Abstract
Objective:
The objective of this study was to determine whether genes that regulate cellular invasion and metastasis are differentially expressed and could serve as diagnostic markers of malignant thyroid nodules.
Summary and Background Data:
Patients whose thyroid nodules have indeterminate or suspicious cytologic features on fine needle aspiration (FNA) biopsy require thyroidectomy because of a 20% to 30% risk of thyroid cancer. Cell invasion and metastasis is a hallmark of malignant phenotype; therefore, genes that regulate these processes might be differentially expressed and could serve as diagnostic markers of malignancy.
Methods:
Differentially expressed genes (2-fold higher or lower) in malignant versus benign thyroid neoplasms were identified by extracellular matrix and adhesion molecule cDNA array analysis and confirmed by real-time quantitative polymerase chain reaction (PCR). The area under the receiver operating characteristic (AUC) curve was calculated to determine diagnostic accuracy of gene expression level cutoffs established by logistic regression analysis.
Results:
By cDNA array analysis, ADAMTS8, ECM1, MMP8, PLAU, SELP, and TMPRSS4 were upregulated, and by quantitative PCR, ECM1, SELP, and TMPRSS4 mRNA expression was higher in malignant (n = 57) than in benign (n = 38) thyroid neoplasms (P< 0.002). ECM1 and TMPRSS4 mRNA expression levels were independent predictors of a malignant thyroid neoplasm (P < 0.003). The AUC was 0.956 for ECM1 and 0.926 for TMPRSS4. Combining both markers improved their diagnostic use (AUC 0.985; sensitivity, 91.7%; specificity, 89.8%; positive predictive value, 85.7%; negative predictive value, 82.8%). ECM1 and TMPRSS4 expression analysis improved the diagnostic accuracy of FNA biopsy in 35 of 38 indeterminate or suspicious results. The level of ECM1 mRNA expression was higher in TNM stage I differentiated thyroid cancers than in stage II and III tumors (P ≤ 0.031).
Conclusions:
ECM1 and TMPRSS4 are excellent diagnostic markers of malignant thyroid nodules and may be used to improve the diagnostic accuracy of FNA biopsy. ECM1 is also a marker of the extent of disease in differentiated thyroid cancers.
Approximately 30% of thyroid nodule fine needle aspiration biopsies are indeterminate, nondiagnostic, or suspicious. To improve diagnostic accuracy, we used cDNA array analysis and quantitative polymerase chain reaction to identify differentially expressed cell invasion and adhesion genes that would be useful diagnostic markers of malignancy. We found that ECM1 and TMPRSS4 were excellent markers of malignant thyroid nodules, and that ECM1 was a marker of extent of disease in differentiated thyroid cancers.
The incidence of thyroid cancer has increased over the last 3 decades and approximately 24,000 new cases of thyroid cancer occurred in 2004.1 The incidence of thyroid nodules is even higher as a result of greater use of more sensitive imaging studies.2,3 Approximately 10% of the U.S. population will develop a significant thyroid nodule during their lifetime.4 Fine needle aspiration (FNA) biopsy has reduced the need for performing diagnostic thyroidectomies to rule out malignant thyroid neoplasms,5–10 but may show indeterminate or suspicious cytologic features in 15% to 20% of all thyroid nodules.5–7 This limitation is commonly the result of the overlapping cytologic features between benign (hyperplastic nodules, follicular adenoma, Hürthle cell adenoma) and malignant (follicular cancer, Hürthle cell cancer, the follicular variants of papillary cancer) follicular thyroid nodules.5,6 Because the risk of malignancy is approximately 20% for follicular and Hürthle tumors and approximately 50% in nodules suspicious for papillary thyroid cancer, thyroidectomy is generally the recommended treatment. Unfortunately, none of the preoperative clinical (age, sex, solitary vs multiple nodules), imaging (tumor size, nodule ultrasound characteristics), and cytologic (atypia, mitotic index) factors studied so far are accurate enough to determine which patients with suspicious or indeterminate FNA cytologic findings should undergo thyroidectomy.11–14
Several genetic alterations (RET/PTC rearrangements, PAX8-PPARγ, BRAF) and molecular markers (galectin-3, telomerase, lactoferrin, CD44, CK19, MET, HMBE-1, p21, p27, DAP4, HMG1, TTF-1, TPO) have been studied to help distinguish benign from malignant thyroid neoplasms.15–39 RET/PTC rearrangements, BRAF mutations, and MET overexpression are present mostly in papillary thyroid cancer and cannot discriminate benign from malignant follicular thyroid tumors.30,31,40–44 Although the initial report that identified the PAX8-PPARγ fusion gene suggested that this chromosomal translocation primarily occurred in follicular thyroid cancer, subsequent studies have shown it to be present in up to 55% of follicular adenomas.35,45,46 Similarly, TPO, TTF1, p53 mutations, p21, and telomerase expression analysis have not been found to discriminate reliably between benign and malignant follicular tumors.47–52 Positive immunostaining for CK19, S-100 protein, HMBE-1, galectin-3, and p27 is helpful for identifying papillary thyroid cancer and its follicular variant.53–59 However, false-positive and false-negative results for these markers occur because there is considerable overlap in gene expression levels, especially in thyroid glands with lymphocytic thyroiditis.60–65
cDNA array expression analysis, which has been used to analyze several human cancers, allows the correlation of gene expression profile with clinical variables, the classification or definition of different tumor types, and the identification of genes or networks of genes involved in carcinogenesis.66–69 cDNA array studies of thyroid cancer have identified 47 to 627 genes that are differentially expressed and distinguish benign from malignant thyroid neoplasms with a sensitivity of 87.5% to 93.0% and a specificity of 87.1% to 100%.23,24,26,70 However, these genes are variable and only a few studies of a limited number of genes confirmed the gene expression levels by RT PCR.16,70 This is important given that some investigators have suggested that cDNA array analysis may incorrectly identify 30% of genes and gene expression levels.71 Furthermore, the clinical use of using cDNA array analysis on FNA samples to discriminate benign from malignant thyroid neoplasms is unclear because more total RNA is required than would be available from FNA samples. The conflicting results of the differentially expressed genes among the different cDNA array studies and platforms used is also a problem, and there appears to be no uniform method of data analysis that has yet been standardized.72,73
Genes that regulate cell–cell and cell–matrix adhesion and degradation of extracellular matrix are critical in tumor cell invasion and metastasis, the hallmarks of malignant phenotype.74 Extracellular matrix and adhesion molecules, therefore, represent excellent candidate genes to study as markers of malignant thyroid nodules. Accordingly, we used an extracellular matrix and adhesion molecule cDNA array to identify differentially expressed genes in thyroid tissue that would represent tumors that are indeterminate or suspicious on FNA cytology. The differentially expressed genes on cDNA array analysis were further evaluated by real-time quantitative polymerase chain reaction (PCR) to determine their diagnostic accuracy.
METHODS
Thyroid Tissue and Fine Needle Biopsy Samples
Thyroid tissue samples and clinical and histopathology data were obtained for 131 patients with informed consent. The Committee on Human Research at the University of California, San Francisco approved the study.
Tissue from patients with hyperplastic nodule (n = 28), follicular adenoma (n = 28), follicular thyroid cancer (n = 25), follicular variant of papillary thyroid cancer (n = 19), papillary thyroid cancer (n = 26), and anaplastic thyroid cancer (n = 5) was studied. All thyroid tissue diagnoses were confirmed by permanent histology. The tissues used in our experiments were snap-frozen in liquid nitrogen at the time of thyroidectomy and stored at −80°C until total RNA was extracted. In 12 patients undergoing thyroidectomy for thyroid nodules, a biopsy of the thyroid nodule was performed with a 25-gauge needle at the time of thyroidectomy. The biopsy samples were immediately stored on ice until total RNA extraction. These samples were evaluated as samples with “unknown” histology.
cDNA Array
A cDNA array containing 96 human extracellular matrix and adhesion molecule genes, 3 endogenous control genes, and 2 negative control genes was used according to the manufacturer's instructions (HS-010 GEarray Superarray kit; Bioscience Co., Fredrick, MD). Equal amounts of total RNA from 5 randomly selected patient samples for each thyroid histology group (hyperplastic nodule, follicular adenoma, follicular cancer, follicular variant of papillary thyroid cancer, and papillary thyroid cancer) were pooled together.75,76 Total RNA was prepared by TRIZOL (Invitrogen, Carlsbad, CA) extraction and quantitated by spectrophotometry. Total RNA (1 μg total of 5 pooled samples for each histology) was prepared, labeled, and the cDNA array blots were hybridized according to the manufacturer's instructions (GEarray; Bioscience Co.). The Alpha image analyzer was used for spot densitometry measurements of the quadruplicate signals per gene. Gene expression levels (ie, density measurements) were normalized to β-actin to control for total RNA and hybridization efficiency differences among the different blots.
Real-Time Quantitative Polymerase Chain Reaction
Genes that were up- or downregulated by greater than 2-fold in malignant thyroid tissue on cDNA array blots were further analyzed by quantitative PCR. Total RNA was prepared by TRIZOL extraction (Invitrogen). Total RNA (125 ng/μL) was reverse-transcribed using the RT script cDNA synthesis kit (USB Corp., Cleveland, OH). Real-time quantitative PCR was used to measure mRNA expression levels relative to β-glucuronidase (GUS) mRNA expression. Normalized gene expression level = 2 −(Ct of gene of interest −Ct of GUS) × 100%, where Ct is the PCR cycle threshold.77 The PCR primers and probes for the genes were purchased from Applied Biosystems (Assay-on-Demand kit, Foster City, CA).
All PCR reactions were performed in a final volume of 20 μL on an ABI PRISM 7900 Sequence Detection System (Applied Biosystems) with 1 μL cDNA template. The PCR condition was 95°C for 12 minutes followed by 40 cycles of 95°C for 15 seconds and 60°C for 1 minute. All quantitative PCR reactions were done in triplicate and repeated at least twice.
Statistical Analysis
Nonparametric Kruskal-Wallis and Mann-Whitney rank-sum tests were used to determine differences in normalized mRNA expression levels. A P value <0.05 was considered statistically significant. Data are presented as mean ± standard deviation unless otherwise stated.
We used logistic regression analysis to develop a scoring model and determine if each gene was an independent marker for malignant thyroid neoplasms and to define a cutoff point. To evaluate the performance of the logistic regression scoring model for gene expression levels as diagnostic markers of malignant thyroid neoplasms, we determined the area under the receiver operating characteristic (ROC) curve (AUC). ROC curves were plotted for individual variables and for scores derived from logistic regression fits. We identified points corresponding to equal penalties for misclassification and points with estimated sensitivity above 95%. Because logistic regression scores were constructed, we identified a cutoff point corresponding to 99% sensitivity. We divided the sample at random into 10 parts and used the same approach (fitting a logistic regression model and identifying the score corresponding to 99% sensitivity) on nine tenths of the data (training data) using the score to identify positive/negative on the remaining tenth of the data (test data), each time producing a table that cross-classified histology with test status. This procedure was repeated 10 times, each time testing on a different tenth. The 10 tables were summed and proportions derived from them were used to get unbiased estimates of sensitivity, specificity, positive predictive value, and negative predictive value.
RESULTS
Extracellular Matrix and Adhesion Molecule cDNA Array Analysis
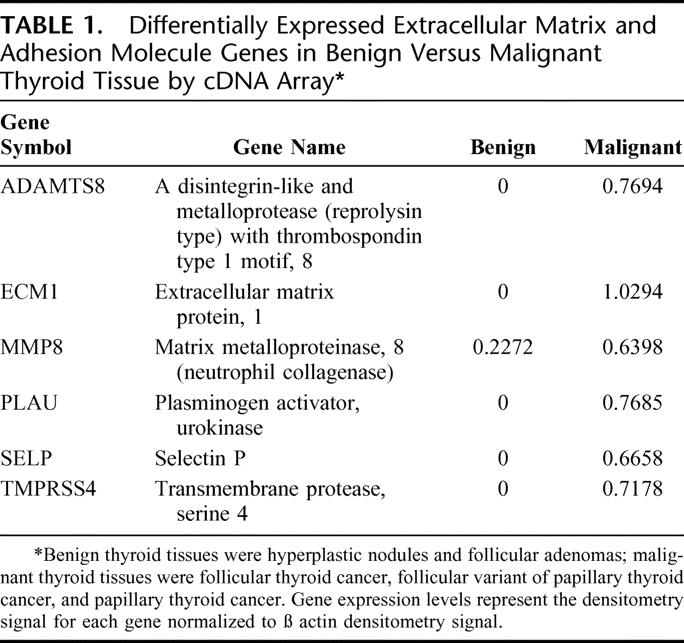
In all of the cDNA array blots, the endogenous control genes were positive and the negative control genes had no hybridization signals. Comparison of cDNA array blots of hyperplastic nodules and follicular adenoma to follicular cancer, follicular variant of papillary thyroid cancer, and papillary thyroid cancer samples identified 6 differentially expressed genes: ADAMTS8, ECM1, MMP8, PLAU, SELP, and TMPRSS4 (Table 1). The intraassay variability of the densitometry measurements was ≤5.0%.
TABLE 1. Differentially Expressed Extracellular Matrix and Adhesion Molecule Genes in Benign Versus Malignant Thyroid Tissue by cDNA Array
Real-Time Quantitative Polymerase Chain Reaction
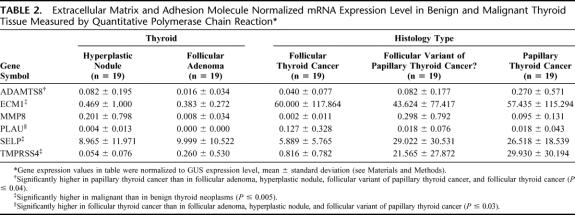
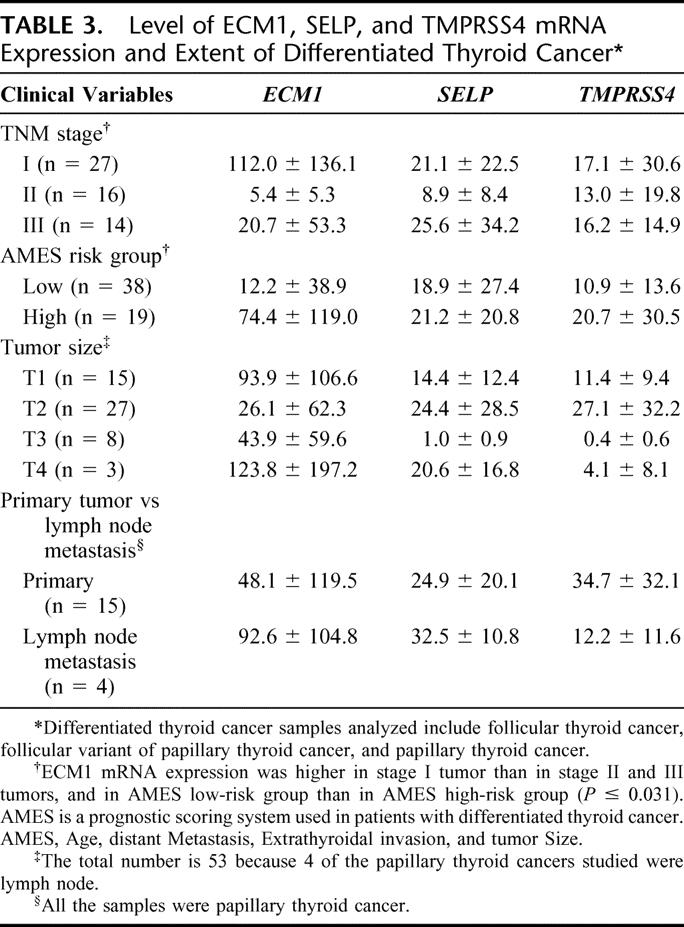
Quantitative PCR measurements of the 6 differentially expressed genes showed that ECM1, SELP, and TMPRSS4 mRNA expression were higher in malignant (follicular cancer, follicular variant of papillary thyroid cancer, and papillary thyroid cancer) than in benign (hyperplastic nodules and follicular adenoma) thyroid neoplasms (P ≤ 0.005) (Table 2). When we excluded cases of papillary thyroid cancer, which usually are accurately diagnosed by FNA biopsy, the levels of ECM1 and TMPRSS4 mRNA expression were higher in follicular thyroid cancers and the follicular variant of papillary thyroid cancers than in hyperplastic nodules and follicular adenomas (P < 0.003). The overlap in gene expression levels between benign and malignant thyroid neoplasms was 12.6% for ECM1 (5 follicular cancer, 2 follicular variant of papillary cancer, 2 papillary cancer), 25.2% for TMPRSS4 (17 follicular cancer, 6 follicular variant of papillary cancer, 2 papillary cancer), and 34.7% for SELP (17 follicular cancer, 8 follicular variant of papillary cancer, 8 papillary cancer) (Fig. 1). The level of ECM1 mRNA expression was higher in TNM stage I differentiated thyroid cancers than in stage II and III tumors, and higher in low-risk AMES than in high-risk AMES differentiated thyroid cancers (Table 3). There was no difference in the level of ECM1, SELP, and TMPRSS4 mRNA expression by tumor size or by primary tumor versus lymph node metastasis (Table 3).
TABLE 2. Extracellular Matrix and Adhesion Molecule Normalized mRNA Expression Level in Benign and Malignant Thyroid Tissue Measured by Quantitative Polymerase Chain Reaction
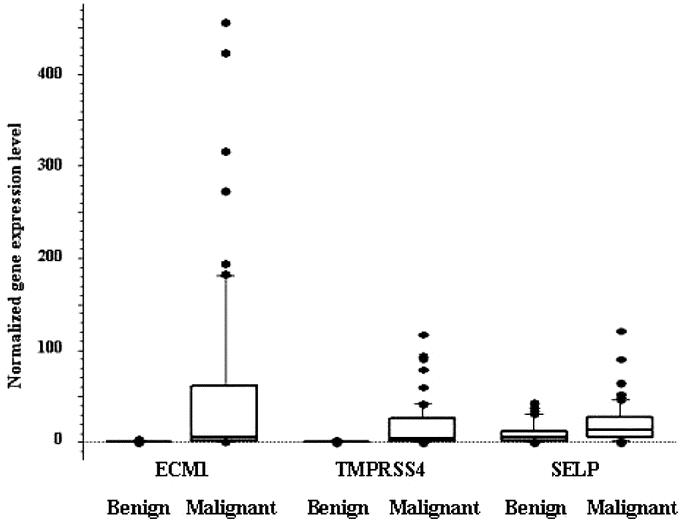
FIGURE 1. Box plot of ECM1, TMPRSS4, and SELP mRNA expression in benign and malignant thyroid neoplasms. The values are normalized ECM1, TMPRSS4, and SELP mRNA expression to GUS mRNA expression. The box represents 50% of data (above 25% percentile and below 75% percentile) and the line within the box is the median value.
TABLE 3. Level of ECM1, SELP, and TMPRSS4 mRNA Expression and Extent of Differentiated Thyroid Cancer
Diagnostic Accuracy of ECM1, SELP, and TMPRSS4 mRNA Expression Level as a Marker for Malignant Thyroid Neoplasms
Logistic regression analysis showed that ECM1 and TMPRSS4 mRNA expression levels were independent markers of malignant thyroid neoplasms but SELP mRNA expression level was not (P < 0.003). The scoring model generated was: total score = 1.78 + 4.234 log10(ECM1 + 0.01) + 2.148 log10(TMPRSS4 + 0.01) with a cutoff point of −1.344, so that when the score is ≥−1.344, the case would be considered malignant. The AUC was 0.956 for ECM1 and 0.926 for TMPRSS4 (Fig. 2). Combining both markers improved their diagnostic use, yielding an AUC of 0.985 with a sensitivity of 91.7%, specificity of 89.8%, positive predictive value of 85.7%, and negative predictive value of 82.8%. Because the diagnostic accuracy of quantitative PCR depends on the reproducibility of the gene expression levels, we also compared the intraassay variability of normalized ECM1 and TMPRSS4 mRNA expression levels. The correlation of normalized ECM1 and TMPRSS4 mRNA levels between experiments was excellent (R2 = 0.938, P < 0.0001).
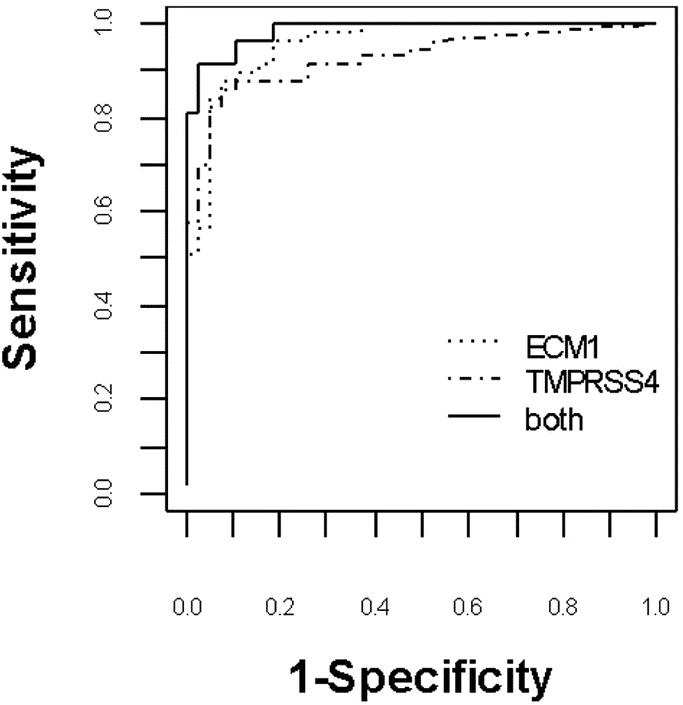
FIGURE 2. Receiver operating characteristic curve of normalized ECM1 and TMPRSS4 mRNA expression for distinguishing benign from malignant thyroid neoplasms. Combined use of both markers was more accurate than one marker alone. Area under the curve of 1.0 represents a “perfect” diagnostic test without any false-negative or false-positive results.
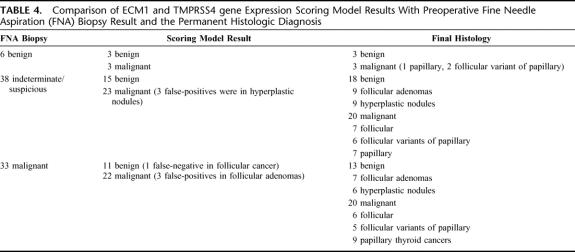
Comparison of the ECM1 and TMPRRS4 scoring model results to the preoperative FNA biopsy results showed that gene expression analysis was more accurate than FNA cytology (Table 4). In 35 of 38 indeterminate or suspicious FNA biopsy results, ECM1 and TMPRSS4 correctly distinguished between benign and malignant thyroid neoplasms (Table 4). Furthermore, 3 of 6 nodules considered benign by FNA biopsy were correctly identified as malignant, and 11 of 32 nodules considered malignant by FNA were correctly identified as benign.
TABLE 4. Comparison of ECM1 and TMPRSS4 gene Expression Scoring Model Results With Preoperative Fine Needle Aspiration (FNA) Biopsy Result and the Permanent Histologic Diagnosis
Feasibility of ECM1 and TMPRSS4 mRNA Measurement in Fine Needle Aspiration Biopsy Samples
The accuracy of ECM1 and TMPRSS4 mRNA expression for distinguishing malignant from benign thyroid neoplasms was determined using frozen thyroid tissue. To ensure that FNA biopsy samples would yield enough total RNA for real-time quantitative PCR gene expression analysis, we evaluated 12 intraoperatively obtained FNA samples of thyroid nodules and were able to obtain 406 ng/μL to 914 ng/μL of total RNA using a 25-gauge needle. This finding suggests that enough thyroid tissue would be available from FNA biopsy to test the accuracy of ECM1 and TMPRSS4 mRNA expression in distinguishing malignant from benign thyroid neoplasms. Most importantly, the scoring model for ECM1 and TMPRSS4 mRNA expression correctly identified 10 of 11 benign (4 hyperplastic nodules, 2 Hürthle cell adenoma, one follicular adenoma [false-positive], and one of one malignant [one papillary thyroid cancer] thyroid nodules).
Validation of Diagnostic Logistic Regression Scoring Model for ECM1 and TMPRSS4 mRNA Expression Levels
The accuracy of the logistic regression scoring model, which used normalized ECM1 and TMPRSS4 mRNA expression cutoff levels to distinguish malignant from benign thyroid neoplasms, was determined in 95 patients with known benign and malignant thyroid histologic diagnoses. A bias may have been introduced in measuring the accuracy because of the lack of blinding to the thyroid tissue histology. We therefore evaluated an additional 36 thyroid samples (9 hyperplastic nodules, 9 follicular adenomas, 6 follicular cancers, 7 papillary cancers, and 5 anaplastic thyroid cancer) as “unknown” to validate the regression scoring model and confirm the diagnostic accuracy of normalized ECM1 and TMPRSS4 mRNA expression levels. In these samples, the model had a sensitivity of 90.0%, a specificity of 92.6%, a positive predictive value of 81.8%, and a negative predictive value of 96.1%.
DISCUSSION
Our study focused on thyroid nodules that generally cannot be accurately diagnosed as benign or malignant neoplasms by preoperative FNA cytology.5,6,78 Of 96 extracellular matrix and adhesion molecule genes, 6 were differentially expressed by more than 2-fold in malignant thyroid neoplasms. However, only ECM1, SELP, and TMPRSS4 mRNA expression were significantly higher in malignant than in benign thyroid neoplasms by quantitative PCR measurement. Of these 3 genes, ECM1 and TMPRSS4 were independent markers of malignant thyroid neoplasms. The diagnostic accuracy of these 2 markers was best when used in combination.
The function of the ECM1, TMPRSS4, and SELP genes in thyroid cancer is unknown. The ECM1 gene encodes an extracellular matrix protein and is located on chromosome 1q21.79 ECM1 has several physiological functions that include bone development and maintenance of the extracellular matrix.80–83 It also regulates cell proliferation and angiogenesis in breast cancer cells. Upregulated ECM1 protein expression has been observed in a variety of human epithelial tumors.80 In a study of serial analysis of gene expression (SAGE) to identify transcripts that may distinguish benign from malignant thyroid neoplasms, elevated ECM1 expression has been observed in thyroid tumors.84 The level of normalized ECM1 mRNA expression was higher in low-risk tumors than in high-risk tumors. This finding is not surprising because more aggressive or transformed cancer cells are capable of growth without interactions between the cell surface and extracellular matrix for survival and cell cycle progression.85 This would suggest then that the decreased ECM1 mRNA expression in more aggressive differentiated thyroid cancer tumors may reflect cell anchorage-independent tumor growth. The TMPRSS4 gene, initially referred to as TMPRSS3, is located on chromosome 11.q23.3 and encodes a member of the serine protease family.86 This gene, to our knowledge, has not been previously reported to be differentially expressed in thyroid neoplasms, but is overexpressed in pancreatic carcinoma.87 SELP is a member of the selectin family adhesion molecules that mediate calcium-dependent cell–cell interactions in endothelial cells and activated platelets88 and is thought to be necessary for tumor cell growth and metastasis.89 Increased expression of SELP has been observed in endothelial cells of colorectal cancers, and elevated serum levels have been reported in patients with breast cancer, lung cancer, lymphoma, and melanoma.90–92 We found that ECM1, TMPRSS4, and SELP were overexpressed in malignant thyroid neoplasms of follicular cell origin. These findings suggest that ECM1, TMPRSS4, and SELP may play a role in thyroid carcinogenesis.
Although numerous molecular markers of thyroid cancer have been studied, they have had little clinical use in follicular tumors, and the clinical feasibility of performing cDNA microarray analysis on FNA samples remains unclear. In this context, the high sensitivity and specificity of ECM1 and TMPRSS4 expression that we observed have important clinical ramifications. The need for “diagnostic” thyroidectomy can be reduced or eliminated if the risk of malignancy of a thyroid nodule can be reliably predicted by measuring ECM1 and TMPRSS4 mRNA expression levels. As a result, some or all of the diagnostic thyroidectomies being performed for benign thyroid nodules could be eliminated, as could the morbidity associated with thyroidectomy. Reliable prediction would also reduce the cost of managing thyroid nodules because a more definitive operation can be performed in the first place without the need for reoperations in patients who are subsequently found to have a malignant thyroid neoplasm on permanent histology.
We have demonstrated in a limited number of FNA samples that quantitative PCR measurement for ECM1 and TMPRSS4 mRNA expression is feasible and that the logistic scoring model is accurate. These results suggest that ECM1 and TMPRSS4 would be useful diagnostic markers as an adjunct to FNA biopsy with a higher accuracy than that of FNA cytology. However, the diagnostic accuracy of these markers needs to be evaluated in Hürthle cell neoplasms, which are indeterminate on FNA cytology. Furthermore, our findings need to be confirmed by others. This should be readily accomplished because quantitative PCR is an easy and accurate technique, the primer and probes used in this study are commercially available, and our results were highly reproducible from experiment to experiment. In addition, quantitative PCR requires only a small amount of RNA, which would be readily available from FNA samples, as we and others have demonstrated.93,94
In summary, we found that ECM1, SELP, and TMPRSS4 are overexpressed in malignant thyroid neoplasms of follicular cell origin. ECM1 and TMPRSS4 appear to be helpful diagnostic markers for distinguishing malignant from benign thyroid follicular neoplasms. ECM1 is a marker of extent of disease for differentiated thyroid cancer.
ACKNOWLEDGMENTS
Supported in part by the University of California Cancer Research Coordinating Committee, the Harold Amos Medical Faculty Development Program of the Robert Wood Johnson Foundation, and a Hellman Family grant.
Discussions
Dr. Richard A. Prinz (Chicago, Illinois): I would like to congratulate and compliment Dr. Kebebew and the San Francisco group for tackling an important clinical problem in surgical endocrinology. Fifteen-twenty% of patients having a diagnostic fine needle aspiration biopsy of a thyroid nodule will have an indeterminate result and require a diagnostic thyroidectomy to differentiate hyperplastic nodules and follicular and Hurthle cell adenomas from follicular and Hurthle cell carcinoma. A long and growing list of methods have been tested to see if this can be decreased. These have included imaging studies such as MRI and PET scanning, immunochemistry of various molecular markers, and, more recently, cDNA microarrays.
Dr. Kebebew used a directed cDNA microarray analysis to identify genes differentially expressed in benign and malignant thyroid nodules. This is followed by realtime quantitative PCR of two genes, ECM1 and TMPRSS4. He suggests that this technology can be used to improve the diagnostic accuracy of fine needle aspiration biopsy.
We know that the results of cytology depend heavily on the skill and experience of the cytologist. Do the results obtained in your study depend on the skill and experience of your lab? In other words, just how transferable are the results that you have shown us today?
Also, in comparing your technique to cytology, did a single experienced cytologist read the biopsies or were multiple cytologists involved?
I believe you are suggesting that this technique is a complement to conventional cytology. Do you have any experience showing that both can be done together in the preoperative evaluation, not just in the operating room? Since the biopsy material has to be handled differently for both tests, does it pose any problems either in the collection of the specimens or the performance of each test?
With regard to your methodology, does overlap in gene expression caused either by sampling issues or concomitant conditions in the thyroid such as thyroiditis diminish its accuracy?
Since the usual clinical issue is to distinguish whether a follicular neoplasm is actually a benign follicular adenoma or a malignant follicular carcinoma, what are your results if you exclude follicular variant of papillary cancer in addition to papillary cancer? Are they just as good?
The ECM1 levels seem to be higher in low risk early-stage thyroid cancers. Does this pose any problem with the identification or diagnosis of late-stage tumors with your methodology?
Finally, have you determined whether these genes have any functional activity either in the pathogenesis or in the progression of follicular thyroid cancer?
Dr. Electron Kebebew (San Francisco, California): I think your first question was whether these results would be reproducible in other laboratories. I believe that it is feasible. One approach would be to use a reference sample to standardize the results amongst different laboratories. We would be happy and interested to have our colleagues that practice endocrine surgery join us in doing a clinical trial to validate and confirm these results.
The second question was whether an experienced cytopathologist looked at all of these slides. And I think that is an important point, because we know when experienced cytopathologists look at the FNA biopsy, the diagnosis or the management approach might change. We have seen this to be the case at our institution.
To answer your question: no, the comparison was made based on what the FNA diagnosis was preoperatively without secondary review by an experienced cytopathologist. Some of these samples were not reviewed by our cytopathologists at our institution and were reviewed by other cytopathologists.
The other issue was, is it technically possible to do concurrently gene expression analysis on FNA biopsy samples? In the intraoperative FNA samples, we only performed four passes, and in general most cytopathologists are doing up to ten passes. Therefore I think it is technically feasible.
Yes, the segregation of AMES low-risk and high-risk differentiated thyroid cancer based on the level of ECM1 gene expression is interesting. We don't have a good explanation for why it is higher in low-risk tumors. But it might be that the more aggressive tumors undergo anchorage independent growth. And there are examples of this in other types of cancer. But we don't really know the functional consequence of the difference in expression levels, and that is certainly something to explore in the future.
I think the other question you asked was, what could account for the false positives? And we were interested in looking at the presence of chronic lymphocytic thyroiditis as a possible explanation for this because it has been reported in other molecular marker studies to account for false positives. Interestingly, we had 22 samples that did have thyroiditis present, but that didn't make a difference on whether the results were false positive, true positive, true negative or false negative.
Dr. Ashok R. Shaha (New York, New York): We looked at the MUC1, and there was survival data, that showed some differences. The question I have is: I was curious, you included some anaplastic thyroid cancers in your study. That should be a different entity, and fine needle biopsy should at least give you some suspicion.
The second question I have is: Did you find any difference between minimally invasive follicular cancer and widely invasive follicular cancer? Because that is where probably the difference would be in the—follicular lesion which is diagnosed on needle biopsy.
Did the size or the age of the patient matter? If the lesion is more than 3 centimeters we are generally going to take it out most of the time no matter what the fine needle aspiration shows, especially if it is a solid thyroid nodule.
The last question: In the initial slide you mentioned this is probably the most rising type of cancer in the human body in the United States. More and more incidentalomas we are going to see now and would this technique help us in evaluating and managing these incidentalomas?
Dr. Electron Kebebew (San Francisco, California): Since we were blinded to the histologic diagnosis, these samples were evaluated as unknowns to validate the scorin model and thus account for the anaplastic thyroid cancer cases. Obviously this was an unintended consequence of being totally blinded to evaluating the unknown samples. As far as the question of minimally invasive versus widely invasive follicular thyroid cancer, I do agree with you that is an important issue. Unfortunately, the samples of follicular thyroid cancer that we studied were widely invasive, so that would be something that we would need to study further to see if it would be useful in distinguishing between minimally and widely invasive follicular thyroid cancer.
We did evaluate the level of expression as related to the age and the tumor size and didn't find a difference, except for ECM1. And surprisingly, we found it was higher in those that were younger than those that were older. And that might account for the segregation or the differences we saw in the AMES high-risk and low-risk tumors.
The last question, I believe, was: could this be useful in the management of incidentalomas? I am sure you are familiar with the work from the Japanese group where they elected to observe some patients with occult papillary thyroid cancers that were less than 1 centimeter in size and found that 70% of these patients didn't have progressive cancers. I think these markers may be a way of segregating out those occult tumors that might be aggressive or not. A study like that probably would not be possible to do here in the U.S. with the standards of our IRB.
Dr. Christopher R. McHenry (Cleveland, Ohio): I wonder if the sensitivity for ECM1 or TMPRSS4 expression varies for specific subsets of patients with an indeterminant fine needle aspiration biopsy, specifically in the patient that has a fine needle aspiration biopsy that is suspicious but not definitive for papillary thyroid cancer. Would you predict similar or better results for distinguishing benign versus malignant disease?
My second question is: Can you improve the sensitivity or usefulness of these markers if you compared neoplastic versus nonneoplastic disease rather than benign versus malignant disease?
Finally, do you foresee there to be any role for evaluating ECM1 and TMPRSS4 expression in patients with a thyroid nodule and a benign fine needle aspiration biopsy to help reduce the incidence of false negative fine needle aspiration biopsy?
Dr. Electron Kebebew (San Francisco, California): I think it is true that the sensitivity and specificity depend upon the histologies that we have evaluated. And if we did exclude those suspicious that would be follicular variant of papillary thyroid cancer, I don't know if it would be as accurate. But the initial analysis did exclude typical papillary thyroid cancer samples. And that is how we arrived at those two genes out of the three that were significantly different between benign and malignant tumors.
It is an interesting question that you asked as far as the difference between neoplastic and nonneoplastic thyroid tissue. Clinically, however, we are dealing with patients that have a nodule either benign or malignant, so we never really are trying to exclude a malignancy in normal thyroid or thyroiditis glands without a tumor. To answer your last question, I think that would potentially be very useful as demonstrated in those 6 patients that had an initial benign FNA result where we found 3 of them were malignant and proven histologically to be malignant but they had other clinical indications that led them to have an operation. So I think that would be useful in that sense and we need to do these studies to see if the accuracy is really superior to FNA biopsy and cytologic examination.
Dr. Herbert Chen (Madison, Wisconsin): I see 1 potential limitation with your technology. Because you are looking for differences in RNA levels, you stick the nodule once for the FNA and a second time for the sample for your analysis, and theoretically you could be giving 2 separate distinct samples. So I was wondering if you have looked at expression levels of the proteins. That way you could possibly get around this by doing immunohistochemistry. You could FNA once, look at it under the microscope, and then stain that same slide to make sure you are actually doing your test on the same sample that you are looking at by FNA.
Dr. Electron Kebebew (San Francisco, California): It is actually something we have thought about, that is a sampling error, whether the cytologic sample is the same as the sample for gene expression analysis. But there are investigators that have demonstrated it is possible to perform PCR on emptied FNA needle samples. This is because only a small amount of RNA is required for real-time quantitative PCR.
Footnotes
Reprints: Electron Kebebew, MD, FACS, Endocrine Surgery and Oncology Program, UCSF Comprehensive Cancer Center, Box 1674, San Francisco, CA 94143-1674. E-mail: kebebewe@surgery.ucsf.edu.
REFERENCES
- 1.Jemal A, Clegg LX, Ward E, et al. Annual report to the nation on the status of cancer, 1975–2001, with a special feature regarding survival. Cancer. 2004;101:3–27. [DOI] [PubMed] [Google Scholar]
- 2.Silver RJ, Parangi S. Management of thyroid incidentalomas. Surg Clin North Am. 2004;84:907–919. [DOI] [PubMed] [Google Scholar]
- 3.Uchino S, Noguchi S, Yamashita H, et al. Detection of asymptomatic differentiated thyroid carcinoma by neck ultrasonographic screening for familial nonmedullary thyroid carcinoma. World J Surg. 2004;10:14. [DOI] [PubMed] [Google Scholar]
- 4.Mazzaferri EL. Management of a solitary thyroid nodule. N Engl J Med. 1993;328:553–559. [DOI] [PubMed] [Google Scholar]
- 5.Greaves TS, Olvera M, Florentine BD, et al. Follicular lesions of thyroid: a 5-year fine-needle aspiration experience. Cancer. 2000;90:335–341. [PubMed] [Google Scholar]
- 6.Baloch ZW, Fleisher S, LiVolsi VA, et al. Diagnosis of ‘follicular neoplasm’: a gray zone in thyroid fine-needle aspiration cytology. Diagn Cytopathol. 2002;26:41–44. [DOI] [PubMed] [Google Scholar]
- 7.Gharib H, Goellner JR. Fine-needle aspiration biopsy of the thyroid: an appraisal. Ann Intern Med. 1993;118:282–289. [DOI] [PubMed] [Google Scholar]
- 8.Cersosimo E, Gharib H, Suman VJ, et al. ‘Suspicious’ thyroid cytologic findings: outcome in patients without immediate surgical treatment. Mayo Clin Proc. 1993;68:343–348. [DOI] [PubMed] [Google Scholar]
- 9.Gharib H, Goellner JR, Johnson DA. Fine-needle aspiration cytology of the thyroid. A 12-year experience with 11,000 biopsies. Clin Lab Med. 1993;13:699–709. [PubMed] [Google Scholar]
- 10.Hooft L, Hoekstra OS, Boers M, et al. Practice, efficacy, and costs of thyroid nodule evaluation: a retrospective study in a Dutch university hospital. Thyroid. 2004;14:287–293. [DOI] [PubMed] [Google Scholar]
- 11.Tuttle RM, Lemar H, Burch HB. Clinical features associated with an increased risk of thyroid malignancy in patients with follicular neoplasia by fine-needle aspiration. Thyroid. 1998;8:377–383. [DOI] [PubMed] [Google Scholar]
- 12.Basu D, Jayaram G. A logistic model for thyroid lesions. Diagn Cytopathol. 1992;8:23–27. [DOI] [PubMed] [Google Scholar]
- 13.Eldar S, Sabo E, Cohen A, et al. The value of histomorphometric nuclear parameters in the diagnosis of well differentiated follicular carcinomas and follicular adenomas of the thyroid gland. Histopathology. 1999;34:453–461. [DOI] [PubMed] [Google Scholar]
- 14.Tyler DS, Winchester DJ, Caraway NP, et al. Indeterminate fine-needle aspiration biopsy of the thyroid: identification of subgroups at high risk for invasive carcinoma. Surgery. 1994;116:1054–1060. [PubMed] [Google Scholar]
- 15.Wreesmann VB, Sieczka EM, Socci ND, et al. Genome-wide profiling of papillary thyroid cancer identifies MUC1 as an independent prognostic marker. Cancer Res. 2004;64:3780–3789. [DOI] [PubMed] [Google Scholar]
- 16.Aldred MA, Huang Y, Liyanarachchi S, et al. Papillary and follicular thyroid carcinomas show distinctly different microarray expression profiles and can be distinguished by a minimum of five genes. J Clin Oncol. 2004;22:3531–3539. [DOI] [PubMed] [Google Scholar]
- 17.Umbricht CB, Conrad GT, Clark DP, et al. Human telomerase reverse transcriptase gene expression and the surgical management of suspicious thyroid tumors. Clin Cancer Res. 2004;10:5762–5768. [DOI] [PubMed] [Google Scholar]
- 18.Volante M, Bozzalla-Cassione F, Orlandi F, et al. Diagnostic role of galectin-3 in follicular thyroid tumors. Virchows Arch. 2004;444:309–312. [DOI] [PubMed] [Google Scholar]
- 19.Weber KB, Shroyer KR, Heinz DE, et al. The use of a combination of galectin-3 and thyroid peroxidase for the diagnosis and prognosis of thyroid cancer. Am J Clin Pathol. 2004;122:524–531. [DOI] [PubMed] [Google Scholar]
- 20.Marques AR, Espadinha C, Frias MJ, et al. Underexpression of peroxisome proliferator-activated receptor (PPAR)gamma in PAX8/PPARgamma-negative thyroid tumours. Br J Cancer. 2004;91:732–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hibi Y, Nagaya T, Kambe F, et al. Is thyroid follicular cancer in Japanese caused by a specific t(2;3) (q13; p25) translocation generating Pax8-PPAR gamma fusion mRNA? Endocr J. 2004;51:361–366. [DOI] [PubMed] [Google Scholar]
- 22.Nikiforova MN, Biddinger PW, Caudill CM, et al. PAX8-PPARgamma rearrangement in thyroid tumors: RT-PCR and immunohistochemical analyses. Am J Surg Pathol. 2002;26:1016–1023. [DOI] [PubMed] [Google Scholar]
- 23.Finley DJ, Zhu B, Barden CB, et al. Discrimination of benign and malignant thyroid nodules by molecular profiling. Ann Surg. 2004;240:425–436; discussion 436-427. [DOI] [PMC free article] [PubMed]
- 24.Finley DJ, Arora N, Zhu B, et al. Molecular profiling distinguishes papillary carcinoma from benign thyroid nodules. J Clin Endocrinol Metab. 2004;89:3214–3223. [DOI] [PubMed] [Google Scholar]
- 25.Herrmann ME, LiVolsi VA, Pasha TL, et al. Immunohistochemical expression of galectin-3 in benign and malignant thyroid lesions. Arch Pathol Lab Med. 2002;126:710–713. [DOI] [PubMed] [Google Scholar]
- 26.Mazzanti C, Zeiger MA, Costouros NG, et al. Using gene expression profiling to differentiate benign versus malignant thyroid tumors. Cancer Res. 2004;64:2898–2903. [DOI] [PubMed] [Google Scholar]
- 27.Rosai J. Immunohistochemical markers of thyroid tumors: significance and diagnostic applications. Tumori. 2003;89:517–519. [DOI] [PubMed] [Google Scholar]
- 28.Raphael SJ. The meanings of markers: ancillary techniques in diagnosis of thyroid neoplasia. Endocr Pathol. 2002;13:301–311. [DOI] [PubMed] [Google Scholar]
- 29.Smida J, Salassidis K, Hieber L, et al. Distinct frequency of ret rearrangements in papillary thyroid carcinomas of children and adults from Belarus. Int J Cancer. 1999;80:32–38. [DOI] [PubMed] [Google Scholar]
- 30.Cheung CC, Carydis B, Ezzat S, et al. Analysis of ret/PTC gene rearrangements refines the fine needle aspiration diagnosis of thyroid cancer. J Clin Endocrinol Metab. 2001;86:2187–2190. [DOI] [PubMed] [Google Scholar]
- 31.Wirtschafter A, Schmidt R, Rosen D, et al. Expression of the RET/PTC fusion gene as a marker for papillary carcinoma in Hashimoto's thyroiditis. Laryngoscope. 1997;107:95–100. [DOI] [PubMed] [Google Scholar]
- 32.Lacroix L, Mian C, Barrier T, et al. PAX8 and peroxisome proliferator-activated receptor gamma 1 gene expression status in benign and malignant thyroid tissues. Eur J Endocrinol. 2004;151:367–374. [DOI] [PubMed] [Google Scholar]
- 33.Volante M, Bozzalla-Cassione F, DePompa R, et al. Galectin-3 and HBME-1 expression in oncocytic cell tumors of the thyroid. Virchows Arch. 2004;445:183–188. [DOI] [PubMed] [Google Scholar]
- 34.Dwight T, Thoppe SR, Foukakis T, et al. Involvement of the PAX8/peroxisome proliferator-activated receptor gamma rearrangement in follicular thyroid tumors. J Clin Endocrinol Metab. 2003;88:4440–4445. [DOI] [PubMed] [Google Scholar]
- 35.Marques AR, Espadinha C, Catarino AL, et al. Expression of PAX8-PPAR gamma 1 rearrangements in both follicular thyroid carcinomas and adenomas. J Clin Endocrinol Metab. 2002;87:3947–3952. [DOI] [PubMed] [Google Scholar]
- 36.Tuccari G, Barresi G. Immunohistochemical demonstration of ceruloplasmin in follicular adenomas and thyroid carcinomas. Histopathology. 1987;11:723–731. [DOI] [PubMed] [Google Scholar]
- 37.Yossie Asato de Camargo R, Longatto Filho A, Alves VA, et al. Lactoferrin in thyroid lesions: immunoreactivity in fine needle aspiration biopsy samples. Acta Cytol. 1996;40:408–413. [DOI] [PubMed] [Google Scholar]
- 38.Ross JS, del Rosario AD, Sanderson B, et al. Selective expression of CD44 cell-adhesion molecule in thyroid papillary carcinoma fine-needle aspirates. Diagn Cytopathol. 1996;14:287–291. [DOI] [PubMed] [Google Scholar]
- 39.Komminoth P, Seelentag WK, Saremaslani P, et al. CD44 isoform expression in the diffuse neuroendocrine system. II. Benign and malignant tumors. Histochem Cell Biol. 1996;106:551–562. [DOI] [PubMed] [Google Scholar]
- 40.Tallini G. Molecular pathobiology of thyroid neoplasms. Endocr Pathol. 2002;13:271–288. [DOI] [PubMed] [Google Scholar]
- 41.Nikiforov YE. RET/PTC rearrangement in thyroid tumors. Endocr Pathol. 2002;13:3–16. [DOI] [PubMed] [Google Scholar]
- 42.Ippolito A, Vella V, La Rosa GL, et al. Immunostaining for Met/HGF receptor may be useful to identify malignancies in thyroid lesions classified suspicious at fine-needle aspiration biopsy. Thyroid. 2001;11:783–787. [DOI] [PubMed] [Google Scholar]
- 43.Zanetti A, Stoppacciaro A, Marzullo A, et al. Expression of Met protein and urokinase-type plasminogen activator receptor (uPA-R) in papillary carcinoma of the thyroid. J Pathol. 1998;186:287–291. [DOI] [PubMed] [Google Scholar]
- 44.Salvatore G, Giannini R, Faviana P, et al. Analysis of BRAF point mutation and RET/PTC rearrangement refines the fine-needle aspiration diagnosis of papillary thyroid carcinoma. J Clin Endocrinol Metab. 2004;89:5175–5180. [DOI] [PubMed] [Google Scholar]
- 45.Kroll TG, Sarraf P, Pecciarini L, et al. PAX8-PPARgamma1 fusion oncogene in human thyroid carcinoma. Science. 2000;289:1357–1360. [DOI] [PubMed] [Google Scholar]
- 46.Cheung L, Messina M, Gill A, et al. Detection of the PAX8-PPAR gamma fusion oncogene in both follicular thyroid carcinomas and adenomas. J Clin Endocrinol Metab. 2003;88:354–357. [DOI] [PubMed] [Google Scholar]
- 47.Ho YS, Tseng SC, Chin TY, et al. p53 gene mutation in thyroid carcinoma. Cancer Lett. 1996;103:57–63. [DOI] [PubMed] [Google Scholar]
- 48.Fabbro D, Di Loreto C, Beltrami CA, et al. Expression of thyroid-specific transcription factors TTF-1 and PAX-8 in human thyroid neoplasms. Cancer Res. 1994;54:4744–4749. [PubMed] [Google Scholar]
- 49.Suzuki S, Fukushima T, Ami H, et al. New attempt of preoperative differential diagnosis of thyroid neoplasms by telomerase activity measurement. Oncol Rep. 2002;9:539–544. [PubMed] [Google Scholar]
- 50.Liou MJ, Chan EC, Lin JD, et al. Human telomerase reverse transcriptase (hTERT) gene expression in FNA samples from thyroid neoplasms. Cancer Lett. 2003;191:223–227. [DOI] [PubMed] [Google Scholar]
- 51.Hashimoto T, Matsubara F, Mizukami Y, et al. Tumor markers and oncogene expression in thyroid cancer using biochemical and immunohistochemical studies. Endocrinol Jpn. 1990;37:247–254. [DOI] [PubMed] [Google Scholar]
- 52.Yoshida K, Hamatani K, Koide H, et al. Preparation of anti-ras Mr 21,000 protein monoclonal antibodies and immunohistochemical analyses on expression of ras genes in human stomach and thyroid cancers. Cancer Res. 1988;48:5503–5509. [PubMed] [Google Scholar]
- 53.Rorive S, Eddafali B, Fernandez S, et al. Changes in galectin-7 and cytokeratin-19 expression during the progression of malignancy in thyroid tumors: diagnostic and biological implications. Mod Pathol. 2002;15:1294–1301. [DOI] [PubMed] [Google Scholar]
- 54.Beesley MF, McLaren KM. Cytokeratin 19 and galectin-3 immunohistochemistry in the differential diagnosis of solitary thyroid nodules. Histopathology. 2002;41:236–243. [DOI] [PubMed] [Google Scholar]
- 55.Mai KT, Bokhary R, Yazdi HM, et al. Reduced HBME-1 immunoreactivity of papillary thyroid carcinoma and papillary thyroid carcinoma-related neoplastic lesions with Hürthle cell and/or apocrine-like changes. Histopathology. 2002;40:133–142. [DOI] [PubMed] [Google Scholar]
- 56.Raphael SJ, McKeown-Eyssen G, Asa SL. High-molecular-weight cytokeratin and cytokeratin-19 in the diagnosis of thyroid tumors. Mod Pathol. 1994;7:295–300. [PubMed] [Google Scholar]
- 57.Nishimura R, Yokose T, Mukai K. S-100 protein is a differentiation marker in thyroid carcinoma of follicular cell origin: an immunohistochemical study. Pathol Int. 1997;47:673–679. [DOI] [PubMed] [Google Scholar]
- 58.Orlandi F, Saggiorato E, Pivano G, et al. Galectin-3 is a presurgical marker of human thyroid carcinoma. Cancer Res. 1998;58:3015–3020. [PubMed] [Google Scholar]
- 59.Saggiorato E, Cappia S, De Giuli P, et al. Galectin-3 as a presurgical immunocytodiagnostic marker of minimally invasive follicular thyroid carcinoma. J Clin Endocrinol Metab. 2001;86:5152–5158. [DOI] [PubMed] [Google Scholar]
- 60.Abu-Alfa AK, Straus FH 2nd, Montag AG. An immunohistochemical study of thyroid Hürthle cells and their neoplasms: the roles of S-100 and HMB-45 proteins. Mod Pathol. 1994;7:529–532. [PubMed] [Google Scholar]
- 61.Erickson LA, Jin L, Wollan PC, et al. Expression of p27kip1 and Ki-67 in benign and malignant thyroid tumors. Mod Pathol. 1998;11:169–174. [PubMed] [Google Scholar]
- 62.Tallini G, Garcia-Rostan G, Herrero A, et al. Downregulation of p27KIP1 and Ki67/Mib1 labeling index support the classification of thyroid carcinoma into prognostically relevant categories. Am J Surg Pathol. 1999;23:678–685. [DOI] [PubMed] [Google Scholar]
- 63.Nascimento MC, Bisi H, Alves VA, et al. Differential reactivity for galectin-3 in Hürthle cell adenomas and carcinomas. Endocr Pathol. 2001;12:275–279. [DOI] [PubMed] [Google Scholar]
- 64.Kovacs RB, Foldes J, Winkler G, et al. The investigation of galectin-3 in diseases of the thyroid gland. Eur J Endocrinol. 2003;149:449–453. [DOI] [PubMed] [Google Scholar]
- 65.Jakubiak-Wielganowicz M, Kubiak R, Sygut J, et al. Usefulness of galectin-3 immunohistochemistry in differential diagnosis between thyroid follicular carcinoma and follicular adenoma. Pol J Pathol. 2003;54:111–115. [PubMed] [Google Scholar]
- 66.Cox JM. Applications of nylon membrane arrays to gene expression analysis. J Immunol Methods. 2001;250:3–13. [DOI] [PubMed] [Google Scholar]
- 67.Jordan B. Historical background and anticipated developments. Ann N Y Acad Sci. 2002;975:24–32. [DOI] [PubMed] [Google Scholar]
- 68.Hess KR, Zhang W, Baggerly KA, et al. Microarrays: handling the deluge of data and extracting reliable information. Trends Biotechnol. 2001;19:463–468. [DOI] [PubMed] [Google Scholar]
- 69.Hornberg JJ, de Haas RR, Dekker H, et al. Analysis of multiple gene expression array experiments after repetitive hybridizations on nylon membranes. Biotechniques. 2002;33:108, 110, 112-113, passim. [DOI] [PubMed] [Google Scholar]
- 70.Barden CB, Shister KW, Zhu B, et al. Classification of follicular thyroid tumors by molecular signature: results of gene profiling. Clin Cancer Res. 2003;9:1792–1800. [PubMed] [Google Scholar]
- 71.Kothapalli R, Yoder SJ, Mane S, et al. Microarray results: how accurate are they? BMC Bioinformatics. 2002;3:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mah N, Thelin A, Lu T, et al. A comparison of oligonucleotide and cDNA-based microarray systems. Physiol Genomics. 2004;16:361–370. [DOI] [PubMed] [Google Scholar]
- 73.Hwang KB, Kong SW, Greenberg SA, et al. Combining gene expression data from different generations of oligonucleotide arrays. BMC Bioinformatics. 2004;5:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cairns RA, Khokha R, Hill RP. Molecular mechanisms of tumor invasion and metastasis: an integrated view. Curr Mol Med. 2003;3:659–671. [DOI] [PubMed] [Google Scholar]
- 75.Agrawal D, Chen T, Irby R, et al. Osteopontin identified as lead marker of colon cancer progression, using pooled sample expression profiling. J Natl Cancer Inst. 2002;94:513–521. [DOI] [PubMed] [Google Scholar]
- 76.Kendziorski CM, Zhang Y, Lan H, et al. The efficiency of pooling mRNA in microarray experiments. Biostatistics. 2003;4:465–477. [DOI] [PubMed] [Google Scholar]
- 77.Ginzinger DG. Gene quantification using real-time quantitative PCR: an emerging technology hits the mainstream. Exp Hematol. 2002;30:503–512. [DOI] [PubMed] [Google Scholar]
- 78.Baloch ZW, LiVolsi VA. The quest for a magic tumor marker: continuing saga in the diagnosis of the follicular lesions of thyroid. Am J Clin Pathol. 2002;118:165–166. [DOI] [PubMed] [Google Scholar]
- 79.Johnson MR, Wilkin DJ, Vos HL, et al. Characterization of the human extracellular matrix protein 1 gene on chromosome 1q21. Matrix Biol. 1997;16:289–292. [DOI] [PubMed] [Google Scholar]
- 80.Wang L, Yu J, Ni J, et al. Extracellular matrix protein 1 (ECM1) is over-expressed in malignant epithelial tumors. Cancer Lett. 2003;200:57–67. [DOI] [PubMed] [Google Scholar]
- 81.Mongiat M, Fu J, Oldershaw R, et al. Perlecan protein core interacts with extracellular matrix protein 1 (ECM1), a glycoprotein involved in bone formation and angiogenesis. J Biol Chem. 2003;278:17491–17499. [DOI] [PubMed] [Google Scholar]
- 82.Han Z, Ni J, Smits P, et al. Extracellular matrix protein 1 (ECM1) has angiogenic properties and is expressed by breast tumor cells. Faseb J. 2001;15:988–994. [DOI] [PubMed] [Google Scholar]
- 83.Deckers MM, Smits P, Karperien M, et al. Recombinant human extracellular matrix protein 1 inhibits alkaline phosphatase activity and mineralization of mouse embryonic metatarsals in vitro. Bone. 2001;28:14–20. [DOI] [PubMed] [Google Scholar]
- 84.Pauws E, Veenboer GJ, Smit JW, et al. Genes differentially expressed in thyroid carcinoma identified by comparison of SAGE expression profiles. Faseb J. 2004;18:560–561. [DOI] [PubMed] [Google Scholar]
- 85.Pawlak G, Helfman DM. Cytoskeletal changes in cell transformation and tumorigenesis. Curr Opin Genet Dev. 2001;11:41–47. [DOI] [PubMed] [Google Scholar]
- 86.Netzel-Arnett S, Hooper JD, Szabo R, et al. Membrane anchored serine proteases: a rapidly expanding group of cell surface proteolytic enzymes with potential roles in cancer. Cancer Metastasis Rev. 2003;22:237–258. [DOI] [PubMed] [Google Scholar]
- 87.Wallrapp C, Hahnel S, Muller-Pillasch F, et al. A novel transmembrane serine protease (TMPRSS3) overexpressed in pancreatic cancer. Cancer Res. 2000;60:2602–2606. [PubMed] [Google Scholar]
- 88.Geng JG, Chen M, Chou KC. P-selectin cell adhesion molecule in inflammation, thrombosis, cancer growth and metastasis. Curr Med Chem. 2004;11:2153–2160. [DOI] [PubMed] [Google Scholar]
- 89.Kim YJ, Borsig L, Varki NM, et al. P-selectin deficiency attenuates tumor growth and metastasis. Proc Natl Acad Sci U S A. 1998;95:9325–9330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Roselli M, Mineo TC, Martini F, et al. Soluble selectin levels in patients with lung cancer. Int J Biol Markers. 2002;17:56–62. [DOI] [PubMed] [Google Scholar]
- 91.Blann AD, Gurney D, Wadley M, et al. Increased soluble P-selectin in patients with haematological and breast cancer: a comparison with fibrinogen, plasminogen activator inhibitor and von Willebrand factor. Blood Coagul Fibrinolysis. 2001;12:43–50. [DOI] [PubMed] [Google Scholar]
- 92.Schadendorf D, Diehl S, Zuberbier T, et al. Quantitative detection of soluble adhesion molecules in sera of melanoma patients correlates with clinical stage. Dermatology. 1996;192:89–93. [DOI] [PubMed] [Google Scholar]
- 93.Winzer R, Schmutzler C, Jakobs TC, et al. Reverse transcriptase-polymerase chain reaction analysis of thyrocyte-relevant genes in fine-needle aspiration biopsies of the human thyroid. Thyroid. 1998;8:981–987. [DOI] [PubMed] [Google Scholar]
- 94.Takano T, Miyauchi A, Matsuzuka F, et al. Preoperative diagnosis of medullary thyroid carcinoma by RT-PCR using RNA extracted from leftover cells within a needle used for fine needle aspiration biopsy. J Clin Endocrinol Metab. 1999;84:951–955. [DOI] [PubMed] [Google Scholar]