Abstract
Marek’s disease virus (MDV) is a herpesvirus that induces T lymphomas in chickens. The aim of this study was to assess the role of the macrophage activator chicken myelomonocytic growth factor (cMGF) in controlling MDV infection. B13/B13 chickens, which are highly susceptible to MD, were either treated with cMGF delivered via a live fowlpox virus (fp/cMGF) or treated with the parent vector (fp/M3) or were left as untreated controls. Seven days later, when challenged with the very virulent RB-1B strain of MDV, the spleens of chickens treated with fp/cMGF showed increased expression of the inducible nitric oxide synthase (iNOS) gene compared to those of control chickens and fp/M3-treated chickens. Increased iNOS gene expression was also accompanied by greater induction of gamma interferon and macrophage inflammatory protein (K203) gene expression, both possible activators of iNOS. fp/cMGF treatment also increased the number of monocytes and systemic NO production in contrast to fp/M3 treatment. Even though cMGF treatment was unable to prevent death for the chickens, it did prolong their survival time, and viremia and tumor incidence were greatly reduced. In addition, cMGF treatment improved the partial protection induced by vaccination with HVT (herpesvirus isolated from turkeys) against RB-1B, preventing 100% mortality (versus 66% with vaccination alone) and greatly reducing tumor development. Treatment with fp/M3 did not have such effects. These results suggest that cMGF may play multiple roles in protection against MD. First, it may enhance the innate immune response by increasing the number and activity of monocytes and macrophages, resulting in increased NO production. Second, it may enhance the acquired immune response, indicated by its ability to enhance vaccine efficacy.
Marek’s disease virus (MDV) is a herpesvirus isolated from chickens. The disease is characterized by strong early immunosuppression and development of T lymphomas. Three serotypes of virus have been identified: serotype 1 includes strains with oncogenic potential, serotype 2 includes nononcogenic MDV strains, and serotype 3 is limited to a herpesvirus isolated from turkeys (HVT) and not pathogenic for chickens. Resistance to disease can be acquired after vaccination. Attenuated, serotype 1 MDV strains, serotype 2 MDV strains, and HVT are used to vaccinate against oncogenic MDV (3). Genetic resistance has also been observed at multiple levels for MD, including one linked to the major histocompatibility complex (1). In genetic and acquired resistance, viral replication and spread are reduced, not prevented. Nevertheless, tumor occurrence is prevented (33, 40). The mechanisms underlying resistance to MDV have not yet been elucidated. Indeed, the continuing emergence of new hypervirulent strains of MDV necessitates improving vaccination efficiency and the development of new prophylactic strategies.
Oncogenic MDV induces an initial phase of viral replication in the first week after infection, which is restricted to B lymphocytes and to a few T lymphocytes. This is followed by a latent phase restricted to T lymphocytes, the final target of the transformation process (33). Most of the T lymphomas exhibit the CD4+ CD8− phenotype (30). Very little is known about innate and acquired immune responses involved in the control of this disease. The majority of studies on immune responses occurring during MD have identified cell-mediated immunity, especially specific and nonspecific cytotoxic responses, mediated by T lymphocytes and NK cells, respectively, as important in early protective mechanisms against viral replication (27, 31).
Macrophages also participate in the host defense response against MD. In particular, in vivo macrophage depletion, either before challenge with MDV or continuously during the disease, increases the incidence of tumors (9, 10). Indeed, macrophages from MDV-infected chickens (17) and activated macrophages (8) are able to inhibit MDV replication in vitro. Nitric oxide (NO), which is produced from l-arginine by macrophages upon activation, is involved in this inhibitory effect (8, 42). The antiviral and antitumoral properties of NO are now well established in mammals (23). Nevertheless, splenic macrophages from MDV-infected chickens also demonstrate immunosuppressive action on lymphocyte proliferation (20). Macrophages may thus have both a beneficial role by limiting MDV replication and a detrimental role by participating in the initial immunosuppression.
Activating macrophages may therefore be beneficial in MDV-infected chickens; they may reduce viral replication and impede initial immunosuppression, and finally reduce tumor development. Treatment with chicken myelomonocytic growth factor (cMGF), a cytokine active on macrophages, might be used to mediate such an effect. cMGF is a glycoprotein of 27 kDa that has been characterized as a hematopoietic growth factor enhancing macrophage and granulocyte colony formation from chicken bone marrow progenitors in vitro (21, 22). To date, the only functional effects of cMGF demonstrated in vivo are enhancement of the number of cells from the monocyte/macrophage lineage and associated macrophage activation properties resulting in increased phagocytosis and NO production (43).
The aim of this study was to define the effects of cMGF on the development of MD. Conventional administration of cytokines by injection has limitations because their short half-life in vivo necessitates repeated injections. However, this problem can be overcome by delivery of cytokines via live viral vectors, thus offering the advantages of prolonged release of cytokines. We therefore used delivery of cMGF via a vaccinal strain of fowlpox virus that has previously been shown to induce a strong and sustained systemic response to the cytokine in chickens (43).
MATERIALS AND METHODS
Chickens.
Two-week-old White Leghorn chickens homozygous for the B13 major histocompatibility complex and born and raised at the Institut National de la Recherche Agronomique (INRA) (Station de Pathologie Aviaire et de Parasitologie [PAP], Nouzilly, France) under specific-pathogen-free (SPF) conditions were used for the experiments. Eggs from an SPF flock (outbred White Leghorn) were the source of 11-day-old embryos needed for preparation of fibroblast cultures.
Virus.
The very virulent strain of MDV, RB-1B, was maintained by successive passages on SPF outbred chickens. HVT vaccine was purchased from Fort-Dodge Santé Animale (Tours, France). For each virus, 1,000 PFU was inoculated intramuscularly into a chicken. FPV vaccine strain vector (fp/M3) and recombinant fp/cMGF (cMGF delivered via a live fowlpox virus) (43) were propagated on monolayers of primary chicken embryo fibroblasts (CEFs), and 105 PFU was inoculated intramuscularly into each chicken.
Experimental design.
In experiment 1, when histocompatible B13/B13 chickens were 2 weeks old, 18 chickens were inoculated with fp/cMGF, 18 were inoculated with the control fp/M3 vector, and 18 were left as noninoculated controls. The number of blood monocytes and the level of nitric oxide (NO2− plus NO3−) in serum were measured 3, 7, and 10 days later, and spleen cytokine mRNA expression was measured 7 days after fp/cMGF or fp/M3 inoculation. In experiment 2, 10 2-week-old B13/B13 chickens were inoculated with fp/cMGF and 10 chickens were inoculated with fp/M3: 5 chickens from each group were then challenged with RB-1B 7 days later to measure RB-1B viremia in blood leukocytes in the fibroblast assay. Five control chickens were inoculated with RB-1B alone. In experiment 3, 12 noninfected B13/B13 chickens were left as controls, and 36 chickens divided into three groups of 12 were inoculated with RB-1B: one group was inoculated with fp/cMGF 7 days before inoculation with RB-1B, one group was inoculated with fp/M3 vector, and the third group was not treated. Spontaneous and lipopolysaccharide (LPS)-induced nitric oxide (NO2− plus NO3−) levels in serum were measured in these chickens 1 and 3 weeks after MDV challenge. In experiment 4, one group of 36 chickens was divided into three groups of 12: one group was inoculated with fp/cMGF, one group was inoculated with fp/M3 vector and the third group was not treated. They were challenged with RB-1B 7 days later. RB-1B viremia was determined by semiquantitative PCR 3 weeks after challenge. Mortality of chickens and incidence of macroscopic tumors were scored. A qualitative tumor score was given to each organ: + for one or a few small tumors to ++++ for numerous and/or large tumors. A second group of 36 chickens was treated identically, except that vaccination with HVT was performed 3 days before RB-1B challenge. All these chickens were sacrificed 13 weeks after RB-1B challenge.
Serum, spleen, and blood cell preparations.
Blood samples were obtained from chickens by cardiac puncture, allowed to clot for 4 h, and centrifuged for 10 min at 3,000 × g. The serum aliquots were stored at −20°C. Sera were used for measurement of nitrate levels. Spleens were removed aseptically. Single-cell suspensions in 1.1× phosphate-buffered saline (pH 7.4) (Gibco, Life Technologies Ltd., Parsley, United Kingdom) were prepared by gently teasing the spleen apart on a steel sieve. Nucleated erythrocytes were eliminated by centrifugation at 400 × g. Isolated splenocytes were used to study cytokine gene expression. Blood leukocytes were obtained after centrifugation of heparinized blood (50 IU/ml) on an lymphocyte separation medium (LSM) 1077 (Eurobio, Les Ulis, France) for 20 min at room temperature and were used to measure viremia.
Assay of nitric oxide (NO2− plus NO3−) level in serum.
The sum of NO2− plus NO3− has been confirmed to be a good indicator of NO production (36). The method described by Hegesh and Shiloah (11) was used with slight modifications. The samples were protein precipitated (with 0.05 ml of 30% zinc sulfate per ml) before analysis. Then 0.5 ml of the sample was incubated for 24 h (and agitated) with granulated cadmium (0.6 g), which had been washed with distilled water, hydrochloric acid (0.1 mol), distilled water, and ammonium hydroxide buffer (0.1 mol; pH 9.6). NO3− is reduced to NO2− by the cadmium. Fifty microliters of each sample was then collected, and the quantity of nitrite was measured using the Griess reaction by adding 50 μl of a freshly prepared mixture (50/50) of 1% sulfanilamide (Sigma, St. Louis, Mo.) in 1.2 N hydrochloric acid and 0.3% N-1 naphthylethylenediamide dihydrochloride (Sigma) in a 96-well flat-bottomed plate. Absorbance at 540 nm was determined after 10-min incubation in the dark. Nitrite concentrations were calculated by reference to a calibration curve prepared using standard solutions of sodium nitrite (starting at 200 μM) (Prolabo, Fontenay-sous-bois, France).
Detection of cytokine mRNA by RT-PCR.
Splenocytes were homogenized in 1 ml of RNAble solution (Eurobio), and total RNA was suspended in RNase-free sterile water. The amount and quality of RNA were determined by spectrophotometry and analyzed by agarose gel electrophoresis. A reverse transcription (RT) procedure was performed to determine the relative quantities of mRNA for chicken gamma interferon (IFN-γ) (7), inducible nitric oxide synthase (iNOS) (24), K203 (34), and β-actin as previously described (5), with a few modifications. Reverse transcription of RNA was performed in a final volume of 25 μl containing 0.5 μg of random oligo(dT) and 2.5 μg of total RNA. The RT reaction mixture was incubated for 5 min at 65°C, and 20 mM (each) deoxynucleoside triphosphate (dNTP), 1× RT buffer (50 mM Tris-HCl [pH 8.3], 75 mM KCl, 3 mM MgCl2, 8 mM dithiothreitol), 4 U of RNase inhibitor, and 200 U of Moloney murine leukemia virus (M-MLV) reverse transcriptase (Promega, Charbonnières, France) were then added. The reaction mixture was heated for 1 h at 37°C and then for 5 min at 85°C to denature the M-MLV reverse transcriptase, cooled on ice for 5 min, and stored at −20°C. The primer sequences for the PCRs are listed in Table 1. PCR conditions were previously defined for each cytokine-primer pair. The PCR mixture contained the following: 1 mM (each) dNTP, 1× PCR buffer (50 mM KCl, 10 mM Tris-HCl), 1 mM MgCl2, 12 pmol (each) primer (sense and antisense primers), 2 μl of cDNA, and Taq polymerase (Promega). After an initial denaturation step of 4 min at 92°C, followed by 1 min at 72°C, temperature cycling was performed as follows: 92°C for 4 min, 61°C for 1 min, and 72°C for 1 min. Samples were stored at 4°C until use. An aliquot of each reaction mixture was subjected to electrophoresis in 2% agarose gels.
TABLE 1.
Primers used for RT-PCR analysis of chicken mRNAs
| Target mRNA | Accession no. | Primer sequence
|
Size of PCR products (bp) | |
|---|---|---|---|---|
| 5′ | 3′ | |||
| β-Actin | L08165 | 5′-CATCACCATTGGCAATGAGAGG-3′ | 5′-GCAAGCAGGAGTACGATGAATC-3′ | 353 |
| IFN-γ | U07868 | 5′-GATGACTTCCAGACTTACAAC-3′ | 5′-AGCAATTGCATCTCCTCTGAGACTG-3′ | 485 |
| iNOS | U46504 | 5′-AGGCCAAACATCCTGGAGGTC-3′ | 5′-TCATAGAGACGCTGCTGCCAG-3′ | 371 |
| K203 | Y18692 | 5′-ATGAAGCTCTCTGCAGTTGTTCT-3′ | 5′-TCAGTCCCGCTTGACGCTCTG-3′ | 269 |
In vitro fibroblast assay for determination of MDV viremia.
Primary CEF cultures were prepared by standard methods in medium 199 (7% fetal calf serum) and seeded (106/well) in six-well culture plates (Falcon; Becton Dickinson Labware, Franklin Lakes, N.J.). CEFs were grown to confluence in six-well plates for 2 days and then infected with blood leukocytes (2 × 106). Cells were then washed 2 days later. RB-1B replication in CEFs was evaluated by counting the number of PFU per well under the microscope after 7 days of culture.
Semiquantitative PCR from total blood to determine MDV viremia.
To extract DNA from blood, 50 μl of blood was mixed with 50 μl of 3% sodium citrate and then mixed with 500 μl of lysis buffer (1% saponin; Sigma). DNA was extracted from pelleted nuclei with 500 μl of TE buffer (10 mM Tris, 1 mM EDTA) and 10 μl of proteinase K (20 mg/ml). The DNA was precipitated with cold ethanol in the presence of 2 M sodium acetate and centrifuged for 15 min at 10,000 × g. The pellet was washed twice with 70% ethanol and dried under vacuum. The DNA was then resuspended in 250 μl of TE buffer and stored at 4°C.
The semiquantitative PCR assay was performed by the method of Bumstead et al. (2) with slight modifications. Primers 1 and 2 were chosen from sequence data for the ICP4 region of the MDV genome. The primers amplify a product of 329 bp from RB-1B. Primer 1 consists of the sequence HEX-GATCGCCCACCACGATTACTACCT, and primer 2 consists of the sequence AATGAGCGAACTGCCTCACACAAC. Control gene primers, primers 3 and 4, were derived from the sequence of chicken Fas (GenBank accession no. J04485) selected to give a product of 147 bp. Primer 3 consists of the sequence TET-CTGATACAAGCAGGCAGAGC, and primer 4 consists of the sequence TGGTTGGATGGAGCAACTGG.
Fluorescent primers (primers 1 and 3), modified by high-pressure liquid chromatography, and nonfluorescent primers (primers 2 and 4) were synthesized by Eurogentec (France). PCR was performed with 5 μl of total blood preparation, 10 pmol of each primer, and 1.25 U of Thermus aquaticus DNA polymerase (Promega) in a total volume of 25 μl. Thirty cycles of amplification were done, with one cycle consisting of 1 min at 94°C, 1 min at 62°C, and 1 min at 72°C. After the final cycle, the elongation phase at 72°C was extended to 10 min. The PCR products (2 μl) were dried and resuspended in 0.5 μl of Tamra 500 (size standards available for fluorescence analysis; Perkin-Elmer, Courtaboeuf, France) and 20 μl of formamide (Pierce, Bezons, France). For quantification analysis of both fluorescence in each tube, the fluorescence peak heights were determined using ABI PRISM Genescan software. A positive RB-1B control extracted from feather follicules was always introduced for comparison and repeatedly gave the same results.
Fluorescence assay of blood smears.
Blood smears were fixed with 75% ethanol-25% acetone. Smears were then stained with the monocyte-specific monoclonal antibody 68.1 (13) diluted 1:10, and staining was revealed with secondary fluorescein isothiocyanate (FITC)-labeled goat anti-mouse polyclonal antibody (Jackson Laboratory, Bar Harbor, Maine). Sections were examined using an Olympus microscope with excitation and barrier filters for FITC. Results were expressed as the mean number of stained cells (± standard error of the mean [SEM]) per microscopic field; the mean values are for five chickens. The number of stained cells given for each chicken was the mean of five different microscopic fields of a blood smear.
Statistical analysis.
For nitrate levels and fluorescence staining, data were expressed as means (± SEMs). Differences in the study group means were tested using the Kruskal-Wallis nonparametric analysis of variance test (Simstat version 3.5b). Thereafter, differences between group means were examined with the Mann-Whitney test. Survival curves were compared at different time points using the Fisher exact test (Epi info version 5.01b). A P value of ≤0.05 was considered statistically significant.
RESULTS
fp/cMGF inoculation enhances the number and function of monocytes.
Inoculation with fp/cMGF (105 PFU per chicken) (experiment 1) quickly induced an increase in the number of blood monocytes (identified by the specific monoclonal antibody 68.1) compared to that of control chickens inoculated with the fp/M3 vector (Table 2). As early as 3 days postinoculation, the number of blood monocytes was increased twofold, and this increase persisted for 7 days postinoculation (P < 0.001). The effects of fp/cMGF and fp/M3 on NO production were examined (Table 3). The level of nitrate in serum reflects systemic NO production (36). Inoculation of fp/cMGF induced a twofold increase in serum nitrate production in the blood compared to that in noninfected controls as early as 3 days postinoculation. This increase was still observed on day 7 postinoculation but was no longer present 10 days after inoculation. In addition, an enhanced response to macrophage-activating stimuli, such as LPS, was strongly exacerbated from 3 to 7 days following inoculation, resulting in a twofold increase in nitrate production in serum. No such enhancement of nitrate production in serum was noted with fp/M3 vector alone. Thus, 7 days after fowlpox virus inoculation was chosen for the time for MDV challenge, when serum NO levels were maximum.
TABLE 2.
Effect of treatment with fp/cMGF or fp/M3 vector on the number of monocytesa
| Days postinoculation | No. of monocytes/microscopic fieldb
|
||
|---|---|---|---|
| Control | fp/M3 | fp/cMGF | |
| 3 | 36.4 ± 4.2 | 41.8 ± 6.8 | 82.2 ± 8.5*** |
| 7 | 42.0 ± 7.1 | 41.2 ± 2.7 | 93.6 ± 5.3*** |
Two-week-old B13/B13 histocompatible chickens were inoculated with fp/cMGF or fp/M3 (105 PFU per chicken) or left untreated (control). Blood samples were taken 3 and 7 days later. Monocytes were labeled on blood smears with monoclonal antibody 68.1, and staining was revealed with goat anti-mouse immunoglobulin G polyclonal antiserum labeled with fluorescein. For each chicken, the number of stained cells was the mean of five different microscopic fields on the blood smear.
Results are expressed as mean values (±SEMs) for five chickens per group and per day. Statistical significance was indicated for fp-inoculated chickens compared to control chickens. (***, P < 0.001).
TABLE 3.
Effects of fp/cMGF on spontaneous and inducible nitric oxide (NO2− plus NO3−) production in seruma
| Days postinoculation | Treatment | Nitric oxide (NO2− + NO3−) level (μM)b
|
||
|---|---|---|---|---|
| Control | fp/M3 | fp/cMGF | ||
| 3 | None | 3.6 (1.1) | 4.4 (1.3) | 9.2 (1.2)** |
| LPS (50 μg) | 132.1 (30.6) | 168.4 (6.4) | 309.9 (11.1)** | |
| 7 | None | 6.2 (3.1) | 5.1 (2.6) | 11.3 (2.8)* |
| LPS (50 μg) | 133.4 (21.9) | 98.1 (6.3) | 257.7 (40.6)** | |
| 10 | None | 8.3 (1.3) | 4.2 (1.3) | 3.6 (0.9)* |
| LPS (50 μg) | 166.3 (18.8) | 196.2 (58.2) | 119.1 (30.8) | |
Two-week-old B13/B13 histocompatible chickens were inoculated with fp/cMGF or fp/M3 (105 PFU per chicken). Three, seven, or ten days later, serum samples were taken before and after intravenous stimulation with LPS (50 μg per chicken) to measure the nitric oxide (NO2− plus NO3−) level. Nitrate (NO3−) was reduced to nitrite (NO2−) by incubation with cadmium, and total nitrite content was measured using Griess reaction.
Results are expressed as mean values (± SEMs) for five chickens per group per day. Statistical significance was indicated for fp-inoculated chickens compared to control chickens (*, P ≤ 0.05; **, P ≤ 0.01).
fp/cMGF induction of iNOS, IFN-γ, and macrophage inflammatory protein (MIP) K203 gene expression in the spleen.
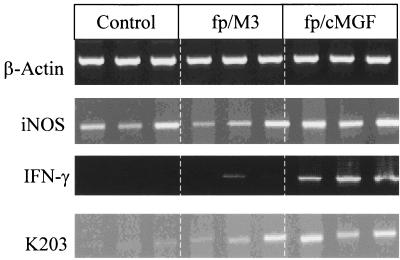
Three chickens per group were tested individually for iNOS, IFN-γ, and K203 gene expression in the spleen (experiment 1). The quantities of cDNA analyzed were adjusted to give comparable amounts of β-actin gene expression for the chickens. Leukocytes isolated from the spleens of control, 3-week-old B13/B13 chickens exhibited iNOS expression under the experimental conditions used (Fig. 1). However, expression of the IFN-γ or K203 gene was not detected. Inoculation of the vector fp/M3 was followed by very weak IFN-γ gene expression 7 days postinoculation in two of three chickens. K203 expression was low in this group, thus demonstrating a limited effect of fp/M3 on cytokine response. fp/M3-inoculated chickens did not show any increase in iNOS gene expression above the background level. In contrast, fp/cMGF inoculation induced strong IFN-γ and K203 gene expression in the spleens of all three chickens tested 7 days postinoculation. iNOS gene expression was strongly increased concomitantly.
FIG. 1.
Qualitative RT-PCR amplification of mRNA of iNOS, IFN-γ, and K203 cytokines from B13/B13 chicken splenocytes 7 days following inoculation of fp/cMGF or fp/M3 vector. The data shown are the results for individual chickens. Thirty amplification cycles were performed for IFN-γ and iNOS, 28 cycles were performed for β-actin, and 34 cycles were performed for K203.
fp/cMGF treatment inhibits MDV replication in vivo.
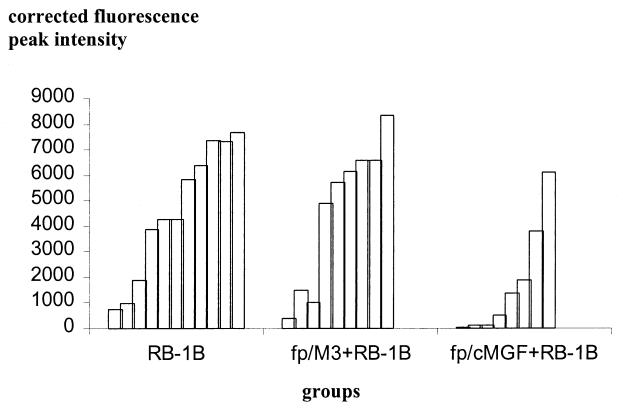
RB-1B viremia was evaluated 3 weeks following inoculation by two methods (experiment 2). Blood leukocytes were first isolated and cultured on CEFs. MDV is characterized by strong T-cell association and is not found in the supernatant or serum (3). The number of PFU was determined 1 week after contact of blood leukocytes with fibroblasts (Table 4). No virus was observed in blood leukocytes from chickens inoculated with both types of fowlpox virus but not challenged with RB-1B. Treatment with fp/cMGF 3 weeks after RB-1B challenge resulted in a significant 2.7-fold reduction in the RB-1B PFU recovered from blood leukocytes. In contrast, the reduction in RB-1B PFU by fp/M3 was weak and not significant. In addition, viremia was tested directly on total blood using a semiquantitative PCR technique (experiment 4). The results (shown in Fig. 2) displayed more heterogeneity than those for isolated blood leukocyte coculture on fibroblasts. Nevertheless, the results led to a similar conclusion. Control chickens infected with MDV and chickens inoculated first with fp/M3 vector and then with RB-1B exhibited the same general pattern of viremia distribution. In contrast, fp/cMGF-treated chickens showed a striking reduction in viremia after RB-1B challenge.
TABLE 4.
Effects of fp/cMGF and fp/M3 on viremia after RB-1B infectiona
| Treatment group | No. of PFU/2 × 106 blood leukocytesb |
|---|---|
| Uninfected | 0 |
| fp/M3 | 0 |
| fp/cMGF | 0 |
| RB-1B | 251 (38) |
| fp/M3 + RB-1B | 200 (41) |
| fp/cMGF + RB-1B | 94 (37)*** |
Two-week-old B13/B13 histocompatible chickens were inoculated with fp/cMGF or fp/M3 (105 PFU per chicken). Seven days later, chickens were challenged with RB-1B (1,000 PFU per chicken). Blood leukocytes were isolated on LSM 1077 21 days after RB-1B inoculation, and viremia level was determined from RB-1B PFU recovery on CEFs. CEFs (106 cells/well) were grown to confluence in six-well plates and infected with blood leukocytes (2 × 106 cells/well). Forty-eight hours later, the CEFs were washed, and virus yield (PFU per well) was measured by PFU unit assay after 7 days of culture.
Values are given as means (± SEMs) (five chickens per group). Statistical significance was indicated for the fp/cMGF and RB-1B treatment group compared to the RB-1B treatment group (***, P ≤ 0.001).
FIG. 2.
Semiquantitative PCR from total blood for determination of MDV viremia. Two-week-old B13/B13 histocompatible chickens were inoculated with fp/cMGF or fp/M3 (105 PFU per chicken). Seven days later, chickens were challenged with RB-1B (1,000 PFU per chicken). Total blood DNA was extracted 21 days after RB-1B inoculation, and viremia levels were determined using specific semiquantitative PCR. Each bar represents a PCR result for one chicken. The bars for the chickens are ordered by increasing PCR value within each group.
fp/cMGF treatment does not alter the nitrate response in MDV-infected chickens.
RB-1B inoculation induced a slight increase in nitrate production (experiment 3) in very susceptible B13/B13 chickens 1 week later (Table 5). Nevertheless, this increase was not significant and disappeared 3 weeks postinoculation. The nitrate levels after intravenous injection of LPS were used to evaluate the systemic response as an indication of the ability of the macrophage to respond to activation (35). LPS dramatically increased nitrate production in sera of noninfected chickens 6 h following intravenous injection (12-fold). This inducing capacity of LPS on serum nitrate production remained unchanged or slightly reduced 1 week after RB-1B challenge in untreated chickens and in chickens pretreated with fowlpox virus. In contrast, RB-1B-inoculated chickens exhibited an exacerbated LPS-stimulated nitrate response in serum 3 weeks following challenge. However, none of the fowlpox virus pretreatments was able to modify such a response.
TABLE 5.
Effect of RB-1B infection on spontaneous and inducible nitric oxide (NO2− plus NO3−) production in seruma
| Wk postinoculation | Treatment | Nitric oxide (NO2− + NO3−) level (μM)b
|
|||
|---|---|---|---|---|---|
| Control | RB-1B | fp/M3 + RB-1B | fp/cMGF + RB-1B | ||
| 1 | None | 11.4 (2.1) | 41.5 (15.0) | 31.2 (3.9) | 25.2 (10.3) |
| LPS (50 μg) | 134.8 (28.5) | 175.5 (37.7) | 90.3 (52.9) | 154.3 (74.4) | |
| 3 | None | 17.5 (0.9) | 18.9 (5.8) | 15.88 (3.7) | 16.1 (4.0) |
| LPS (50 μg) | 156.6 (15.2) | 402.2 (33.7) | 446.5 (151.4) | 371.5 (103.1) | |
Serum samples were obtained from B13/B13 chickens, 1 and 3 weeks after inoculation with RB-1B. Pretreatment with fp/cMGF or fp/M3 was performed 7 days before RB-1B challenge. Nitric oxide (NO2− + NO3−) level in serum was measured before and after intravenous stimulation with LPS. Nitrate (NO3−) was reduced to nitrite (NO2−) by incubation with cadmium, and total nitrite content was measured using Griess reaction.
Results are expressed as mean values (± SEMs) for five chickens per group per day. No significant difference was observed for fp-inoculated chickens compared to untreated chickens after RB-1B challenge.
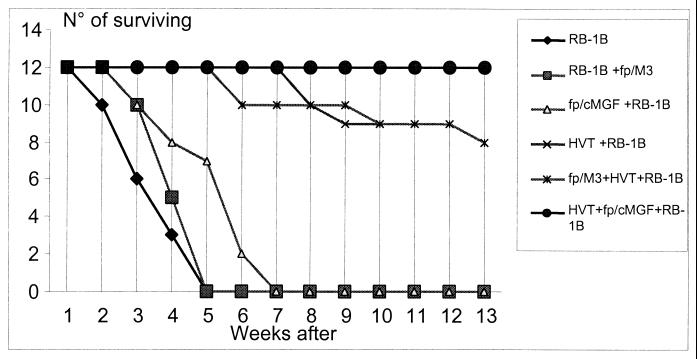
fp/cMGF treatment reduces tumor incidence and increases survival time following MDV infection.
All B13/B13 chickens inoculated with RB-1B had died by 5 weeks following infection (experiment 4) (Fig. 3). fp/M3 inoculation 7 days before MDV challenge slightly increased survival time, with 50% of the chickens dead 1 week later than for control chickens. However, all these chickens had died by 5 weeks postchallenge. fp/cMGF pretreatment increased survival time even further, with 50% of the chickens dead 2 weeks after control chickens. Nevertheless, all fp/cMGF chickens had died by 7 weeks after RB-1B challenge. The survival curve for fp/cMGF chickens infected with RB-1B was significantly different from the survival curves for control and fp/M3 chickens (P < 0.002 at 7 weeks postinoculation). Tumor incidence was also affected by fp/cMGF treatment, and the changes closely reflected the effects seen with survival times. Ninety-two percent of RB-1B-infected control chickens had macroscopic tumors, with the spleen, liver, and kidneys being the major sites of tumors (Table 6). The overall picture of tumor distribution was not greatly modified following pretreatment with fp/M3, except for reduction in spleen tumor burden at 5 weeks. In contrast, the growth of tumors in the spleens and livers of fp/cMGF-treated chickens was greatly reduced (fivefold) from that of control infected chickens, but tumor occurrence in kidneys was not changed.
FIG. 3.
Effects of fp/cMGF and fp/M3 on survival of HVT-vaccinated chickens and unvaccinated chickens inoculated with RB-1B. Two-week-old B13/B13 chickens (12 chickens per group) were inoculated with fp/cMGF or fp/M3 vector (105 PFU per chicken) or left untreated. Vaccination with HVT (1,000 PFU per chicken) was performed 4 days after fowlpox virus inoculation, and RB-1B was inoculated 3 days later (i.e., 7 days after inoculation). All nonvaccinated chickens died by 5 weeks after RB-1B challenge. All remaining HVT-vaccinated chickens were sacrificed 13 weeks after RB-1B challenge.
TABLE 6.
Effects of fp/cMGF and fp/M3 on tumor incidence in RB-1B inoculated chickensa
| Time and treatment | Tumor incidence (%) (tumor score)b
|
||||
|---|---|---|---|---|---|
| Total | Liver | Spleen | Kidney | Other | |
| 5 wks postinoculation | |||||
| RB-1B | 92 | 42 (++) | 50 (+++) | 68 (++++) | 17 (+) |
| fp/M3 + RB-1B | 84 | 34 (++) | 20 (+) | 73 (++++) | 27 (++) |
| fp/cMGF + RB-1B | 60 | 9 (+) | 9 (++) | 60 (+++) | 27 (+) |
| 13 wks postinoculation | |||||
| HVT + RB-1B | 50 | 11 (+) | 11 (+) | 55 (++) | 0 |
| fp/M3 + HVT + RB-1B | 58 | 0 | 44 (+) | 60 (++) | 11 (+) |
| fp/cMGF + HVT + RB-1B | 8 | 0 | 0 | 8 (+) | 0 |
Two-week-old B13/B13 chickens (12 chickens per group) were inoculated with fp/cMGF or fp/M3 vector (105 PFU per chicken) or left untreated. Vaccination with HVT (1,000 PFU per chicken) was performed 4 days after fp inoculation and RB-1B was inoculated 3 days later (i.e., 7 days after fp inoculation). All nonvaccinated chickens died by 5 weeks after RB-1B challenge. All remaining HVT-vaccinated chickens were sacrificed 13 weeks after RB-1B challenge. Macroscopic tumors were located mainly in the liver, spleen, kidneys, and other sites (heart, gonads, and inoculation site).
A qualitative tumor score was given to each organ as follows: from + (one or few small tumors) to ++++ (numerous and/or large tumors).
Vaccination with HVT 3 days before RB-1B challenge greatly reduced the mortality rate, but tumor incidence reached about 50% in both control and fp/M3-treated chickens. The chickens treated with fp/cMGF all survived. The survival curves for fp/cMGF-treated chickens and vaccinated chickens infected with RB-1B were significantly different from the survival curves of control and fp/M3-treated vaccinated chickens (P < 0.04 at 13 weeks postinoculation). The incidence of tumors was affected dramatically, with a sixfold reduction in total incidence compared to that for control vaccinated chickens, and in particular, the absence of tumor development in the spleen and liver.
DISCUSSION
We investigated the effects of the avian cytokine cMGF on viral replication and tumoral development following infection by a chicken-specific herpesvirus that transforms T lymphocytes in vivo. cMGF is a 27-kDa glycoprotein that was first characterized according to its ability to stimulate the growth of macrophage and granulocyte colonies from avian bone marrow progenitor cells in vitro (21). cMGF also stimulates the proliferation of retrovirally transformed avian myeloblasts and monocytes (22). cMGF has no known homologue in mammals. Molecular cloning of the cMGF gene showed 54% homology to mammalian granulocyte colony-stimulating factor (G-CSF) and 40% homology to interleukin 6 (IL-6) at the amino acid level (22). Nevertheless, its biologic activity on avian macrophages in vitro and in vivo is similar to that of macrophage colony-stimulating activity (M-CSF) and granulocyte-macrophage colony-stimulating factor (GM-CSF) (43). In the present study, cMGF was delivered via a live replicating fowlpox virus vector which is specific to the chicken. This system has previously been shown to provide continuous, prolonged exposure to the cytokine in chickens (43). Our results here demonstrate for the first time that a cytokine was able to modify tumor development in chickens infected with a highly virulent oncogenic strain of MDV. Treatment of MDV-susceptible chickens with fp/cMGF was shown to reduce the viremia caused by the very virulent strain RB-1B during the 3 weeks following challenge. In addition, tumor incidence and burden were decreased, particularly in the liver, and the time of death was delayed compared to that of birds treated with the fp vector alone. It has not yet been determined whether the effect of cMGF treatment on tumor incidence in MDV-infected chickens is direct (a result of enhancement of killing of tumor cells) or indirect (acting through inhibition of MDV replication via activation of innate immune mechanisms, resulting in decreased tumor formation) (3).
The production of interferon, either IFN-γ or IFN-α, is the first line of nonspecific defense against viruses. To gain some insight into the immune mechanisms triggered by treatment with fp/cMGF in chickens, we analyzed the cytokine expression profile in the spleen just before challenge with RB-1B. In parallel to the induction of strong iNOS gene expression in the spleen, fp/cMGF clearly induced the expression of IFN-γ and MIP K203 genes in all chickens tested 7 days postinoculation, but IFN-α was not expressed (data not shown). In comparison, the effects of fp/M3 were very weak and incomplete. Studies on protective mechanisms against various herpesviruses point to IFN-γ production as a key immune response (4), and IFN-γ has also been hypothesized to be involved in host resistance mechanisms against MDV according to recent findings on genetic resistance to the disease (41). Studies have confirmed the potentially inhibiting role of chicken IFN-γ on MDV replication in vitro, either as a direct effect on embryo fibroblasts or an indirect effect through NO production by macrophages (8, 42). Thus, the effective triggering of IFN-γ response by fp/cMGF at the time of MDV challenge might interfere with MDV replication. IFN-γ has also been shown to be a cytokine with antitumor activities in mammals (15). No such role has yet been proved for MD. Mammalian M-CSF exhibits strong inductive capacity on IFN-γ (29) and chemokine production such as MIP-1β and IL-8 in vivo (16), chemokines known to be involved in initiation of the immune response. In contrast, mammalian GM-CSF favors in vivo IFN-γ production but did not induce MIP-1β (16). The coinduction of IFN-γ and MIP chemokines appears to be a sign of development of the Th1 type of immune response in viral infection in mammals (37). K203 has been identified as one member of the chicken MIP family and presents a higher degree of amino acid identity with mammalian MIP-1β than with mammalian MIP-1α (34). To date, no functional studies have established the roles of these chicken chemokines. However, interestingly, both IFN-γ and chemokines MIP-1α and -1β are strong inducers of iNOS activity in mammals (38). Chicken MGF delivered via a viral vector was thus able to induce a strong cytokine response with some Th1 characteristics that may be able to favor NO production and trigger adequate immune surroundings able to facilitate ultimately a cytotoxic CD8+ response, which has been shown to participate in antiviral defense mechanisms against MDV (27). Interestingly, this pattern of expression of iNOS, IFN-γ, and MIPs has been shown to be triggered shortly after challenge with MDV in the spleens from chickens able to display later resistance to MD, either genetically (41; also our unpublished data) or after vaccination (our unpublished data).
Macrophages, which have been identified as a major target of cMGF, might be important in mediating the antiviral and antitumoral effects of fp/cMGF in vivo. Mammalian M-CSF and GM-CSF have indeed demonstrated antitumor activity through their ability to activate macrophages in various tumor systems (14, 44). Macrophages have been shown to participate substantially in host defense immune responses against eventual tumor development in MD in chickens. In vivo depletion of macrophages prior to MDV inoculation using antimacrophage polyclonal serum (10), levamisole (18), and repeated treatment with silica (9) increases viremia, tumor incidence, and mortality caused by MD. Interestingly, as we also observed, increased tumor burden mostly affects the spleen and liver. Chicken macrophages, either activated or obtained from tumors, are indeed able to display killing activity on MDV-transformed cells and to inhibit their proliferation in vitro (10, 19, 32). Activated peritoneal macrophages from MDV-infected chickens are also able to reduce MDV replication efficiently in chick kidney cells (17) and lower the virus isolation rate from spleen cells in vitro (19). Treatment with intramuscularly injected fp/cMGF significantly increased the number of blood monocytes from 3 to 7 days following inoculation, declining to normal levels by 10 days. These findings are similar to those of York et al. (43), although the effects of fp/cMGF treatment appeared to be of longer duration in their study. Moreover, we observed that treatment with fp/cMGF was able to increase nitrate levels in serum, which is taken as evidence of systemic NO production (36), either directly or after injection of the NO inducer LPS (24), thus proving the overall activation state of macrophages in cMGF-treated chickens. No such effects were observed with the fp vector.
NO is a molecule that displays antiviral and antitumoral activities. High levels of NO can be produced by macrophages from the l-arginine substrate through activation of iNOS. There has been extensive work done with NO in mammals showing that NO plays an important role in the nonspecific immune defense mechanisms against herpesviruses (23). Interestingly, chemical NO donors inhibit the replication of MDV in embryo fibroblasts in vitro (8, 42). NO production is one of the major mechanisms responsible for the inhibitory activity of IFN-γ-activated chicken macrophages on MDV replication in vitro (8). Moreover, injection of a chemical inhibitor of iNOS in vivo results in significant enhancement in MDV replication (42). Because cMGF is able to activate NO productive capacities in macrophages in vivo just before MDV challenge, NO production might be a mechanism involved in the inhibition of viremia in MDV-infected chickens. Stronger iNOS expression was established in the spleen 1 week following the inoculation of fp/cMGF compared with the vector alone, parallel to the strong increase in the systemic NO production capacity. Thus, NO production might have been an active mechanism effectively inhibiting MDV replication at the time of MDV inoculation, particularly in the blood.
In mammals, induction of NO production through macrophage activation is one mechanism by which cytokine GM-CSF has been shown to exert an antitumoral effect (14). However, chickens treated with fp/cMGF in our study did not show an additional and significant increase in serum nitrate levels after MDV inoculation compared to those of infected control chickens and chickens treated with fp vector. An increase in the LPS response appeared 3 weeks after MDV infection, but it was seen in all chickens. However, rather than indicating activation of macrophages on appearance of tumors, this increase in LPS-induced nitrate production might reflect the deterioration in renal function in all MDV-infected chickens due to tumoral injury, leading to a modified rate of nitrate excretion (12). Thus, it cannot be demonstrated that NO production was a mechanism triggered in a sustained way by cMGF during the course of the disease and thus able to be effective on developing tumors.
Therefore, action through activation of macrophages might not be the sole mechanism by which cytokine cMGF acts. Precursors of granulocytes have been identified as potential targets of cMGF activity, at least in vitro (22). However, York et al. (43) were unable to demonstrate any increase in circulating granulocytes after treatment with fp/cMGF in vivo. Nevertheless, at present, we cannot exclude a possible effect on granulocytes in MDV-infected chickens that might mediate part of the resulting antitumoral effect. Results from studies with mammals have attributed some antiviral and antitumoral properties to granulocytes (6, 25). However, tumor rejection through activation of granulocytes appears effective only when granulocytes are targeted to the tumor through local cytokine expression in the tumor cells themselves (6). Recent studies on mammalian M-CSF have in addition demonstrated involvement of this cytokine in NK1.1 cell activity, more by favoring proliferation from progenitors than by directly increasing the NK function (28). The increase in NK activity following M-CSF injection in mice leads to reduced tumor development (26). We also observed that cMGF treatment induced an early greater increase of IFN-γ expression in the spleen than treatment with fp vector. This increase might result partly from activation of splenic NK cells. Moreover, we observed that cMGF treatment resulted in considerable tumor reduction compared to control and fp/M3 treatment; this was observed mainly in the liver as well as in the spleen but not in the kidneys. NK cells are localized mainly in the spleen and liver. In addition to action through macrophages and NO production, cMGF might thus increase NK cell activity, possibly with antitumoral and antiviral activity concomitant with IFN-γ production. Additional studies are necessary to investigate this point.
Protection against MD can be achieved by vaccination. Nonpathogenic HVT is widely used as a vaccine, but protection through vaccination may be partial against very virulent strains of MDV (39). Our results showed improved vaccinal protection and reduced tumor burden when cMGF treatment was associated with HVT vaccination in chickens genetically highly susceptible to MDV. Therefore, it might be worth considering delivering cMGF in combination with vaccinal viral strains to increase protection against MD.
In conclusion, for the first time, we were able to demonstrate a beneficial effect of pretreatment with a cytokine on the course of MD in chickens. The overall consequence of treatment with cMGF was reduction in viral replication and tumor burden accompanied by prolonged survival time. There is evidence that an enhanced innate immune response is at least one of the mechanisms involved in resistance to MDV. cMGF might exert part of its action through increased monocyte/macrophage numbers and activation, leading to increased NO production with antiviral and antitumoral activity. Nevertheless, further studies are needed to clarify the possible involvement of other cells, such as granulocytes and NK cells, which are still poorly characterized in chickens.
Acknowledgments
We thank Jacques Cabaret for valuable assistance in statistical analysis; Doreen Raine for revision of the English; and Bruno Campone, Alain Potier, and Jean Méry from Animal Husbandry for excellent technical assistance.
Aouatef Djeraba was supported by grants from the Algerian Education and Research Ministry and the French Academy of Medicine.
REFERENCES
- 1.Briles, W. E., R. W. Briles, D. L. Pollock, and M. Pattison. 1982. Marek’s disease resistance of B (MHC) heterozygotes in a cross of purebred Leghorn lines. Poult. Sci. 61:205–211. [DOI] [PubMed] [Google Scholar]
- 2.Bumstead, N., J. Sillibourne, M. Rennie, N. Ross, and F. Davison. 1997. Quantification of Marek’s disease virus in chicken lymphocytes using the polymerase chain reaction with fluorescence detection. J. Virol. Methods 65:75–81. [DOI] [PubMed] [Google Scholar]
- 3.Calnek, B. W. 1986. Marek’s disease-a model for herpesvirus oncology. Crit. Rev. Microbiol. 12:293–320. [DOI] [PubMed] [Google Scholar]
- 4.Cantin, E., B. Tanamachi, H. Openshaw, J. Mann, and K. Clarke. 1999. Gamma interferon (IFN-γ) receptor null-mutant mice are more susceptible to herpes simplex virus type 1 infection than IFN-γ ligand null-mutant mice. J. Virol. 73:5196–5200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cetre, C., C. Cocude, C. Pierrot, C. Godin, A. Capron, M. Capron, and J. Khalife. 1998. In vivo expression of cytokine mRNA in rats infected with Schistosoma mansoni. Parasite Immunol. 20:135–142. [DOI] [PubMed] [Google Scholar]
- 6.Colombo, M. P., A. Modesti, G. Parmiani, and G. Forni. 1992. Local cytokine availability elicits tumor rejection and systemic immunity through granulocyte-T-lymphocyte cross-talk. Cancer Res. 52:4853–4857. [PubMed] [Google Scholar]
- 7.Digby, M. R., and J. W. Lowenthal. 1995. Cloning and expression of the chicken interferon-gamma gene. J. Interferon Cytokine Res. 15:939–945. [DOI] [PubMed] [Google Scholar]
- 8.Djeraba, A., N. Bernardet, G. Dambrine, and P. Quere. 2000. Nitric oxide inhibits Marek’s disease virus replication but is not the single decisive factor in interferon-gamma-mediated viral inhibition. Virology 277:58–65. [DOI] [PubMed] [Google Scholar]
- 9.Gupta, M. K., H. V. Chauhan, G. J. Jha, and K. K. Singh. 1989. The role of the reticuloendothelial system in the immunopathology of Marek’s disease. Vet. Microbiol. 20:223–234. [DOI] [PubMed] [Google Scholar]
- 10.Haffer, K., M. Sevoian, and M. Wilder. 1979. The role of the macrophages in Marek’s disease: in vitro and in vivo studies. Int. J. Cancer 23:648–656. [DOI] [PubMed] [Google Scholar]
- 11.Hegesh, E., and J. Shiloah. 1982. Blood nitrates and infantile methemoglobinemia. Clin. Chim. Acta 125:107–115. [DOI] [PubMed] [Google Scholar]
- 12.Heller, J., H. Kristeleit, K. A. Brensing, R. P. Woitas, U. Spengler, and T. Sauerbruch. 1999. Nitrite and nitrate levels in patients with cirrhosis of the liver: influence of kidney function and fasting state. Scand. J. Gastroenterol. 34:297–302. [DOI] [PubMed] [Google Scholar]
- 13.Jeurissen, S. H., E. M. Janse, G. Koch, and G. F. de Boer. 1988. The monoclonal antibody CVI-ChNL-68.1 recognizes cells of the monocyte-macrophage lineage in chickens. Dev. Comp. Immunol. 12:855–864. [DOI] [PubMed] [Google Scholar]
- 14.Ju, D. W., X. Cao, and B. Acres. 1997. Intratumoral injection of GM-CSF gene encoded recombinant vaccinia virus elicits potent antitumor response in a mixture melanoma model. Cancer Gene Ther. 4:139–144. [PubMed] [Google Scholar]
- 15.Kaplan, D. H., V. Shankaran, A. S. Dighe, E. Stockert, M. Aguet, L. J. Old, and R. D. Schreiber. 1998. Demonstration of an interferon gamma-dependent tumor surveillance system in immunocompetent mice. Proc. Natl. Acad. Sci. USA 95:7556–7561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim, J. J., J. S. Yang, D. J. Lee, D. M. Wilson, L. K. Nottingham, L. Morrison, A. Tsai, J. Oh, K. Dang, T. Dentchev, M. G. Agadjanyan, J. I. Sin, A. A. Chalian, and D. B. Weiner. 2000. Macrophage colony-stimulating factor can modulate immune responses and attract dendritic cells in vivo. Hum. Gene Ther. 11:305–321. [DOI] [PubMed] [Google Scholar]
- 17.Kodama, H., T. Mikami, M. Inoue, and H. Izawa. 1979. Inhibitory effects of macrophages against Marek’s disease virus plaque formation in chicken kidney cell cultures. J. Natl. Cancer Inst. 63:1267–1271. [PubMed] [Google Scholar]
- 18.Kodama, H., T. Mikami, and H. Izawa. 1980. Effects of levamisole on pathogenesis of Marek’s disease. J. Natl. Cancer Inst. 65:155–159. [PubMed] [Google Scholar]
- 19.Lee, L. F. 1979. Macrophage restriction of Marek’s disease virus replication and lymphoma cell proliferation. J. Immunol. 123:1088–1091. [PubMed] [Google Scholar]
- 20.Lee, L. F., J. M. Sharma, K. Nazerian, and R. L. Witter. 1978. Suppression of mitogen-induced proliferation of normal spleen cells by macrophages from chickens inoculated with Marek’s disease virus. J. Immunol. 120:1554–1559. [PubMed] [Google Scholar]
- 21.Leutz, A., H. Beug, and T. Graf. 1984. Purification and characterization of cMGF, a novel chicken myelomonocytic growth factor. EMBO J. 3:3191–3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leutz, A., K. Damm, E. Sterneck, E. Kowenz, S. Ness, R. Frank, H. Gausepohl, Y. C. Pan, J. Smart, M. Hayman, et al. 1989. Molecular cloning of the chicken myelomonocytic growth factor (cMGF) reveals relationship to interleukin 6 and granulocyte colony stimulating factor. EMBO J. 8:175–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liew, F. Y., and F. E. Cox. 1991. Nonspecific defence mechanism: the role of nitric oxide. Immunol. Today 12:A17–A21. [DOI] [PubMed] [Google Scholar]
- 24.Lin, A. W., C. C. Chang, and C. C. McCormick. 1996. Molecular cloning and expression of an avian macrophage nitric-oxide synthase cDNA and the analysis of the genomic 5′-flanking region. J. Biol. Chem. 271:11911–11919. [DOI] [PubMed] [Google Scholar]
- 25.Milligan, G. N. 1999. Neutrophils aid in protection of the vaginal mucosae of immune mice against challenge with herpes simplex virus type 2. J. Virol. 73:6380–6386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Misawa, E., T. Sakurai, M. Yamada, H. Hayasawa, and K. Motoyoshi. 2000. Effects of macrophage colony-stimulating factor and interleukin-2 administration on NK1.1(+) cells in mice. Int. J. Immunopharmacol. 22:967–977. [DOI] [PubMed] [Google Scholar]
- 27.Omar, A. R., and K. A. Schat. 1996. Syngeneic Marek’s disease virus (MDV)-specific cell-mediated immune responses against immediate early, late, and unique MDV proteins. Virology 222:87–99. [DOI] [PubMed] [Google Scholar]
- 28.Sakurai, T., E. Misawa, M. Yamada, H. Hayasawa, and K. Motoyoshi. 2000. Effects of macrophage-colony-stimulating factor on cyclophosphamide-injected mouse NK1.1+ cell activity. Cancer Immunol. Immunother. 49:94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sakurai, T., N. Wakimoto, M. Yamada, S. Shimamura, and K. Motoyoshi. 1998. Effect of macrophage colony-stimulating factor (M-CSF) on mouse immune responses in vivo. Immunopharmacol. Immunotoxicol. 20:79–102. [DOI] [PubMed] [Google Scholar]
- 30.Schat, K. A., C. L. Chen, B. W. Calnek, and D. Char. 1991. Transformation of T-lymphocyte subsets by Marek’s disease herpesvirus. J. Virol. 65:1408–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharma, J. M. 1981. Natural killer cell activity in chickens exposed to Marek’s disease virus: inhibition of activity in susceptible chickens and enhancement of activity in resistant and vaccinated chickens. Avian Dis. 25:882–893. [PubMed] [Google Scholar]
- 32.Sharma, J. M. 1983. Presence of adherent cytotoxic cells and non-adherent natural killer cells in progressive and regressive Marek’s disease tumors. Vet. Immunol. Immunopathol. 5:125–140. [DOI] [PubMed] [Google Scholar]
- 33.Shek, W. R., B. W. Calnek, K. A. Schat, and C. H. Chen. 1983. Characterization of Marek’s disease virus-infected lymphocytes: discrimination between cytolytically and latently infected cells. J. Natl. Cancer Inst. 70:485–491. [PubMed] [Google Scholar]
- 34.Sick, C., K. Schneider, P. Staeheli, and K. C. Weining. 2000. Novel chicken CXC and CC chemokines. Cytokine 12:181–186. [DOI] [PubMed] [Google Scholar]
- 35.Smith, S. R., D. Manfra, L. Davies, C. Terminelli, G. Denhardt, and J. Donkin. 1997. Elevated levels of NO in both unchallenged and LPS-challenged C. parvum-primed mice are attributable to the activity of a cytokine-inducible isoform of iNOS. J. Leukoc. Biol. 61:24–32. [DOI] [PubMed] [Google Scholar]
- 36.Stuehr, D. J., and M. A. Marletta. 1985. Mammalian nitrate biosynthesis: mouse macrophages produce nitrite and nitrate in response to Escherichia coli lipopolysaccharide. Proc. Natl. Acad. Sci. USA 82:7738–7742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trumpfheller, C., K. Tenner-Racz, P. Racz, B. Fleischer, and S. Frosch. 1998. Expression of macrophage inflammatory protein (MIP)-1alpha, MIP-1beta, and RANTES genes in lymph nodes from HIV+ individuals: correlation with a Th1-type cytokine response. Clin. Exp. Immunol. 112:92–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Villalta, F., Y. Zhang, K. E. Bibb, J. C. Kappes, and M. F. Lima. 1998. The cysteine-cysteine family of chemokines RANTES, MIP-1α, and MIP-1β induce trypanocidal activity in human macrophages via nitric oxide. Infect. Immun. 66:4690–4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Witter, R. L. 1997. Increased virulence of Marek’s disease virus field isolates. Avian Dis. 41:149–163. [PubMed] [Google Scholar]
- 40.Witter, R. L., J. M. Sharma, and L. Offenbecker. 1976. Turkey herpesvirus infection in chickens: induction of lymphoproliferative lesions and characterization of vaccinal immunity against Marek’s disease. Avian Dis. 20:676–692. [PubMed] [Google Scholar]
- 41.Xing, Z., and K. A. Schat. 2000. Expression of cytokine genes in Marek’s disease virus-infected chickens and chicken embryo fibroblast cultures. Immunology 100:70–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xing, Z., and K. A. Schat. 2000. Inhibitory effects of nitric oxide and gamma interferon on in vitro and in vivo replication of Marek’s disease virus. J. Virol. 74:3605–3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.York, J. J., A. D. Strom, T. E. Connick, P. G. McWaters, D. B. Boyle, and J. W. Lowenthal. 1996. In vivo effects of chicken myelomonocytic growth factor: delivery via a viral vector. J. Immunol. 156:2991–2997. [PubMed] [Google Scholar]
- 44.Yoshioka, H., S. Hama, T. Sadatomo, E. Taniguchi, K. Harada, K. Sugiyama, F. Kimura, K. Motoyoshi, and K. Kurisu. 1998. Transformation of rat glioma cells with the M-CSF gene inhibits tumorigenesis in vivo. J. Neurooncol. 40:197–204. [DOI] [PubMed] [Google Scholar]