Abstract
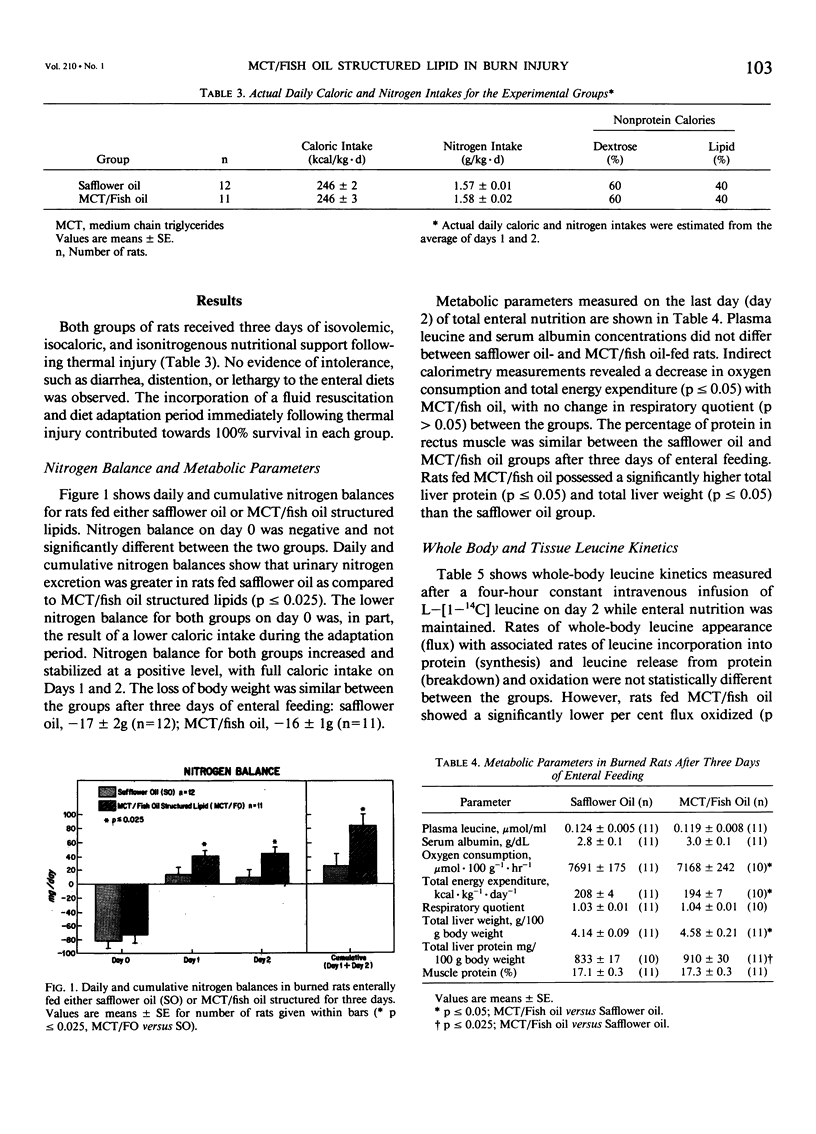
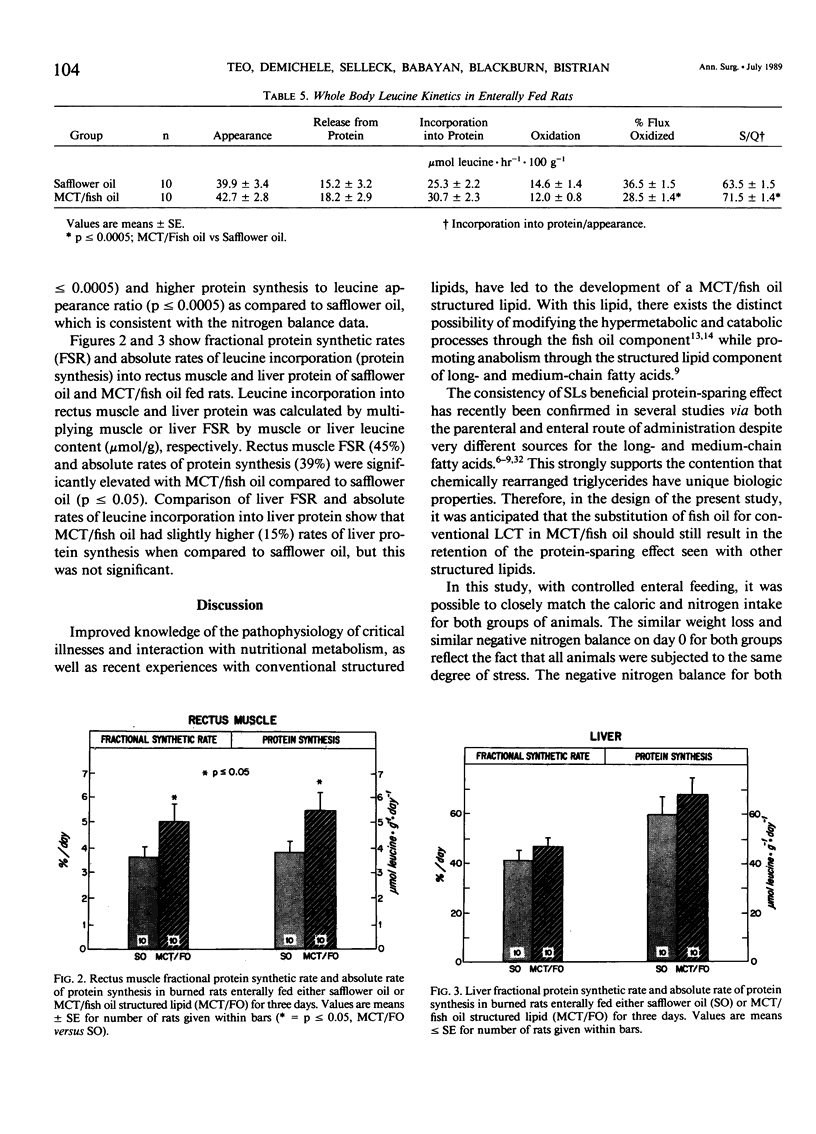
The effects of enteral feeding with safflower oil or a structured lipid (SL) derived from 60% medium-chain triglyceride (MCT) and 40% fish oil (MCT/fish oil) on protein and energy metabolism were compared in gastrostomy-fed burned rats (30% body surface area) by measuring oxygen consumption, carbon dioxide production, nitrogen balance, total liver protein, whole-body leucine kinetics, and rectus muscle and liver protein fractional synthetic rates (FSR, %/day). Male Sprague-Dawley rats (195 +/- 5g) received 50 ml/day of an enteral regimen containing 50 kcal, 2 g amino acids, and 40% nonprotein calories as lipid for three days. Protein kinetics were estimated by using a continuous L-[1-14C] leucine infusion technique on day 2. Thermally injured rats enterally fed MCT/fish oil yielded significantly higher daily and cumulative nitrogen balances (p less than or equal to 0.025) and rectus muscle (39%) FSR (p less than or equal to 0.05) when compared with safflower oil. MCT/fish oil showed a 22% decrease (p less than or equal to 0.005) in per cent flux oxidized and a 7% (p less than or equal to 0.05) decrease in total energy expenditure (TEE) versus safflower oil. A 15% increase in liver FSR was accompanied by a significant elevation (p less than or equal to 0.025) in total liver protein with MCT/fish oil. This novel SL shares the properties of other structured lipids in that it reduces the net protein catabolic effects of burn injury, in part, by influencing tissue protein synthetic rates. The reduction in TEE is unique to MCT/fish oil and may relate to the ability of fish oil to diminish the injury response.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberti K. G., Batstone G. F., Foster K. J., Johnston D. G. Relative role of various hormones in mediating the metabolic response to injury. JPEN J Parenter Enteral Nutr. 1980 Mar-Apr;4(2):141–146. doi: 10.1177/014860718000400214. [DOI] [PubMed] [Google Scholar]
- Alexander J. W., Saito H., Trocki O., Ogle C. K. The importance of lipid type in the diet after burn injury. Ann Surg. 1986 Jul;204(1):1–8. doi: 10.1097/00000658-198607000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach A. C., Babayan V. K. Medium-chain triglycerides: an update. Am J Clin Nutr. 1982 Nov;36(5):950–962. doi: 10.1093/ajcn/36.5.950. [DOI] [PubMed] [Google Scholar]
- Baracos V., Rodemann H. P., Dinarello C. A., Goldberg A. L. Stimulation of muscle protein degradation and prostaglandin E2 release by leukocytic pyrogen (interleukin-1). A mechanism for the increased degradation of muscle proteins during fever. N Engl J Med. 1983 Mar 10;308(10):553–558. doi: 10.1056/NEJM198303103081002. [DOI] [PubMed] [Google Scholar]
- Berkenbosch F., van Oers J., del Rey A., Tilders F., Besedovsky H. Corticotropin-releasing factor-producing neurons in the rat activated by interleukin-1. Science. 1987 Oct 23;238(4826):524–526. doi: 10.1126/science.2443979. [DOI] [PubMed] [Google Scholar]
- Bernton E. W., Beach J. E., Holaday J. W., Smallridge R. C., Fein H. G. Release of multiple hormones by a direct action of interleukin-1 on pituitary cells. Science. 1987 Oct 23;238(4826):519–521. doi: 10.1126/science.2821620. [DOI] [PubMed] [Google Scholar]
- Blalock J. E., Smith E. M. A complete regulatory loop between the immune and neuroendocrine systems. Fed Proc. 1985 Jan;44(1 Pt 1):108–111. [PubMed] [Google Scholar]
- Border J. R., Burns G. P., Rumph C., Schenk W. G., Jr Carnitine levels in severe infection and starvation: a possible key to the prolonged catabolic state. Surgery. 1970 Jul;68(1):175–179. [PubMed] [Google Scholar]
- Chang T. W., Goldberg A. L. The origin of alanine produced in skeletal muscle. J Biol Chem. 1978 May 25;253(10):3677–3684. [PubMed] [Google Scholar]
- Deitch E. A., Maejima K., Berg R. Effect of oral antibiotics and bacterial overgrowth on the translocation of the GI tract microflora in burned rats. J Trauma. 1985 May;25(5):385–392. doi: 10.1097/00005373-198505000-00002. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A. An update on human interleukin-1: from molecular biology to clinical relevance. J Clin Immunol. 1985 Sep;5(5):287–297. doi: 10.1007/BF00918247. [DOI] [PubMed] [Google Scholar]
- Durkot M. J., Wolfe R. R. Effects of adrenergic blockade on glucose kinetics in septic and burned guinea pigs. Am J Physiol. 1981 Sep;241(3):R222–R227. doi: 10.1152/ajpregu.1981.241.3.R222. [DOI] [PubMed] [Google Scholar]
- FLECK A., MUNRO H. N. THE DETERMINATION OF ORGANIC NITROGEN IN BIOLOGICAL MATERIALS. A REVIEW. Clin Chim Acta. 1965 Jan;11:2–12. doi: 10.1016/0009-8981(65)90083-5. [DOI] [PubMed] [Google Scholar]
- Fischer G. W., Hunter K. W., Wilson S. R., Mease A. D. Diminished bacterial defences with intralipid. Lancet. 1980 Oct 18;2(8199):819–820. doi: 10.1016/s0140-6736(80)90171-3. [DOI] [PubMed] [Google Scholar]
- Fischer S., Weber P. C. Prostaglandin I3 is formed in vivo in man after dietary eicosapentaenoic acid. Nature. 1984 Jan 12;307(5947):165–168. doi: 10.1038/307165a0. [DOI] [PubMed] [Google Scholar]
- Garlick P. J., Millward D. J., James W. P. The diurnal response of muscle and liver protein synthesis in vivo in meal-fed rats. Biochem J. 1973 Dec;136(4):935–945. doi: 10.1042/bj1360935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D. W., Pickett W. C., Goetzl E. J. Human neutrophil chemotactic and degranulating activities of leukotriene B5 (LTB5) derived from eicosapentaenoic acid. Biochem Biophys Res Commun. 1983 Nov 30;117(1):282–288. doi: 10.1016/0006-291x(83)91572-3. [DOI] [PubMed] [Google Scholar]
- Herold P. M., Kinsella J. E. Fish oil consumption and decreased risk of cardiovascular disease: a comparison of findings from animal and human feeding trials. Am J Clin Nutr. 1986 Apr;43(4):566–598. doi: 10.1093/ajcn/43.4.566. [DOI] [PubMed] [Google Scholar]
- Jandacek R. J., Whiteside J. A., Holcombe B. N., Volpenhein R. A., Taulbee J. D. The rapid hydrolysis and efficient absorption of triglycerides with octanoic acid in the 1 and 3 positions and long-chain fatty acid in the 2 position. Am J Clin Nutr. 1987 May;45(5):940–945. doi: 10.1093/ajcn/45.5.940. [DOI] [PubMed] [Google Scholar]
- Karlstad M. D., DeMichele S. J., Istfan N., Blackburn G. L., Bistrian B. R. Effect of burn and first-pass splanchnic leucine extraction on protein kinetics in rats. Am J Physiol. 1988 Aug;255(2 Pt 2):R303–R309. doi: 10.1152/ajpregu.1988.255.2.R303. [DOI] [PubMed] [Google Scholar]
- Kelley V. E., Ferretti A., Izui S., Strom T. B. A fish oil diet rich in eicosapentaenoic acid reduces cyclooxygenase metabolites, and suppresses lupus in MRL-lpr mice. J Immunol. 1985 Mar;134(3):1914–1919. [PubMed] [Google Scholar]
- Kremer J. M., Bigauoette J., Michalek A. V., Timchalk M. A., Lininger L., Rynes R. I., Huyck C., Zieminski J., Bartholomew L. E. Effects of manipulation of dietary fatty acids on clinical manifestations of rheumatoid arthritis. Lancet. 1985 Jan 26;1(8422):184–187. doi: 10.1016/s0140-6736(85)92024-0. [DOI] [PubMed] [Google Scholar]
- Laurent B. C., Moldawer L. L., Young V. R., Bistrian B. R., Blackburn G. L. Whole-body leucine and muscle protein kinetics in rats fed varying protein intakes. Am J Physiol. 1984 May;246(5 Pt 1):E444–E451. doi: 10.1152/ajpendo.1984.246.5.E444. [DOI] [PubMed] [Google Scholar]
- Lee T. H., Hoover R. L., Williams J. D., Sperling R. I., Ravalese J., 3rd, Spur B. W., Robinson D. R., Corey E. J., Lewis R. A., Austen K. F. Effect of dietary enrichment with eicosapentaenoic and docosahexaenoic acids on in vitro neutrophil and monocyte leukotriene generation and neutrophil function. N Engl J Med. 1985 May 9;312(19):1217–1224. doi: 10.1056/NEJM198505093121903. [DOI] [PubMed] [Google Scholar]
- Ling P. R., Bistrian B. R., Blackburn G. L., Istfan N. Effect of fetal growth on maternal protein metabolism in postabsorptive rat. Am J Physiol. 1987 Mar;252(3 Pt 1):E380–E390. doi: 10.1152/ajpendo.1987.252.3.E380. [DOI] [PubMed] [Google Scholar]
- Lokesh B. R., Hsieh H. L., Kinsella J. E. Peritoneal macrophages from mice fed dietary (n-3) polyunsaturated fatty acids secrete low levels of prostaglandins. J Nutr. 1986 Dec;116(12):2547–2552. doi: 10.1093/jn/116.12.2547. [DOI] [PubMed] [Google Scholar]
- Maiz A., Yamazaki K., Sobrado J., Babayan V. K., Moldawer L. L., Bistrian B. R., Blackburn G. L. Protein metabolism during total parenteral nutrition (TPN) in injured rats using medium-chain triglycerides. Metabolism. 1984 Oct;33(10):901–909. doi: 10.1016/0026-0495(84)90243-9. [DOI] [PubMed] [Google Scholar]
- Mochizuki H., Trocki O., Dominioni L., Brackett K. A., Joffe S. N., Alexander J. W. Mechanism of prevention of postburn hypermetabolism and catabolism by early enteral feeding. Ann Surg. 1984 Sep;200(3):297–310. doi: 10.1097/00000658-198409000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok K. T., Maiz A., Yamazaki K., Sobrado J., Babayan V. K., Moldawer L. L., Bistrian B. R., Blackburn G. L. Structured medium-chain and long-chain triglyceride emulsions are superior to physical mixtures in sparing body protein in the burned rat. Metabolism. 1984 Oct;33(10):910–915. doi: 10.1016/0026-0495(84)90244-0. [DOI] [PubMed] [Google Scholar]
- Morita I., Saito Y., Chang W. C., Murota S. Effects of purified eicosapentaenoic acid on arachidonic acid metabolism in cultured murine aortic smooth muscle cells, vessel walls and platelets. Lipids. 1983 Jan;18(1):42–49. doi: 10.1007/BF02534689. [DOI] [PubMed] [Google Scholar]
- Needleman P., Raz A., Minkes M. S., Ferrendelli J. A., Sprecher H. Triene prostaglandins: prostacyclin and thromboxane biosynthesis and unique biological properties. Proc Natl Acad Sci U S A. 1979 Feb;76(2):944–948. doi: 10.1073/pnas.76.2.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninnemann J. L., Stockland A. E. Participation of prostaglandin E in immunosuppression following thermal injury. J Trauma. 1984 Mar;24(3):201–207. doi: 10.1097/00005373-198403000-00003. [DOI] [PubMed] [Google Scholar]
- Nordenström J., Askanazi J., Elwyn D. H., Martin P., Carpentier Y. A., Robin A. P., Kinney J. M. Nitrogen balance during total parenteral nutrition: glucose vs. fat. Ann Surg. 1983 Jan;197(1):27–33. [PMC free article] [PubMed] [Google Scholar]
- Rodemann H. P., Goldberg A. L. Arachidonic acid, prostaglandin E2 and F2 alpha influence rates of protein turnover in skeletal and cardiac muscle. J Biol Chem. 1982 Feb 25;257(4):1632–1638. [PubMed] [Google Scholar]
- Rodriguez N., Schwenk W. F., Beaufrere B., Miles J. M., Haymond M. W. Trioctanoin infusion increases in vivo leucine oxidation: a lesson in isotope modeling. Am J Physiol. 1986 Sep;251(3 Pt 1):E343–E348. doi: 10.1152/ajpendo.1986.251.3.E343. [DOI] [PubMed] [Google Scholar]
- Roh M. S., Drazenovich K. A., Barbose J. J., Dinarello C. A., Cobb C. F. Direct stimulation of the adrenal cortex by interleukin-1. Surgery. 1987 Aug;102(2):140–146. [PubMed] [Google Scholar]
- Sapolsky R., Rivier C., Yamamoto G., Plotsky P., Vale W. Interleukin-1 stimulates the secretion of hypothalamic corticotropin-releasing factor. Science. 1987 Oct 23;238(4826):522–524. doi: 10.1126/science.2821621. [DOI] [PubMed] [Google Scholar]
- Shangraw R. E., Turinsky J. Effect of disuse and thermal injury on protein turnover in skeletal muscle. J Surg Res. 1982 Oct;33(4):345–355. doi: 10.1016/0022-4804(82)90048-8. [DOI] [PubMed] [Google Scholar]
- Sobrado J., Moldawer L. L., Pomposelli J. J., Mascioli E. A., Babayan V. K., Bistrian B. R., Blackburn G. L. Lipid emulsions and reticuloendothelial system function in healthy and burned guinea pigs. Am J Clin Nutr. 1985 Nov;42(5):855–863. doi: 10.1093/ajcn/42.5.855. [DOI] [PubMed] [Google Scholar]
- Trocki O., Heyd T. J., Waymack J. P., Alexander J. W. Effects of fish oil on postburn metabolism and immunity. JPEN J Parenter Enteral Nutr. 1987 Nov-Dec;11(6):521–528. doi: 10.1177/0148607187011006521. [DOI] [PubMed] [Google Scholar]
- WEIR J. B. DE B. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol. 1949 Aug;109(1-2):1–9. doi: 10.1113/jphysiol.1949.sp004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wannemacher R. W., Jr, Kaminski M. V., Jr, Dinterman R. E., McCabe T. R. Use of lipid calories during pneumococcal sepsis in the Rhesus monkey. JPEN J Parenter Enteral Nutr. 1982 Mar-Apr;6(2):100–105. doi: 10.1177/0148607182006002100. [DOI] [PubMed] [Google Scholar]
- Waterlow J. C., Stephen J. M. The measurement of total lysine turnover in the rat by intravenous infusion of L-[U-14C]lysine. Clin Sci. 1967 Dec;33(3):489–506. [PubMed] [Google Scholar]
- Wilmore D. W., Long J. M., Mason A. D., Jr, Skreen R. W., Pruitt B. A., Jr Catecholamines: mediator of the hypermetabolic response to thermal injury. Ann Surg. 1974 Oct;180(4):653–669. doi: 10.1097/00000658-197410000-00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woloski B. M., Smith E. M., Meyer W. J., 3rd, Fuller G. M., Blalock J. E. Corticotropin-releasing activity of monokines. Science. 1985 Nov 29;230(4729):1035–1037. doi: 10.1126/science.2997929. [DOI] [PubMed] [Google Scholar]
- Yamazaki K., Maiz A., Sobrado J., Babayan V., Moldawer L. L., Bistrian B. R., Blackburn G. L. Hypocaloric lipid emulsions and amino acid metabolism in injured rats. JPEN J Parenter Enteral Nutr. 1984 Jul-Aug;8(4):360–366. doi: 10.1177/0148607184008004360. [DOI] [PubMed] [Google Scholar]


