymphatic basins. Arch Surg. 1999;134:43–49. [DOI] [PubMed] [Google Scholar]
- 17.Albertini JJ, Cruse CW, Rapaport D, et al. Intraoperative radio-lympho-scintigraphy improves sentinel lymph node identification for patients with melanoma. Ann Surg. 1996;223:217–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krag DN, Meijer SJ, Weaver DL, et al. Minimal-access surgery for staging of malignant melanoma. Arch Surg. 1995;130:654–658. [DOI] [PubMed] [Google Scholar]
- 19.Thompson JF, Niewind P, Uren RF, et al. Single-dose isotope injection for both preoperative lymphoscintigraphy and intraoperative sentinel lymph node identification in melanoma patients. Melanoma Res. 1997;7:500–506. [DOI] [PubMed] [Google Scholar]
- 20.Morton DL, Giuliano AE, Reintgen DS, et al. Symposium: lymphatic mapping and sentinel node biopsy in patients with breast cancer and melanoma. Contemporary Surg. 1998;53:281–298 (part 1); 353–361 (part 2).
- 21.Albertini JJ, Lyman GH, Cox C, et al. Lymphatic mapping and sentinel node biopsy in the patient with breast cancer. JAMA. 1996;276:1818–1822. [PubMed] [Google Scholar]
- 22.Giuliano AE, Kirgan DM, Guenther JM, et al. Lymphatic mapping and sentinel lymphadenectomy for breast cancer. Ann Surg. 1994;220:391–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martinez SR, Bilchik AJ. Lymphatic mapping and sentinel node analysis in colon cancer. Clin Colorectal Cancer. 2005;4:320–324. [DOI] [PubMed] [Google Scholar]
- 24.Faries MB, Bleicher RJ, Ye X, et al. Lymphatic mapping and sentinel lymphadenectomy for primary and metastatic pulmonary malignant neoplasms. Arch Surg. 2004;139:870–876. [DOI] [PubMed] [Google Scholar]
- 25.Bilchik AJ, Giuliano A, Essner R, et al. Universal application of intraoperative lymphatic mapping and sentinel lymphadenectomy in solid neoplasms. Cancer J Sci Am. 1998;4:351–358. [PubMed] [Google Scholar]
- 26.Glass EC, Essner R, Morton DL. Kinetics of three lymphoscintigraphic agents in patients with cutaneous melanoma. J Nucl Med. 1998;39:1185–1190. [PubMed] [Google Scholar]
- 27.Morton DL. Intraoperative lymphatic mapping and sentinel lymphadenectomy: community standard care or clinical investigation? Cancer J Sci Am. 1997;3:328–330. [PubMed] [Google Scholar]
- 28.Wrightson WR, Wong SL, Edwards MJ, et al. Sunbelt Melanoma Trial Study Group. Complications associated with sentinel lymph node biopsy for melanoma. Ann Surg Oncol. 2003;10:676–680. [DOI] [PubMed] [Google Scholar]
- 29.Zogakis TG, Essner R, Wang HJ, et al. Melanoma recurrence patterns after negative selective lymphadenectomy. Arch Surg. In press. [DOI] [PubMed]
- 30.Morton DL, Hoon DS, Cochran AJ, et al. Lymphatic mapping and sentinel lymphadenectomy for early-stage melanoma: therapeutic utility and implications of nodal microanatomy and molecular staging for improving the accuracy of detection of nodal micrometastases. Ann Surg. 2003;238:538–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spanknebel K, Coit DG, Bieligk SC, et al. Characterization of micrometastatic disease in melanoma sentinel lymph nodes by enhanced pathology: recommendations for standardizing pathologic analysis. Am J Surg Pathol. 2005;29:305–317. [DOI] [PubMed] [Google Scholar]
- 32.Cook MG, Green MA, Anderson B, et al. EORTC Melanoma Group. The development of optimal pathological assessment of sentinel lymph nodes for melanoma. J Pathol. 2003;200:314–319. [DOI] [PubMed] [Google Scholar]
- 33.Shivers SC, Wang X, Li W, et al. Molecular staging of malignant melanoma: correlation with clinical outcome. JAMA. 1998;280:1410–1415. [DOI] [PubMed] [Google Scholar]
- 34.Takeuchi H, Morton DL, Kuo C, et al. Prognostic significance of molecular upstaging of paraffin-embedded sentinel lymph nodes in melanoma patients. J Clin Oncol. 2004;22:2671–2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uren RF, Howman-Giles R, Thompson JF. Patterns of lymphatic drainage from the skin in patients with melanoma. J Nucl Med. 2003;44:570–582. [PubMed] [Google Scholar]
- 36.Beitsch P, Balch C. Operative morbidity and risk factor assessment in melanoma patients undergoing inguinal lymph node dissection. Am J Surg. 1992;164:462–466. [DOI] [PubMed] [Google Scholar]
- 37.Baas PC, Koops HS, Hoekstra HJ, et al. Groin dissection in the treatment of lower-extremity melanoma. Short-term and long-term morbidity. Arch Surg. 1992;127:281–286. [DOI] [PubMed] [Google Scholar]
- 38.Shaw JHF, Rumball EM. Complications and local recurrence following lymphadenectomy. Br J Surg. 1990;77:760–764. [DOI] [PubMed] [Google Scholar]
- 39.Lee JH, Essner R, Torisu-Itakura H, et al. Factors predictive of tumor-positive nonsentinel lymph nodes after tumor-positive sentinel lymph node dissection for melanoma. J Clin Oncol. 2004;22:3677–3684. [DOI] [PubMed] [Google Scholar]
- 40.Cochran AJ, Wen DR, Huang RR, et al. Prediction of metastatic melanoma in nonsentinel nodes and clinical outcome based on the primary melanoma and the sentinel node. Mod Pathol. 2004;17:747–755. [DOI] [PubMed] [Google Scholar]
- 41.Cochran AJ, Roberts A, Wen DR, et al. Update on lymphatic mapping and sentinel node biopsy in the management of patients with melanocytic tumours. Pathology. 2004;36:478–484. [DOI] [PubMed] [Google Scholar]