Abstract
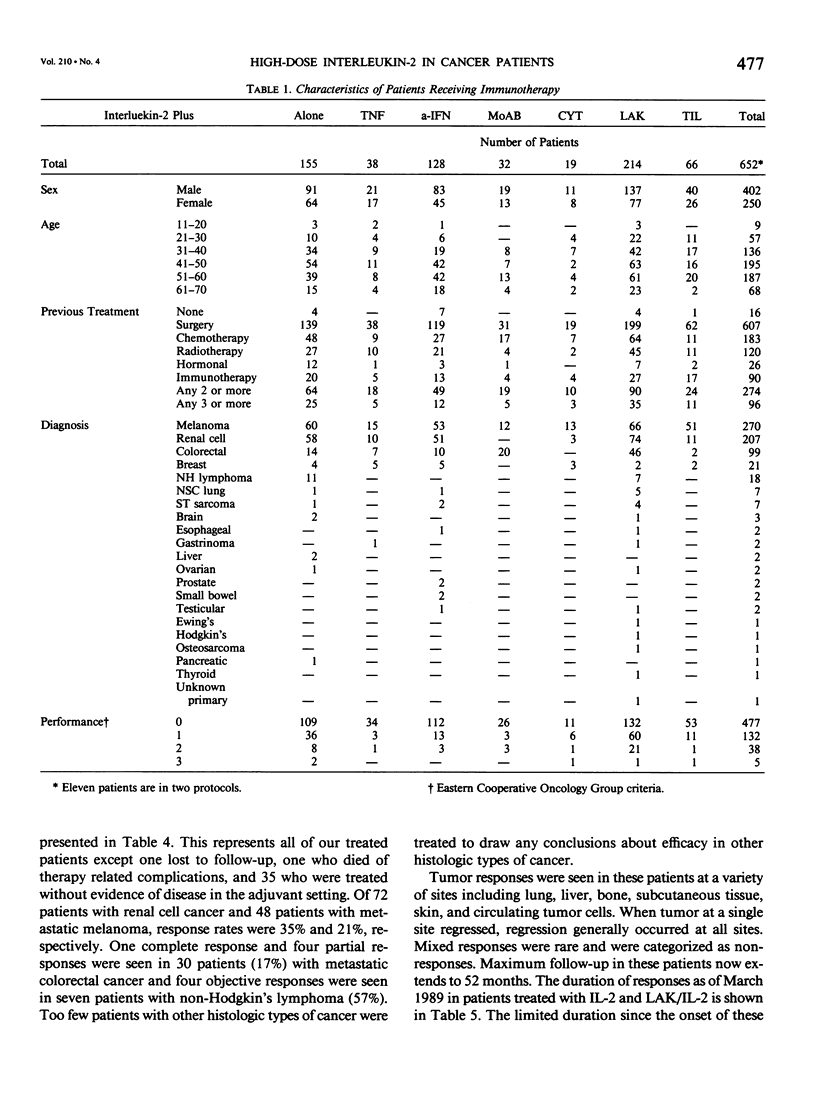
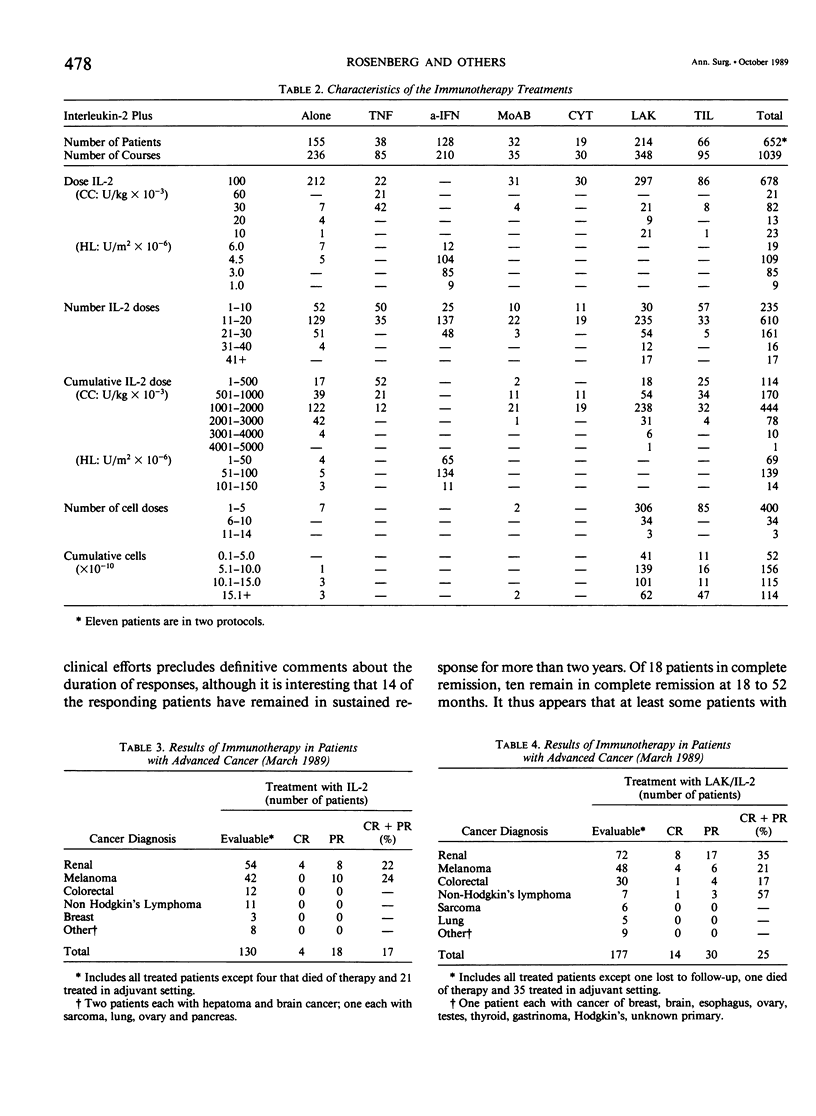
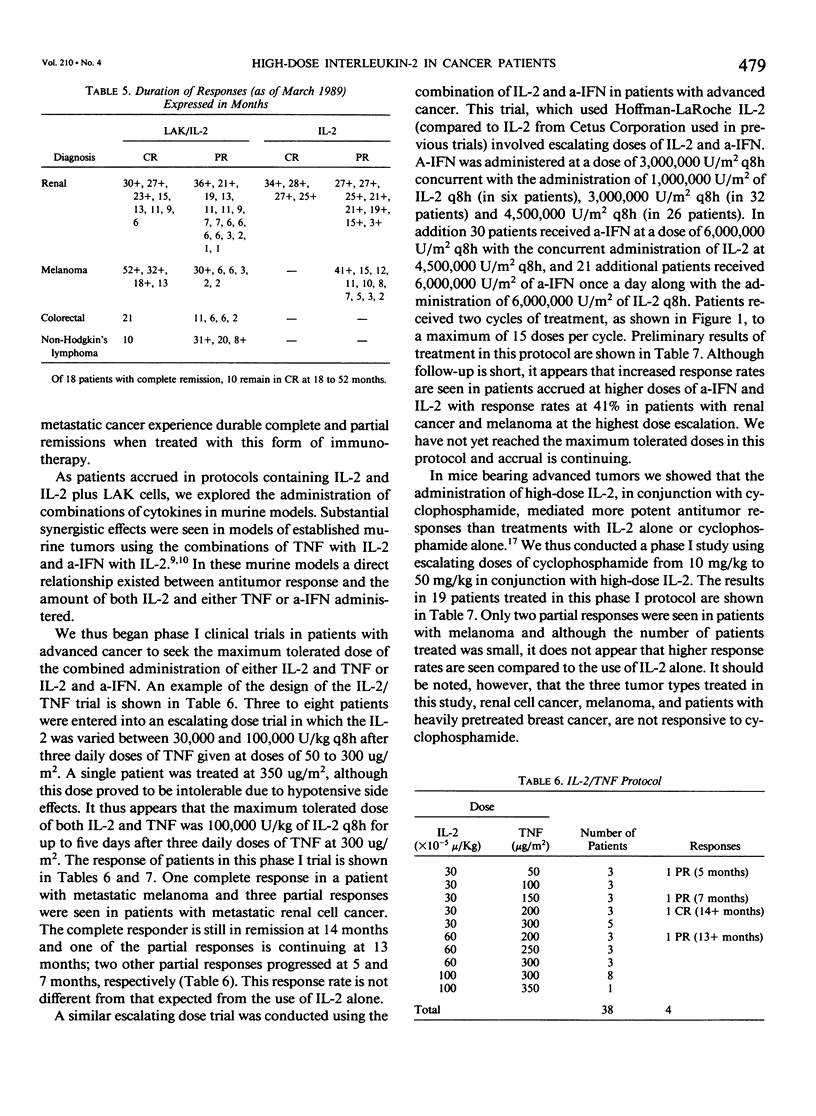
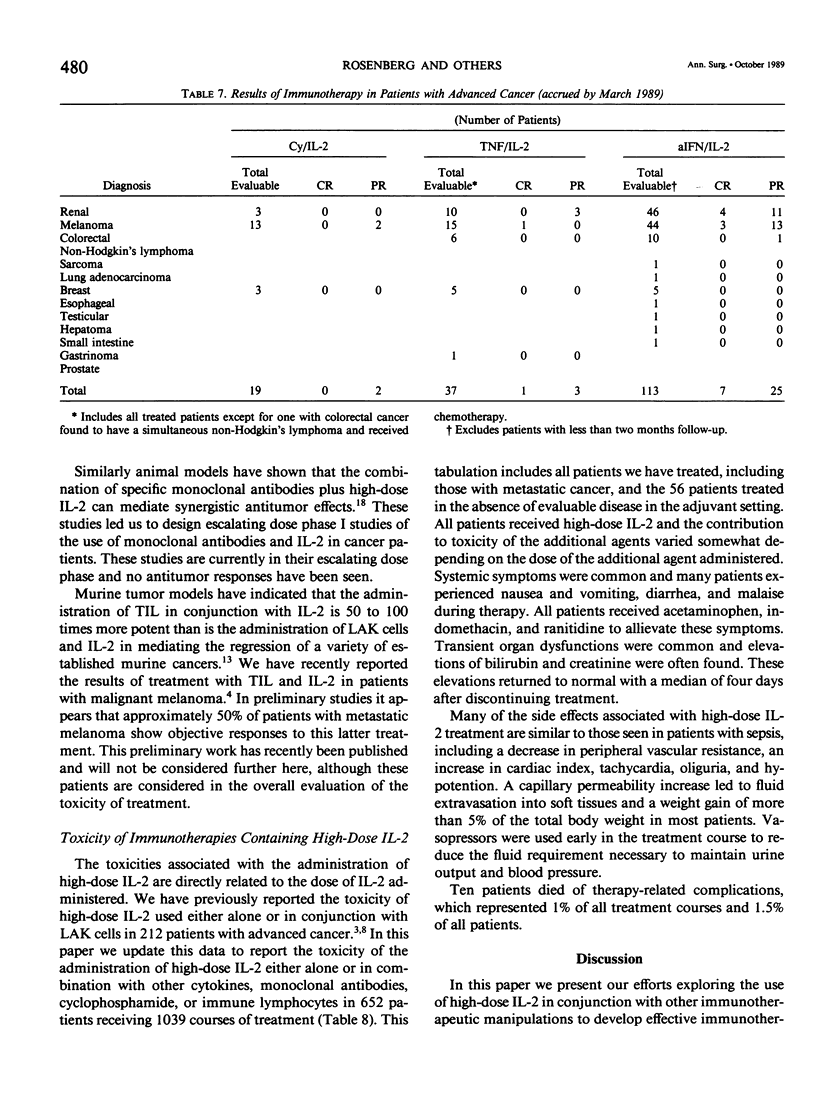
We have administered 1039 courses of high-dose interleukin-2 (IL-2) to 652 cancer patients. Five hundred ninety-six patients had metastatic cancer that either had failed standard effective therapies or had disease for which no standard effective therapy existed, and 56 patients were treated in the absence of evaluable disease in the adjuvant setting. IL-2 was administered either alone (155 patients) or in conjunction with activated immune cells such as lymphokine activated killer (LAK) cells (214 patients) or tumor infiltrating lymphocytes (TIL) (66 patients), with other cytokines such as alpha interferon (a-IFN)(128 patients) or tumor necrosis factor (TNF)(38 patients), with monoclonal antibodies (32 patients), or with the chemotherapeutic agent cyclophosphamide (19 patients). Initial results with the treatment of high-dose IL-2 alone or in conjunction with LAK cells have indicated that objective regressions of cancer can be achieved in 20% to 35% of patients with selected advanced metastatic cancers. Although most responses have been seen in patients with metastatic renal cell cancer, melanoma, colorectal cancer, and non-Hodgkin's lymphoma, many histologic types of cancer have not been treated in significant numbers. These regressions can be durable; of 18 patients achieving a complete response, ten have not experienced recurrence at intervals from 18 to 52 months. Although combinations of IL-2 with TNF do not appear to result in increased responses, there is a suggestion in our initial phase I studies that the combination of a-IFN and IL-2 is more effective than the administration of cytokine alone and this combination deserves further study. Similarly the adoptive transfer of TIL in conjunction with IL-2 also appears to be more effective than the use of IL-2 alone. The toxic side effects in patients treated with high-dose IL-2 are presented and include malaise, nausea and vomiting, hypotension, fluid retention, and organ dysfunction. Treatment-related deaths were seen in 1% of all treatment courses and in 1.5% of patients. These studies demonstrate that a purely immunologic manipulation can mediate the regression of advanced cancers in selected patients and may provide a base for the development of practical, effective biologic treatments for some cancer patients.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cameron R. B., McIntosh J. K., Rosenberg S. A. Synergistic antitumor effects of combination immunotherapy with recombinant interleukin-2 and a recombinant hybrid alpha-interferon in the treatment of established murine hepatic metastases. Cancer Res. 1988 Oct 15;48(20):5810–5817. [PubMed] [Google Scholar]
- Eisenthal A., Cameron R. B., Uppenkamp I., Rosenberg S. A. Effect of combined therapy with lymphokine-activated killer cells, interleukin 2 and specific monoclonal antibody on established B16 melanoma lung metastases. Cancer Res. 1988 Dec 15;48(24 Pt 1):7140–7145. [PubMed] [Google Scholar]
- Ettinghausen S. E., Lipford E. H., 3rd, Mulé J. J., Rosenberg S. A. Systemic administration of recombinant interleukin 2 stimulates in vivo lymphoid cell proliferation in tissues. J Immunol. 1985 Aug;135(2):1488–1497. [PubMed] [Google Scholar]
- Fahey J. L., Sarna G., Gale R. P., Seeger R. Immune interventions in disease. Ann Intern Med. 1987 Feb;106(2):257–274. doi: 10.7326/0003-4819-106-2-257. [DOI] [PubMed] [Google Scholar]
- Fisher B., Packard B. S., Read E. J., Carrasquillo J. A., Carter C. S., Topalian S. L., Yang J. C., Yolles P., Larson S. M., Rosenberg S. A. Tumor localization of adoptively transferred indium-111 labeled tumor infiltrating lymphocytes in patients with metastatic melanoma. J Clin Oncol. 1989 Feb;7(2):250–261. doi: 10.1200/JCO.1989.7.2.250. [DOI] [PubMed] [Google Scholar]
- Frei E., 3rd, Spriggs D. Tumor necrosis factor: still a promising agent. J Clin Oncol. 1989 Mar;7(3):291–294. doi: 10.1200/JCO.1989.7.3.291. [DOI] [PubMed] [Google Scholar]
- Gemlo B. T., Palladino M. A., Jr, Jaffe H. S., Espevik T. P., Rayner A. A. Circulating cytokines in patients with metastatic cancer treated with recombinant interleukin 2 and lymphokine-activated killer cells. Cancer Res. 1988 Oct 15;48(20):5864–5867. [PubMed] [Google Scholar]
- Lafreniere R., Rosenberg S. A. Adoptive immunotherapy of murine hepatic metastases with lymphokine activated killer (LAK) cells and recombinant interleukin 2 (RIL 2) can mediate the regression of both immunogenic and nonimmunogenic sarcomas and an adenocarcinoma. J Immunol. 1985 Dec;135(6):4273–4280. [PubMed] [Google Scholar]
- Lotze M. T., Chang A. E., Seipp C. A., Simpson C., Vetto J. T., Rosenberg S. A. High-dose recombinant interleukin 2 in the treatment of patients with disseminated cancer. Responses, treatment-related morbidity, and histologic findings. JAMA. 1986 Dec 12;256(22):3117–3124. [PubMed] [Google Scholar]
- Lotze M. T., Matory Y. L., Ettinghausen S. E., Rayner A. A., Sharrow S. O., Seipp C. A., Custer M. C., Rosenberg S. A. In vivo administration of purified human interleukin 2. II. Half life, immunologic effects, and expansion of peripheral lymphoid cells in vivo with recombinant IL 2. J Immunol. 1985 Oct;135(4):2865–2875. [PubMed] [Google Scholar]
- McIntosh J. K., Mulé J. J., Merino M. J., Rosenberg S. A. Synergistic antitumor effects of immunotherapy with recombinant interleukin-2 and recombinant tumor necrosis factor-alpha. Cancer Res. 1988 Jul 15;48(14):4011–4017. [PubMed] [Google Scholar]
- Muul L. M., Nason-Burchenal K., Carter C. S., Cullis H., Slavin D., Hyatt C., Director E. P., Leitman S. F., Klein H. G., Rosenberg S. A. Development of an automated closed system for generation of human lymphokine-activated killer (LAK) cells for use in adoptive immunotherapy. J Immunol Methods. 1987 Aug 3;101(2):171–181. doi: 10.1016/0022-1759(87)90148-7. [DOI] [PubMed] [Google Scholar]
- Muul L. M., Spiess P. J., Director E. P., Rosenberg S. A. Identification of specific cytolytic immune responses against autologous tumor in humans bearing malignant melanoma. J Immunol. 1987 Feb 1;138(3):989–995. [PubMed] [Google Scholar]
- Papa M. Z., Mulé J. J., Rosenberg S. A. Antitumor efficacy of lymphokine-activated killer cells and recombinant interleukin 2 in vivo: successful immunotherapy of established pulmonary metastases from weakly immunogenic and nonimmunogenic murine tumors of three district histological types. Cancer Res. 1986 Oct;46(10):4973–4978. [PubMed] [Google Scholar]
- Papa M. Z., Yang J. C., Vetto J. T., Shiloni E., Eisenthal A., Rosenberg S. A. Combined effects of chemotherapy and interleukin 2 in the therapy of mice with advanced pulmonary tumors. Cancer Res. 1988 Jan 1;48(1):122–129. [PubMed] [Google Scholar]
- Rosenberg S. A. Adoptive immunotherapy of cancer using lymphokine activated killer cells and recombinant interleukin-2. Important Adv Oncol. 1986:55–91. [PubMed] [Google Scholar]
- Rosenberg S. A., Grimm E. A., McGrogan M., Doyle M., Kawasaki E., Koths K., Mark D. F. Biological activity of recombinant human interleukin-2 produced in Escherichia coli. Science. 1984 Mar 30;223(4643):1412–1414. doi: 10.1126/science.6367046. [DOI] [PubMed] [Google Scholar]
- Rosenberg S. A., Lotze M. T., Muul L. M., Chang A. E., Avis F. P., Leitman S., Linehan W. M., Robertson C. N., Lee R. E., Rubin J. T. A progress report on the treatment of 157 patients with advanced cancer using lymphokine-activated killer cells and interleukin-2 or high-dose interleukin-2 alone. N Engl J Med. 1987 Apr 9;316(15):889–897. doi: 10.1056/NEJM198704093161501. [DOI] [PubMed] [Google Scholar]
- Rosenberg S. A., Lotze M. T., Muul L. M., Leitman S., Chang A. E., Ettinghausen S. E., Matory Y. L., Skibber J. M., Shiloni E., Vetto J. T. Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N Engl J Med. 1985 Dec 5;313(23):1485–1492. doi: 10.1056/NEJM198512053132327. [DOI] [PubMed] [Google Scholar]
- Rosenberg S. A., Packard B. S., Aebersold P. M., Solomon D., Topalian S. L., Toy S. T., Simon P., Lotze M. T., Yang J. C., Seipp C. A. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. N Engl J Med. 1988 Dec 22;319(25):1676–1680. doi: 10.1056/NEJM198812223192527. [DOI] [PubMed] [Google Scholar]
- Rosenberg S. A., Spiess P., Lafreniere R. A new approach to the adoptive immunotherapy of cancer with tumor-infiltrating lymphocytes. Science. 1986 Sep 19;233(4770):1318–1321. doi: 10.1126/science.3489291. [DOI] [PubMed] [Google Scholar]
- Rosenberg S. A. The development of new immunotherapies for the treatment of cancer using interleukin-2. A review. Ann Surg. 1988 Aug;208(2):121–135. doi: 10.1097/00000658-198808000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg S. Lymphokine-activated killer cells: a new approach to immunotherapy of cancer. J Natl Cancer Inst. 1985 Oct;75(4):595–603. [PubMed] [Google Scholar]
- Rosenstein M., Ettinghausen S. E., Rosenberg S. A. Extravasation of intravascular fluid mediated by the systemic administration of recombinant interleukin 2. J Immunol. 1986 Sep 1;137(5):1735–1742. [PubMed] [Google Scholar]
- Schoof D. D., Gramolini B. A., Davidson D. L., Massaro A. F., Wilson R. E., Eberlein T. J. Adoptive immunotherapy of human cancer using low-dose recombinant interleukin 2 and lymphokine-activated killer cells. Cancer Res. 1988 Sep 1;48(17):5007–5010. [PubMed] [Google Scholar]
- Smith K. A. Interleukin-2: inception, impact, and implications. Science. 1988 May 27;240(4856):1169–1176. doi: 10.1126/science.3131876. [DOI] [PubMed] [Google Scholar]
- Topalian S. L., Muul L. M., Solomon D., Rosenberg S. A. Expansion of human tumor infiltrating lymphocytes for use in immunotherapy trials. J Immunol Methods. 1987 Aug 24;102(1):127–141. doi: 10.1016/s0022-1759(87)80018-2. [DOI] [PubMed] [Google Scholar]
- West W. H., Tauer K. W., Yannelli J. R., Marshall G. D., Orr D. W., Thurman G. B., Oldham R. K. Constant-infusion recombinant interleukin-2 in adoptive immunotherapy of advanced cancer. N Engl J Med. 1987 Apr 9;316(15):898–905. doi: 10.1056/NEJM198704093161502. [DOI] [PubMed] [Google Scholar]


