Abstract
Objective:
The objective of this study was to characterize the patient population with respect to patient selection, assess surgical morbidity and graft failures, and analyze the contribution of perioperative clinical factors to recipient outcome in adult living donor liver transplantation (ALDLT).
Summary Background Data:
Previous reports have been center-specific or from large databases lacking detailed variables. The Adult-to-Adult Living Donor Liver Transplantation Cohort Study (A2ALL) represents the first detailed North American multicenter report of recipient risk and outcome aiming to characterize variables predictive of graft failure.
Methods:
Three hundred eighty-five ALDLT recipients transplanted at 9 centers were studied with analysis of over 35 donor, recipient, intraoperative, and postoperative variables. Cox regression models were used to examine the relationship of variables to the risk of graft failure.
Results:
Ninety-day and 1-year graft survival were 87% and 81%, respectively. Fifty-one (13.2%) grafts failed in the first 90 days. The most common causes of graft failure were vascular thrombosis, primary nonfunction, and sepsis. Biliary complications were common (30% early, 11% late). Older recipient age and length of cold ischemia were significant predictors of graft failure. Center experience greater than 20 ALDLT was associated with a significantly lower risk of graft failure. Recipient Model for End-stage Liver Disease score and graft size were not significant predictors.
Conclusions:
This multicenter A2ALL experience provides evidence that ALDLT is a viable option for liver replacement. Older recipient age and prolonged cold ischemia time increase the risk of graft failure. Outcomes improve with increasing center experience.
This US multicenter study demonstrates that adult living donor liver transplantation is a viable option for liver replacement, with outcomes that improve with center experience. Characterization of recipient, donor, and graft clinical variables also revealed that increasing recipient age and prolonged cold ischemia time increase the risk of graft failure.
Liver transplantation has become the accepted standard of care for many patients with end-stage liver disease. In the United States, over 5000 patients receive a liver transplant each year.1 With limitations in the supply of deceased donor livers, the waitlist grew rapidly through the 1990s, and although the size of the waiting list has stabilized around 17,000 for the past couple of years, the availability of donors still limits the number of patients who can receive this potentially lifesaving intervention. Efforts to expand the donor pool have resulted in increased use of expanded-criteria donors2–4 and recent application of living donation to the adult population.5–9 First reported in the United States in 1998,10 the use of adult-to-adult living donor transplants (ALDLT) has now been reported in over 1600 patients and has been performed in 94 centers across the United States.
The transplant procedure remains a technically complex operation, and the application of living donation to the adult recipient population has added another layer of difficulty to an already challenging procedure. Unlike living donor transplantation in the pediatric population or in kidney transplantation,11,12 there has yet to emerge a clear advantage of ALDLT over transplantation of the liver from deceased donors, beyond the obvious expansion of the donor pool. The extension of living donation to adult recipients requires a major hepatic resection in a healthy person to obtain an adequate graft, engendering controversy regarding both donor risk and increased complexity of the recipient operation.13–16 Most analyses to date have been confined to single-center reports focusing on surgical technique, complications, and selection. Brown et al17 collected the US experience, but this analysis depended on voluntary reporting of programmatic and outcome data. Recent reports utilizing national data from the United Network for Organ Sharing and from the Scientific Registry of Transplant Recipients (SRTR) demonstrate worse outcome of right lobe grafts compared with whole grafts from deceased donors,18,19 but detailed data regarding many donor and recipient variables that may contribute to this outcome are not available from such databases.
Limited cases are performed at any one center and approaches to the recipient and donor are too diverse across centers to provide reliable information on outcomes that can be generalized. Therefore, the National Institutes of Health, with supplemental funding from the American Society of Transplant Surgeons and the Health Resources and Services Administration, US Department of Health and Human Services, organized a consortium of 9 leading liver transplantation centers and a data coordinating center to accrue and follow sufficient numbers of patients being considered for and undergoing right lobe ALDLT to provide results from adequately powered studies. This group, the Adult-to-Adult Living Donor Liver Transplantation Cohort Study (A2ALL),20 is conducting both retrospective and prospective studies, with a primary goal of providing information on donor and recipient outcomes of ALDLT over a decade from 1998 to 2008. Although the main outcomes of the A2ALL prospective study will not be available for at least 5 years, insights can be gained from the retrospective cohort study of over 700 donors and recipients treated between 1998 and 2003.
This report analyzes detailed recipient outcomes of a large US multicenter experience of 385 right lobe ALDLT grafts. Specific aims included the characterization of the study population with respect to patient selection, the assessment of surgical morbidity and graft failures, and an analysis of the contribution of perioperative clinical factors to surgical outcomes. While this report focuses on first-year outcomes and complications in the recipient, analyses of outcomes and complications among ALDLT donors will be the focus of future reports from the A2ALL Study Group.
METHODS
A2ALL Retrospective Cohort Study Protocol
Data for this study came from the A2ALL Retrospective Cohort Study and were supplemented by data from the 9 A2ALL transplant centers made available through a data use agreement with the SRTR.
The primary objective of the A2ALL Retrospective Cohort Study is to determine the survival benefit of ALDLT. Starting at the time a potential ALDLT recipient identified a potential donor (defined as the date of initial potential donor history and physical examination), survival of those who eventually received an ALDLT will be compared with those who continued on the waiting list for a deceased donor liver transplant. Comparisons of ALDLT recipients to recipients of deceased donor liver transplants will be reported in future A2ALL publications. For the purposes of the current study, data collected for the overall study objective were used to focus on details of the early outcomes of recipients of right lobe grafts from living donors.
Data Collection and Conventions
The A2ALL Retrospective Cohort Study data include 821 patients who had a potential living donor evaluated from January 1, 1998, until February 28, 2003. Of these 821 potential recipients, 385 received an ALDLT at one of the 9 centers between April 1998 and August 2003. Domino liver transplant recipients (n = 2), patients whose transplant procedure was aborted (n = 3), and recipients with no donor match in the study (n = 2) were excluded from the analysis. Extensive chart reviews were conducted and a secure web-based electronic data entry system was used.
The Model for End-stage Liver Disease score (MELD) was calculated as previously described21,22 and was capped at 40. Standard liver volume (SLV) was calculated as 1072.8 × body surface area − 345.7.23 Right lobe weight (postresection) in the operating room was used for graft volume, where available. If this was not available, right lobe weight estimated by preoperative volume imaging was used. If neither was available, 0.6 × SLV was used, based on excellent correlation between graft size and the formula estimate among cases where both had been measured. Cold ischemia time was defined as time from donor cross-clamp to graft removal from ice. The number of ALDLTs previously performed at a center was defined as the case number for each ALDLT transplant.
Human Subjects Protection
The study was approved by the institutional review boards and privacy boards of the University of Michigan Data Coordinating Center and each of the 9 participating transplant centers.
Statistical Analysis
The cohort was characterized using descriptive statistics. Potential predictors of time to graft failure (retransplant or death) were tested using Cox regression. Over 35 donor, recipient, transplant center, anatomic, and intraoperative variables were evaluated. All analyses were performed using SAS version 9.1.
RESULTS
Characteristics of the A2ALL Transplant Centers
The 9 A2ALL transplant centers are diverse in their size, ratio of living donors to deceased donors, and geography. Four A2ALL centers began performing ALDLT in 1998, and 4 more began in 1999. The number of ALDLT performed per year by A2ALL centers and included in the retrospective study is depicted in Figure 1.
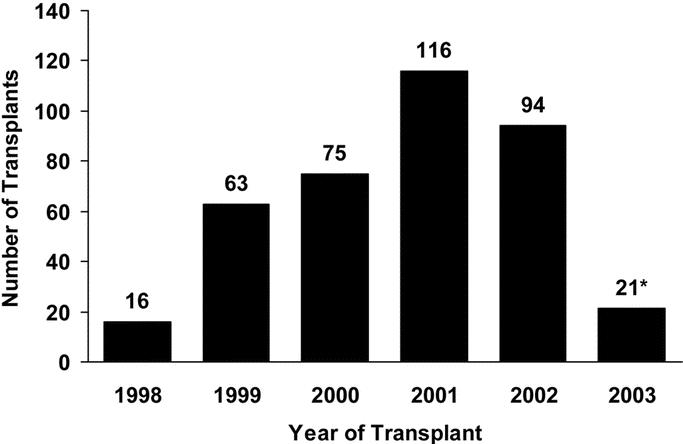
FIGURE 1. Annual ALDLT activity at A2ALL transplant centers. *Denotes partial year.
Characteristics of the Recipients
Pretransplant characteristics of the 385 ALDLT recipients, including demographics, diagnosis, and disease severity as measured by calculated MELD score at time of transplantation, are listed in Table 1. The mean age of the recipients was 49 ± 11 years, and the majority were male, non-Hispanic, and white. The most common diagnosis was hepatitis C cirrhosis (46%), followed by cholestatic liver disease (18%) and alcoholic cirrhosis (13%). Hepatocellular carcinoma (HCC) was recorded as a primary or secondary diagnosis in 16% of recipients. Only 4% of recipients were in fulminant failure due to acute hepatic necrosis.
TABLE 1. Characteristics of 385 Recipients of Living Donor Liver Transplants
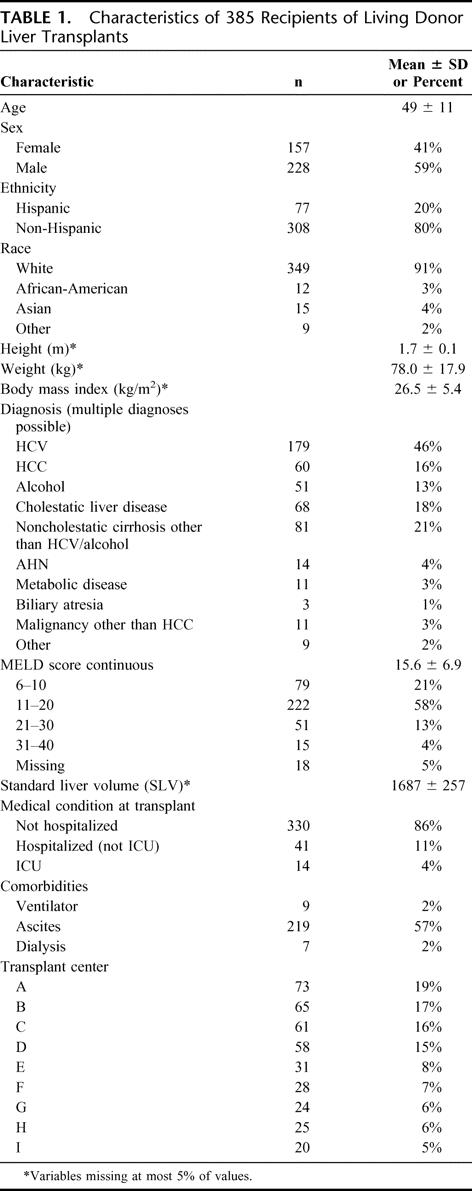
In general, the medical status of the ALDLT recipient group was relatively well compensated. The mean MELD score at the time of transplant was 15.6 ± 6.9 (range, 6–40), with only 4% having a MELD greater than 30. Only 41 were hospitalized (11% of all recipients) or in the intensive care unit (ICU) (4%). Comorbid conditions included the presence of ascites (57%), requirement for pretransplant dialysis (2%), and pretransplant ventilator dependence (2%).
Characteristics of Donors
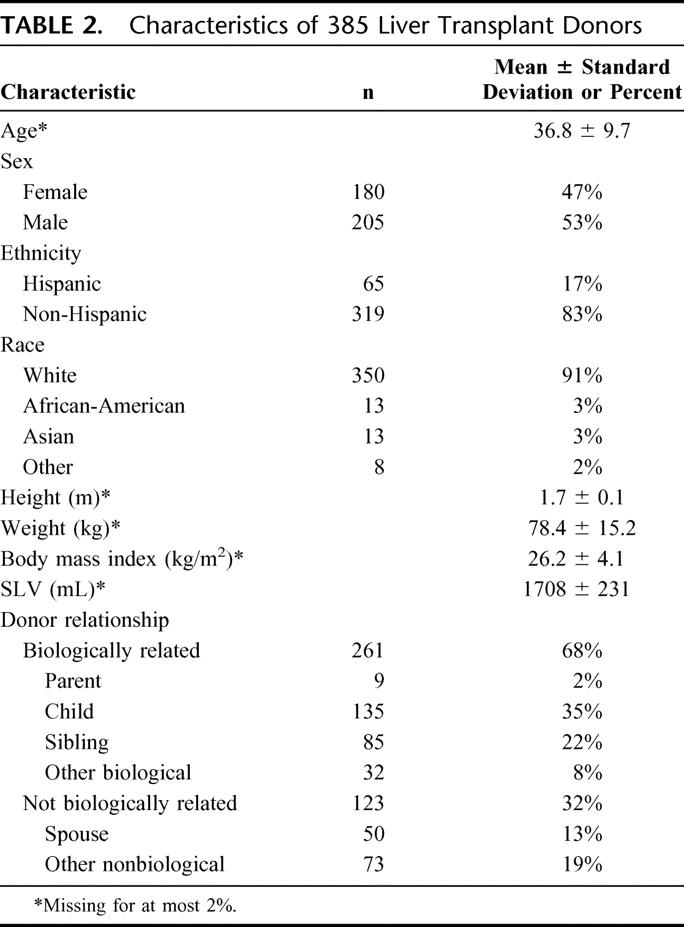
Living donors were generally younger than recipients with a mean of 37 ± 10 years (range, 18–57) (Table 2). The majority of donors were male, non-Hispanic and white. Calculated mean SLV was 1708 ± 231 mL, similar to the recipient SLV (1687 ± 257 mL). Most donors were biologically related to their recipient (usually adult children or siblings). However, 32% were biologically unrelated, and spouses comprised 13% of all donors.
TABLE 2. Characteristics of 385 Liver Transplant Donors
Characteristics of Donor Grafts and Perioperative Variables
The size and anatomic variants of the graft and intraoperative details that may contribute to early outcomes following ALDLT are listed in Table 3. The mean graft weight (or volume) was 966 g (or mL), with a range of 470 to 1729. Mean graft weight to recipient weight ratio (GWRWR) was 1.29. The majority of grafts (94%) were greater than the previously published recommended minimum GWRWR of 0.8%, and 92% exceeded the recommended minimum graft volume: recipient SLV ratio of 0.4.24 Multiple bile ducts were noted in 43%. Thirty-six percent had multiple hepatic veins, 11% had multiple portal veins to the right lobe, and 5% had multiple hepatic arteries.
TABLE 3. Perioperative Variables for 385 Living Donor Liver Transplants
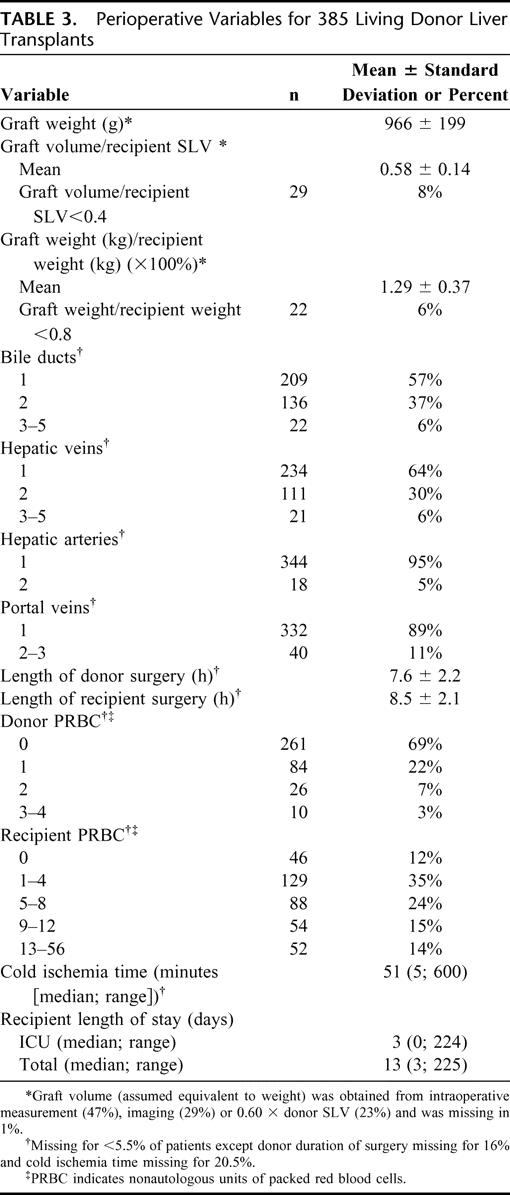
The donor operations averaged 7.6 ± 2.2 hours in duration. Thirty-two percent of donors received autologous blood transfusions. No donor received more than 4 units of nonautologous packed red blood cells (PRBC), with most requiring no PRBC (most centers routinely use a cell-saver device for the donor operation, and these units were not counted). The mean duration of the recipient operations was 8.5 ± 2.1 hours. About one-half of the ALDLT recipients received 0–4 units of PRBC, and 14% required more than 12 units of blood. The median cold ischemia time, from donor cross-clamp to graft removal from ice, was 51 minutes, with a range of 5 to 600 minutes. Cold ischemia decreased with increasing experience at A2ALL centers. In the first 10 cases, 25% of grafts had cold ischemic time greater than 120 minutes. This decreased to 17% in cases 11–20, 13% in cases 21–30, 7% in cases 31–40, 3% in cases 41–50, and none in later cases. Recipients of living donor grafts stayed in the ICU a median of 3 days (range, 0–224) with a median total length of stay of 13 days (range, 3–225).
Graft and Recipient Outcomes
Graft and recipient outcomes following ALDLT are outlined in Table 4. Outcomes were categorized as early (first 90 days), or late (91 to 365 days). There were 42 deaths in the first year after transplant. Kaplan-Meier estimates of patient survival were 94% and 89% at 90 days and 1 year, respectively. The largest number of deaths was due to infection and sepsis (43%), followed by multiorgan failure, graft failure, and cardiopulmonary causes. Survival with the original functioning ALDLT graft was 87% and 81% at 90 days and 1 year, respectively. There were 72 graft failures in the first year, 71% occurring in the first 90 days. The major causes of graft failure were hepatic artery or portal vein thromboses (n= 19) and primary nonfunction (n = 12), and 21 patients died with a functioning graft. Of note, in the first year, only 3 grafts were lost to recurrent hepatitis. One graft loss was primarily attributed to acute rejection, and this was felt to be a secondary cause of graft loss in 3 others. Thirty-seven patients were retransplanted, of whom 86% were retransplanted in the first 90 days. Of the retransplanted patients, 7 died.
TABLE 4. Graft and Recipient Outcomes

Complications
The majority of complications were documented in the first 90 days following transplantation (Table 5). The largest numbers of early complications were infectious, with 32% first experiencing an infection in the initial 3 months after ALDLT. Most early infections were bacterial, but there was a 9% incidence of fungal infections. Pulmonary complications were relatively common, such as pleural effusions (18%) and pulmonary edema (10%).
TABLE 5. Recipient Complications After ALDLT
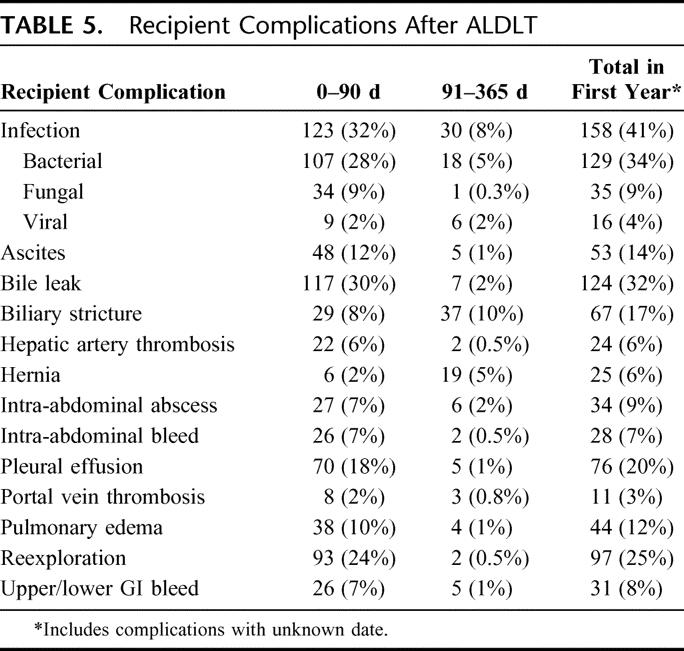
Technical complications were also common in the early postoperative period. Early bile leaks were seen in 30% of recipients and biliary strictures in 8%. Hepatic artery thrombosis occurred in 6% of recipients, and portal vein thrombosis in 2%. Seven percent experienced an intra-abdominal bleed, and 24% of patients required reexploration soon after transplant. The influence of center experience on the occurrence of technical complications (bile leak, hepatic artery thrombosis, and portal vein thrombosis) was analyzed. The incidence of bile leak in the first 90 days decreased from 38% in cases 1–20 to 24% in cases 21 and later (P = 0.004). There was also a decrease in hepatic artery thrombosis (8% to 4%) and portal vein thrombosis (3% to 1%), but these differences were not statistically significant.
There were fewer complications after the initial 90 days, the most common being biliary strictures (10%), infections (8%), and hernias (5%).
Factors Influencing Allograft Survival
Of the 35 variables that were tested, 3 were associated with a significant independent risk of graft failure (retransplantation or death): recipient age, cold ischemia time, and center experience (Table 6). Each 10-year increase in recipient age was associated with a 41% higher risk (P = 0.0008) of graft failure. Longer cold ischemia time was associated with 19% increased risk of graft failure per hour (P = 0.002). Early center experience (case number 20 or less) was associated with an 83% higher risk of graft failure (P = 0.0045). In addition, transplants from biologically related donors had better outcomes (hazard ratio, 0.67; P = 0.069). Increasing donor age was associated with a 13% elevated risk of graft loss per 10 years but was not statistically significant.
TABLE 6. Factors Associated With Graft Failure/Death Based on Cox Regression
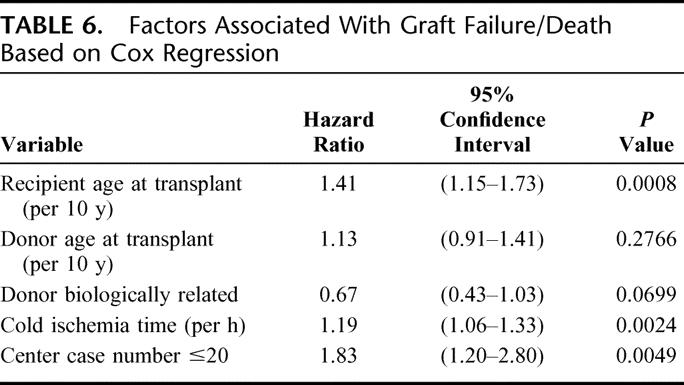
Recipient medical status at time of transplant did not appear to affect outcome, although the numbers of patients hospitalized (11%), in the ICU (4%), on dialysis (2%), or with MELD scores greater than 30 (4%) were small, and these factors were not significant in the Cox model.
Neither graft size nor GWRWR was significantly associated with graft failure risk. However, only 22 grafts (6%) were less than 0.8% GWRWR, and only 29 (8%) were less than 40% of the SLV of the recipient. When adjusted for recipient age, there was a suggestion of such an association between higher GWRWR and lower risk of graft failure, but the relationship had a wide confidence interval (CI) and was clearly not statistically significant (hazard ratio, 0.86; 95% CI, 0.49 to 1.52; P = 0.5979). In separate analyses, combinations of small graft weight or low GWRWR with other variables (such as MELD score, donor age, donor biologic relation, and case number) did not reveal any significant associations with outcome (data not shown). Similarly, no significant associations were found between recipient blood loss, anomalous donor arterial and venous anatomy, and multiplicity of bile ducts and the risk of graft failure.
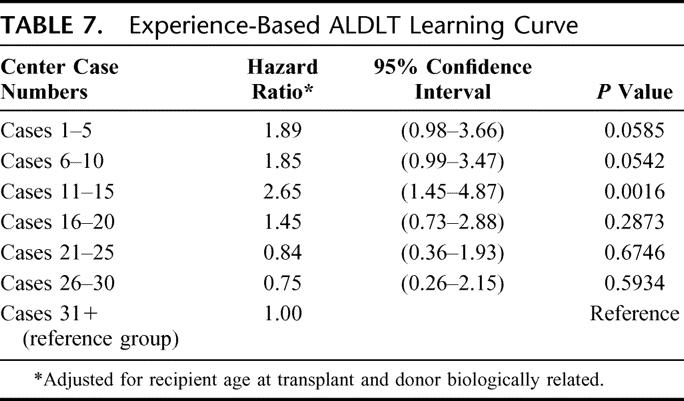
To further investigate the shape of the experience-based learning curve in ALDLT, we examined the risk of graft failure by case number within each center by 5-case increments (Table 7). Compared with case numbers above 30, very early cases (≤10) were associated with 85%-89% higher risk (P < 0.06). Cases 11 to 15 were associated with particularly high risk (hazard ratio, 2.65; P = 0.0017). Thereafter, the hazard ratio decreased and was not significantly different than for the ALDLT case numbers above 30.
TABLE 7. Experience-Based ALDLT Learning Curve
DISCUSSION
While results of transplantation of a right lobe from a living donor into an adult have been reported from large centers and consortiums from Europe and Asia, this is the first major report of a large multicenter nonregistry-based experience of ALDLT in North American patients. The importance of perioperative variables on ALDLT outcome has never been published in a large multicenter experience. The A2ALL Study Group has collected the retrospective data necessary to assess variables that contribute to early graft loss in ALDLT. This retrospective experience from 9 centers demonstrates acceptable outcomes of this approach to liver transplantation. The multivariable analysis of risk factors for graft failure revealed the importance of the learning curve, recipient age, and cold ischemia time as predictive of graft failure.
Living donor liver transplantation has emerged as a surgical technical achievement designed to increase the organ supply, with the increasing waiting list morbidity and mortality among transplant candidates as a justification for expansion to the adult population. This surgical strategy evolved rapidly, with the appearance of new challenges that have significant impact on liver transplantation, as well as hepatobiliary surgery. The principal surgical challenges include the procurement of an allograft with sufficient liver volume to meet the metabolic needs of the recipient, optimization of vascular inflow, venous outflow, and biliary drainage, plus an appreciation of anatomic variations that may necessitate complex biliary or vascular reconstruction. Initial reports have not demonstrated a clear advantage of ALDLT over deceased donor transplantation in the adult population, in contrast to the excellent outcomes reported in pediatric LDLT.11,12,18,19 This difference may be due to possible differences in the surgical procedure and the wider spectrum of donor and recipient candidates involved in ALDLT compared with pediatric recipients of living donors. ALDLT includes a more heterogeneous donor population, and adult recipients have a wide range of disease severity and higher incidence of medical comorbidities not seen in pediatric recipients. In addition, the use of a partial graft in an adult recipient predisposes the recipient to a unique set of potential technical and anatomic complications that are not prevalent in whole deceased donor grafts. Careful analysis of variables that affect early graft outcome in ALDLT is necessary to determine methods that may be manipulated to improve outcome.
No formal registry of ALDLT has been successfully implemented despite widespread calls for its creation.25,26 Although the outcome of this procedure is available through national data collected from all transplant centers, detailed information regarding perioperative variables is not included in these databases, so risk analysis is limited. Thus, accurate detailed data on the performance of ALDLT in the United States exists only as individual reports in the literature.
Analysis of graft and patient survival in this report parallels previous reports in Europe, Asia, and North America. The European experience of ALDLT summarized by Broelsch et al27 reported on 11 centers in 8 countries that performed 105 pediatric and 123 adult living donations, 111 of which were right lobe allografts. Recipient and allograft survival were 86% and 83%, respectively. Two large single center reports from France and Germany reported 1-year graft survival ranging from 75% to 85%.28,29 In Asia, the lack of deceased donor organs has pushed the progress of ALDLT beyond that of Europe or North America. Todo et al30 of Hokkaido University in Japan reported an 80% actual allograft survival in an initial series of 21 right lobe allografts. Regional multicenter summary data have been published that mirror individual center data, with 1-year graft survival ranging from 85% to 90%.31–34
In North America, Marcos35 reported 80% recipient survival in the first 20 recipients that improved to 95% for the second group of 20 recipients, and Bak et al36 of the University of Colorado reported 85% recipient survival in an initial series of 20 right lobe allografts. Miller et al37 reported the largest North American series from Mount Sinai Medical Center in New York, and multiple detailed reviews from the United States have since been published with similar outcomes.35,38–40 There have been 2 reports from A2ALL centers detailing surgical outcomes in ALDLT.41,42 An analyses by Abt et al19 of US data including all ALDLT from 1998 to 2001 (731 patients with complete data) revealed 1-year patient survival of 87.4%, with 12% undergoing retransplantation. Our A2ALL results in this series showed similar 1-year patient survival of 89%, with 9.6% undergoing retransplantation. The primary cause of graft failure in the first 90 days was vascular thrombosis, followed by primary nonfunction, whereas the main cause of graft failure in the later time period was death of the recipient with a functioning graft. The primary cause of recipient death in this A2ALL report was infection and sepsis, a fatal complication not clearly identified in other large series.
In this A2ALL series, perioperative complications were numerous, occurred in the first 3 months posttransplant for the most part, and were similar in scope to previous single-center reports. The most commonly identified complications were early infections and bile leaks. Late complications were infrequent by comparison and were dominated by biliary strictures. In a blinded survey sponsored by the American Society of Transplant Surgeons of 30 North American liver transplant centers that had performed a total of 208 ALDLT within the United States and Canada, an overall incidence of complications of 30% was reported.43 The 3 most frequent complications reported were biliary (18%), vascular (6% allograft loss), and primary allograft nonfunction (4% allograft loss). A later survey by Brown et al17 of US transplant programs reported data on 433 ALDLT. Recipient and allograft survival were not reported, but the reported incidences of biliary and vascular complications were 23% and 8%, respectively.
Identification and characterization of the “learning curve” effect for this difficult procedure is a principal finding in this study. Significant improvement in graft outcome was noted after 20 cases, as well as decreased incidence of bile leaks. Although all the A2ALL centers were very experienced in deceased donor liver transplantation, where procedure volume has been correlated with outcome,44,45 and many were experienced in split liver transplantation and pediatric living donor liver transplantation, there was still the finding of a relatively steep learning curve with this procedure. The number of cases required to gain expertise in a center is not well defined in the literature, and several centers have compared early and late results demonstrating significant improvement in outcome with increased expertise.31,36,42,46 A potential interpretation of the data from Table 7, admittedly somewhat speculative, would be that the first 10 cases in each center were chosen fairly carefully, with outcomes only slightly worse than the reference group (>30 cases). Cases 11–15 may reflect broadening of indications or relaxation of selection criteria, resulting in significantly higher risk (hazard ratio, 2.65) for these recipients. From case 16 onward, the combination of yet more experience, perhaps combined with a return to a more conservative approach, may have led to better results. This may demonstrate better judgment in patient selection, more efficient perioperative management, and improved technical results learned from prior experience and past mistakes. The learning curve and subsequent improvement in outcomes is a continuous process, and further experience may be accompanied by even better outcomes than those shown by the later cases in this series.
Increasing recipient age was significantly associated with adverse outcome and should be an important clinical factor in the choice of appropriate recipients for this procedure. These results support findings from national data where LDLT recipients older than 57 years had an increased risk of graft failure due to patient death.19 Numerous other reports have emphasized the importance of the age and medical condition of the recipient in deceased donor liver transplantation.47–49 Transplantation of a partial graft in critically ill patients with chronic liver disease or fulminant hepatic failure has been correlated with poor outcome.50–52 Interestingly, our study did not demonstrate a significant effect of high MELD score or other markers of recipient status and medical comorbidities on patient and graft survival. This may reflect the small number of patients in each of these categories or a trend by A2ALL centers to use conservative judgment in the cases chosen for ALDLT.
Despite very short periods of cold ischemia compared with what is typical in deceased donor liver transplantation, the duration of cold ischemia had a significant association with graft outcome. The relative risk of graft failure in whole organ deceased donor transplants is not significantly increased unless the cold ischemia time is in excess of 8 hours.53 However, the combination of a partial graft and cold ischemia may be interactive, and has been suggested by animal models.54,55 Longer cold ischemia times in some of our recipients may have been due to inability to conduct temporally overlapping donor and recipient surgical procedures or to unanticipated difficulties during the recipient operation. The former should be able to be overcome with appropriate logistical planning, coordinated operating rooms, and 2 operating teams. Improved operative efficiency was demonstrated in this series by a steady decrease in the incidence of grafts with cold ischemia times exceeding 120 minutes as center volume increased.
It was surprising that several other variables, particularly donor age and graft size, were not statistically significant in the multivariable model. One report of national data demonstrates increasing rates of graft failure as donor age increases.19 We did show increased risk with every additional 10 donor years, and the lack of formal statistical significance may be due to the inclusion of few older donors.
While the ability of a small graft to support a large recipient has been a concern to most surgeons performing ALDLT, we did not demonstrate poor early graft function secondary to insufficient transplanted allograft volume.24,56–58 Our series did not see any correlation of graft size with outcome, but the A2ALL centers also avoided the extremes of size mismatch, with very few grafts below the recommended minimum volume. Whether smaller graft size contributes to the high incidence of infectious complications remains to be determined.
In summary, the results of this study demonstrate that ALDLT is a procedure where center experience plays a significant role in outcome, as do recipient age and cold ischemia time of the right lobe graft. In addition, there was a trend toward increasing risk with older-aged donors. There continues to be a significant number of perioperative complications, particularly infectious, biliary, and vascular, and these are a challenge for centers performing these procedures. This large North American multicenter experience provides evidence that ALDLT is a viable option for liver replacement with outcomes that continue to improve with experience.
ACKNOWLEDGMENTS
The A2ALL Steering Committee and coinvestigators of the A2ALL Study Group were instrumental in the design of this retrospective cohort study. The authors gratefully acknowledge the hard work and dedication of the study coordinators at the 9 A2ALL transplant centers and the staff of the Data Coordinating Center. The assistance of Karen Wisniewski, MS, in data analysis is greatly appreciated.
The supplemental data included here have been supplied by the University Renal Research and Education Association (URREA) as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the SRTR or the U.S. Government.
Discussions
Dr. Charles M. Miller (Cleveland, Ohio): Once again at this meeting, adult-to-adult living donor liver transplantations are being shown now, in a large multicenter U.S. trial, to be an extremely valuable and viable option for liver replacement therapy. Yet, as pointed out in the manuscript, the procedure remains controversial, and the volume of cases has not continued to grow for many reasons.
The controversies that surround this procedure center on donor safety, proper candidate selection both in terms of etiology and the severity of disease and co-morbidities, technical details of the donor surgery and recipient operation, inclusion or not of the middle hepatic vein, inflow modification, the avoidance of functional small-for-size syndrome, biliary reconstructions of complications, and now variations in the relative need for this procedure as we explore extended criteria donors and the UNOS allocation system evolves and improves. In this context, I would like to ask you four questions.
You found the primary cause for graft failure to be primary non-function and vascular thrombosis, but there was no mention of small-for-size syndrome or functional small-for-size syndrome. Personally, I have actually never seen a case of primary non-function in living donors and I don't know that it exists. And I think what is being reported as primary non-function is actually a form of a functional small-for-size syndrome, too small volumetrically, but functional small-for-size syndrome, that causes severe early graft dysfunction and often vascular thrombosis and biliary ischemia. Can you comment on this?
In that vein, you state the majority of early complications were infectious. Were these infections seen in conjunction with early graft dysfunction or other technical complications, or seen de novo?
As you know, there is a vigorous debate especially around the use of adult living donors for hepatitis C and how the rate and severity may differ with regard to rapid recurrence. In the 179 patients that were transplanted with hepatitis C, what was the rate and severity of recurrence? Can you shed some light on this debate?
Finally, one of the most interesting and maybe important findings of the manuscript is the clear, concise definition of the learning curve. One of the questions I am frequently asked is: Who should be doing this procedure and who shouldn't be? I have actually never answered it clearly. If our goal is to use living donation to dramatically reduce the waiting list morbidity and mortality and make up the gap between supply and demand, and in taking into consideration the new developments in deceased donor allocation, who do you think should be doing adult live donors? How many new centers do we need? And what are your suggestions for shortening the learning curve and making it less dramatic?
Dr. Kim M. Olthoff (Philadelphia, Pennsylvania): Thank you, Dr. Miller. I think your question about the functional small-for-size graft is an important one, and we struggled to answer with the data that we had in this retrospective study. Any retrospective study is limited by the data available. We all thought that graft size would have played an important role. We looked at all the combinations possible, such as a small graft in a large recipient, or a smaller graft in a very sick recipient with a higher MELD score, or older age, or medical co-morbidities, and we were not able to find a statistical correlation with graft size. Saying that, there is difficulty in defining the small-for-size syndrome functionally in our retrospective study. I do believe that some of the causes of graft failures may fall into that category, such as the infectious complications, the primary nonfunctions, and the vascular thromboses. Unfortunately, with the retrospective data we didn't see a statistically significant association with graft size. I hope that with the prospective study where we are getting more detailed data that this might be answered.
With regard to the question about infectious complications, we were not able to see a correlation between the technical complications and the infectious complications, although there seemed to be a great deal of overlap in many of these recipients. Whether the infectious complications are a surrogate marker of a functional small-for-size syndrome is a question that remains to be answered. I think that is a possibility.
The question of recurrent hepatitis C has been a controversial one with numerous centers reporting single center results saying that recurrent hepatitis C may recur faster in living donor grafts, with others reporting that it is not. We actually only had three graft losses in the first year for recurrent hepatitis C, so it was not a significant problem within the first year. In the prospective study we will be obtaining protocol biopsies and viral loads to better answer this question.
The learning curve was an interesting finding that came out of the data, and I think it is an important question to ask, particularly since UNOS is now imposing regulations on living donor centers across the country, requiring a certain amount of experience. The number of cases that were chosen for the UNOS requirements were relatively arbitrary. I think this is important to show this data to see that there is a cut-off point in the range 5 to 20 in centers that were already very experienced in split livers, deceased donors, pediatric living donors.
Which centers should be doing living donors is a hard question to answer, because it might be that it is the personnel that are experienced and not the center. In all the A2ALL centers the personnel stayed the same during the day period, so I can't say that it was a center-specific finding or personnel finding, or both. But that finding is definitely there and I believe that there should be the requirement for some experience prior to living donation being set up at a specific center.
Dr. Raymond Pollak (Peoria, Illinois): This is a very important paper. In evaluating the novel technologies, it would be helpful to know if you had a contemporaneous control of matched recipients for age, gender and ethnicity in order to be able to evaluate the utility of this new technology.
Dr. Kim M. Olthoff (Philadelphia, Pennsylvania): This is a very important question and is actually a significant goal of the A2ALL study. For every living donor recipient that is being followed there is also a matched control patient receiving a deceased donor liver transplant. Early findings demonstrate a benefit from the time of the decision to pursue living donation. That analysis is not yet complete and is currently ongoing. Yes, it is a very important comparison.
Dr. Charles B. Huddleston (St. Louis, Missouri): My compliments on an outstanding series. Dr. Miller alluded to issues of donor safety but there were no comments or questions from him about that and none in your presentation. I think for living donor transplantation in general, regardless of the organ involved, we have to assure that the donors are maintained with a very low morbidity and mortality. Could you comment on that in this very large series?
Dr. Kim M. Olthoff (Philadelphia, Pennsylvania): This study focused on the early surgical outcomes and graft failures in the recipients alone. The donor outcomes regarding safety is a very important aspect of the A2ALL study. These data have been collected on all the donors for each of these 385 retrospective recipients and will be out in a separate report. More importantly will be the results of the prospective cohort where we are also looking at detailed analyses of donor outcome as far as quality of life and long-term results, which has not been looked at previously in a large multicenter trial. So we hope to have answers for that in the future aswell.
Dr. Nancy L. Ascher (San Francisco, California): Along those lines, Dr. Olthoff, I think one of the main concerns of the community has been how we ascertain that the donor actually understands what he or she is going through. Can you just review for the group what the informed consent process is for the A2ALL study?
Dr. Kim M. Olthoff (Philadelphia, Pennsylvania): At the time that the recipient is listed for liver transplantation, the option of living donor is presented to the recipient. It is then up to the recipient and their potential donors to come forward, out of free will, so to speak, to pursue a living donation evaluation and potential surgery.
It is a very complete evaluation, including a computerized question-and-answer program so we know that they are very much aware of what is involved. It also involves a one-on-one meeting with one of the surgeons going over all the potential risks and benefits. We also have a patient advocate to go over potential risks and benefits of the procedure, allowing the potential donor to withdraw at any time.
The evaluation itself involves a complete medical evaluation, as well as detailed imaging, and liver biopsies in some cases. There is a time period where they can consider whether they wish to proceed or not, and have the opportunity to back out if they wish to. Several stages of consent are obtained.
Dr. Byers W. Shaw, Jr. (Omaha, Nebraska): I have two quick questions. One of them is whether or not this is open for other centers to enroll their patients or whether it is a closed study; because the more data you get, the better, obviously. And the second is a curiosity about this learning curve issue, which was also present in the paper that preceded yours. Will you be able to get any information out of this study about what people learned between that 15th and 20th case that makes such a huge difference?
Dr. Kim M. Olthoff (Philadelphia, Pennsylvania): The answer to the first question is, yes, it would be nice, and beneficial, to have more centers involved but I think funding by the NIH probably limits that. To answer the second question, we proposed what our A2ALL learning curve meant and reflected: that in the first ten cases we were all very careful in choosing our recipients. We then may have gotten a little more cavalier with the cases in the 10 to 15 range, thinking all was going well. Then reality hit when we saw that there were some problems with technical situations or perhaps our recipient and donor choices improved. I personally think it is probably the choice of the recipient and the donor that has the most impact on outcome, and is reflected in the learning curve.
Dr. Robert M. Mentzer, Jr. (Lexington, Kentucky): I have just a brief question. Assuming that graft and patient survival rates are similar for living-related and cadaveric donors, what effect, if any, do you believe the MELD scoring allocation system will have on the current rate of living-related donation?
Dr. Kim M. Olthoff (Philadelphia, Pennsylvania): I think the MELD system has already had an impact in this choice, in that many of the early living donor recipients were those with hepatocellular carcinoma, and when these patients received exception points in the MELD system, many of them did not need the living donor option.
The key with the MELD system and living donation will be in those areas where a high MELD is required to get a deceased donor organ, and it will be the living donor option that will allow us to transplant the more mid-range or the lower MELD system patients, the ones that have a high risk of death while waiting or poor quality of life and little chance of receiving a deceased donor.
Footnotes
Supported in part by the National Institutes of Health (NIDDK grant numbers U01-DK62536, U01-DK62444, U01-DK62467, U01-DK62483, U01-DK62484, U01-DK62494, U01-DK62496, U01-DK62498, U01-DK62505, U01-DK62531), the American Society of Transplant Surgeons, and the US Department of Health and Human Services, Health Resources and Services Administration.
Presented in part at the American Surgical Association, Palm Beach, Florida, April 2005.
This is publication number 1 of the Adult-to-Adult Living Donor Liver Transplantation Cohort Study.
Reprints: Robert M. Merion, MD, University of Michigan, 315 West Huron Street, Suite 240, Ann Arbor, MI 48103-4262. E-mail: merionb@umich.edu.
Reprints: Kim M. Olthoff, MD, Hospital of the University of Pennsylvania, 2 Dulles, 3400 Spruce Street, Philadelphia, PA 19104. E-mail: kim.olthoff@uphs.upenn.edu.
REFERENCES
- 1.Hanto DW, Fishbein TM, Pinson CW, et al. Liver and intestine transplantation: summary analysis, 1994–2003. Am J Transplant. 2005;5(4 pt 2):916–933. [DOI] [PubMed] [Google Scholar]
- 2.Busuttil RW, Tanaka K. The utility of marginal donors in liver transplantation. Liver Transpl. 2003;9:651–663. [DOI] [PubMed] [Google Scholar]
- 3.Amin MG, Wolf MP, TenBrook JA Jr, et al. Expanded criteria donor grafts for deceased donor liver transplantation under the MELD system: a decision analysis. Liver Transpl. 2004;10:1468–1475. [DOI] [PubMed] [Google Scholar]
- 4.Feng S, Bragg-Gresham JL, Dykstra DM, et al. Definitions and outcomes of transplants using expanded criteria donor livers. Hepatology. 2003;38(4 suppl 1):158A.12829998 [Google Scholar]
- 5.Marcos A, Fisher RA, Ham JM, et al. Right lobe living donor liver transplantation. Transplantation. 1999;68:798–803. [DOI] [PubMed] [Google Scholar]
- 6.Makuuchi M, Miller CM, Olthoff K, et al. Adult-adult living donor liver transplantation. J Gastrointest Surg. 2004;8:303–312. [DOI] [PubMed] [Google Scholar]
- 7.Miller CM, Delmonico FL. Transplantation of liver grafts from living donors into adults. N Engl J Med. 2001;345:923. [PubMed] [Google Scholar]
- 8.Trotter JF, Wachs M, Everson GT, et al. Adult-to-adult transplantation of the right hepatic lobe from a living donor. N Engl J Med. 2002;346:1074–1082. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka K, Kiuchi T. Living-donor liver transplantation in the new decade: perspective from the twentieth to the twenty-first century. J Hepatobiliary Pancreat Surg. 2002;9:218–222. [DOI] [PubMed] [Google Scholar]
- 10.Wachs ME, Bak TE, Karrer FM, et al. Adult living donor liver transplantation using a right hepatic lobe. Transplantation. 1998;66:1313–1316. [DOI] [PubMed] [Google Scholar]
- 11.Abt PL, Rapaport-Kelz R, Desai NM, et al. Survival among pediatric liver transplant recipients: impact of segmental grafts. Liver Transpl. 2004;10:1287–1293. [DOI] [PubMed] [Google Scholar]
- 12.Roberts JP, Hulbert-Shearon TE, Merion RM, et al. Influence of graft type on outcomes after pediatric liver transplantation. Am J Transplant. 2004;4:373–377. [DOI] [PubMed] [Google Scholar]
- 13.American Society of Transplant Surgeons. American Society of Transplant Surgeons’ position paper on adult-to-adult living donor liver transplantation. Liver Transpl. 2000;6:815–817. [DOI] [PubMed] [Google Scholar]
- 14.Surman OS. The ethics of partial-liver donation. N Engl J Med. 2002;346:1038. [DOI] [PubMed] [Google Scholar]
- 15.Miller CM. Regulation and oversight of adult living donor liver transplantation. Liver Transpl. 2003;9(10 suppl 2):S69–72. [DOI] [PubMed] [Google Scholar]
- 16.Hirano I, Blei AT. Deaths after living related liver transplantation. Liver Transpl. 2000;6:250. [DOI] [PubMed] [Google Scholar]
- 17.Brown RS Jr, Russo MW, Lai M, et al. A survey of liver transplantation from living adult donors in the United States. N Engl J Med. 2003;348:818–825. [DOI] [PubMed] [Google Scholar]
- 18.Thuluvath PJ, Yoo HY. Graft and patient survival after adult live donor liver transplantation compared to a matched cohort who received a deceased donor transplantation. Liver Transpl. 2004;10:1263–1268. [DOI] [PubMed] [Google Scholar]
- 19.Abt PL, Mange KC, Olthoff KM, et al. Allograft survival following adult-to-adult living donor liver transplantation. Am J Transplant. 2004;4:1302–1307. [DOI] [PubMed] [Google Scholar]
- 20.National Institutes of Health. Adult to adult living donor liver transplantation cohort study. Available at: http://www.nih-a2all.org.
- 21.UNOS. MELD-PELD calculator. Available at: http://www.unos.org/waitlist/includes_local/pdfs/meld_peld_calculator.pdf.
- 22.Freeman RB Jr, Wiesner RH, Roberts JP, et al. Improving liver allocation: MELD and PELD. Am J Transplant. 2004;4(suppl 9):114–131. [DOI] [PubMed] [Google Scholar]
- 23.Heinemann A, Wischhusen F, Puschel K, et al. Standard liver volume in the Caucasian population. Liver Transpl Surg. 1999;5:366–368. [DOI] [PubMed] [Google Scholar]
- 24.Lo CM, Fan ST, Liu CL, et al. Minimum graft size for successful living donor liver transplantation. Transplantation. 1999;68:1112–1116. [DOI] [PubMed] [Google Scholar]
- 25.Abecassis M, Adams M, Adams P, et al. Consensus statement on the live organ donor. JAMA. 2000;284:2919–2926. [DOI] [PubMed] [Google Scholar]
- 26.Shiffman ML, Brown RS Jr, Olthoff KM, et al. Living donor liver transplantation: summary of a conference at The National Institutes of Health. Liver Transpl. 2002;8:174–188. [DOI] [PubMed] [Google Scholar]
- 27.Broelsch CE, Malago M, Testa G, et al. Living donor liver transplantation in adults: outcome in Europe. Liver Transpl. 2000;6(6 suppl 2):S64–65. [DOI] [PubMed] [Google Scholar]
- 28.Boillot O, Belghiti J, Azoulay D, et al. Initial French experience in adult-to-adult living donor liver transplantation. Transplant Proc. 2003;35:962–963. [DOI] [PubMed] [Google Scholar]
- 29.Malago M, Testa G, Frilling A, et al. Right living donor liver transplantation: an option for adult patients: single institution experience with 74 patients. Ann Surg. 2003;238:853–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Todo S, Furukawa H, Jin MB, et al. Living donor liver transplantation in adults: outcome in Japan. Liver Transpl. 2000;6(6 suppl 2):S66–72. [DOI] [PubMed] [Google Scholar]
- 31.Lo CM, Fan ST, Liu CL, et al. Lessons learned from one hundred right lobe living donor liver transplants. Ann Surg. 2004;240:151–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen CL, Fan ST, Lee SG, et al. Living-donor liver transplantation: 12 years of experience in Asia. Transplantation. 2003;75(3 suppl):S6–11. [DOI] [PubMed] [Google Scholar]
- 33.Sugawara Y, Makuuchi M. Advances in adult living donor liver transplantation: a review based on reports from the 10th anniversary of the adult-to-adult living donor liver transplantation meeting in Tokyo. Liver Transpl. 2004;10:715–720. [DOI] [PubMed] [Google Scholar]
- 34.Lee SG, Park KM, Lee YJ, et al. 157 Adult-to-adult living donor liver transplantation. Transplant Proc. 2001;33:1323–1325. [DOI] [PubMed] [Google Scholar]
- 35.Marcos A. Right lobe living donor liver transplantation: a review. Liver Transpl. 2000;6:3–20. [DOI] [PubMed] [Google Scholar]
- 36.Bak T, Wachs M, Trotter J, et al. Adult-to-adult living donor liver transplantation using right-lobe grafts: results and lessons learned from a single-center experience. Liver Transpl. 2001;7:680–686. [DOI] [PubMed] [Google Scholar]
- 37.Miller CM, Gondolesi GE, Florman S, et al. One hundred nine living donor liver transplants in adults and children: a single-center experience. Ann Surg. 2001;234:301–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Humar A. Donor and recipient outcomes after adult living donor liver transplantation. Liver Transpl. 2003;9(10 suppl 2):S42–44. [DOI] [PubMed] [Google Scholar]
- 39.Pomfret EA. Early and late complications in the right-lobe adult living donor. Liver Transpl. 2003;9(10 suppl 2):S45–49. [DOI] [PubMed] [Google Scholar]
- 40.Russo MW, Brown RS Jr. Adult living donor liver transplantation. Am J Transplant. 2004;4:458–465. [DOI] [PubMed] [Google Scholar]
- 41.Ghobrial RM, Saab S, Lassman C, et al. Donor and recipient outcomes in right lobe adult living donor liver transplantation. Liver Transpl. 2002;8:901–909. [DOI] [PubMed] [Google Scholar]
- 42.Maluf DG, Stravitz RT, Cotterell AH, et al. Adult living donor versus deceased donor liver transplantation: a 6-year single center experience. Am J Transplant. 2005;5:149–156. [DOI] [PubMed] [Google Scholar]
- 43.Renz JF, Busuttil RW. Adult-to-adult living-donor liver transplantation: a critical analysis. Semin Liver Dis. 2000;20:411–424. [DOI] [PubMed] [Google Scholar]
- 44.Axelrod DA, Guidinger MK, McCullough KP, et al. Association of center volume with outcome after liver and kidney transplantation. Am J Transplant. 2004;4:920–927. [DOI] [PubMed] [Google Scholar]
- 45.Edwards EB, Roberts JP, McBride MA, et al. The effect of the volume of procedures at transplantation centers on mortality after liver transplantation. N Engl J Med. 1999;341:2049–2053. [DOI] [PubMed] [Google Scholar]
- 46.Miller C. Living donor liver transplantation: overview after 178 cases. Transplant Proc. 2003;35:964–965. [DOI] [PubMed] [Google Scholar]
- 47.Narayanan Menon KV, Nyberg SL, Harmsen WS, et al. MELD and other factors associated with survival after liver transplantation. Am J Transplant. 2004;4:819–825. [DOI] [PubMed] [Google Scholar]
- 48.Ghobrial RM, Gornbein J, Steadman R, et al. Pretransplant model to predict posttransplant survival in liver transplant patients. Ann Surg. 2002;236:315–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Desai NM, Mange KC, Crawford MD, et al. Predicting outcome after liver transplantation: utility of the model for end-stage liver disease and a newly derived discrimination function. Transplantation. 2004;77:99–106. [DOI] [PubMed] [Google Scholar]
- 50.Testa G, Malago M, Nadalin S, et al. Right-liver living donor transplantation for decompensated end-stage liver disease. Liver Transpl. 2002;8:340–346. [DOI] [PubMed] [Google Scholar]
- 51.Zamir G, Olthoff KM, Desai N, et al. Toward further expansion of the organ pool for adult liver recipients: splitting the cadaveric liver into right and left lobes. Transplantation. 2002;74:1757–1761. [DOI] [PubMed] [Google Scholar]
- 52.Kam I. Adult-adult right hepatic lobe living donor liver transplantation for status 2a patients: too little, too late. Liver Transpl. 2002;8:347–349. [DOI] [PubMed] [Google Scholar]
- 53.DebRoy M, Dykstra D, Roberts J, et al. The impact of cold ischemic time and donor age on liver transplant outcome. Am J Transplant. 2003;3(suppl 5):A#1167.
- 54.Selzner N, Selzner M, Tian Y, et al. Cold ischemia decreases liver regeneration after partial liver transplantation in the rat: a TNF-alpha/IL-6-dependent mechanism. Hepatology. 2002;36(4 pt 1):812–818. [DOI] [PubMed] [Google Scholar]
- 55.Debonera F, Wang G, Xie J, et al. Severe preservation injury induces Il-6/STAT3 activation with lack of cell cycle progression after partial liver graft transplantation. Am J Transplant. 2004;4:1964–1971. [DOI] [PubMed] [Google Scholar]
- 56.Emond JC, Renz JF, Ferrell LD, et al. Functional analysis of grafts from living donors: implications for the treatment of older recipients. Ann Surg. 1996;224:544–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kiuchi T, Tanaka K, Ito T, et al. Small-for-size graft in living donor liver transplantation: how far should we go? Liver Transpl. 2003;9:S29–35. [DOI] [PubMed] [Google Scholar]
- 58.Tanaka K, Ogura Y. “Small-for-size graft” and “small-for-size syndrome” in living donor liver transplantation. Yonsei Med J. 2004;45:1089–1094. [DOI] [PubMed] [Google Scholar]


