Abstract
The hepatitis C virus (HCV) core protein is among the most conserved proteins in HCV and is known to induce sensitization of cytotoxic T lymphocytes (CTL). Therefore, it is a prime candidate for a component of a potential HCV vaccine. The HCV core protein has, however, been reported to exert multiple effects on cell functions, raising questions as to its suitability for this purpose. This question was investigated here with mice into which replication-deficient adenoviruses expressing core protein of an HCV genotype 1b isolate were injected. We show that induction of cytokines in response to the infection, infiltration of lymphocytes into the infected liver, priming of virus-specific CTL, and liver injury are not modulated by expression of the core protein in the liver. Moreover, no changes in the sensitivity to tumor necrosis factor alpha- or Fas-mediated liver injury are demonstrable. A similar lack of demonstrable effects of the core protein on immune functions has also been obtained using transgenic mice expressing another HCV genotype 1b core protein. It is concluded that the HCV core protein of genotype 1b has no modulatory effects on induction of virus-specific immune responses and may therefore be a suitable component of an HCV vaccine.
Hepatitis C virus (HCV) is the leading cause of chronic infectious hepatitis, affecting more than 100 million people worldwide. Despite induction of cell-mediated and humoral immunity to the virus, the infection may persist over years, leading to liver cirrhosis and development of hepatomas (23). The large number of people infected and the seriousness of sequelae call for increased efforts in the development of curative treatments and protective vaccines. Current treatment protocols using alpha interferon (IFN-α) and ribavirin are successful in only a proportion of patients; therefore, developing alternative treatment modalities is an urgent goal.
Evidence has been accumulating that cytotoxic T lymphocytes (CTL) specific for HCV epitopes play a decisive role in the successful outcome of IFN-α and ribavirin therapy (15). For example, in patients shown to have eliminated the infection, a significant and persistent CTL response to the virus has been documented (9). Therefore, stimulating induction of HCV-specific CTL may be a promising approach to induce viral elimination. Indeed, a correlation between the presence of HCV core protein-specific CTL in infected individuals and their ability to respond to IFN-α therapy has been reported (15). Therefore, CTL recognizing HCV core epitopes may play an important role in elimination of the infection.
The core protein-encoding sequence is among the most conserved genes in HCV genome, making it a prime candidate for a component of a vaccine. There are, however, concerns about this. The core protein has been reported to exert multiple effects on cell functions, including modulation of Fas- and tumor necrosis factor alpha (TNF-α)-mediated signals and suppression of cell-mediated immune responses (8, 14, 18, 19, 28). Therefore, use of the core protein or its gene sequence in a vaccine could lead to undesirable effects, such as immunosuppression, immune deviation, or increased liver injury. Because of these concerns, it is important to examine whether the HCV core protein may increase liver injury or modulate immune responses.
Here we investigated this question with mice, using an infectious replication-deficient adenovirus construct expressing the core protein of HCV genotype 1b (5) as well as mice expressing HCV core as a transgene. We show that induction of cytokines in response to the infection, migration of lymphocytes into the infected liver, priming of virus-specific CTL, and liver injury are not modulated by expression of the HCV core protein in the liver. We conclude that the HCV core protein, of genotype 1b, has no modulatory effects on induction of virus-specific immune responses and may therefore be a suitable component of an HCV vaccine.
MATERIALS AND METHODS
Preparation of recombinant adenovirus-expressing HCV core protein.
An adenovirus with deletions in the E1 and E3 region and containing the green fluorescent protein (GFP) gene (adeno-GFP virus) was used to generate the construct expressing the HCV core protein. The procedure for generating the recombinant adenovirus was modified from methods published previously (5). HCV cDNA encoding the core protein was cloned from a genotype lb isolate (pCV-J4L6S) (25) by PCR using the Expand High Fidelity System (Roche). The following oligonucleotides were used as primers: sense, GGTCTCGTCGACCGTGCACCATGAGCACG; antisense, CGGTCTAGACTTCCTAAG CGGAAGCTGGG (restriction sites are underlined). The amplified products were digested with SalI and XbaI and inserted into a SalI- and XbaI-digested pAdTrack-CMV shuttle vector (Q. Biogene). Control adenovirus was made from the empty pAdTrack-CMV. The resultant constructs were linearized with PmeI and cotransformed with adenoviral backbone vector pAdeasy-1 (Q. Biogene) into Escherichia coli BJ5183 by electroporation to achieve recombination. The adenoviral recombinants were analyzed by restriction analysis, amplified in E. coli DH10B, and purified using a Midiprep kit (Qiagen). To produce virus, the recombinants were linearized by digestion with PacI to release the viral genome, which was transfected into human embryonic kidney HEK-293 cells using Lipofectamine (Life Technologies). Recombinant adenoviruses were harvested from cells after three cycles of freezing and thawing on day 7 to 10 after transfection and were further propagated by serial infection of HEK-293 cells.
Immunoblotting for HCV core and GFP and reverse transcription-PCR (RT-PCR) for cytokine expression.
To examine expression of the HCV core protein, HEK-293 cells were infected with recombinant adenoviruses at a multiplicity of infection (MOI) of 50. After 24 h, at which time there was maximum GFP expression, cells were washed with ice-cold Tris-buffered saline (50 mM Tris-HCl, 150 mM NaCl [pH 8.0]) and then incubated with lysis buffer (50 mM Tris-HCl [pH 8.0], 150 mM NaCl, 2 mM EDTA, 0.5% NP-40, 1 mM dithiothreitol) containing proteinase inhibitors on ice for 30 min. To prepare liver extracts, livers from mice injected intravenously with 2 × 109 PFU of virus were harvested 3 days after injection, and lysates were prepared by homogenizing liver tissue in the same buffer system. Cell lysates were cleared by centrifugation at 10,000 × g for 5 min. For immunoblots, 150 μg of protein per lane was separated on sodium dodecyl sulfate-12% polyacrylamide gels by electrophoresis. Resolved proteins were transferred onto nitrocellulose membranes, which were blocked with 5% skim milk in Tris-buffered saline containing 0.1% Triton X-100. Membranes were then incubated with HCV core-specific mouse monoclonal antibody Al/3DI (Anogen, Mississauga, Ontario, Canada) or polyclonal rabbit anti-GFP antibody (Clontech, Palo Alto, Calif.) followed by horseradish peroxidase-conjugated secondary antibodies (American Qualex). Antibodies were visualized with a membrane-bound antibody enhanced chemiluminescence kit (Amersham).
To assay cytokines in liver tissue, livers were harvested and total RNA was extracted by the phenol-chloroform method using an RNAzol B kit (Tel-Test, Inc., Friendswood, Tex.) (11, 12). Two micrograms of RNA was reverse transcribed to cDNA in a 50-μl reaction mixture using Superscript II RNase H− reverse transcriptase and random primers (Life Technologies). For PCR, the equivalent amount of cDNA product (5 μl) was amplified in a 50-μl reaction mixture containing 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 1.5 mM MgCl2, 0.2 mM deoxynucleoside triphosphates, 2.5 U of Taq DNA polymerase (Perkin-Elmer, Norwalk, Conn.), and a 1 μM concentration of each specific primer. Amplification was performed in a DNA Thermal Cycler 480 (Perkin-Elmer) set for 1 min each at 94, 58, and 72°C for 35 cycles for IFN-γ, interleukin-12 (IL-12) p40, and IL-18 and 30 cycles for β-actin, followed by an extension for 7 min at 72°C. After amplification, PCR products were electrophoresed on a 2% agarose gel and visualized by ethidium bromide staining under UV illumination. Primers for β-actin and IFN-γ were purchased from Stratagene (La Jolla, Calif.), and those for IL-12 p40 and IL-18 were designed from published sequences (22) and synthesized at the University of Southern California (USC) microchemical facility.
Animals, immunizations, assays for serum ALT, and fluorometric cell analyses.
Pathogen-free female C57BL/6 (H-2b) (B6) and B6.MRL-Faslpr (B6 lpr) mice, 6 to 12 weeks of age, were obtained from the Jackson Laboratory (Bar Harbor, Maine). C57BL/6/TNFR1/R2 double-deficient (B6 TNFR1,2−/−) mice (11) were generously provided by Chaim Jacob (Department of Medicine, USC Keck School of Medicine). C57BL/6 mice expressing the HCV core 1b gene (Taiwan isolate) (1) under control of the human elongation factor (EF) 1α promoter (7) were generated and bred at the USC transgenic mouse facility (unpublished data).
For immunization, type 5 adenoviruses with deletions in the E1 and E3 regions and coding for both GFP and the HCV core protein (adeno-core virus) or only GFP were used (5, 11). The recombinant adenoviruses were propagated in HEK-293 cells (5, 11). In all experiments, animals were primed by injection of 5 × 108 to 2 × 109 PFU of virus into the tail vein on day 0. Serum alanine aminotransferase (ALT) was assayed as described previously (11, 12), using a commercial assay kit (Sigma Diagnostics, St. Louis, Mo.). Experiments were performed in accordance with guidelines from the USC Institutional Animal Care and Use Committee.
For fluorometric analysis of liver-infiltrating lymphocytes, mononuclear cells were isolated from livers as described previously (11, 12). Liver tissue was passed through a 200-gauge stainless-steel mesh in serum-free Hanks balanced salt solution, and mononuclear cells were purified by Percoll gradient centrifugation. Red blood cells in this cell preparation were lysed with ammonium chloride. For fluorescence-activated cell sorter (FACS) analysis, cells were stained with monoclonal antibodies and analyzed as described previously (11, 12). The following antibodies were used: anti-mouse phycoerythrin- or fluorescein isothiocyanate-conjugated anti-CD3 (145-2C11), anti-CD4 (GK1.5), anti-CD8 (53-6.7), and anti-NK1.1 (PK136), all purchased from PharMingen (San Diego, Calif.). Cells were washed, stained with antibody at 4°C for 30 min, and analyzed by FACS on a FACStarplus (Becton Dickinson, Mountain View, Calif.). The numbers of CD3+, CD4+, CD8+, NK1.1+, and NK1.1+ CD3+ cells per liver were calculated by multiplying the percentage of each subpopulation by the total number of mononuclear cells per liver.
Induction of in vitro CTL responses and assay of cell death by TNF-α and Fas cross-linking.
To induce cytotoxic T cells, spleen cells from adenovirus-primed mice were harvested 10 days after infection. Spleen cells were cultured in 2-ml Linbro plates for 5 days in complete RPMI 1640 medium and restimulated with virus at an MOI of 5 (12). Cytotoxicity was assayed on 51Cr-labeled C57SV (H-2b) targets which had been cultured for 24 h with virus at an MOI of 50 (6, 12, 26).
To assay the sensitivity of cells to TNF-α-mediated apoptosis, B/C10ME cells (28) were infected at an MOI of 50 with adenovirus overnight. Cells were dispensed into 96-well plates (2 × 104 cells per well) and incubated with increasing concentrations of recombinant murine TNF-α (PharMingen) for 20 h at 37°C. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (Sigma) was added at a concentration of 1 mg per ml to wells, followed by incubation for 3 h, at which time the medium was replaced with 200 μl of dimethyl sulfoxide and the mixture was incubated for 20 min at room temperature. The optical density at 560 nm was read, and the percentage of dead cells was calculated (3, 28).
To assay Fas-mediated cell death, C57SV cells (12) were infected with adenovirus at an MOI of 50 overnight and concomitantly labeled with 5 μCi of [3H]thymidine per 2 × 106 cells/ml. Cells (2 × 105/well) were incubated with 0.25 μg of anti-mouse Fas antibody (PharMingen) per ml for 20 h at 37°C. Cultures were harvested onto glass fiber filters for determination of radioactivity. Cytotoxicity was calculated from radioactivity incorporated into cells and radioactivity recovered from the filter (2).
RESULTS
Recombinant adenovirus coding for HCV core protein directs copious core protein synthesis in the mouse liver.
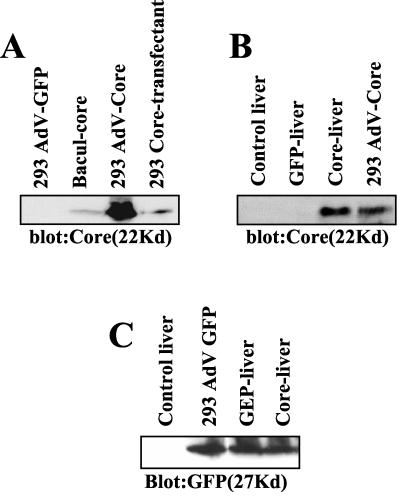
Intravenous injection of adenoviruses into mice causes infection and viral gene expression in the liver (6, 11, 12, 26). The immune response to virus antigens causes a dramatic increase of lymphocytes in the liver and appearance of liver enzymes in the serum (11, 12). This model of virus-induced hepatitis was therefore deemed suitable to examine whether the HCV core protein exerts modulatory effects on immunity to a viral infection of the liver. The core gene was inserted under control of the cytomegalovirus promoter into an adenovirus with coexpression of GFP (5). Because of deletions in the E1 and E3 regions, this virus is replication deficient, requiring propagation in HEK-293 cells to complement the defect in the E1 gene (26). To examine expression of core protein by this construct, extracts from infected cells were assayed by immunoblotting. The results in Fig. 1A reveal copious amounts of core protein in cells infected with the adeno-core virus but not in cells infected with adeno-GFP control virus. A comparison of adeno-core virus-infected cells with cells stably transfected with the HCV core gene under control of an EF promoter or expressed from a recombinant baculovirus reveals substantially larger amounts of core protein in the adeno-core virus-infected cells (Fig. 1A). We conclude that adeno-core virus is able to mediate high-level expression of core protein in cell lines.
FIG. 1.
Expression of HCV core protein in adenovirus-infected HEK-293 cells and expression of core protein and GFP in the livers of virus-infected mice. (A) One hundred micrograms of protein from cell extracts of adeno-GFP virus (AdV-GFP)- or adeno-core virus (AdV-Core)-infected HEK-293 cells was compared to that from HEK-293 cells stably transfected with HCV core (293 Core-transfectant) and a baculovirus-core control (Bacul-core). The gel was probed for HCV core protein by immunoblotting. (B) C57BL/6 mice were injected with 2 × 109 PFU of adeno-GFP virus (GFP-liver) or adeno-core virus (Core-liver) on day 0. Livers were harvested on day 3, and 150 μg of protein from liver extracts per lane was assayed for HCV core protein by immunoblotting. As a comparison, 150 μg of protein of cell extract from adeno-core virus-infected HEK-293 cells is shown (293 AdV-Core). (C) Mice were injected as for panel B, and 150 μg of protein of liver extract per lane was assayed for expression of GFP by immunoblotting. Shown are results from livers of mice injected with adeno-core or GFP control virus as well from 293 cells infected with GFP control virus.
To examine the ability of this construct to express HCV core protein in the liver, 2 × 109 PFU of virus was injected into the tail veins of mice, and livers were harvested 3 days later. It is seen in Fig. 1B that there is copious expression of core protein in liver extracts, although the exact quantitative comparison of the expression level of core protein in the mouse liver and the cell line is difficult, since liver tissue includes many cells and tissues other than hepatocytes. Because there is evidence that the core protein may regulate expression of viral and/or cellular genes (17), the relative expression of the adenovirus-encoded GFP in livers of mice infected with adeno-core or control virus was assayed by immunoblotting. It is seen in Fig. 1C that there is no difference in the expression of GFP in adeno-core virus-infected livers compared to control virus-infected livers. These results show that expression of the HCV core protein under control of the cytomegalovirus promoter in an adenovirus construct directs the production of copious amounts of core protein in the mouse liver. Moreover, the expression of core protein does not modulate expression of the virus-encoded GFP in the liver.
HCV core does not modulate induction of IL-12, IL-18, and IFN-γ in the liver.
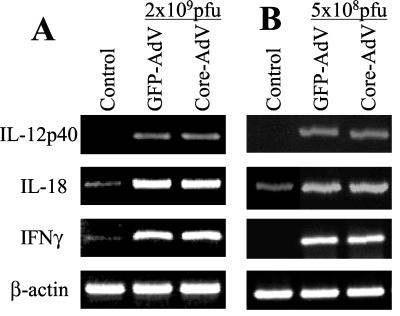
Infection of the liver with adenovirus causes induction of cytokines that activate innate and adaptive immunity (11, 12). Among these are IL-12, IL-18, and IFN-γ, all of which activate natural killer (NK) and T-cell-mediated immune responses (16, 21, 27). We examined the effects of the core protein on expression of these cytokines by semiquantitative RT-PCR. The results in Fig. 2A show that infection with 2 × 109 PFU of virus led to a strong induction of IL-12 p40, IL-18, and IFN-γ transcripts in the liver. Interestingly, there was no apparent difference in cytokine transcript levels induced by the GFP control and adeno-core virus. It is possible that regulation of these cytokines by the core protein was obscured by the high dose of virus used for infection. To examine this possibility, livers from mice infected with the lowest virus dose (5 × 108 PFU) found to induce reproducible serum ALT levels and CTL priming were tested. The results in Fig. 2B show that even at this lower dose, there was no apparent difference between cytokine levels induced by adeno-core virus- and GFP control virus-injected livers. Therefore, within the limitations of this assay, expression of HCV core protein in the liver does not modulate the virus-dependent induction of these three important cytokines.
FIG. 2.
Induction of IL-12 p40, IL-18, and IFN-γ in virus-infected mice. C57BL/6 mice were injected with 2 × 109 PFU (A) or 5 × 108 PFU (B) of adeno-GFP (GFP-AdV) or adeno-core (Core-AdV) virus on day 0. Livers were harvested on day 6, and RNA was extracted and assayed for IL-12 p40, IL-18, IFN-γ, and β-actin transcripts by RT-PCR.
Adeno-core virus-infected mice show normal accumulation of T cells in the liver and unaltered ALT levels in the serum.
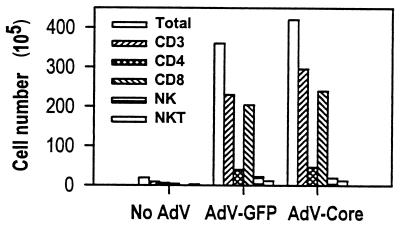
One of the earliest manifestations of a cell-mediated immune response in the infected liver is the increase of lymphoid cells in this organ. Therefore, analysis of mononuclear cells in the liver provides a useful tool to assess cell-mediated immune responses to the infection. To examine cell infiltration in the liver, mice were injected with the recombinant viruses, and livers were harvested on day 6. Mononuclear cells were isolated, stained for NK cell and T-cell markers, and analyzed by FACS. The results in Fig. 3 show that there is a large increase of lymphoid cells in the liver, a majority of which are T cells. Among those, the greatest increase is seen in the CD8+ cell population, while CD4+ cells increase to a lesser degree. The increase in NK cells and NK T cells is also moderate. Injection of adeno-core virus induces an increase very similar to that seen with control virus. Therefore, expression of the HCV core gene does not modulate virus-induced recruitment of T cells, NK cells, or NK T cells into the infected liver.
FIG. 3.
Recruitment of lymphocytes into the virus-infected liver. Groups of three C57BL/6 mice were injected with 2 × 109 PFU of adeno-GFP (AdV-GFP) or adeno-core (AdV-Core) virus on day 0. Livers were harvested on day 6, and mononuclear cells were isolated from pooled organs from each group. Cells were analyzed by FACS staining for T cells (CD3+ cells), CD4+ cells, CD8+ cells, NK cells (NK1.1+ cells), and NK T cells (NK1.1+ CD3+ cells). The calculated number of cells per liver for each cell population is shown. The results are from one of two repeat experiments.
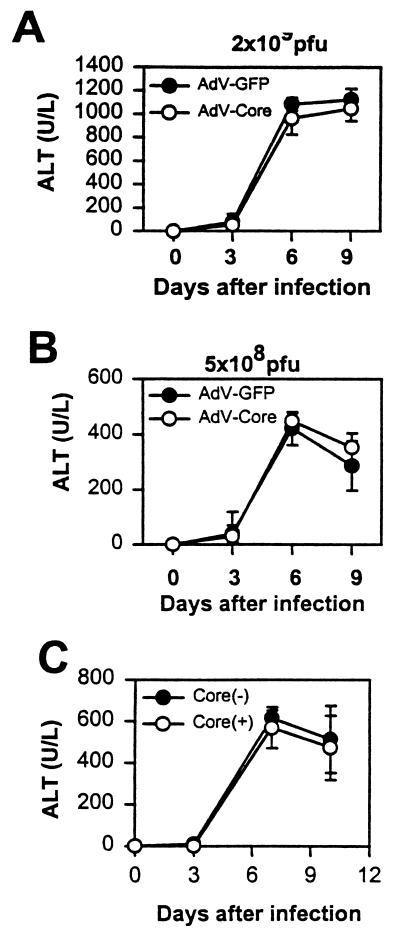
The finding that HCV core gene expression has no demonstrable effect on either cytokine production or cell infiltration in the liver predicts that cell-mediated immunity to the infection is unaffected. Therefore, serum ALT levels in mice injected with adeno-core and control viruses should be similar. Indeed, the results in Fig. 4A show that a dose of 2 × 109 PFU of adeno-core virus induces serum ALT levels indistinguishable from those of mice injected with adeno-GFP control virus. At the lower dose of 5 × 108 PFU of virus per mouse, ALT levels are lower, as expected, but again there is no difference between mice injected with adeno-core or adeno-GFP control virus (Fig. 4B).
FIG. 4.
Serum ALT levels in mice infected with adeno-core and adeno-GFP virus and in core transgenic mice infected with adeno-lacZ virus. (A and B) C57BL/6 mice were injected with 2 × 109 PFU (A) or 5 × 108 PFU (B) of adeno-GFP (AdV-GFP) or adeno-core (AdV-Core) virus on day 0, and sera were collected from groups of four mice on the days indicated. (C) Core transgenic (+) and control (−) mice were injected with 109 PFU of adeno-lacZ virus. Error bars indicate standard deviations.
It is possible that expression of the HCV core in the adenovirus construct interferes with its regulatory effects in the animal. Therefore, we used another strategy to examine the effects of the core protein in vivo. For this purpose, HCV core transgenic mice, which express the core protein (derived from a Taiwan isolate of genotype 1b) (28) in the liver as well as other organs constitutively under the EF 1α promoter (unpublished data), were injected with adenovirus and then assayed for ALT levels. The results in Fig. 4C show that there is no demonstrable difference in serum ALT levels between core transgenic and control mice. These results demonstrate that expression of the core protein either as a transgene or in an adenoviral construct has no effects on cellular infiltration into the liver and the resulting liver injury assayed by serum ALT levels.
The HCV core protein has no demonstrable effect on TNF-α- and Fas-mediated liver injury.
Previous experiments with the adenovirus model have shown that TNF-α- and Fas-mediated pathways constitute the principal mechanisms of liver injury and therefore are responsible for increased ALT levels in the serum (11). This and the demonstration that expression of the HCV core gene may change the sensitivity of cell lines to TNF-α- and Fas-mediated cell death (4, 14, 17, 19, 20, 28) predict that injection of the adeno-core virus results in ALT levels different from those in mice infected with control virus. That this is not seen could be due to the countereffects of core protein-mediated stimulation of cell death via the TNF-α pathway and simultaneous inhibition of the Fas-mediated cell death pathway.
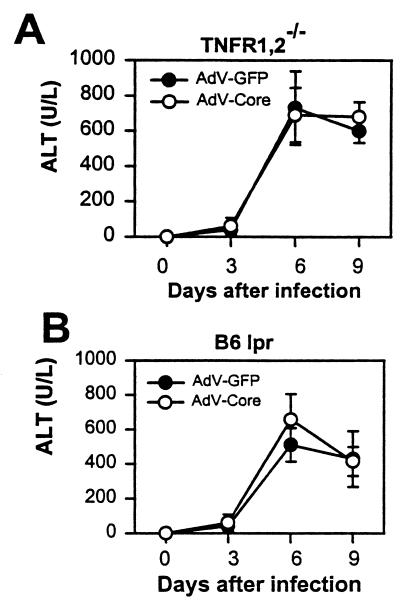
To examine this possibility, animals in which one of the respective pathways is defective were tested. C57BL/6 lpr mice were used because of their lack of Fas receptors, and C57BL/6 TNF receptor 1 and receptor 2 double-deleted (B6 TNFR1,2−/−) mice were used to block the action of TNF-α (11). Figure 5 shows that injection of adenovirus induces increased serum ALT levels in both types of mice, consistent with the previous demonstration that liver injury may proceed by either the Fas or the TNF-α pathway (11). The results also show that adeno-core and adeno-GFP control viruses induce virtually identical ALT levels. Therefore, even in a setting in which only one of the pathways of liver injury is functional, no modulatory effect of the HCV core protein expressed in the virus is demonstrable.
FIG. 5.
Serum ALT levels in TNF receptor- and Fas-deficient mice. (A) C57BL/6 mice defective for TNF receptor 1 and 2 (B6 TNFR1,2−/−) were injected with 2 × 109 PFU of adeno-core (AdV-Core) or adeno-GFP (AdV-GFP) virus on day 0, and sera were collected from groups of four mice on the days indicated. (B) Fas receptor-deficient C57BL/6 lpr mice (B6 lpr) were injected with virus and assayed as for panel A. Error bars indicate standard deviations. Data are from one of two similar experiments.
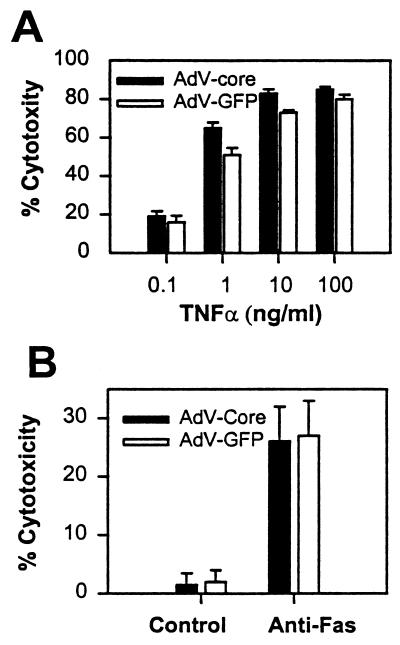
These results then raise the question whether virus-mediated expression of the HCV core protein causes modulation of TNF-α- and Fas-mediated cell death in tissue culture cell lines, as previously observed using cell lines stably or transiently transfected with the core-expressing plasmids (4, 14, 18, 19, 20, 28). To reexamine this question, a fibrosarcoma cell line, B/C10ME, was infected with adeno-GFP or adeno-core virus at a high MOI, and cells were assayed for sensitivity to TNF-α in the absence of protein synthesis inhibitors. The results in Fig. 6A show that TNF-α at concentrations of between 1 and 100 ng per ml caused significant cell death in B/C10ME cells. There is a small increase in TNF-α sensitivity in cells infected with adeno-core virus compared to adeno-GFP virus, consistent with the previous findings from our laboratories (28). However, this increase, while repeatable in several independent experiments, is much smaller than the effects previously observed when the same core protein sequence was expressed from a plasmid by transfection (28). To assay sensitivity to Fas-mediated apoptosis, C57SV cells were infected with virus, followed by incubation with anti-Fas antibody. The results in Fig. 6B show that Fas cross-linking induces cell death which is identical in cells infected with control or adeno-core virus. Therefore, Fas-mediated cell death is not modulated in a setting in which expression of the HCV core protein is mediated by an adenovirus construct in vitro or in vivo, whereas there is a small effect of core expression on TNF-α-mediated cell death in vitro but not in vivo.
FIG. 6.
Sensitivity of virus-infected cell lines to TNF-α- and Fas-mediated apoptosis. (A) B/C10ME cells were infected at an MOI of 50 with adeno-core (AdV-Core) or adeno-GFP (AdV-GFP) virus followed by incubation for 20 h with various concentrations of TNF-α. Cell death was assayed by the MTT assay. (B) C57SV cells were infected with virus as for panel A and labeled with [3H]thymidine, followed by incubation with anti-Fas antibody for 20 h. Cell death was assayed by the DNA fragmentation assay. Error bars indicate standard deviations. Data are from one of three experiments.
HCV core gene expression does not suppress the induction of virus-specific CTL.
Previous experiments had shown that the initial liver injury induced by adenovirus infection and assayed by serum ALT levels is, by and large, caused by NK cells (12). Therefore, the failure of HCV core gene expression to modulate ALT levels in the serum suggests that the core protein has no effects on the early innate immune response. This, however, leaves open the possibility that the core protein is able to mediate effects on the induction of virus-specific CTL. Indeed, published data had shown that recombinant vaccinia viruses expressing HCV core protein, when injected into mice, induce a poor CTL response to vaccinia virus antigens (8).
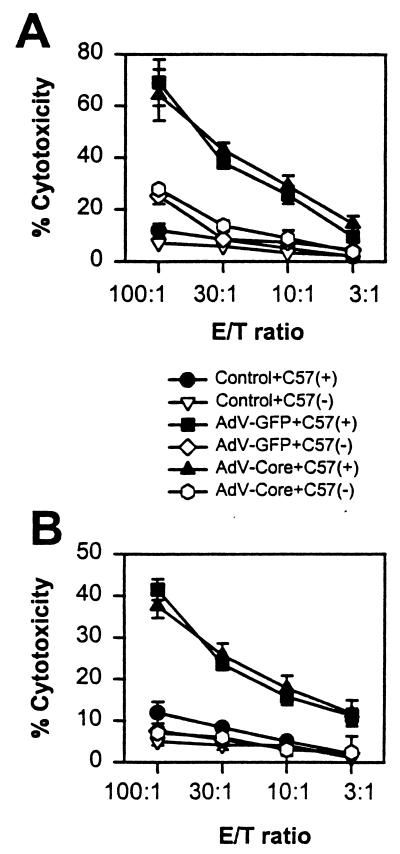
It is therefore important to examine whether priming of CTL to adenovirus is inhibited in mice infected with the adeno-core virus. Spleen cells from virus-infected mice were restimulated in vitro with virus and then tested for virus-specific cytolytic activity (6, 26) on infected or uninfected C57SV (H-2b) targets (13). Figure 7A shows that cytolytic activities in cultures from spleen cells of mice primed with 2 × 109 PFU of virus and restimulated with the respective adeno-GFP or adeno-core virus are essentially identical. A similar result is seen in cultures from adeno-GFP virus- or adeno-core virus-primed mice which were restimulated with adeno-lacZ virus in vitro (Fig. 7B).
FIG. 7.
Priming of virus-specific CTL in adeno-GFP and adeno-core virus-infected mice. (A) C57BL/6 mice were injected with 2 × 109 PFU of adeno-GFP (AdV-GFP) or adeno-core (AdV-Core) virus on day 0, and spleen cells were harvested on day 10 and restimulated in tissue culture for 5 days with the virus used for priming. Cells were harvested and assayed for lytic activity on uninfected [C57(−)] or adeno-lacZ virus-infected [C57(+)] C57SV cells at the effector-to-target cell (E/T) ratios shown. Controls were spleen cells from unprimed mice stimulated with virus in vitro. (B) Mice were primed as for panel A, but spleen cells were restimulated in vitro with adeno-lacZ virus and then assayed for lytic activity on normal or adeno-lacZ virus-infected C57SV cells. Error bars indicate standard deviations. Results are from one of two experiments.
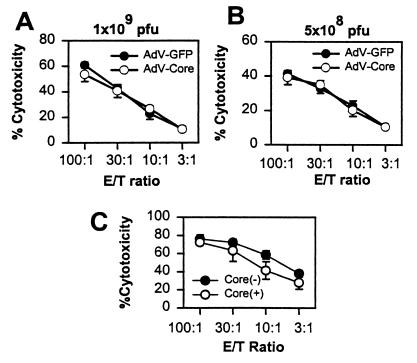
It is possible that the putative regulatory effects of the core are masked by the high virus dose used in these experiments and may be demonstrable only at low virus doses. The results in Fig. 8A and B show that at doses of 1 × 109 and 5 × 108 PFU per mouse, there is again no difference between adeno-core- and control GFP-primed spleen cells in their ability to generate CTL activity. Lower virus doses do not lead to reproducible priming of CTL and therefore could not be tested. It therefore appears that expression of the HCV core gene by adenovirus does not inhibit priming of adenovirus-specific CTL.
FIG. 8.
Induction of CTL priming by low-dose virus injection and in normal and core transgenic mice. (A and B) C57BL/6 mice were injected with 1 × 109 PFU (A) or 5 × 108 PFU (B) of adeno-GFP (AdV-GFP) or adeno-core (AdV-Core) virus on day 0 as indicated, and spleen cells were harvested on day 10 and restimulated in tissue culture for 5 days with the virus used for priming. Cells were harvested and assayed for lytic activity as for Fig. 7 on adeno-lacZ virus-infected C57SV targets. (C) Core transgenic (+) or control (−) mice were injected with 109 PFU of adeno-lacZ virus, and spleen cells were harvested on day 10 and restimulated with adeno-lacZ virus for 5 days, at which time cytolytic activity was assayed on adeno-lacZ virus-infected C57SV cells. Error bars indicate standard deviations. Results are from one of two experiments.
To examine the possibility that the core modulates priming of CTL when it is expressed endogenously, core transgenic mice and control mice were infected with virus and spleens were assayed for CTL priming. The results in Fig. 8C show that there is no significant difference in CTL priming between control and core transgenic mice.
DISCUSSION
We and others had previously reported that the HCV core protein expressed in stable transfectants modulates TNF-α-mediated signaling in tissue culture cell lines (14, 18, 19, 28). This observation could have important implications not only in the progression of HCV-induced hepatitis but also with respect to the utility of HCV core protein in HCV vaccines. TNF-α has been shown to be an important mediator in experimental liver injury models in animals (10, 13). However, this cytokine is also known as a growth factor for hepatocytes (24). TNF-α induces apoptosis in hepatocytes in the presence of protein synthesis inhibitors, but in their absence it induces synthesis of laminin and stimulates DNA synthesis (10, 24). Laminin is an important extracellular matrix protein which plays a role in liver regeneration. Therefore, induction of laminin synthesis by TNF-α in conjunction with stimulation of TNF-α receptor signaling by the HCV core protein could have an antagonistic effect in virus-induced liver injury. It is also known that TNF-α induces c-Jun and NF-κB in hepatocytes (14), which are thought to be essential in the proliferation-signaling cascade. Stimulation of TNF-α signaling by the HCV core protein, therefore, could have beneficial as well as destructive effects in the virus-infected liver.
We show here that injection of adeno-core virus induces production of copious amounts of HCV core protein in the liver that are detectable by immunoblotting. Despite this, serum ALT levels induced by this virus are identical to those induced by control virus. Concordant results are seen in mice expressing or not expressing the HCV core as a transgene and infected with adeno-lacZ virus. Even in animals in which either Fas- or TNF-α-mediated apoptosis pathways are nonfunctional, no differences in ALT levels between mice injected with adeno-core or adeno-GFP control virus were discernible. These results do not support the notion that the HCV core protein stimulates or inhibits signaling of TNF-α- or Fas-mediated death pathways in the liver.
Using two fibrosarcoma-derived cell lines, i.e., B/C10ME, which expresses TNF receptors, and C57SV, which expresses Fas, we examined whether the HCV core protein has demonstrable effects on TNF-α- or Fas-mediated induction of apoptosis. In a departure from our previous experiments (28), the present study was performed in the absence of protein synthesis inhibitors, as this is more reflective of events which would occur in the liver during virus infection. We show that cell death induced by TNF-α was slightly more pronounced in cells infected with adeno-core compared to adeno-GFP virus, and Fas-mediated cell death was indistinguishable in cells infected with the two viruses. These results, while consistent with the above in vivo experiments, are at variance with some of the results obtained with stable cell lines, which showed that core protein can strongly modulate the cellular response to TNF-α or Fas ligand (4, 14, 18, 19, 20). It should be pointed out that the virus strains used in various published studies and in different laboratories were different. However, in our studies, we used the same core protein constructs for tissue culture and animal studies, yet we saw at least some modulatory effects of the core protein on the TNF-α response in vitro but no effects in vivo. Furthermore, two core sequences derived from different genotype 1b isolates were used for the adenovirus constructs and in the transgenic mice, yet neither of them showed any demonstrable effects of the core protein in vivo. Our results thus suggest that the observed effects of the core protein in tissue culture cell lines may not be a true reflection of the reactions occurring in the animal. Therefore, the effects of the HCV core protein on TNF-α and Fas signaling may be demonstrable only under certain experimental conditions, one of which could be inhibition of protein synthesis.
Our results showing that ALT levels in the sera of adeno-core virus- and adeno-GFP virus-infected mice are identical suggest that the HCV core protein has no effects on activation of the cell-mediated immune response to the infection. Additional data provided here are consistent with this interpretation. The induction of cytokines IL-12 p40, IL-18, and IFN-γ, assayed by RT-PCR in whole liver tissue, is unchanged, as are the recruitment of T cells into the infected liver and the priming of CTL specific for adenovirus antigens. It therefore appears that the HCV core protein of genotype 1b, expressed by an adenovirus in the liver, does not modulate the cell-mediated immune response which is mounted against this infection. Clearly these results are at variance with data in which the HCV core protein, expressed in a vaccinia virus construct, was reported to inhibit induction of vaccinia virus-specific CTL, leading to death of the infected mice (8). Presently it is not known what the reason for this difference might be, and experiments are needed to examine whether the HCV core protein alters vaccinia virus pathogenesis or modulates the induction of CTL responses to vaccinia virus but not adenovirus antigens.
In conclusion, our results show that expression of the HCV core protein of genotype 1b in an adenovirus construct leads to expression of core protein in the virus-infected mouse liver, yet this expression does not cause a modulation of the cell-mediated immune response to the infection, nor is there a change in liver injury reflected in serum ALT levels. Concordant results are obtained with mice in which the core is expressed endogenously as a transgene. Therefore, this study suggests that the core protein may be a suitable candidate to be included in potential HCV DNA vaccines.
Acknowledgments
This work was supported by research grants AI 43954 and U19 AI40038 from the National Institutes of Health. J.-W. He is a Research Associate and M. M. C. Lai is an Investigator of the Howard Hughes Medical Institute.
The transgenic mice used in this study were generated with the help of the core facility of the USC/Norris Comprehensive Cancer Center.
REFERENCES
- 1.Chen, P. J., M. H. Lin, K. F. Tai, P. C. Liu, C. J. Lin, and D. S. Chen. 1992. The Taiwanese hepatitis C virus genome: sequence determination and mapping the 5′ termini of viral genomic and antigenomic RNA. Virology 188:102–113. [DOI] [PubMed] [Google Scholar]
- 2.Chow, S. C., D. J. McConkey, S. Orrenius, and M. Jondal. 1989. Quantitation of DNA fragmentation using fiberglass filters. Anal. Biochem. 183:42–45. [DOI] [PubMed] [Google Scholar]
- 3.Green, L. M., J. L. Reade, and C. F. Ware. 1984. Rapid colorimetric assay for cell viability: application to the quantitation of cytotoxic and growth inhibitory lymphokines. J. Immunol. Methods 70:257–268. [DOI] [PubMed] [Google Scholar]
- 4.Hahn, C. S., Y. G. Cho, B. S. Kang, I. M. Lester, and Y. S. Hahn. 2000. The HCV core protein acts as a positive regulator of fas-mediated apoptosis in a human lymphoblastoid T cell line. Virology 276:127–137. [DOI] [PubMed] [Google Scholar]
- 5.He, T. C., S. Zhou, L. T. da Costa, J. Yu, K. W. Kinzler, and B. Vogelstein. 1998. A simplified system for generating recombinant adenoviruses. Proc. Natl. Acad. Sci. USA 95:2509–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Juillard, V., P. Villefroy, D. Godfrin, A. Pavirani, A. Venet, and J. G. Guillet. 1995. Long-term humoral and cellular immunity induced by a single immunization with replication-defective adenovirus recombinant vector. Eur. J. Immunol. 25:3467–3473. [DOI] [PubMed] [Google Scholar]
- 7.Kim, D. W., T. Uetsuki, Y. Kaziro, N. Yamaguchi, and S. Sugano. 1990. Use of the human elongation factor 1 alpha promoter as a versatile and efficient expression system. Gene 16:217–223. [DOI] [PubMed] [Google Scholar]
- 8.Large, M. K., D. J. Kittlesen, and Y. S. Hahn. 1999. Suppression of host immune response by the core protein of hepatitis C virus: possible implications for hepatitis C virus persistence. J. Immunol. 162:931–938. [PubMed] [Google Scholar]
- 9.Lechner, F., D. K. Wong, P. R. Dunbar, R. Chapman, R. T. Chung, P. Dohrenwend, G. Robbins, R. Phillips, P. Klenerman, and B. D. Walker. 2000. Analysis of successful immune responses in persons infected with hepatitis C virus. J. Exp. Med. 191:1499–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leist, M., F. Gantner, I. Bohlinger, P. G. Germann, G. Tiegs, and A. Wendel. 1994. Murine hepatocyte apoptosis induced in vitro and in vivo by TNF-alpha requires transcriptional arrest. J. Immunol. 153:1778–1788. [PubMed] [Google Scholar]
- 11.Liu, Z. X., S. Govindarajan, S. Okamoto, and G. Dennert. 2000. Fas- and tumor necrosis factor receptor 1-dependent but not perforin-dependent pathways cause injury in livers infected with an adenovirus construct in mice. Hepatology 31:665–673. [DOI] [PubMed] [Google Scholar]
- 12.Liu, Z. X., S. Govindarajan, S. Okamoto, and G. Dennert. 2000. NK cells cause liver injury and facilitate the induction of T cell-mediated immunity to a viral liver infection. J. Immunol. 164:6480–6486. [DOI] [PubMed] [Google Scholar]
- 13.Liu, Z. X., S. Govindarajan, S. Okamoto, and G. Dennert. 2001. Fas-mediated apoptosis causes elimination of virus-specific cytotoxic T cells in the virus-infected liver. J. Immunol. 166:3035–3041. [DOI] [PubMed] [Google Scholar]
- 14.Marusawa, H., M. Hijikata, T. Chiba, and K. Shimotohno. 1999. Hepatitis C virus core protein inhibits Fas- and tumor necrosis factor alpha-mediated apoptosis via NF-kappa B activation. J. Virol. 73:4713–4720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nelson, D. R., C. G. Marousis, T. Ohno, G. L. Davis, and J. Y. Lau. 1998. Intrahepatic hepatitis C virus-specific cytotoxic T lymphocyte activity and response to interferon alfa therapy in chronic hepatitis C. Hepatology 28:225–230. [DOI] [PubMed] [Google Scholar]
- 16.Okamura, H., H. Tsutsui, S. Kashiwamura, T. Yoshimoto, and K. Nakanishi. 1998. Interleukin-18: a novel cytokine that augments both innate and acquired immunity. Adv. Immunol. 70:281–312. [DOI] [PubMed] [Google Scholar]
- 17.Ray, R. B., L. M. Lagging, K. Meyer, R. Steele, and R. Ray. 1995. Transcriptional regulation of cellular and viral promoters by the hepatitis C virus core protein. Virus Res. 37:209–220. [DOI] [PubMed] [Google Scholar]
- 18.Ray, R. B., K. Meyer, and R. Ray. 1996. Suppression of apoptotic cell death by hepatitis C virus core protein. Virology 226:176–182. [DOI] [PubMed] [Google Scholar]
- 19.Ray, R. B., K. Meyer, R. Steele, A. Shrivastava, B. B. Aggarwal, and R. Ray. 1998. Inhibition of tumor necrosis factor (TNF-alpha)-mediated apoptosis by hepatitis C virus core protein. J. Biol. Chem. 273:2256–2259. [DOI] [PubMed] [Google Scholar]
- 20.Ruggieri, A., T. Harada, Y. Matsuura, and T. Miyamura. 1997. Sensitization to Fas-mediated apoptosis by hepatitis C virus core protein. Virology 229:68–76. [DOI] [PubMed] [Google Scholar]
- 21.Trinchieri, G. 1995. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu. Rev. Immunol. 13:251–276. [DOI] [PubMed] [Google Scholar]
- 22.Tsutsui, H., K. Matsui, N. Kawada, Y. Hyodo, N. Hayashi, H. Okamura, K. Higashino, and K. Nakanishi. 1997. IL-18 accounts for both TNF-alpha- and Fas ligand-mediated hepatotoxic pathways in endotoxin-induced liver injury in mice. J. Immunol. 159:3961–3967. [PubMed] [Google Scholar]
- 23.Uchida, T. 1994. Pathology of hepatitis C. Intervirology 37:126–132. [DOI] [PubMed] [Google Scholar]
- 24.Watanabe, Y., H. Osaki, and T. Akaike. 1997. TNF-alpha bifunctionally induces proliferation in primary hepatocytes: role of cell anchorage and spreading. J. Immunol. 159:4840–4847. [PubMed] [Google Scholar]
- 25.Yanagi, M., M. St. Claire, M. Shapiro, S. U. Emerson, R. H. Purcell, and J. Bukh. 1998. Transcripts of a chimeric cDNA clone of hepatitis C virus genotype lb are infectious in vivo. Virology 244:161–172. [DOI] [PubMed] [Google Scholar]
- 26.Yang, Y., H. C. Ertl, and J. M. Wilson. 1994. MHC class I-restricted cytotoxic T lymphocytes to viral antigens destroy hepatocytes in mice infected with E1-deleted recombinant adenoviruses. Immunity 1:433–442. [DOI] [PubMed] [Google Scholar]
- 27.Yoshimoto, T., K. Takeda, T. Tanaka, K. Ohkusu, S. Kashiwamura, H. Okamura, S. Akira, and K. Nakanishi. 1998. IL-12 up-regulates IL-18 receptor expression on T cells, Th1 cells, and B cells: synergism with IL-18 for IFN-gamma production. J. Immunol. 161:3400–3407. [PubMed] [Google Scholar]
- 28.Zhu, N., A. Khoshnan, R. Schneider, M. Matsumoto, G. Dennert, C. Ware, and M. M. Lai. 1998. Hepatitis C virus core protein binds to the cytoplasmic domain of tumor necrosis factor (TNF) receptor 1 and enhances TNF-induced apoptosis. J. Virol. 72:3691–3697. [DOI] [PMC free article] [PubMed] [Google Scholar]