Abstract
Objectives:
Assess outcomes following intrathoracic leaks after esophagectomy from 1970 to 2004 to evaluate the impact of evolving surgical and perioperative techniques on leak-associated mortality (LAM).
Summary Background Data:
An intrathoracic leak following esophagectomy has historically been considered a catastrophic event, with mortality as high as 71%. Concerns about this complication often affect choice of surgical approach for esophagectomy.
Methods:
A retrospective review of all esophagectomies for cancer from 1970 to 2004 (n = 1223) was performed. Outcomes following intrathoracic anastomoses (n = 621) were analyzed by era: historical 1970–1986 (n = 145) and modern 1987–2004 (n = 476).
Results:
There was no difference in the frequency of leak between the time intervals (4.8% versus 6.3%, P = 0.5). Despite a significant increase in the use of preoperative chemoradiation (1% versus 42%, P < 0.001) in the historical versus modern era, the overall mortality decreased from 11% to 2.5% (P < 0.001). The LAM was markedly reduced from 43% to 3.3% (P = 0.016). Factors associated with LAM included failure to use enteral nutrition (HR 13.22, CI 1.8–96.8) and era in which the surgery was performed (HR 18.3, 1.9–180). Other differences included an increased proportion of successful reoperations for leak control (11/30 versus 0/7, P = 0.08) and use of reinforcing muscle flaps (7/11). In the modern era, perioperative mortality is not significantly different for patients with or without intrathoracic leaks (3.3% versus 2.5%, P = 0.55), nor is long-term survival (P = 0.16).
Conclusions:
Modern surgical management of intrathoracic leaks results in no increased mortality and has no impact on long-term survival. Clinical decisions regarding the use of intrathoracic anastomoses should not be affected by concerns of increased mortality from leak.
An intrathoracic leak following esophagectomy has historically been considered a catastrophic event, with mortality as high as 71%. This retrospective review of 621 intrathoracic esophagectomies shows a decrease in leak-associated mortality from 43% to 3.3%. Clinical decisions regarding the use of intrathoracic anastomoses should not be affected by concerns of increased mortality from leak.
Surgical resection is currently the most successful approach to treat localized esophageal cancer. Great controversy, however, exists regarding the best surgical approach to remove and reconstruct the esophagus. The 2 most common methods are transhiatal esophagectomy and transthoracic, or Ivor Lewis,1 esophagectomy. Three prospective randomized studies have been unable to demonstrate a survival benefit for one approach over the other.2–4 However, each technique has its proponents. The most compelling argument against an intrathoracic anastomosis is that while the rate of anastomotic leak is lower in the transthoracic approach (3%–12%5–8 versus 10%–25%9,10 in cervical anastomoses), the leak-associated mortality rate (LAM) from intrathoracic leaks has historically been much higher (50%–71%5,6,11 versus <20%) in cervical leaks.6,10 Previous studies from our group have suggested, however, that operative mortality has dropped significantly with time and that esophageal resection in the modern era at a high-volume center is <3%. We, therefore, reevaluated the outcome and treatment strategy of intrathoracic leaks to determine if improvements in LAM may have been achieved in the modern era. We observed that while intrathoracic leaks still occur, management has improved and the mortality from this complication has declined significantly from 43% to 3.3% (P = 0.016).
METHODS
Patients
We performed a retrospective review of all esophagectomies performed for esophageal cancer for in the time period January 1970 through June 2004. Twelve hundred twenty-three patients were identified; 621 had a chest anastomosis. The charts of these patients were examined in detail for clinical events. We identified 37 patients who developed an intrathoracic leak. To determine the evolution of the occurrence and management of this problem over time, we split the period of evaluation into 2 eras: historical (1970–1986) (n = 145) and modern (1987–2004) (n = 476). We then ascertained the overall intrathoracic leak rate in each time period and the associated mortality rate in those patients who sustained a leak (LAM). Medical records of the 37 patients who had an anastomotic leak were further reviewed specifically focusing on surgical technique at the original operation and the management strategy used once the leak occurred. Our institutional review board approved this study.
Surgery
All patients with an intrathoracic anastomosis had an Ivor Lewis esophagectomy. The stomach was used to replace the esophagus in 615 (99%) cases, with small bowel in 4 (0.2%) and colon in 2 (0.3%) of the remaining cases. Choice of anastomotic technique was at the discretion of the surgeon. Gastric drainage procedures (pyloroplasty or pyloromyotomy) were done in nearly every case. Nasogastric tubes were routinely passed under direct vision prior to completing the anastomosis. The anastomosis was checked for leaks intraoperatively by filling the chest with saline, then insufflating the conduit with air via the nasogastric tube and checking for air bubbles from any suture lines. Thus, reinforcement sutures could be placed if needed. In the modern era, most patients had a feeding jejunostomy tube placed electively at the time of surgery to allow for postoperative enteral nutrition.
Leak Definitions
Leaks were defined as clinical or subclinical and contained or uncontained. If there was any suspicion of leak noted in the medical record—based on the clinician's interpretation of fever or other signs of sepsis, characteristics of chest-tube drainage, chest wound infection, bedside methylene blue contrast study, or findings suggestive of tracheoesophageal fistula—the diagnosis was considered to be clinical. In most cases, this was confirmed with a radiographic contrast esophagram. Subclinical diagnosis was defined as any leak determined solely on routine contrast esophagography, in the absence of clinical suspicion of a leak. This was typically performed on postoperative day 7. The extent of leak was categorized as contained or uncontained based upon appearance on imaging studies. A contained leak was defined as a relatively small area of contrast extravasation that was contained by mediastinal structures. Uncontained leak was defined as a large leak with contrast freely flowing into the pleural space. Uncontained leaks have been felt by some authors to be more serious events.12 LAM was defined as death that could be attributed to the occurrence of a leak.
Statistical Analysis
Association between categorical variables was assessed using the χ2 statistic or Fisher exact test. Independent sample means were compared with Student t test. Survival was estimated from date of surgery to date of death and graphically displayed using the Kaplan-Meier method. Predictors for death from intrathoracic leak were determined using univariate followed by multivariable Cox regression analysis. A 2-tailed P value < 0.05 was considered statistically significant for all results. Data entry and analysis was performed with SPSS (Chicago, IL) software, version 13.0.
RESULTS
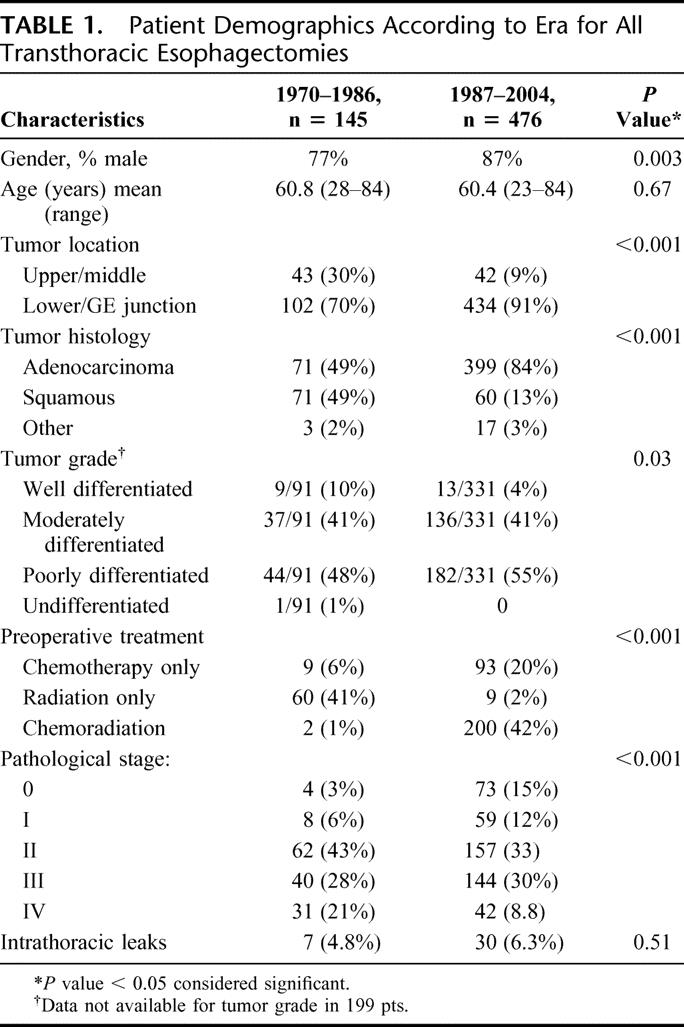
Demographic data and clinical characteristics of all transthoracic esophagectomy patients are listed in Table 1. Median potential follow-up was 134.8 months. There was no difference in the frequency of intrathoracic leak observed between the 2 eras. Conduit choice (P = 0.63) and patient age were similar. More patients in the historical era had advanced-stage cancer, but this likely related to inaccurate clinical staging that improved with better diagnostic testing available in the modern era. Despite a significant increase in the use of preoperative chemoradiotherapy in the modern era, the overall perioperative mortality decreased from 11% to 2.5% (P < 0.001) over time. Length of stay decreased as well, from a median of 18 days to 13 days (P < 0.001).
TABLE 1. Patient Demographics According to Era for All Transthoracic Esophagectomies
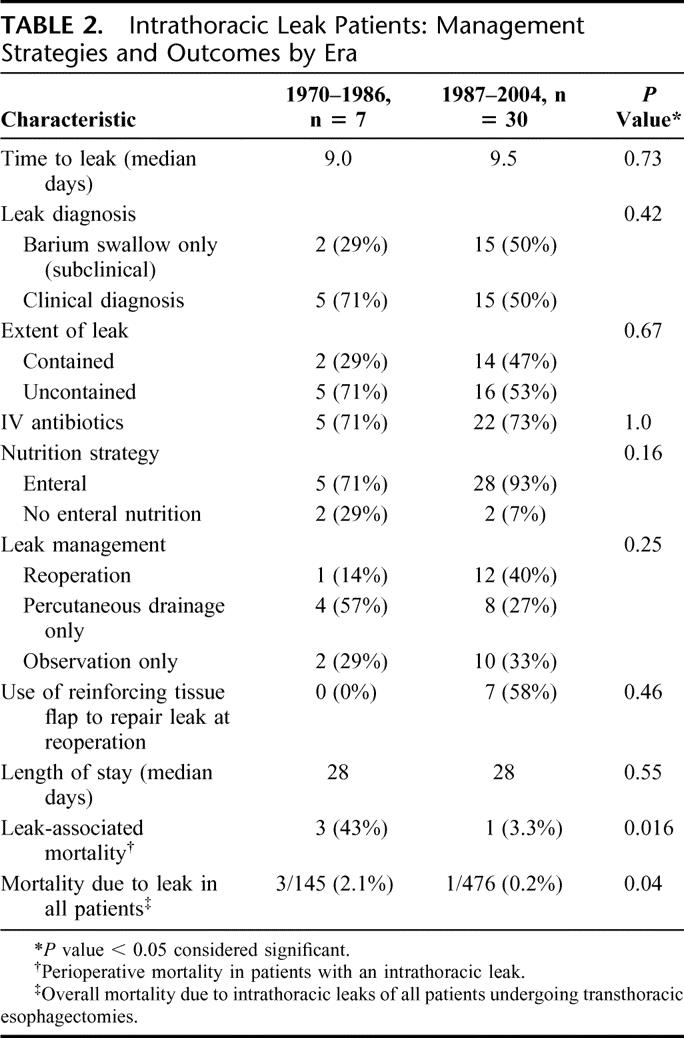
Results among patients who developed an intrathoracic leak are listed in Table 2. The frequency of comorbidities (diabetes mellitus, coronary artery disease, history of cigarette smoking, serum albumin <3.5 g/dL) and Zubrod performance status were similar in the 2 time periods (data not shown). The proportion of patients with preoperative weight loss was higher in the historical era (86% historical, 37% modern, P = 0.03), although the percent body weight lost was similar in the 2 eras (7.2% versus 5.7% of body weight, P = 0.48). Choice of anastomotic technique (stapled or hand-sewn) did not vary significantly over time (P = 0.14). The time to diagnosis, distribution of subclinical and clinical leaks, and extent of leak were also similar between the 2 eras. Despite these similarities, the LAM dramatically decreased from 43% to 3.3% in the modern era (P = 0.016). Of note, all deaths in patients with leaks were determined to be due to the leak. The decrease in LAM may have been due in part to increased reoperations for leak (40% versus 14%), and increased use of reinforcing tissue flaps (58% versus 0% of reoperated patients) in the modern era.
TABLE 2. Intrathoracic Leak Patients: Management Strategies and Outcomes by Era
Leaks were managed by a variety of techniques. Observation only was used when a small, contained leak was noted on barium swallow and the patient was asymptomatic; antibiotic use and NPO status were applied at the discretion of the surgeon. Percutaneous drainage refers to any nonoperative strategy involving mediastinal or pleural drainage, whether by a preexisting chest tube, new chest tube placement for the purpose of draining empyema from a leak, or image-guided drain insertion. Reoperation is defined as any return to the operating room for surgery related to a leak. The surgical methods employed included simple decortication, irrigation and placement of chest tubes (wide drainage), takedown of the anastomosis with creation of an end-esophagostomy and return of conduit to the abdomen, and primary repair of the anastomosis or conduit perforation, with or without the use of a reinforcing flap (such as pleura, omentum, pericardial fat, or transposed chest wall muscle). The reoperation was labeled as successful if the intervention resulted in control of leak and cessation of mediastinal contamination and unsuccessful if it did not.
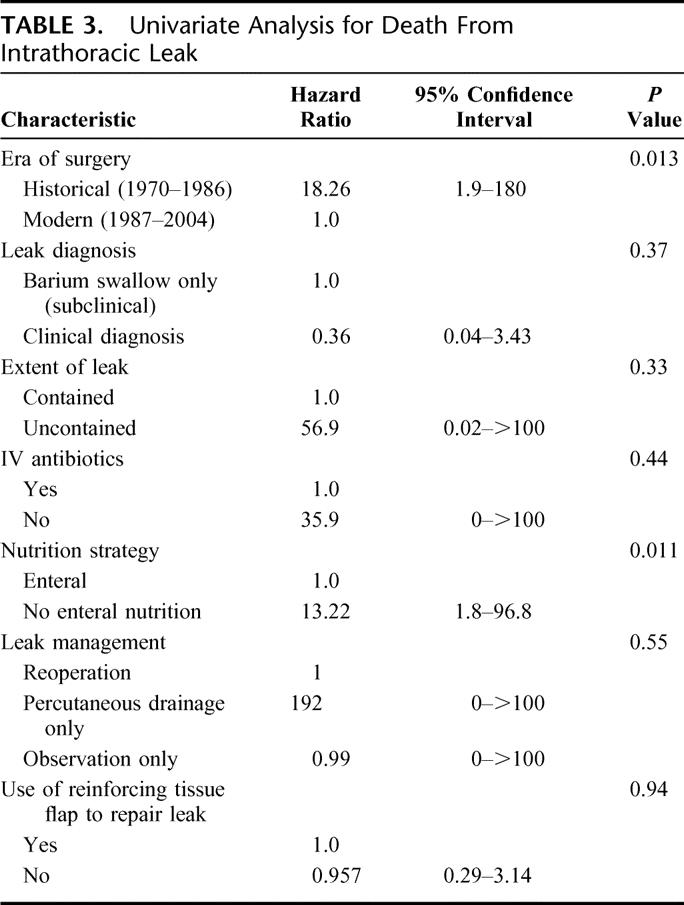
To ascertain risk factors associated with death due to intrathoracic leak, we performed univariate analysis with multiple clinically relevant risk factors for death after leak (Table 3). The era of surgery and postoperative nutritional strategy were significant risk factors for death following a leak. Other risk factors not listed in the table that were not predictive of death from leak include age, tumor stage, use of preoperative chemotherapy or radiation, performance status, preoperative weight loss, medical comorbidities, surgical blood loss, or splenectomy. All factors with a P value < 0.250 were incorporated into a multivariable model and analyzed by Cox regression; no independent risk factors were identified.
TABLE 3. Univariate Analysis for Death From Intrathoracic Leak
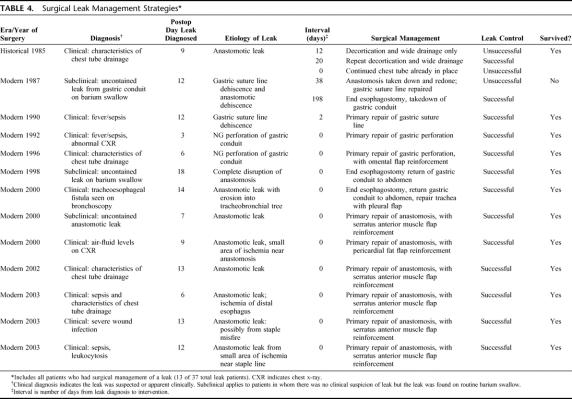
The proportion of patients undergoing successful reoperation for leak control tended to increase from the historical to the modern era (0/7 versus 11/30, P = 0.08). As is often the case with a rare disease or complication, the small number of patients who developed a leak throughout the 34-year time period limited the statistical power to detect a significant differences among potentially contributory clinical variables. Accordingly, for the purposes of description, we tabulated our surgical management of leak (Table 4). The pattern suggests that prompt intervention with control of the leak resulted in improved survival. In 7 cases, all in the modern era, a reinforcing tissue flap was employed, allowing for repair of the anastomosis or conduit perforation and preserving GI tract continuity.
TABLE 4. Surgical Leak Management Strategies
To determine if intrathoracic leak altered the overall mortality rate for esophagectomy, we compared combined 30-day and hospital mortality for all leak patients and all nonleak patients in the modern era. The difference between the 2 groups (1/30, or 3.3%, versus 11/446, or 2.5%) was not significant (P = 0.546). Furthermore, to examine the possibility that leak patients survive their hospitalization but expire soon after discharge, we compared long-term survival in leak versus nonleak patients in the modern era (Fig. 1), which again was not significantly different (P = 0.17). A recent publication12 suggested that the extent of leak predicts long-term survival. We analyzed our study population in the modern era and compared contained with uncontained leaks (Fig. 2); we did not observe a significant survival difference (P = 0.16).
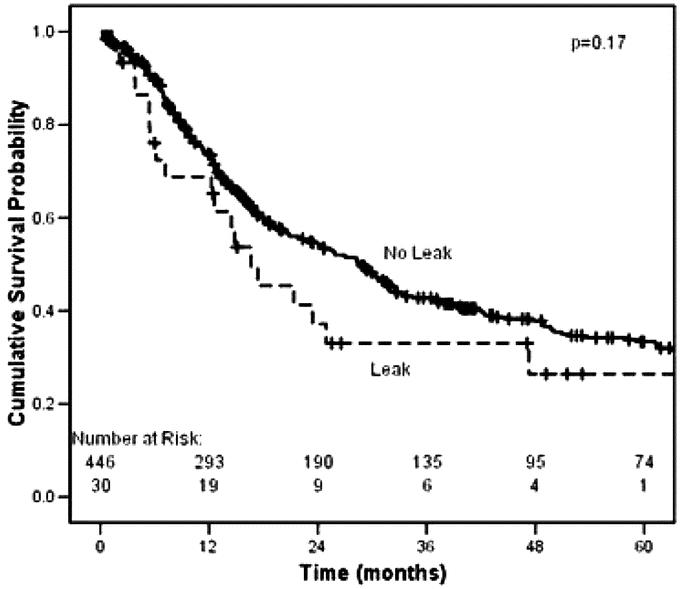
FIGURE 1. Kaplan-Meier survival analysis in the modern era comparing intrathoracic leak (n = 30) to no leak (n = 446). Includes perioperative mortality. Median survival for patients with leak, 16.6 months; for no leak, 28.9 months (P = 0.17).
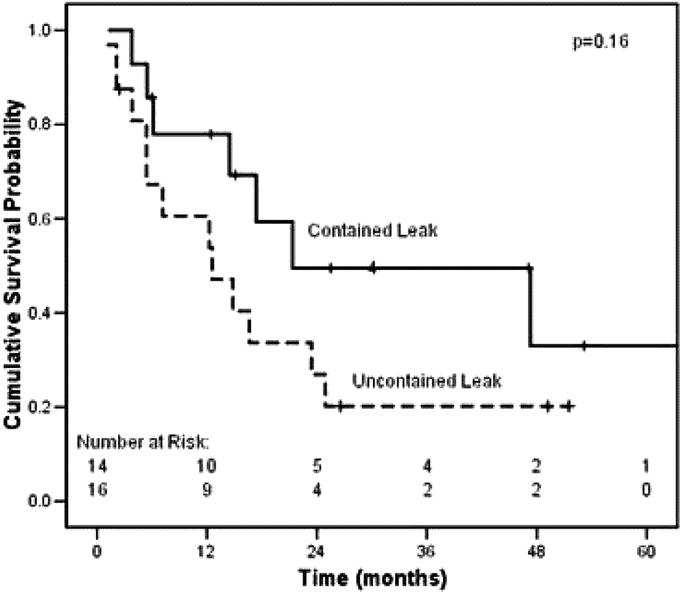
FIGURE 2. Kaplan Meier survival for intrathoracic leaks in the modern era, comparing contained (n = 14) versus uncontained leaks (n = 16). Median survival for contained leak, 21.3 months; for uncontained, 12.6 months (P = 0.16).
For patients who were able to resume oral intake and follow-up information was available (n = 26), 5 (19%) complained of dysphagia. There was no difference in dysphagia rates by time period (P = 0.49) or by leak management strategy (P = 0.20). Four patients required dilation a median of 4.5 times. No patients required reoperation for dysphagia.
DISCUSSION
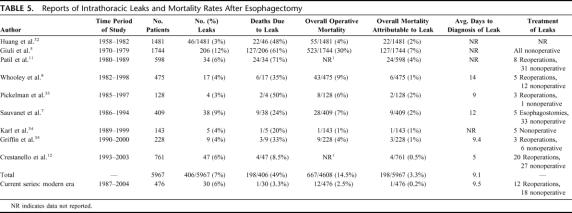
Intrathoracic leaks after esophagectomy have traditionally been viewed as a catastrophic event. Historically, intrathoracic leak rates have ranged from 3%–12%, with up to 71% of these patients dying from the leak (Table 5). Our study, however, demonstrates that in the modern era at a high-volume esophageal cancer center, mortality after intrathoracic leaks is much lower. Despite a leak rate similar to previous studies (6%), we noted a leak mortality rate of only 3.3% in the last 476 transthoracic esophageal resections. Additionally, overall perioperative mortality attributable to transthoracic leaks was only 0.2% in the modern era, and long-term survival was not impacted by an intrathoracic leak. Because the number of intrathoracic leaks in this paper was relatively small (n = 37), the power to find a statistically significant difference for any risk factor or management strategy was limited. Nevertheless, we attempted to evaluate differences over time to try to identify which factors may have contributed to such a remarkable improvement in mortality.
TABLE 5. Reports of Intrathoracic Leaks and Mortality Rates After Esophagectomy
It is important to emphasize that the limitations of this study include the fact that it was a retrospective study performed over a long time period. During the study period the histology of the patients changed from squamous cell carcinoma to adenocarcinoma. This reflects an increasing frequency of esophageal adenocarcinoma and gastroesophageal cancers in the United States first noted by Blot and colleagues in the early 1990s.13 Univariate analysis suggested that this histologic shift did not account for the improvements in LAM over time. It is also important to note that a significantly higher proportion of patients in the modern era were treated with preoperative chemoradiation, which has been suggested by some as a risk factor for postoperative complications.14,15 Despite the increased use of preoperative chemoradiation, our study suggests no change in the percentage of esophageal leaks and a decrease rather than an increase in LAM.
Although the number of patients with esophageal leaks in this study was small, several trends appeared to emerge. In the modern era, an aggressive postoperative enteral nutritional strategy was followed, with routine placement of jejunostomy tubes for postoperative enteral supplementation. Univariate analysis (Table 3) indicated that enteral nutrition (via jejunostomy tube) was associated with improved survival. Several studies have reported the importance of nutritional support in esophagectomy patients, who often have significant malnutrition prior to such a major operation,16–18 and many studies have documented the superiority of enteral nutrition over the parenteral route.19,20 With the added catabolic stress of an intrathoracic leak, reoperation, and sepsis, adequate nutrition may be even more important. Our results suggest that enteral nutrition is an important factor that may aid recovery from transthoracic esophageal leaks, perhaps by preventing prolonged periods of nutritional deficiency (Table 3).
In the modern era, we also noted a tendency towards a more aggressive approach for uncontained esophageal leaks. Regardless of the era, success at reoperation appeared to depend on controlling the leak at the time of surgery. Two patients (1 in the historical group and 1 in the modern era) were reexplored, but the procedures did not stop the leak. Drainage and decortication was performed in one case and esophageal leak repair without tissue reinforcement was performed in the other. Both patients failed to improve and required repeated interventions; one of the patients died. Currently, we emphasize a more aggressive approach at the time of reoperation with esophageal diversion for necrotic or unsalvageable conduits. In uncontained leaks with viable conduits, aggressive leak control with surgical repair and muscle flap reinforcement is attempted and may be responsible for improved leak control and reduced sepsis. Other authors have stressed the importance of tissue flap reinforcement of esophageal leaks with pleura, pericardium, omentum, or muscle.10,12,21 Our preference is to use a rotational flap based on the serratus anterior or latissimus dorsi muscle flap. This strategy depends in part on a muscle-sparing thoracotomy (of serratus anterior and/or latissimus dorsi) at the initial operation to allow the use of these flaps in subsequent reoperations. The utility of muscle flaps to treat a variety of intrathoracic infections through control of local contamination and dead space may occur through the reestablishment of local blood flow with increased delivery of oxygen and white blood cells.22,23
It is also important to emphasize that an aggressive reoperative strategy is not always required for success if the leak is well contained. Many contained leaks can be managed without reoperation, as first described by Cameron et al24 for esophageal perforation. In fact, in our series, 12 of 37 patients with contained leaks were successfully managed only with observation and cessation of oral intake, with leak resolution demonstrated on follow-up contrast studies. A recent publication25 has put forward an alternative approach using esophageal stents. In this study, 24 patients were treated with covered stents to speed oral intake and hospital discharge. Unfortunately, this strategy resulted in 6 in-hospital deaths and 2 patients in whom the stent placement worsened the leak. Additionally, 9 cases of late stent dislocation required reintervention. The average length of hospitalization was 46 days compared with our median length of only 28 days. Because of these results, we do not recommend the use of covered stents but instead favor a leak management strategy outline in Figure 3 in which contained leaks are often observed and uncontained leaks are reoperated early with tissue-flap reinforcement of the esophageal leak repairs.
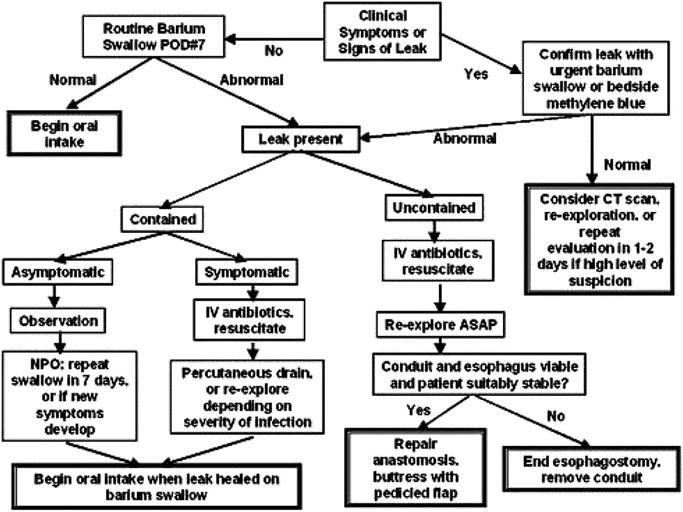
FIGURE 3. Flowchart for esophageal leak management.
Another important point is that much of the reduction in LAM noted in our study may have been due to the experience and resources available at a high-volume esophageal referral center. As suggested by several studies, esophagectomy mortality correlates closely with the number of esophageal resections performed at the institution.26–28 Medicare data suggest that overall operative mortality for an esophageal resection can approach 21% at low-volume esophageal centers as opposed to 2% at high-volume centers.29 It is important therefore to emphasize that the low LAM noted in this study may not be possible to achieve at low-volume esophageal centers.
Many authors have advocated transhiatal esophagectomies and cervical anastomoses on the basis of the low LAM compared with transthoracic anastomoses.30 Despite these excellent results, cervical anastomoses are not without complications,31 such as anastomotic stricture, conduit necrosis, and death. Over the same time period as this study, we performed a similar number of esophagectomies with a cervical anastomosis (n = 618). Leaks occurred in 15.4% of these patients, and LAM was 11%. Not all these deaths could be attributed to the leak, but nonetheless, cervical leaks were still a serious complication, especially if associated with conduit necrosis. At our institution, similar mortality rates can be obtained following intrathoracic or cervical anastomoses. We therefore feel that clinical factors such as location of the tumor and experience and familiarity of the surgeon with the operative approach should dictate the choice of anastomotic location.
In summary, an intrathoracic leak following an esophagectomy remains a significant adverse event that deserves prompt and focused attention. In the current era, timely, aggressive surgical management of uncontained leaks, combined with improvements in perioperative care, can be credited with the significant improvements in LAM over time. Our results suggest that clinical decisions regarding the use of intrathoracic anastomoses should no longer be affected by concerns of increased mortality from leak when the transthoracic resection is performed at a high-volume esophageal referral center.
Discussions
Dr. Lawrence W. Way (San Francisco, California): This report describes the management of intrathoracic leaks from esophageal anastomoses over the 35-year period from 1970 to 2004. Many others have published observations and recommendations on this subject, and the current report should be viewed in this broader context. It might also be useful to reflect on the progress in management of risky visceral anastomoses (eg, esophagogastric; pancreaticojejunal; gastrojejunal) over the past 35 years, since the pattern is similar—fewer leaks and less mortality from leaks when they do occur.
In a publication 10 years ago, Swisher (Am J Surg 1995;169–609) noted that the rate of anastomotic leaks following esophagectomy had declined from 12% of cases during an earlier period to 5% in the most recent decade, and the mortality rate of these leaks had declined from 30% to 10%. The current publication describes what has happened since, in a period where a more aggressive treatment strategy, involving early reoperation and the use of a serratus anterior muscle flap to protect the repair of a leaking anastomosis, has dropped the leak-related mortality rate to even lower levels.
The ideas are rational. Intrathoracic leaks are especially dangerous because of their exposed position in the chest, where there is a paucity of healthy flexible tissue to help plug the healing leak. Opposite conditions exist in the abdominal cavity, which largely explains the propensity for intestinal and other abdominal visceral leaks to close without additional surgery. The muscle flap, as used by Drs. Martin and Swisher, creates for an esophageal anastomosis similar to the more propitious conditions found in the abdomen. A key to their success was early operative intervention for patients with uncontained symptomatic leaks.
Three other articles report similarly low leak-related mortality rates, variably attributable to a decreased leak rate or decreased death rate of a leak: Crestanello et al (J Thorac Cardiovasc Surg 2005;129:254); Karl et al (Ann Surg 2000;231:635); and Cerfolio et al (Chest 2004;126:1187). As a group, these four articles suggest that we may have reached a point where in high volume centers, leaks will no longer have a major effect on the outcome of esophagectomies. The question is just what aspects of practice have got us here? Theauthors argue that early enteral feeding for all postoperative patients was important, but this was not done in the other centers just mentioned, and the literature does not particularly support the theory that early enteral feeding should be administered as a routine. We dropped the use of routine feeding jejunostomies some years ago and saw our patients recovery faster; they are now discharged on postoperative day 9 (median), just when routine jejunal feedings would be getting into full swing.
What else? It is clear that success is possible using a wide variety of anastomotic techniques. Other variables, such as preserving the gastric blood supply are obvious. In my opinion there are no certain explanations for the progress, which probably results from a multitude of factors with indirect effects, such as better preoperative health of the patients, better control of concomitant cardiopulmonary disease, improvements of diagnostic radiology, interventional radiology, and anesthesia, etc. Pellegrini and I came to similar conclusions with regard to the declining mortality rate of leaks after Whipple procedures (Arch Surg 1989;124:778).
I have the following questions. Data in the paper indicate that two-thirds of the 37 patients with subclinical, contained leaks were treated in some way other than observation. What was done in these cases other than observation and why? Secondly, can you reconcile your strong advocacy of routine early tube feeding for all postoperative patients with the observations that others have achieved similar results without it?
In summary, this important contribution strongly supports the contention that prompt reoperation and an anastomotic repair buttressed with healthy tissue, such as a muscle flap, substantially decreases the death rate of uncontained esophageal leaks.
Dr. Stephen G. Swisher (Houston, Texas): I would like to agree with your observation that it is not clear that enteral nutrition is the cause of the decreased mortality in the modern day thoracic leaks. With the very small number of patients that died from leaks, statistical analysis of course was very difficult. Although univariate analysis demonstrated an association with enteral nutrition and mortality there certainly weren't enough patients to do this in a multivariate fashion. The association may therefore be due simply to correlation with the time period in which enteral nutrition was used rather than enteral nutrition itself.
In our own practice we have a number of surgeons performing esophageal operations who place a J-tube at the time of surgery, but do not utilize enteral nutrition and outcome has improved for them as well following thoracic leaks. This suggests that the time period rather than enteral nutrition itself may be the critical factor.
The factors that probably are most critical are the ones that you alluded to such as the importance of performing the surgery at a high volume center. It is not simply the surgeon but it is the whole team, which is important in a high volume center including the radiologist, intensivist, and radiologic interventionalist. Reevaluation with repeat CT scans is also very important so the overall good outcome is due in large part to a team approach. I am not sure that these good outcomes following intrathoracic leaks could be achieved in a low volume center.
Your final question about the management of contained asymptomatic patients. In our review there were 13 patients with contained asymptomatic leaks and 10 of them were observed. The other 3 were percutaneously drained when they started to develop fevers. It is difficult to know exactly how to treat these patients since many simply may be barium swallow abnormalities rather than true clinical leaks. Nevertheless, our group has adopted a conservative approach and we do not allow our patients to orally eat again until they demonstrate healing of these contained leaks on a repeat barium swallow.
Dr. John Wong (Hong Kong, China): May I give a brief international perspective. At the M.D. Anderson and a small number of centers in the United States and in centers like Munich and Hong Kong, these high volume specialized centers have demonstrated a decreased mortality over time, which is associated with increased survival in the long term. This decreased mortality is not entirely related to decreased complications. Complications still occurred, but mortality related to complications has reduced significantly. One of the major complications is anastomotic leakage, which is the subject of today's paper.
I would like to ask three questions. The first is: Because there is a change in the incidence of squamous cancer and adenocarcinoma over the historical period and the modern period in M.D. Anderson, has the site of the anastomosis changed over time?
The second is: What modifications have been made to the technique of the anastomosis which, together with other factors, may have led to a lower leakage rate?
The final question is: You showed an algorithm of early exploration in the uncontained leaks. In our center, any leak within three days of operation would be re-explored on the grounds that it is a serious technical fault or because of gangrene of the conduit. Any leak beyond three days can be investigated to find out what type of leak it is and then managed individually. Do you have any specific recommendations?
Dr. Stephen G. Swisher (Houston, Texas): There has been a definite shift over time in the percentage of patients we have that are adenocarcinoma; whereas before it was predominantly squamous cell carcinoma, it is now primarily adenocarcinoma.
In terms of technical differences, we tend to use a variety of stapled techniques. We either use an ILS stapler or a modified Orringer technique that was recently described in the neck to perform the anastomosis in the chest. Whether stapled techniques are contributing to the good outcome is not clear. We have not been able to see a correlation with technique in the past when we have evaluated this question.
With regards to our approach to patients that have a leak within the chest that is uncontained and symptomatic. Our approach usually is to be very aggressive up front at resuscitating these patients and once they are fully resuscitated to proceed to the operating room and resect the conduit if it is necrotic. If the conduit is not necrotic then we repair the leak and reinforce the repair with a muscle flap. These patients are usually able to tolerate one operation but not two so the initial intervention is critical.
Dr. Alex G. Little (Dayton, Ohio): I just want to emphasize somewhat the point that Dr. Wong made which has to do with volume. There are two equal time periods here but the number of patients operated on in the second time period with more favorable outcomes was three times as many as that in the first group. Obviously hospital experience has tripled, and unless you tripled the number of surgeons, increased volume per surgeon.
So what I think we have been provided with today is a window into the performance, if you will, quality improvement process that occurs when volume increases and explains why outcomes improved. So we have gone beyond simply identifying that outcomes for certain types of patients, diseases and operations, are better in high volume institutions and now seeing a window into why that occurs with the opportunity to transfer the lessons learned beyond the high volume institutions to lower volume centers. It is a great presentation and I think a lot of people will learn from it.
Dr. Stephen G. Swisher (Houston, Texas): M.D. Anderson really has always been a high volume center throughout the study period so our improved outcome is not completely explained by the fact that we have an increased number of cases in the recent time period.
Dr. Richard J. Finley (Vancouver, British Columbia, Canada): I have two questions. Recognizing that the time to leak was nine days, when do you do your contrast study? And have you had any false negatives?
Dr. Stephen G. Swisher (Houston, Texas): That is a very good point. That was the median time to leak. We usually do our contrast study on day seven. In our study of the 30 patients that had a leak, 2 of them had a false negative. So one needs to continue to be vigilant despite a negative swallow and if one suspects a leak one needs to restudy the patient with another barium swallow.
Dr. Richard J. Finley (Vancouver, British Columbia, Canada): My second question relates to the patients who had leaks. Do you have any information about late strictures or diverticular in those groups, particularly the ones that you had the serratus flaps on?
Dr. Stephen G. Swisher (Houston, Texas): Of the patients who had the serratus flaps, we have not seen strictures occurring. We recently had a patient who we placed a latissimus muscle flap on for another problem, and this did end up leading to a problem with dysphagia because the latissimus muscle strictured down and pulled the esophagus over. But we have not seen that with the serratus. And if it does not not wrap around the conduit, we have not seen stricture as a problem.
Dr. Harold J. Wanebo (Providence, Rhode Island): Dr. Swisher, my question is related to the issue of preventing complications and leakage, and I would like to hear what your algorithm is for that.
For example, as I see from your presentation about half of the patients had reconstruction in the chest, and I wonder how the other 600 patients were treated. Did the rest have their anastomoses primarily done in the neck? If so, this raises the question of selection of the site for reconstruction.
The other question I have is: Are there any other ongoing algorithms that your group performs? For example, do all of your patients have a pyloroplasty or pyloromyotomy? Then there are questions related to the use of nasal gastric drainage, long-term use of NG tubes, or stints to reduce leaks. I would like to hear how you actually prevent these complications, as obviously your leak rate is quite low overall.
Dr. Stephen G. Swisher (Houston, Texas): Over time our leak rate has stayed relatively the same for anastomoses in the chest, about 6%. So despite multiple different changes that we have made in technique, our leak rate has remained approximately the same.
We have a number of different surgeons at our institution and several of them favor transhiatal esophagectomies; therefore, over this time period we did do a comparison with the 600 patients who had a transhiatal esophagectomy with a cervical anastomosis. The percentage of leaks for a cervical anastomosis was dramatically higher, about 15%, and the overall mortality for a patient who had a leak in the neck was actually 11%. But this was usually not directly attributable to the leak. Only two deaths were directly attributable to the leak, and that was a mortality of about 2% per cervical leak, which was similar to risk of a leak in the chest.
The one complication that we observe more frequently with a cervical anastomosis is complete conduit necrosis. We have not seen complete conduit necrosis in the chest, primarily because we are often removing the top part of the stomach and therefore the enhanced blood supply to the lower part doesn't lead to conduit necrosis. Yet in the neck we have approximately 1-2% of patients that will have conduit necrosis, and that certainly is a major complication.
Footnotes
Commercial Associations: We have no commercial associations that might pose a conflict of interest in connection with the submitted article, “Intrathoracic Leaks Following Esophagectomy Are No Longer Associated With Increased Mortality.”
Reprints: Stephen G. Swisher, MD, Department of Thoracic and Cardiovascular Surgery, Unit 445, UTMD Anderson Cancer Center, PO Box 301402, Houston, TX 77230-1402. E-mail: sswisher@mdanderson.org.
REFERENCES
- 1.Lewis I. The surgical treatment of carcinoma of the esophagus with special reference to a new operation for growth of the middle third. Br J Surg. 1946;2:18–31. [DOI] [PubMed] [Google Scholar]
- 2.Goldminc M, Maddern G, Le Prise E, et al. Oesophagectomy by a transhiatal approach or thoracotomy: a prospective randomized trial. Br J Surg. 1993;80:367–370. [DOI] [PubMed] [Google Scholar]
- 3.Hulscher JB, van Sandick JW, de Boer AG, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N Engl J Med. 2002;347:1662–1669. [DOI] [PubMed] [Google Scholar]
- 4.Walther B, Johansson J, Johnsson F, et al. Cervical or thoracic anastomosis after esophageal resection and gastric tube reconstruction: a prospective randomized trial comparing sutured neck anastomosis with stapled intrathoracic anastomosis. Ann Surg. 2003;238:803–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giuli R, Gignoux M. Treatment of carcinoma of the esophagus: retrospective study of 2,400 patients. Ann Surg. 1980;192:44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muller JM, Erasmi H, Stelzner M, et al. Surgical therapy of oesophageal carcinoma. Br J Surg. 1990;77:845–857. [DOI] [PubMed] [Google Scholar]
- 7.Sauvanet A, Baltar J, Le Mee J, et al. Diagnosis and conservative management of intrathoracic leakage after oesophagectomy. Br J Surg. 1998;85:1446–1449. [DOI] [PubMed] [Google Scholar]
- 8.Whooley BP, Law S, Alexandrou A, et al. Critical appraisal of the significance of intrathoracic anastomotic leakage after esophagectomy for cancer. Am J Surg. 2001;181:198–203. [DOI] [PubMed] [Google Scholar]
- 9.Orringer MB, Marshall B, Iannettoni MD. Transhiatal esophagectomy: clinical experience and refinements. Ann Surg. 1999;230:392–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Urschel JD. Esophagogastrostomy anastomotic leaks complicating esophagectomy: a review. Am J Surg. 1995;169:634–640. [DOI] [PubMed] [Google Scholar]
- 11.Patil PK, Patel SG, Mistry RC, et al. Cancer of the esophagus: esophagogastric anastomotic leak: a retrospective study of predisposing factors. J Surg Oncol. 1992;49:163–167. [DOI] [PubMed] [Google Scholar]
- 12.Crestanello JA, Deschamps C, Cassivi SD, et al. Selective management of intrathoracic anastomotic leak after esophagectomy. J Thorac Cardiovasc Surg. 2005;129:254–260. [DOI] [PubMed] [Google Scholar]
- 13.Blot WJ, Devesa SS, Kneller RW, et al. Rising incidence of adenocarcinoma of the esophagus and gastric cardia. JAMA. 1991;265:1287–1289. [PubMed] [Google Scholar]
- 14.Doty JR, Salazar JD, Forastiere AA, et al. Postesophagectomy morbidity, mortality, and length of hospital stay after preoperative chemoradiation therapy. Ann Thorac Surg. 2002;74:227–231. [DOI] [PubMed] [Google Scholar]
- 15.Zacherl J, Sendler A, Stein HJ, et al. Current status of neoadjuvant therapy for adenocarcinoma of the distal esophagus. World J Surg. 2003;27:1067–1074. [DOI] [PubMed] [Google Scholar]
- 16.Aiko S, Yoshizumi Y, Sugiura Y, et al. Beneficial effects of immediate enteral nutrition after esophageal cancer surgery. Surg Today. 2001;31:971–978. [DOI] [PubMed] [Google Scholar]
- 17.Baigrie RJ, Devitt PG, Watkin DS. Enteral versus parenteral nutrition after oesophagogastric surgery: a prospective randomized comparison. Aust N Z J Surg. 1996;66:668–670. [DOI] [PubMed] [Google Scholar]
- 18.Mercer CD, Mungara A. Enteral feeding in esophageal surgery. Nutrition. 1996;12:200–201. [DOI] [PubMed] [Google Scholar]
- 19.Bozzetti F, Braga M, Gianotti L, et al. Postoperative enteral versus parenteral nutrition in malnourished patients with gastrointestinal cancer: a randomised multicentre trial. Lancet. 2001;358:1487–1492. [DOI] [PubMed] [Google Scholar]
- 20.Braga M, Gianotti L, Vignali A, et al. Artificial nutrition after major abdominal surgery: impact of route of administration and composition of the diet. Crit Care Med. 1998;26:24–30. [DOI] [PubMed] [Google Scholar]
- 21.Paul S, Bueno R. Section VI: complications following esophagectomy: early detection, treatment, and prevention. Semin Thorac Cardiovasc Surg. 2003;15:210–215. [PubMed] [Google Scholar]
- 22.Arnold PG, Pairolero PC. Intrathoracic muscle flaps: a 10-year experience in the management of life-threatening infections. Plast Reconstr Surg. 1989;84:92–98. [PubMed] [Google Scholar]
- 23.Harris SU, Nahai F. Intrathoracic muscle transposition: surgical anatomy and techniques of harvest. Chest Surg Clin North Am. 1996;6:501–518. [PubMed] [Google Scholar]
- 24.Cameron JL, Kieffer RF, Hendrix TR, et al. Selective nonoperative management of contained intra-thoracic esophageal disruptions. Ann Thorac Surg. 1979;27:404–408. [DOI] [PubMed] [Google Scholar]
- 25.Langer FB, Wenzl E, Prager G, et al. Management of postoperative esophageal leaks with the Polyflex self-expanding covered plastic stent. Ann Thorac Surg. 2005;79:398–403. [DOI] [PubMed] [Google Scholar]
- 26.Dimick JB, Pronovost PJ, Cowan JA, et al. Surgical volume and quality of care for esophageal resection: do high-volume hospitals have fewer complications? Ann Thorac Surg. 2003;75:337–341. [DOI] [PubMed] [Google Scholar]
- 27.Patti MG, Corvera CU, Glasgow RE, et al. A hospital's annual rate of esophagectomy influences the operative mortality rate. J Gastrointest Surg. 1998;2:186–192. [DOI] [PubMed] [Google Scholar]
- 28.van Lanschot JJB, Hulscher JBF, Buskens CJ, et al. Hospital volume and hospital mortality far esophagectomy. Cancer. 2001;91:1574–1578. [DOI] [PubMed] [Google Scholar]
- 29.Swisher SG, DeFord L, Merriman KW, et al. Effect of operative volume on morbidity, mortality, and hospital use after esophagectomy for cancer. J Thorac Cardiovasc Surg. 2000;119:1126–1134. [DOI] [PubMed] [Google Scholar]
- 30.Orringer MB, Marshall B, Iannettoni MD. Transhiatal esophagectomy for treatment of benign and malignant esophageal disease. World J Surg. 2001;25:196–203. [PubMed] [Google Scholar]
- 31.Orringer MB, Marshall B, Iannettoni MD. Eliminating the cervical esophagogastric anastomotic leak with a side-to-side stapled anastomosis. J Thorac Cardiovasc Surg. 2000;119:277–288. [DOI] [PubMed] [Google Scholar]
- 32.Huang GJ, Wang LJ, Liu JS, et al. Surgery of esophageal carcinoma. Semin Surg Oncol. 1985;1:74–83. [DOI] [PubMed] [Google Scholar]
- 33.Pickleman J, Watson W, Cunningham J, et al. The failed gastrointestinal anastomosis: an inevitable catastrophe? J Am Coll Surg. 1999;188:473–482. [DOI] [PubMed] [Google Scholar]
- 34.Karl RC, Schreiber R, Boulware D, et al. Factors affecting morbidity, mortality, and survival in patients undergoing Ivor Lewis esophagogastrectomy. Ann Surg. 2000;231:635–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Griffin SM, Shaw IH, Dresner SM. Early complications after Ivor Lewis subtotal esophagectomy with two-field lymphadenectomy: risk factors and management. J Am Coll Surg. 2002;194:285–297. [DOI] [PubMed] [Google Scholar]