Abstract
Reverse genetics was used to develop a two-component, trivalent live attenuated vaccine against human parainfluenza virus type 3 (HPIV3) and respiratory syncytial virus (RSV) subgroups A and B. The backbone for each of the two components of this vaccine was the attenuated recombinant bovine/human PIV3 (rB/HPIV3), a recombinant BPIV3 in which the bovine HN and F protective antigens are replaced by their HPIV3 counterparts (48). This chimera retains the well-characterized host range attenuation phenotype of BPIV3, which appears to be appropriate for immunization of young infants. The open reading frames (ORFs) for the G and F major protective antigens of RSV subgroup A and B were each placed under the control of PIV3 transcription signals and inserted individually or in homologous pairs as supernumerary genes in the promoter proximal position of rB/HPIV3. The level of replication of rB/HPIV3-RSV chimeric viruses in the respiratory tract of rhesus monkeys was similar to that of their parent virus rB/HPIV3, and each of the chimeras induced a robust immune response to both RSV and HPIV3. RSV-neutralizing antibody titers induced by rB/HPIV3-RSV chimeric viruses were equivalent to those induced by infection with wild-type RSV, and HPIV3-specific antibody responses were similar to, or slightly less than, after infection with the rB/HPIV3 vector itself. This study describes a novel vaccine strategy against RSV in which vaccine viruses with a common attenuated backbone, specifically rB/HPIV3 derivatives expressing the G and/or F major protective antigens of RSV subgroup A and of RSV subgroup B, are used to immunize by the intranasal route against RSV and HPIV3, which are the first and second most important viral agents of pediatric respiratory tract disease worldwide.
Respiratory syncytial virus (RSV) is the most common cause of acute viral lower respiratory disease in infants and young children, followed by human parainfluenza virus type 3 (HPIV3) as the second most important viral respiratory pathogen. In the United States, RSV and HPIV3 are responsible for approximately one-third of all pediatric respiratory tract disease leading to hospitalization (13, 20, 45), and RSV alone is estimated to account for between 73,000 and 126,000 annual hospitalizations of infants younger than 1 year of age (51). Worldwide, acute lower respiratory tract disease is the leading cause of mortality due to infectious diseases (63), and in infants and young children RSV is the most commonly isolated viral pathogen in this disease entity (59). To reduce the burden of disease caused by RSV and HPIV3, vaccines that are safe and immunogenic are clearly needed.
The first RSV vaccine candidate, a formalin-inactivated vaccine developed in the 1960s, failed to provide protection against RSV infection and resulted in immune-mediated enhanced disease upon subsequent infection by wild-type RSV (40). Enhancement of RSV disease does not occur after natural RSV infection and has not been seen following immunization with an intranasally administered, live attenuated RSV vaccine candidate (65). This is an important factor in favor of a topically administered live attenuated RSV vaccine. To date, several live attenuated RSV vaccine candidates have been evaluated in clinical trials (33, 39, 64, 65), but a licensed RSV vaccine is still not available. The most challenging aspect of developing a live attenuated RSV vaccine is to achieve an appropriate balance between attenuation and immunogenicity in the young infant, in whom immune responses are reduced due to immunologic immaturity and the immunosuppressive effects of maternally derived virus-specific serum immunoglobulin G (65). Mucosal immunization provides a partial escape from immunosuppression by serum antibodies (44), and therefore topically administered live attenuated vaccines seem ideal for immunization of young infants. However, all live attenuated RSV vaccine candidates tested to date have been either overattenuated and insufficiently immunogenic (34, 64) or underattenuated (36, 65) in this age group.
Protection against reinfection with RSV and HPIV3 is mainly conferred by serum and mucosal antibodies directed against their viral surface glycoproteins (16). The RSV G and F proteins and the HPIV3 HN and F proteins are the only viral proteins that have been shown to induce neutralizing antibodies and thus are the major protective antigens. Although cytotoxic CD8+ T lymphocytes are important in clearing RSV and HPIV3 infections, resistance to reinfection conferred by cellular immune responses seems to be short-lived (16, 42, 55).
Two live attenuated vaccine candidates for use against HPIV3 have undergone clinical evaluation and appear to be satisfactorily attenuated and immunogenic in 1- to 2-month-old infants, namely, the cold-passaged temperature-sensitive cp45 derivative of HPIV3 (17, 38) and the Kansas strain of bovine PIV3 (BPIV3) (12, 13, 18, 35, 37). BPIV3 is a Jennerian vaccine, because it is a wild-type animal counterpart of a human pathogen, i.e., HPIV3, that is attenuated for replication in humans, chimpanzees, and rhesus monkeys due to a natural host range restriction. Experience with Jennerian and modified Jennerian vaccines (i.e., chimeras made by mixing genes of animal and human counterpart viruses) is extensive and includes the licensed smallpox and rotavirus vaccines (10, 32) as well as candidate vaccines against influenza A virus (9, 53, 54), PIV1 (29), and hepatitis A virus (24). The attenuation phenotype of human Jennerian vaccines is based on differences between the human and nonhuman viruses that developed during evolution due to the adaptation of viruses to their respective hosts. This attenuation phenotype appears to be very stable. Sequence comparison between BPIV3 and HPIV3 identified a large number of host-specific nucleotide and amino acid differences throughout the genome that likely contribute to the host range restriction (1, 2). The view that host range restriction is based on a large number of sequence differences involving many genomic loci would provide an explanation for the observed phenotypic stability. Exchange of the nucleocapsid gene between HPIV3 and BPIV3 viruses attenuated the HPIV3 for rhesus monkeys (1), indicating that this gene possesses some of the host range attenuating sequences in BPIV3. Our laboratory is currently pursuing studies to characterize the contribution of each BPIV3 gene to the host range attenuation phenotype.
We previously recovered BPIV3 from cDNA and replaced its F and HN glycoprotein genes with those of HPIV3 in order to improve the vaccine candidate’s immunogenicity against HPIV3. This recombinant chimeric bovine/human PIV3 (rB/HPIV3) retained the attenuation phenotype of BPIV3 and was highly immunogenic in rhesus monkeys (48). It was found previously that rB/HPIV3 could express either the G or the F open reading frame (ORF) of RSV subgroup A from a single additional, supernumerary gene insert under the control of PIV3 transcription signals and that rB/HPIV3 bearing these single RSV gene inserts retained their infectivity and immunogenicity for hamsters. A promoter-proximal position preceding the N ORF of rB/HPIV3 was chosen for insertion of each RSV glycoprotein gene in order to maximize its level of expression (49). Here, rB/HPIV3 was modified to express both the G and F ORFs of RSV subgroup A or B as additional genes singly or as homologous subgroup pairs from the same promoter-proximal site that was used in the construction of the single-insert rB/HPIV3-RSV chimeras, and the level of attenuation and immunogenicity of the single- and double-gene rB/HPIV3-RSV chimeric recombinant viruses were evaluated in rhesus monkeys. Our findings indicate that rB/HPIV3-RSV chimeras expressing one or two additional RSV glycoprotein ORFs are infectious and immunogenic in rhesus monkeys.
MATERIALS AND METHODS
Viruses and cells.
HEp-2, LLC-MK2 and Vero cells were maintained as previously described (49).
The rHPIV3, the rBPIV3 containing HPIV3 glycoprotein genes (rB/HPIV3), and rB/HPIV3 expressing the G or F ORF of RSV subgroup A were described previously (21, 48, 49). The last two viruses were originally designated rB/HPIV3-G1 and -F1 (49) and in the present work are designated rB/HPIV3-GA and rB/HPIV3-FA, respectively. RSV strains A2 and B1 represent antigenic subgroups A and B, respectively. PIVs were propagated at 32°C in LLC-MK2 cells or Vero cells, as previously described (27). The modified vaccinia strain Ankara (MVA) recombinant virus that expresses bacteriophage T7 RNA polymerase (MVA-T7) was generously provided by L. Wyatt and B. Moss (66).
Construction of antigenomic cDNAs encoding chimeric rB/HPIV3 viruses bearing the RSV G and F ORFs as additional genes.
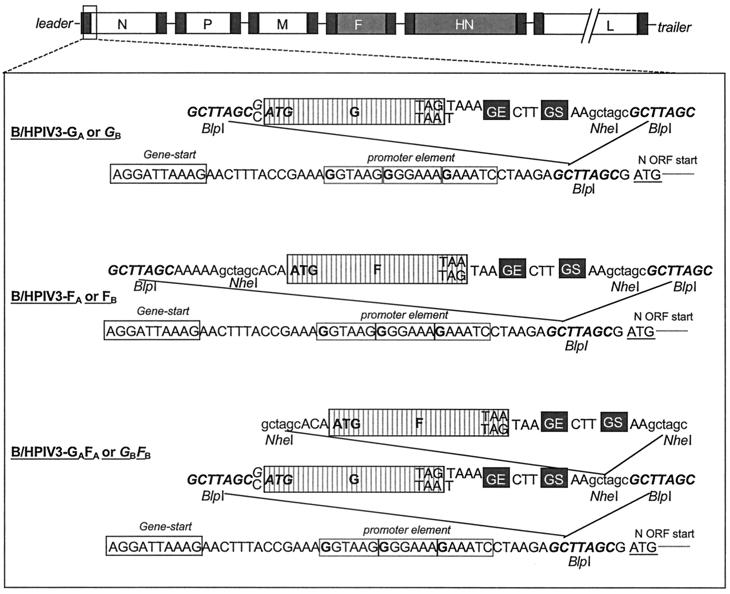
The antigenomic cDNA of rB/HPIV3 (48) was modified previously to contain a unique BlpI restriction enzyme recognition site preceding the N ORF (nucleotides 103 to 109) (49). The BlpI insertion site followed the gene start signal of the N gene and the putative promoter elements (56) at nucleotides 79 to 95 (Fig. 1). This site was used to insert either or both of the RSV G and F glycoprotein genes.
FIG. 1.
Insertion of the G and F ORFs of RSV subgroup A or B into rB/HPIV3 as additional, promoter-proximal genes. The rB/HPIV3 antigenomic cDNA was modified previously by introduction of a unique BlpI site (boldface italic type) in the nontranslated region of the N gene preceding the start codon of the N ORF (49). PCR mutagenesis was used to add a PIV3 gene end (GE) (5′-AAGTAAGAAAAA), intergenic region (5′-CTT), and gene start (GS) (5′-AGGATTAAAG) signal immediately downstream of the stop codon of the G and F ORFs of subgroups A and B. An NheI site was added to the downstream end of each G ORF and on either side of each F ORF, and BlpI sites were added on either side of each insert. The modifications to the subgroup A ORFs were performed in previous work (49), and the subgroup B ORFs were modified in the same way in the present study. Each insert was cloned into the BlpI site in the rB/HPIV3 cDNA to create constructs containing the four single-gene insert viruses, namely, rB/HPIV3-GA, rB/HPIV3-FA, rB/HPIV3-GB, and rB/HPIV3-FB. To create the double-insert constructs rB/HPIV3-GAFAand rB/HPIV3-GBFB the NheI fragment bearing each F ORF was excised from the rB/HPIV3-FA and rB/HPIV3-FB antigenomic cDNA and inserted into the NheI site of the rB/HPIV3-GA and rB/HPIV3-GB cDNAs, respectively. Elements of the viral promoter that, by analogy to Sendai virus (56), are present in the N gene nontranslated region are indicated by the three boxed hexamers, with important G residues shown in boldface type. Nucleotides that differ between the subgroup A and subgroup B constructs are shown on separate lines (top line for subgroup A constructs and bottom line for subgroup B constructs).
In previous work, the G or F ORF of RSV subgroup A was modified using PCR by addition of a BPIV3 gene end signal, intergenic region, and gene start signal at the downstream end of each ORF (Fig. 1). In addition, this PCR step introduced an NheI site at the downstream end of the G ORF, NheI sites at each end of the F ORF, and BlpI sites at each end of each ORF (Fig. 1). The resulting GA and FA inserts were cloned individually into the BlpI site of the antigenomic cDNA of rB/HPIV3 to yield the plasmids pB/HPIV3-GA and pB/HPIV3-FA.
Here, to insert both genes into a single virus, the RSV F ORF was excised from pB/HPIV3-FA using the NheI sites on either side of the insert and cloned into the unique NheI site of pB/HPIV3-GA, yielding the double-insert construct pB/HPIV3-GAFA. The G and F glycoprotein inserts of RSV subgroup B were generated by PCR analogous to the RSV subgroup A inserts, using cDNAs of the G and F genes from the B1 strain of subgroup B RSV (GenBank accession no. AF013254) (62). For the G ORF of RSV B, the forward PCR primer used was (5′ to 3′) TATATAGCTTAGCCATGTCCAAACACAAGAATCAACGCACTGCCAGGACTCTAGAAAAGACC and the reverse primer was (5′ to 3′) CATCGCTAAGCGCTAGCTTctttaatcctAAGtttttcttacttATTAGGCGTGGGACTGAGTGTTCTGAGTAGAGTTGGATGTAGAGGG (the two BlpI sites are underlined, the single NheI site is italicized, translation initiation and termination triplets of the G ORF are in boldface type, gene end and gene start signals are in lowercase type, and the intergenic sequence is in boldface italic type). For the F ORF of RSV B, the forward PCR primer used was (5′ to 3′) AATTAGCTTAGCAAAAAGCTAGCACAATGGAGCTGCTGATCCACAGGTTAAGTGC and the reverse primer was (5′ to 3′) TATATGCTAAGCGCTAGCTTctttaatcctAAGtttttcttacttTTACTATTTGCTGAATGCAATATTATTGATTCCACTTAGTTGGTCTTTGC (see G primers for annotation). The resulting GB and FB inserts were cloned into the BlpI site of the rB/HPIV3 antigenomic cDNA (pB/HPIV3) to yield pB/HPIV3-GB and pB/HPIV3-FB. Excision of the F ORF from pB/HPIV3-FB using NheI and cloning into the NheI site of pB/HPIV3-GB yielded the RSV subgroup B double-insert construct pB/HPIV3-GBFB.
Recovery of chimeric rB/HPIV3-RSV viruses from cDNA.
All six chimeric rB/HPIV3-RSV viruses (rB/HPIV3-GA, rB/HPIV3-FA, rB/HPIV3-GAFA, rB/HPIV3-GB, rB/HPIV3-FB, and rB/HPIV3-GBFB) were produced in HEp-2 cells using a plasmid-based recovery system as previously described (49). Virus present in the supernatant was biologically cloned by three sequential terminal dilutions prior to amplification and characterization.
Northern and Western blots.
HEp-2 cell monolayers (1.5 × 106 cells per well) were infected at a multiplicity of infection (MOI) of 5 and incubated at 37°C. Cells were harvested at 12-h intervals until 48 h postinfection, when all monolayers showed extensive cytopathic effects and more than half of the cells were detached from the bottom of the well. Cells were pelleted, resuspended in phosphate-buffered saline, and aliquoted for Northern (90% of the suspension) and Western (10% of the suspension) blot analysis. For Northern blot analysis, total cellular RNA was extracted with TRIzol reagent (Life Technologies) according to the supplier’s protocol, except that the RNAs were extracted with phenol-chloroform and ethanol precipitated after the isopropanol precipitation. RNA was analyzed by electrophoresis in a 1.5% agarose gel containing 0.44 M formaldehyde, transferred to nitrocellulose membranes (Schleicher & Schuell), and fixed by UV cross-linking (Stratagene). 32P-labeled RSV G- and F-specific DNA probes were generated using the Amersham megaprime DNA labeling system according to the supplier’s protocol.
For Western blot analysis cell pellets from infected cells were disrupted by addition of 1× Tris-glycine sample buffer (Novex) and centrifugation through Qiashredders (Qiagen). Approximately 8 × 103 cell equivalents of each cell extract was subjected to electrophoresis on sodium dodecyl sulfate-polyacrylamide gels (4 to 12% acrylamide) and transferred to a nitrocellulose membrane. The blots were incubated with rabbit antisera raised against a synthetic peptide containing the last 13 residues of the cytoplasmic domain of the RSV A2 F protein and against a synthetic peptide containing amino acids 186 to 201 of the RSV A2 G protein. RSV glycoproteins were visualized by incubation with horseradish peroxidase-labeled goat anti-rabbit immunoglobulin G antibodies (Kirkegaard & Perry Laboratories) and chemiluminescence (NEN Life Science).
Monkey studies.
Rhesus monkeys, which were seronegative for PIV3 as determined by hemagglutination-inhibition (HAI) assay (12) and for RSV as determined by RSV glycoprotein-specific enzyme-linked immunosorbent assay (ELISA) (15), were inoculated intranasally and intratracheally in groups of four animals (two animals for rHPIV3) with 105 50% tissue culture infective doses (TCID50) per ml of each PIV listed in Table 1 or 105 PFU of RSV. Nasopharyngeal swab (NP) specimens were collected daily from day 0 (prior to inoculation on the same day) to day 11 and on day 13 postinoculation. Tracheal lavage (TL) samples were collected on days 2, 4, 6, 8, and 10 postinoculation. Duplicate aliquots were flash-frozen and stored at −70°C until all samples were available for titration. PIV in the NP and TL specimens was titrated on LLC-MK2 cell monolayers in 24- and 96-well plates as previously described (22), and RSV was titrated on HEp-2 cell monolayers in 24-well plates as previously described (11, 49). For comparison of mean peak and mean daily virus titers, 12 rB/HPIV3-infected and 8 rHPIV3-infected rhesus monkeys from previous studies (references 1 and 48) and unpublished data were included in the statistical analysis.
TABLE 1.
rB/HPIV3 expressing the G and/or F glycoproteins of RSV subgroup A or B from additional genes in the promoter-proximal position are attenuated in the respiratory tract of rhesus monkeys
| Immunizing virusa | No. of animals | Mean peak virus titerb (log10 TCID50/ml ± SEc) in:
|
|
|---|---|---|---|
| NP swab | Tracheal lavage fluid | ||
| rB/HPIV3-GA | 4 | 2.0 ± 0.3 A | 2.5 ± 0.5 AB |
| rB/HPIV3-FA | 4 | 2.4 ± 0.5 A | 2.2 ± 0.6 AB |
| rB/HPIV3-GA + FAd | 4 | 2.0 ± 0.3 A | 2.5 ± 0.3 AB |
| rB/HPIV3-GAFA | 4 | 1.5 ± 0.2 A | 2.0 ± 0.1 AB |
| rB/HPIV3-GB | 4 | 1.9 ± 0.5 A | 2.7 ± 0.3 AB |
| rB/HPIV3-FB | 4 | 1.5 ± 0.2 A | 2.3 ± 0.6 AB |
| rB/HPIV3-GBFB | 4 | 1.7 ± 0.6 A | 2.2 ± 0.4 AB |
| rB/HPIV3e | 16 | 3.2 ± 0.3 AB | 2.1 ± 0.2 A |
| rHPIV3e | 10 | 4.4 ± 0. B | 3.3 ± 0.3 B |
Monkeys were inoculated intranasally and intratracheally with 105 TCID50 in a 1-ml inoculum at each site.
Mean of the peak titer for each animal in its group irrespective of sampling day. Mean virus titers were assigned to similar groups (A and B) by the Tukey-Kramer test. Within each column, mean titers with different letters are statistically different (P < 0.05). Titers indicated with two letters are not significantly different from those indicated with either letter.
Virus titrations were performed on LLC-MK2 cells at 32°C. The detection limit was 10 TCID50/ml.
Each animal received a mixture of 105 TCID50 of rB/HPIV3-GA and rB/HPIV3-FA.
Serum samples collected prior to infection and 27 days postinfection were assayed for HPIV3 HN-specific antibodies by HAI assay (12) and for RSV neutralizing antibodies by 60% plaque reduction neutralization test (11, 49). The RSV neutralization assays were performed with the homologous subgroup strain. In addition, serum antibody titers specific for the G and F glycoproteins of RSV were determined by ELISA, as previously described (15). For the F-specific antibody titer, the F protein of the A2 strain of subgroup A virus was used as antigen to test sera of animals infected with rB/HPIV3-RSV bearing either subgroup A or B glycoprotein gene inserts since the F proteins of RSV subgroup A and B are highly related antigenically (3, 28, 31). G-specific responses were assayed with G protein from the homologous antigenic subgroup, specifically strain A2 for subgroup A and strain 18537 for subgroup B, since the G glycoproteins of subgroup A and B are very divergent in amino acid sequence and share only 5% or less antigenic relatedness (28, 31).
RESULTS
Construction of antigenomic cDNAs encoding chimeric rB/HPIV3 viruses bearing the G and F ORFs of RSV subgroup A or B as additional gene inserts.
rB/HPIV3 was previously used to express the G or F ORF of RSV subgroup A as a single additional gene (49). In that study, the RSV subgroup A G or F ORF had been placed under the control of PIV3 transcription signals and inserted into a unique BlpI site that had been introduced into the upstream nontranslated region of the N gene in the rB/HPIV3 antigenomic cDNA (Fig. 1). The BlpI site did not disturb any known cis-acting transcription or replication signal, and its promoter-proximal location was chosen to maximize the expression of the foreign gene and to avoid changing the relative levels of expression of the PIV3 genes (23). In the present study, the G or F ORF of RSV subgroup B was modified in the same way by addition of a BPIV3 gene end signal, an intergenic region and a gene start signal at the downstream end as well as appropriate NheI and BlpI sites for cloning purposes (Fig. 1). To prepare RSV subgroup A and subgroup B constructs containing both G and F, the F ORF was excised from pB/HPIV3-FA or pB/HPIV3-FB using the NheI sites on either side of the insert and cloned into the NheI site of pB/HPIV3-GA or pB/HPIV3-GB, yielding the rB/HPIV3-RSV double-insert constructs pB/HPIV3-GAFA and pB/HPIV3-GBFB. In each case, each ORF was under the control of a separate set of PIV3 transcription signals and would be expressed as an additional, separate mRNA. All constructs were designed to comply with the “rule of six” (7) and to maintain hexamer spacing of transcription signals throughout the genome (41).
Chimeric rB/HPIV3-RSV viruses can be recovered from cDNA.
All six chimeric PIV-RSV viruses were recovered from HEp-2 cells transfected with the respective antigenomic cDNA together with BPIV3 N, P, and L support plasmids. The recovered recombinant viruses were cloned biologically by three sequential terminal dilutions in Vero cells prior to characterization. The presence of the inserted RSV G and F ORFs in the backbone of each recovered recombinant virus was confirmed by reverse transcription-PCR of viral RNA followed by restriction enzyme digestion and DNA sequencing of the gene junctions. The generation of each PCR product was dependent upon the inclusion of reverse transcriptase, indicating that each was derived from viral RNA and not from contaminating cDNA. The sequence of the inserted gene and flanking regions in each of the recovered recombinant viruses was identical to that of the starting antigenomic cDNA (data not shown).
Chimeric rB/HPIV-RSV viruses replicate efficiently in cell culture.
The ability of rB/HPIV3-FA and rB/HPIV3-GA to replicate in LLC-MK2 cells was previously compared to that of BPIV3, HPIV3, and rB/HPIV3 (49). The rB/HPIV3-GA virus grew as efficiently as its rB/HPIV3 and BPIV3 parents, while the growth of rB/HPIV3-FA was reduced approximately eightfold. Nonetheless, the final titer achieved for each of the PIV-RSV chimeric viruses was greater than 107 TCID50/ml. The additional chimeric viruses that were recovered from cDNAs in the present study, i.e., rB/HPIV3-GAFA, rB/HPIV3-GB, rB/HPIV3-FB, and rB/HPIV3-GBFB, grew in Vero cells to a final titer of 107.7, 107.5, 107.1, and 107.7 TCID50/ml, respectively. Similar to rB/HPIV3-FA, rB/HPIV3-FB grew to a slightly lower titer, but the double-insert chimeras were not reduced in their replicative efficacy. This indicates that each virus replicates efficiently in cell culture, which is essential for efficient vaccine manufacture.
The RSV G glycoprotein gene inserted in the first position of the rB/HPIV3 genome is transcribed more efficiently than in the RSV genome.
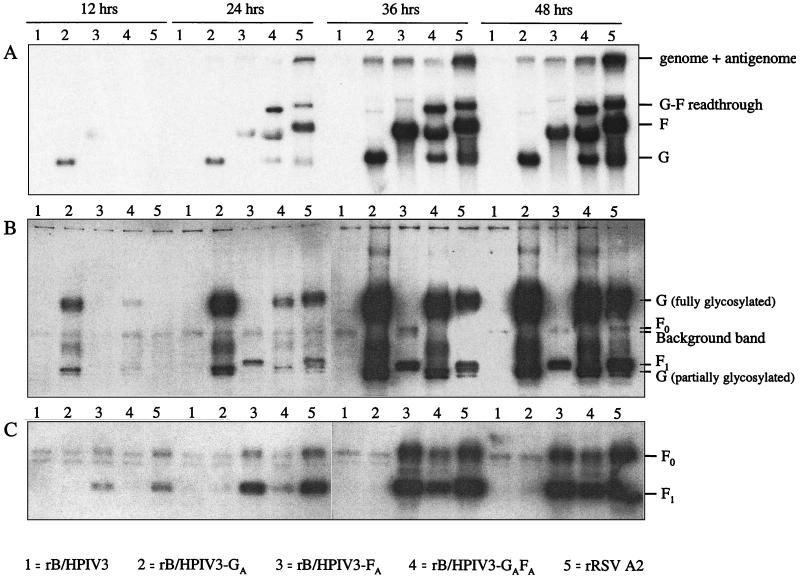
Total intracellular accumulation of the RSV G and F mRNAs was evaluated by Northern blot analysis of cell extracts from HEp-2 cell monolayers infected at an MOI of 5 with rB/HPIV3, rB/HPIV3-GA, rB/HPIV3-FA, rB/HPIV3-GAFA, and wild-type RSV (Fig. 2A). Replicate cell monolayers were harvested at 12, 24, 36, and 48 h postinfection and analyzed by hybridization with double-strand DNA probes against the G and F genes. At 12 h postinfection, RSV G mRNA was detected in rB/HPIV3-GA-infected cells but not in rB/HPIV3-GAFA- or RSV-infected cells (Fig. 2A). At 12, 24, and 36 h postinfection the amount of RSV G mRNA in rB/HPIV3-GA-infected cells was markedly higher than that in RSV- or in rB/HPIV3-GAFA-infected cells. RSV F mRNA was detected at 24 h postinfection in rB/HPIV3-FA-, rB/HPIV3GAFA-, and RSV-infected cells (Fig. 2A). The total amount of F mRNA in rB/HPIV3-FA-infected cells surpassed that in RSV-infected cells at the 36-h time point but not at the 12- and 48-h time points. It should be noted that the F mRNA and the G-F readthrough mRNA transcribed by the rB/HPIV3-RSV chimeras migrate more rapidly than their RSV-transcribed counterparts, which was expected since most of the downstream noncoding region of the F gene was deleted during construction of the chimeras (Fig. 1). Also, it should be noted that this particular DNA probe provided a stronger hybridization signal with the F mRNA than the G mRNA, as can be seen from the mRNA pattern of RSV-infected cells (Fig. 2A, lane 5).
FIG. 2.
Transcription and translation of RSV G and F glycoprotein genes in PIV-RSV subgroup A chimeric viruses and in RSV. Accumulation of RSV G and F mRNAs (A) and proteins (B and C) was examined in HEp-2 cells infected with rB/HPIV3 (lanes 1), rB/HPIV3-GA (lanes 2), rB/HPIV3-FA (lanes 3), rB/HPIV3-GAFA (lanes 4), and RSV (lanes 5) at an MOI of 5, and cells were harvested at the indicated time points postinfection. mRNAs were detected with a mixture of RSV G and F specific 32P-labeled DNA probes (A). The F mRNA transcribed from rB/HPIV3-FA and rB/HPIV3-GAFA is shorter than that transcribed from RSV because the 5′ nontranslated region of F was deleted in the chimeric viruses. G and F glycoproteins were detected by a mixture of antisera raised against the cytoplasmic domain of RSV A2 F protein and against the ectodomain of the RSV A2 G protein (B). Glycoproteins were visualized by incubation with horseradish peroxidase-labeled goat anti-rabbit IgG antibodies. Since the F1 subunit of the fusion glycoprotein and the partially glycosylated G glycoprotein have similar electrophoretic mobilities, an additional Western blot was stained with F-specific antiserum only (C).
Single-insert rB/HPIV-RSV subgroup A chimeras express the RSV G glycoprotein more efficiently than the double-insert chimera or wild-type RSV A2.
Accumulation of the G and F glycoproteins in infected cells was evaluated by Western blot analysis of aliquots of HEp-2 cell extracts (Fig. 2B and C) from the same experiment described for Fig. 2A. Fully glycosylated G protein was detected earlier and in larger quantities in cells infected with the single-insert chimera rB/HPIV3-GA than in cells infected with RSV or with the double-insert chimera rB/HPIV3-GAFA (Fig. 2B). Specifically, glycosylated RSV G protein was readily detectable at 12 h postinfection in cells infected with rB/HPIV3-GA, whereas in cells infected with RSV glycosylated G protein first became apparent at 24 h postinfection. At all time points, the amount of G protein expressed by chimera rB/HPIV3-GA exceeded that expressed by RSV severalfold (Fig. 2B), consistent with the greater accumulation of G mRNA (Fig. 2A). Accumulation of the F protein occurred at approximately the same rate in rB/HPIV3-FA-infected and RSV-infected cells (Fig. 2B and C). Cells infected with the double-insert chimera rB/HPIV3-GAFA accumulated more G glycoprotein at the 36- and 48-h time points than RSV-infected cells (Fig. 2B), but F protein was accumulated more slowly in rB/HPIV3-GAFA-infected cells than in rB/HPIV3-FA-infected or RSV-infected cells (Fig. 2C).
Chimeric rB/HPIV3-RSV viruses are attenuated in the respiratory tract of rhesus monkeys.
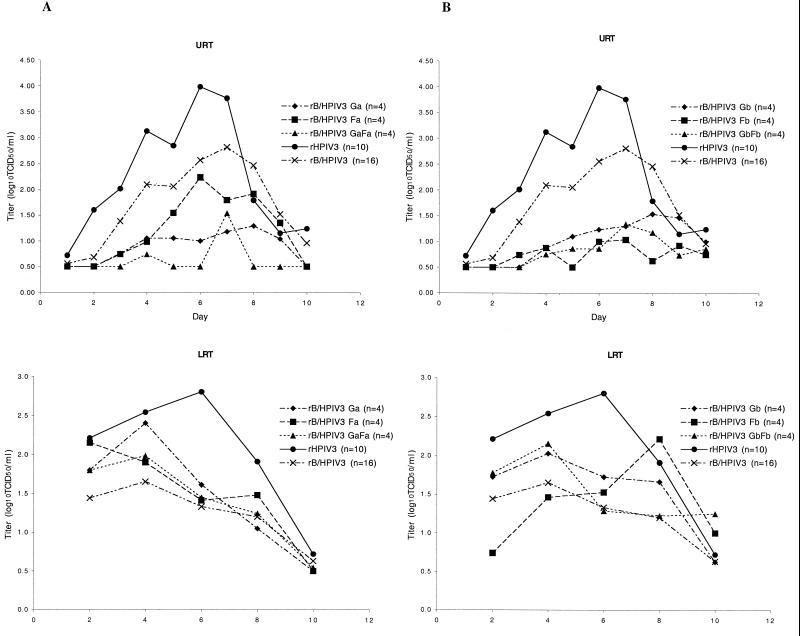
The chimeric rB/HPIV3-RSV viruses were evaluated for their ability to replicate in the upper and lower respiratory tract of rhesus monkeys following intranasal and intratracheal inoculation of 105 TCID50 of each virus per inoculation site. Although the rB/HPIV3-FA and rB/HPIV3-GA viruses had been evaluated in hamsters, none of the rB/HPIV3-RSV chimeras had been evaluated in rhesus monkeys, the primate model in which the host range attenuation phenotype of BPIV3 seen in humans is reproduced. The parental viruses, rB/HPIV3 and rHPIV3, were compared in parallel as controls. Mean peak titers are shown in Table 1 while day-to-day titers are displayed graphically in Fig. 3. In the upper respiratory tract, rB/HPIV3-RSV subgroup A chimeras replicated less well than their parent virus rB/HPIV3. Specifically, rB/HPIV3-FA, rB/HPIV3-GA, and rB/HPIV3-GAFA were from 6- to 15-fold more restricted in replication in the upper respiratory tract than rB/HPIV3 (Table 1). Similarly, all three rB/HPIV3-RSV subgroup B viruses were more attenuated than their rB/HPIV3 parent virus in the upper respiratory tract, and the double-insert chimera rB/HPIV3-GBFB was recovered on fewer days than the single-insert chimeras (Table 1 and Fig. 2B, top panel). In contrast, in the lower respiratory tract, the rB/HPIV3-RSV chimeras replicated at least as efficiently as their parent virus rB/HPIV3 (Fig. 2, bottom panels, and Table 1). In summary, the presence of the 0.9-kb (G), 1.8-kb (F), or 2.7-kb (G and F) RSV inserts just downstream of the genomic promoter slightly increased the attenuation of rB/HPIV3 in the upper respiratory tract of rhesus monkeys but had no further attenuating effect on the level of replication in the lower respiratory tract.
FIG. 3.
Replication of rB/HPIV3-RSV subgroup A (panels A) and subgroup B (panels B) chimeras in the respiratory tract of rhesus monkeys. Monkeys were inoculated with one of the indicated rB/HPIV3-RSV chimeras, rB/HPIV3, or rHPIV3, as described in the text. The number (n) of animals in each group is indicated in parentheses. Mean daily titers in NP swab and TL fluid were determined by serial dilution on LLC-MK2 cell cultures, and the presence of virus in the culture was determined by hemadsorption. Titers are expressed as mean TCID50 per milliliter, and the detection limit was 10 TCID50/ml. Titers for rB/HPIV3-RSV subgroup A chimeras are shown in panels A, and those for subgroup B chimeras are shown in panels B. Top panels represent NP swabs (upper respiratory tract [URT]), and bottom panels represent TLs (lower respiratory tract [LRT]). The titers for the rHPIV3 and rB/HPIV3 viruses are included in both graphs for comparison. Specimens in which virus was not detected were assigned a value of 100.5 TCID50/ml.
rB/HPIV3-RSV chimeric viruses induce serum antibodies to both HPIV3 and RSV.
Serum samples collected prior to infection and 27 days postinfection were assayed for HPIV3 HN-specific antibodies by HAI assay and for RSV neutralizing antibodies by 60% plaque reduction neutralization test (Table 2). Serum antibody titers specific for the RSV G and F glycoproteins were determined by glycoprotein-specific ELISA (Table 3).
TABLE 2.
Immunization of rhesus monkeys with rB/HPIV3 expressing the G and/or F glycoproteins of RSV subgroup B or subgroup A induces neutralizing serum antibodies against RSV and hemagglutination-inhibiting antibodies against HPIV3
| Immunizing virusa | Neutralizing serum antibody response to homologous RSVb (mean reciprocal log2 ± SE)
|
Serum hemagglutination-inhibiting antibody response to HPIV3c (mean reciprocal log2 ± SE)
|
||
|---|---|---|---|---|
| Pre | Day 27 | Pre | Day 27 | |
| RSV A2d | ≤3.3 | 8.8 ± 0.5 | <2 | <2 |
| rB/HPIV3-GA | ≤3.3 | 7.3 ± 1.4 | <2 | 9.3 ± 0.3 |
| rB/HPIV3-FA | ≤3.3 | 7.3 ± 0.0 | <2 | 8.8 ± 0.3 |
| rB/HPIV3-GA + FAe | ≤3.3 | 7.3 ± 0.8 | <2 | 8.8 ± 0.3 |
| rB/HPIV3-GAFA | ≤3.3 | 8.8 ± 1.0 | <2 | 9.5 ± 0.3 |
| RSV B1d | ≤3.3 | 7.8 ± 0.5 | <2 | <2 |
| rB/HPIV3-GB | ≤3.3 | 7.8 ± 1.0 | <2 | 8.3 ± 0.8 |
| rB/HPIV3-FB | ≤3.3 | 6.8 ± 0.5 | <2 | 7.5 ± 0.3 |
| rB/HPIV3-GBFB | ≤3.3 | 7.3 ± 0.8 | <2 | 6.5 ± 0.3 |
| rB/HPIV3 | ≤3.3 | ≤3.3 | <2 | 10.0 ± 0.0 |
Monkeys (n = 4 for each immunization group) were inoculated intranasally and intratracheally with 105 TCID50 (PIV3) or 105 PFU (RSV) in a 1-ml inoculum at each site.
Serum samples were taken on day 0 prior to immunization (Pre) and on day 27 postinoculation, and antibody titers were determined by 60% plaque reduction neutralization test using the homologous subgroup A (strain A2) or subgroup B (strain B1) virus.
Serum samples were taken on day 0 prior to immunization (Pre) and on day 27 postinoculation, and antibody titers were determined by HAI test.
The B1 strain of RSV subgroup B virus replicated to a mean peak titer of 100.9 PFU/ml in NP swab fluid and to 101.0 PFU/ml in TL fluid. The A2 strain of RSV subgroup A virus replicated to a mean peak titer of 101.0 PFU/ml in NP swab fluid and to 102.8 PFU/ml in TL fluid.
Each animal received a mixture of 105 TCID50 of rB/HPIV3-GA and rB/HPIV3-FA.
TABLE 3.
Glycoprotein-specific serum antibody response of rhesus monkeys infected with rB/HPIV3 expressing the G and/or F glycoproteins of RSV subgroup A and subgroup B
| Immunizing virusa | Serum IgG ELISA titer (mean reciprocal log2 ± SE) against homologous RSVb
|
|||||
|---|---|---|---|---|---|---|
| G protein
|
F protein
|
|||||
| Prec | Day 27 | log2 increase (fold) | Prec | Day 27 | log2 increase (fold) | |
| RSV A2 | 4.8 ± 1.0 | 9.8 ± 0.5 | 5.0 | ≤3.3 | 10.8 ± 0.5 | 7.5 |
| rB/HPIV3-GA | 4.3 ± 1.0 | 9.8 ± 0.5 | 5.5 | ≤3.3 | ≤3.3 | 0.0 |
| rB/HPIV3-FA | 4.3 ± 1.0 | 4.8 ± 1.0 | 0.5 | ≤3.3 | 11.3 ± 1.4 | 8.0 |
| rB/HPIV3-GA+FAd | 4.8 ± 0.5 | 10.3 ± 0.6 | 5.5 | 4.3 ± 0.6 | 9.8 ± 0.5 | 5.5 |
| rB/HPIV3-GAFA | 4.3 ± 0.6 | 9.8 ± 0.5 | 5.5 | ≤3.3 | 11.3 ± 0.8 | 8.0 |
| RSV B1 | ≤3.3 | 7.8 ± 1.0 | 4.5 | 5.3 ± 0.8 | 8.3 ± 0.6 | 3.0 |
| rB/HPIV3-GB | 4.3 ± 1.0 | 9.8 ± 0.5 | 5.5 | 5.7 ± 1.5 | 6.8 ± 0.5 | 1.1 |
| rB/HPIV3-FB | 4.3 ± 1.0 | 4.8 ± 1.0 | 0.5 | 4.3 ± 1.0 | 8.3 ± 1.3 | 4.0 |
| rB/HPIV3-GBFB | 4.3 ± 0.6 | 9.8 ± 0.5 | 5.5 | 4.8 ± 1.0 | 6.3 ± 1.9 | 1.5 |
| rB/HPIV3 | 4.8 ± 1.5 | 4.3 ± 1.0 | −0.5 | ≤3.3 | ≤3.3 | 0.0 |
Monkeys were inoculated intranasally and intratracheally with 105 TCID50 in a 1-ml inoculum at each site.
Serum samples were taken on day 0 prior to immunization (Pre) and on day 27 postinoculation, and antibody titers were determined by glycoprotein-specific ELISA for antibodies against RSV G or F protein, as indicated. Purified F protein from strain A2 was used in all tests, whereas purified G protein was from the homologous subgroup (see Materials and Methods).
Titers in the preserum specimen represent nonspecific background optical density levels.
Each animal received a mixture of 105 TCID50 of rB/HPIV3-GA and rB/HPIV3-FA.
Immunization of rhesus monkeys with rB/HPIV3-RSV subgroup A chimeras induced RSV-neutralizing antibody titers that were statistically indistinguishable (Tukey-Kramer test, P < 0.05) from those induced by wild-type RSV infection (Table 2). While mean neutralizing antibody titers induced by single-insert chimeras (rB/HPIV3-GA, rB/HPIV3-FA, or a mixture of rB/HPIV3-GA and rB/HPIV3-FA) were threefold lower than the mean titer induced by RSV infection, there was no difference in mean antibody titer between animals infected with wild-type RSV A and those infected with the double-insert chimera (rB/HPIV3-GAFA) (Table 2). G-specific or F-specific antibody titers induced by each individual rB/HPIV3-RSV A single- or double-insert chimera were as high as the titer induced by infection with wild-type RSV, but monkeys immunized with a mixture of rB/HPIV3-GA and rB/HPIV3-FA mounted a slightly weaker F-specific antibody response (Table 3). With regard to the response to the HPIV3 backbone glycoproteins, each of the rB/HPIV3-RSV subgroup A viruses induced a serum PIV3-specific HAI antibody titer that was comparable to that induced by its parent virus rB/HPIV3 (Table 2).
Infection with rB/HPIV3-RSV subgroup B chimeras elicited a neutralizing antibody response against RSV subgroup B that was similar to that elicited by infection with RSV subgroup B wild-type virus (Table 2). Mean neutralizing antibody titers ranged from 6.8 for rB/HPIV3-FB to 7.8 (reciprocal log2) for both rB/HPIV3-GB and RSV subgroup B virus. The RSV subgroup B glycoprotein G-specific responses were similar for animals infected with RSV B1, rB/HPIV3-GB, and rB/HPIV3-GBFB (Table 3). The double-insert chimera rB/HPIV3-GBFB, however, induced a weaker mean F-specific antibody response than did rB/HPIV3-FB and RSV B1 (Table 3). With regard to the HPIV3 backbone glycoproteins, the PIV3-specific antibody titer that was induced by rB/HPIV3-RSV subgroup B chimera was 3-fold (rB/HPIV3-GB) to 11-fold (rB/HPIV3-GBFB) lower than that induced by rB/HPIV3 (Table 2).
DISCUSSION
RSV and PIV3 remain the most important viral respiratory tract pathogens in infants (51), and acute lower respiratory tract infections remain the leading cause of mortality worldwide due to infectious diseases (63). The development of a safe and immunogenic pediatric vaccine against RSV in particular has proved to be a formidable task that is complicated by host-related factors, including the young age of the target vaccinees and the attendant issues of immunological immaturity and maternal antibody-mediated immunosuppression, as well as by virus-related factors such as the poor growth of RSV in vitro and the instability of RSV infectivity. Although a number of promising RSV subgroup A vaccine candidates have been evaluated in phase I trials, none of the experimental vaccines tested to date was found to be appropriately attenuated and acceptable as a vaccine for seronegative infants younger than 2 months of age (65). In the present study, we examined whether or not rB/HPIV3 could be used as a vector for the G and F glycoproteins of RSV subgroup A or B to generate a live attenuated intranasal vaccine able to protect against RSV subgroup A, RSV subgroup B, and PIV3.
The strategy of using PIV3 as a vector to express RSV protective antigens has the advantage that the PIV3 backbone provides greater efficiency of growth and stability of infectivity compared to RSV and thus would obviate problems inherent in manipulating RSV itself to develop and produce attenuated derivatives. rB/HPIV3 in particular was an attractive choice as a vector because it combined the host range restriction phenotype of its parent, BPIV3, with the major antigenic determinants of one of the target viruses, HPIV3. Importantly, the BPIV3 parent has been shown to have an appropriate level of attenuation and to be phenotypically stable in seronegative infants (35, 37). Its rB/HPIV3 derivative, bearing the HPIV3 HN and F protective antigens, exhibited a similar level of attenuation in rhesus monkeys, and its immunogenicity against HPIV3 was greater than that of the BPIV3 vaccine candidate itself (48). Our present observations indicate that rB/HPIV3 can accept two added, supernumerary gene inserts of up to 2.7 kb in the promoter-proximal position without losing its ability to replicate efficiently in cell culture, a property that is essential for vaccine production. Previously, we demonstrated that gene inserts in the promoter-proximal position of up to 1.8 kb were tolerated without a significant reduction of replication in vitro (49), and HPIV3 was shown to accommodate a gene insert of 3.9 kb with little effect on replication in vitro and only a modest attenuation effect in vivo (52), findings indicative of the flexibility of PIVs as vectors. However, the addition of a second supernumerary gene reduced the amount of protein expressed from the two additional ORFs.
Although we did not formally determine the stability of the RSV inserts in the rB/HPIV3-RSV chimeras, we did not detect loss of the inserts in biological clones of the rB/HPIV3-RSV chimeras after eight passages in cell culture (data not shown). This observation agrees with previously published reports on the stability of the inserts in recombinant parainfluenza viruses expressing foreign glycoproteins (23, 55). Inserts of foreign sequence have been found to be surprisingly stable in mononegaviruses in general (6, 50) in sharp contrast to the instability that is characteristic of positive-strand RNA viruses.
Rhesus monkeys were used in this study because the host-range restriction of BPIV3 and rB/HPIV3 is manifest in this species (12, 48). The observed restriction of replication in the upper and lower respiratory tract of rhesus monkeys correlates well with the restriction of replication and the absence of upper and lower respiratory tract disease in BPIV3-infected seronegative infants (35, 37). In rhesus monkeys, the increased gene number and genome length did not greatly alter the infectivity of the rB/HPIV3-RSV chimeras, since each infected animal shed virus from both the upper and lower respiratory tract over multiple days. The single- and double-gene insert rB/HPIV3 chimeric recombinants appeared more restricted in replication than rB/HPIV3 in the upper respiratory tract, with the double-insert chimeras being slightly more restricted in replication than the single-insert chimeras, but this increase in attenuation was not apparent in the lower respiratory tract. The ability of the rB/HPIV3-RSV vaccine candidates to replicate efficiently in vivo is an important property because the level of attenuation conferred by the host range restriction of BPIV3 has been shown to confer desirable properties of safety and immunogenicity in young children (35, 37). A significant decrease in the ability of an rB/HPIV3-RSV chimera to replicate might run the risk of overattenuation and insufficient immunogenicity, as was reported previously for bovine-human RSV chimeras (5). The present study found that the rB/HPIV3-RSV chimeras retained a high level of immunogenicity in rhesus monkeys against both the HPIV3 backbone glycoproteins and the RSV G and F insert glycoproteins. We did note that, for reasons that are not understood at this time, the immunogenicity against HPIV3 of the rB/HPIV3-RSV subgroup B chimeras was lower than that of the rB/HPIV3-RSV subgroup A chimeras. Additional studies will be necessary to see if this is a consistent observation. In any event, the fact that the rB/HPIV3 subgroup A and B chimeras would be administered together would augment the immune response against the HPIV3 HN and F glycoproteins present in both chimeras. Since long-term immunity to RSV is primarily mediated by a neutralizing antibody response to the G and F glycoproteins of RSV (14) and since each rB/HPIV3-RSV efficiently induced moderate to high levels of such protective antibodies, it is reasonable to suggest that infection of humans with rB/HPIV3-RSV candidate vaccine viruses might confer resistance to illness caused by RSV. We did not challenge the rB/HPIV3-RSV-immunized animals with wild-type RSV subgroup A (A2 strain) or B (B1 strain) virus because RSV replicates only to a low level in rhesus monkeys (see reference 4 and footnote d in Table 2) and thus does not provide a stringent test of protective immunity. Extensive previous experience indicates, however, that the titer of RSV-neutralizing antibodies correlates well with protection against replication of RSV challenge virus and protection against RSV disease (30, 47).
It was previously reported that coexpression of two distinct viral antigens from a single vaccinia viral vector induced a more robust antibody response than coadministration of two virus vectors expressing one antigen each (43). In our study, immunization with the RSV subgroup A double-insert chimera (rB/HPIV3-GAFA) induced, in spite of a lower level of G and F glycoprotein expression in vitro, a threefold-higher neutralizing antibody titer than simultaneous immunization with the two single-insert chimeras rB/HPIV3-GA and rB/HPIV3-FA. This difference was not statistically significant, however. G protein-specific antibody responses to rB/HPIV3-GAFA were equivalent to responses induced by simultaneous immunization with rB/HPIV3-GA and rB/HPIV3-FA or infection with wild-type RSV. The F-specific response, however, was weaker in monkeys infected with a mixture of rB/HPIV3-GA and rB/HPIV3-F A than in animals infected with RSV (Table 3). Thus, there might be a small increase in immunogenicity associated with expression of F and G by a single rB/HPIV3 virus versus two viruses, possibly because G and F are closely associated with one another if both proteins are expressed within the same cell. Although coexpression from a single vector provides the additional advantage of reducing the number of necessary vaccine viruses, this has to be weighed against the reduced level of G and F expression in vitro. In young adult rhesus monkeys we did not observe a reduction of immunogenicity associated with rB/HPIV3-GAFA vaccination, but it remains to be seen whether in human infants immunization with this double-insert chimera is as immunogenic as coimmunization with rB/HPIV3-GA and rB/HPIV3-FA.
Use of the double-insert chimeras rB/HPIV3-GAFA and rB/HPIV3-GBFB as a vaccine to protect against HPIV3, RSV-A, and RSV-B could obviate additional problems that have been encountered in developing a live attenuated RSV vaccine. Live attenuated RSV vaccines tested to date often rely on a small number of codon changes that confer an attenuation (att) and/or temperature sensitivity (ts) phenotype (61, 65). Partial reversion of these attenuated RSV mutants has been observed in chimpanzees (19) and in humans (65). This likely does not pose a significant safety issue, since the observed revertants were only recovered sporadically and retained substantial attenuation. However, it would be preferable to have a vaccine that was completely stable phenotypically. The attenuation phenotype of rB/HPIV3-RSV chimeras, in contrast to that of previously tested RSV vaccine candidates, is based on a host range restriction that is likely conferred by many of the nucleotide and amino acid differences between BPIV3 and HPIV3 that are present in multiple genes (1, 2, 48). This should ensure phenotypic stability and greatly decrease the likelihood of reversion to a wild-type phenotype. Our laboratory currently is characterizing the contribution of each BPIV3 gene to the host range restriction phenotype. The Jennerian vaccines studied to date (9, 10, 24, 29, 32, 53, 54) appear to be phenotypically stable. Although this will need to be confirmed in larger studies for BPIV3, clinical studies to date suggest that the attenuation phenotype is indeed stable following replication in vivo.
An existing, conventional strategy to prepare a live attenuated vaccine against RSV-A, RSV-B, and HPIV3 would be to develop a separate attenuated strain for each virus and combine them to make a three-component trivalent vaccine. Considerable progress has been made towards developing each component (8, 38, 58, 60–62), but the actual RSV-A and RSV-B strains that would constitute such a vaccine are still under development. Furthermore, combining several different viruses into a single vaccine can result in complex interactive events leading to interference of replication of one or more of the components (46). The results reported here provide an alternative strategy to formulating a trivalent vaccine against RSV-A, RSV-B and HPIV3 in which the proposed individually attenuated HPIV3, RSV-A, and RSV-B vaccine components are replaced by a vaccine consisting of rB/HPIV3-GAFA and rB/HPIV3-GBFB, thus involving only two components based on a single attenuated backbone. The advantages of this two-component, single-backbone vaccine include the following: (i) attenuation is based on the host range restriction phenotype of the BPIV3 virus as described above; (ii) the use of PIV3-based vaccine viruses instead of attenuated RSV strains will obviate the problems of poor growth in vitro (57) and physical instability (26); (iii) the use of a single rB/HPIV3 backbone in place of three different viruses should preclude problems resulting from viral interference (46) and should simplify the formulation and administration of this vaccine; (iv) the promoter-proximal locations of the RSV G and F inserts should provide a higher level of expression (25) compared to RSV vaccine candidates with a conventional gene order, in which the G and F genes are the seventh and eighth genes in the map relative to the promoter; (v) the presence of HPIV3 HN and F backbone glycoprotein genes would induce a more effective immune response against HPIV3 than would the moderately divergent BPIV3 HN and F genes in the BPIV3 attenuated parent virus (48); and (vi) the rB/HPIV3-GAFA and rB/HPIV3-GBFB vaccine viruses, or their single-gene insert counterparts, are now ready for clinical evaluation. The fact that the viruses are cDNA derived will make further modification possible should that prove necessary based on the results of clinical evaluation.
In summary, the in vitro and in vivo characteristics of the single- or double-insert chimeras indicate that these viruses are promising vaccine candidates. They are based on a well-characterized attenuated backbone, they provide a satisfactory level of attenuation and immunogenicity, and they can be used in a simplified single-backbone vaccine strategy to protect against three major pathogens, namely, HPIV3, RSV-A, and RSV-B. Taking into consideration that all live attenuated RSV vaccine candidates generated to date have been either overattenuated and lacking immunogenicity (34, 64) or underattenuated and pathogenic to some degree (36, 65), vectored RSV vaccines like the ones presented here might be helpful in achieving the fine balance between attenuation and immunogenicity that is needed.
Acknowledgments
We thank Robert Chanock for his careful review of the manuscript. We also thank Fatemeh Davoodi, Ernest Williams, and Teresa Mulaikal for their excellent technical support. Christine Krempl, Stephen Whitehead, Michael Teng, and Mario Skiadopoulos provided help and advice at various stages.
This research is part of a continuing program of research and development with Wyeth-Lederle Vaccines and Pediatrics through CRADA grants AI-000030 and AI-000087. D.R.W. is a Howard Hughes Medical Institute-NIH Research Scholar.
REFERENCES
- 1.Bailly, J. E., J. M. McAuliffe, A. P. Durbin, W. R. Elkins, P. L. Collins, and B. R. Murphy. 2000. A recombinant human parainfluenza virus type 3 (PIV3) in which the nucleocapsid N protein has been replaced by that of bovine PIV3 is attenuated in primates. J. Virol. 74:3188–3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bailly, J. E., J. M. McAuliffe, M. H. Skiadopoulos, P. L. Collins, and B. R. Murphy. 2000. Sequence determination and molecular analysis of two strains of bovine parainfluenza virus type 3 that are attenuated for primates. Virus Genes 20:173–182. [DOI] [PubMed] [Google Scholar]
- 3.Beeler, J. A., and K. van Wyke Coelingh. 1989. Neutralization epitopes of the F glycoprotein of respiratory syncytial virus: effect of mutation upon fusion function. J. Virol. 63:2941–2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belshe, R. B., L. S. Richardson, W. T. London, D. L. Sly, J. H. Lorfeld, E. Camargo, D. A. Prevar, and R. M. Chanock. 1977. Experimental respiratory syncytial virus infection of four species of primates. J. Med. Virol. 1:157–162. [DOI] [PubMed] [Google Scholar]
- 5.Buchholz, U. J., H. Granzow, K. Schuldt, S. S. Whitehead, B. R. Murphy, and P. L. Collins. 2000. Chimeric bovine respiratory syncytial virus with glycoprotein gene substitutions from human respiratory syncytial virus (HRSV): effects on host range and evaluation as a live-attenuated HRSV vaccine. J. Virol. 74:1187–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bukreyev, A., E. Camargo, and P. L. Collins. 1996. Recovery of infectious respiratory syncytial virus expressing an additional, foreign gene. J. Virol. 70:6634–6641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calain, P., and L. Roux. 1993. The rule of six, a basic feature for efficient replication of Sendai virus defective interfering RNA. J. Virol. 67:4822–4830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng, X., H. Zhou, R. S. Tang, M. G. Munoz, and H. Jin. 2001. Chimeric subgroup A respiratory syncytial virus with the glycoproteins substituted by those of subgroup B and RSV without the M2-2 gene are attenuated in African green monkeys. Virology 283:59–68. [DOI] [PubMed] [Google Scholar]
- 9.Clements, M. L., E. K. Subbarao, L. F. Fries, R. A. Karron, W. T. London, and B. R. Murphy. 1992. Use of single-gene reassortant viruses to study the role of avian influenza A virus genes in attenuation of wild-type human influenza A virus for squirrel monkeys and adult human volunteers. J. Clin. Microbiol. 30:655–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clements-Mann, M. L., M. K. Makhene, J. Mrukowicz, P. F. Wright, Y. Hoshino, K. Midthun, E. Sperber, R. Karron, and A. Z. Kapikian. 1999. Safety and immunogenicity of live attenuated human-bovine (UK) reassortant rotavirus vaccines with VP7-specificity for serotypes 1, 2, 3 or 4 in adults, children and infants. Vaccine 17:2715–2725. [DOI] [PubMed] [Google Scholar]
- 11.Coates, H. V., B. R. Forsyth, and R. M. Chanock. 1966. Biophysical studies of respiratory syncytial virus. I. Density of respiratory syncytial virus and associated complement-fixing antigens in cesium chloride density gradient. J. Bacteriol. 91:1263–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coelingh, K., C. C. Winter, E. L. Tierney, W. T. London, and B. R. Murphy. 1988. Attenuation of bovine parainfluenza virus type 3 in nonhuman primates and its ability to confer immunity to human parainfluenza virus type 3 challenge. J. Infect. Dis. 157:655–662. [DOI] [PubMed] [Google Scholar]
- 13.Collins, P. L., R. M. Chanock, and K. McIntosh. 1996. Parainfluenza viruses, p. 1205–1243. In B. N. Fields, D. M. Knipe, P. M. Howley, R. M. Chanock, J. L. Melnick, T. P. Monath, B. Roizman, and S. E. Straus (ed.), Fields virology, 3rd ed., vol. 1. Lippincott-Raven Publishers, Philadelphia, Pa.
- 14.Collins, P. L., K. McIntosh, and R. M. Chanock. 1996. Respiratory syncytial virus, p. 1313–1352. In B. N. Fields, D. M. Knipe, P. M. Howley, R. M. Chanock, J. L. Melnick, T. P. Monath, B. Roizman, and S. E. Straus (ed.), Fields virology, 3rd ed., vol. 2. Lippincott-Raven, Philadelphia, Pa.
- 15.Connors, M., P. L. Collins, C. Y. Firestone, A. V. Sotnikov, A. Waitze, A. R. Davis, P. P. Hung, R. M. Chanock, and B. R. Murphy. 1992. Cotton rats previously immunized with a chimeric RSV FG glycoprotein develop enhanced pulmonary pathology when infected with RSV, a phenomenon not encountered following immunization with vaccinia-RSV recombinants or RSV. Vaccine 10:475–484. [DOI] [PubMed] [Google Scholar]
- 16.Connors, M., A. B. Kulkarni, P. L. Collins, C. Y. Firestone, K. L. Holmes, H. C. Morse III, and B. R. Murphy. 1992. Resistance to respiratory syncytial virus (RSV) challenge induced by infection with a vaccinia virus recombinant expressing the RSV M2 protein (Vac-M2) is mediated by CD8+ T cells, while that induced by Vac-F or Vac-G recombinants is mediated by antibodies. J. Virol. 66:1277–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crookshanks-Newman, F. K., and R. B. Belshe. 1986. Protection of weanling hamsters from experimental infection with wild-type parainfluenza virus type 3 (para 3) by cold-adapted mutants of para 3. J. Med. Virol. 18:131–137. [DOI] [PubMed] [Google Scholar]
- 18.Crowe, J. E., Jr. 1995. Current approaches to the development of vaccines against disease caused by respiratory syncytial virus (RSV) and parainfluenza virus (PIV). A meeting report of the W. H. O. Programme for Vaccine Development. Vaccine 13:415–421. [DOI] [PubMed] [Google Scholar]
- 19.Crowe, J. E., Jr., P. L. Collins, W. T. London, R. M. Chanock, and B. R. Murphy. 1993. A comparison in chimpanzees of the immunogenicity and efficacy of live attenuated respiratory syncytial virus (RSV) temperature-sensitive mutant vaccines and vaccinia virus recombinants that express the surface glycoproteins of RSV. Vaccine 11:1395–1404. [DOI] [PubMed] [Google Scholar]
- 20.Dudas, R. A., and R. A. Karron. 1998. Respiratory syncytial virus vaccines. Clin. Microbiol. Rev. 11:430–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Durbin, A. P., S. L. Hall, J. W. Siew, S. S. Whitehead, P. L. Collins, and B. R. Murphy. 1997. Recovery of infectious human parainfluenza virus type 3 from cDNA. Virology 235:323–332. [DOI] [PubMed] [Google Scholar]
- 22.Durbin, A. P., J. M. McAuliffe, P. L. Collins, and B. R. Murphy. 1999. Mutations in the C, D, and V open reading frames of human parainfluenza virus type 3 attenuate replication in rodents and primates. Virology 261:319–330. [DOI] [PubMed] [Google Scholar]
- 23.Durbin, A. P., M. H. Skiadopoulos, J. M. McAuliffe, J. M. Riggs, S. R. Surman, P. L. Collins, and B. R. Murphy. 2000. Human parainfluenza virus type 3 (PIV3) expressing the hemagglutinin protein of measles virus provides a potential method for immunization against measles virus and PIV3 in early infancy. J. Virol. 74:6821–6831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Emerson, S. U., S. A. Tsarev, S. Govindarajan, M. Shapiro, and R. H. Purcell. 1996. A simian strain of hepatitis A virus, AGM-27, functions as an attenuated vaccine for chimpanzees. J. Infect. Dis. 173:592–597. [DOI] [PubMed] [Google Scholar]
- 25.Flanagan, E. B., L. A. Ball, and G. W. Wertz. 2000. Moving the glycoprotein gene of vesicular stomatitis virus to promoter-proximal positions accelerates and enhances the protective immune response. J. Virol. 74:7895–7902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gupta, C. K., J. Leszczynski, R. K. Gupta, and G. R. Siber. 1996. Stabilization of respiratory syncytial virus (RSV) against thermal inactivation and freeze-thaw cycles for development and control of RSV vaccines and immune globulin. Vaccine 14:1417–1420. [DOI] [PubMed] [Google Scholar]
- 27.Hall, S. L., A. Stokes, E. L. Tierney, W. T. London, R. B. Belshe, F. C. Newman, and B. R. Murphy. 1992. Cold-passaged human parainfluenza type 3 viruses contain ts and non-ts mutations leading to attenuation in rhesus monkeys. Virus Res. 22:173–184. [DOI] [PubMed] [Google Scholar]
- 28.Hendry, R. M., J. C. Burns, E. E. Walsh, B. S. Graham, P. F. Wright, V. G. Hemming, W. J. Rodriguez, H. W. Kim, G. A. Prince, K. McIntosh, et al. 1988. Strain-specific serum antibody responses in infants undergoing primary infection with respiratory syncytial virus. J. Infect. Dis. 157:640–647. [DOI] [PubMed] [Google Scholar]
- 29.Hurwitz, J. L., K. F. Soike, M. Y. Sangster, A. Portner, R. E. Sealy, D. H. Dawson, and C. Coleclough. 1997. Intranasal Sendai virus vaccine protects African green monkeys from infection with human parainfluenza virus-type one. Vaccine 15:533–540. [DOI] [PubMed] [Google Scholar]
- 30.IMpact-RSV Study Group. 1998. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics 102:531–537. [PubMed] [Google Scholar]
- 31.Johnson, P. R., Jr., R. A. Olmsted, G. A. Prince, B. R. Murphy, D. W. Alling, E. E. Walsh, and P. L. Collins. 1987. Antigenic relatedness between glycoproteins of human respiratory syncytial virus subgroups A and B: evaluation of the contributions of F and G glycoproteins to immunity. J. Virol. 61:3163–3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kapikian, A. Z., T. Vesikari, T. Ruuska, H. P. Madore, C. Christy, R. Dolin, J. Flores, K. Y. Green, B. L. Davidson, M. Gorziglia, et al. 1992. An update on the “Jennerian” and modified “Jennerian” approach to vaccination of infants and young children against rotavirus diarrhea. Adv. Exp. Med. Biol. 327:59–69. [DOI] [PubMed] [Google Scholar]
- 33.Karron, R. A., and D. M. Ambrosino. 1998. Respiratory syncytial virus vaccines. Pediatr. Infect. Dis. J. 17:919–920. [DOI] [PubMed] [Google Scholar]
- 34.Karron, R. A., D. A. Buonagurio, A. F. Georgiu, S. S. Whitehead, J. E. Adamus, M. L. Clements-Mann, D. O. Harris, V. B. Randolph, S. A. Udem, B. R. Murphy, and M. S. Sidhu. 1997. Respiratory syncytial virus (RSV) SH and G proteins are not essential for viral replication in vitro: clinical evaluation and molecular characterization of a cold-passaged, attenuated RSV subgroup B mutant. Proc. Natl. Acad. Sci. USA 94:13961–13966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karron, R. A., M. Makhene, K. Gay, M. H. Wilson, M. L. Clements, and B. R. Murphy. 1996. Evaluation of a live attenuated bovine parainfluenza type 3 vaccine in two- to six-month-old infants. Pediatr. Infect. Dis. J. 15:650–654. [DOI] [PubMed] [Google Scholar]
- 36.Karron, R. A., P. F. Wright, J. E. Crowe, Jr., M. L. Clements, J. Thompson, M. Makhene, R. Casey, and B. R. Murphy. 1997. Evaluation of two live, cold-passaged, temperature-sensitive respiratory syncytial virus (RSV) vaccines in chimpanzees, adults, infants and children. J. Infect. Dis. 176:1428–1436. [DOI] [PubMed] [Google Scholar]
- 37.Karron, R. A., P. F. Wright, S. L. Hall, M. Makhene, J. Thompson, B. A. Burns, S. Tollefson, M. C. Steinhoff, M. H. Wilson, D. O. Harris, et al. 1995. A live attenuated bovine parainfluenza virus type 3 vaccine is safe, infectious, immunogenic, and phenotypically stable in infants and children. J. Infect. Dis. 171:1107–1114. [DOI] [PubMed] [Google Scholar]
- 38.Karron, R. A., P. F. Wright, F. K. Newman, M. Makhene, J. Thompson, R. Samorodin, M. H. Wilson, E. L. Anderson, M. L. Clements, B. R. Murphy, and R. B. Belshe. 1995. A live human parainfluenza type 3 virus vaccine is attenuated and immunogenic in healthy infants and children. J. Infect. Dis. 172:1445–1450. [DOI] [PubMed] [Google Scholar]
- 39.Kim, H. W., J. O. Arrobio, G. Pyles, C. D. Brandt, E. Camargo, R. M. Chanock, and R. H. Parrott. 1971. Clinical and immunological response of infants and children to administration of low-temperature adapted respiratory syncytial virus. Pediatrics 48:745–755. [PubMed] [Google Scholar]
- 40.Kim, H. W., J. G. Canchola, C. D. Brandt, G. Pyles, R. M. Chanock, K. Jensen, and R. H. Parrott. 1969. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am. J. Epidemiol. 89:422–434. [DOI] [PubMed] [Google Scholar]
- 41.Kolakofsky, D., T. Pelet, D. Garcin, S. Hausmann, J. Curran, and L. Roux. 1998. Paramyxovirus RNA synthesis and the requirement for hexamer genome length: the rule of six revisited. J. Virol. 72:891–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kulkarni, A. B., P. L. Collins, I. Bacik, J. W. Yewdell, J. R. Bennink, J. E. Crowe, Jr., and B. R. Murphy. 1995. Cytotoxic T cells specific for a single peptide on the M2 protein of respiratory syncytial virus are the sole mediators of resistance induced by immunization with M2 encoded by a recombinant vaccinia virus. J. Virol. 69:1261–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kutinova, L., P. Hainz, V. Ludvikova, L. Maresova, and S. Nemeckova. 2001. Immune response to vaccinia virus recombinants expressing glycoproteins gE, gB, gH, and gL of varicella-zoster virus. Virology 280:211–220. [DOI] [PubMed] [Google Scholar]
- 44.Murphy, B. R., P. L. Collins, L. Lawrence, J. Zubak, R. M. Chanock, and G. A. Prince. 1989. Immunosuppression of the antibody response to respiratory syncytial virus (RSV) by pre-existing serum antibodies: partial prevention by topical infection of the respiratory tract with vaccinia virus-RSV recombinants. J. Gen. Virol. 70:2185–2190. [DOI] [PubMed] [Google Scholar]
- 45.Murphy, B. R., G. A. Prince, P. L. Collins, K. Van Wyke Coelingh, R. A. Olmsted, M. K. Spriggs, R. H. Parrott, H. W. Kim, C. D. Brandt, and R. M. Chanock. 1988. Current approaches to the development of vaccines effective against parainfluenza and respiratory syncytial viruses. Virus Res. 11:1–15. [DOI] [PubMed] [Google Scholar]
- 46.Patriarca, P. A., P. F. Wright, and T. J. John. 1991. Factors affecting the immunogenicity of oral poliovirus vaccine in developing countries: review. Rev. Infect. Dis. 13:926–939. [DOI] [PubMed] [Google Scholar]
- 47.Prince, G. A., V. G. Hemming, R. L. Horswood, and R. M. Chanock. 1985. Immunoprophylaxis and immunotherapy of respiratory syncytial virus infection in the cotton rat. Virus Res. 3:193–206. [DOI] [PubMed] [Google Scholar]
- 48.Schmidt, A. C., J. M. McAuliffe, A. Huang, S. R. Surman, J. E. Bailly, W. R. Elkins, P. L. Collins, B. R. Murphy, and M. H. Skiadopoulos. 2000. Bovine parainfluenza virus type 3 (BPIV3) fusion and hemagglutinin-neuraminidase glycoproteins make an important contribution to the restricted replication of BPIV3 in primates. J. Virol. 74:8922–8929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schmidt, A. C., J. M. McAuliffe, B. R. Murphy, and P. L. Collins. 2001. Recombinant bovine/human parainfluenza virus type 3 (B/HPIV3) expressing the respiratory syncytial virus (RSV) G and F proteins can be used to achieve simultaneous mucosal immunization against RSV and HPIV3. J. Virol. 75:4594–4603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schnell, M. J., L. Buonocore, M. A. Whitt, and J. K. Rose. 1996. The minimal conserved transcription stop-start signal promotes stable expression of a foreign gene in vesicular stomatitis virus. J. Virol. 70:2318–2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shay, D. K., R. C. Holman, R. D. Newman, L. L. Liu, J. W. Stout, and L. J. Anderson. 1999. Bronchiolitis-associated hospitalizations among US children, 1980–1996. JAMA 282:1440–1446. [DOI] [PubMed] [Google Scholar]
- 52.Skiadopoulos, M. H., S. R. Surman, A. P. Durbin, P. L. Collins, and B. R. Murphy. 2000. Long nucleotide insertions between the HN and L protein coding regions of human parainfluenza virus type 3 yield viruses with temperature-sensitive and attenuation phenotypes. Virology 272:225–234. [DOI] [PubMed] [Google Scholar]
- 53.Subbarao, E. K., M. Perkins, J. J. Treanor, and B. R. Murphy. 1992. The attenuation phenotype conferred by the M gene of the influenza A/Ann Arbor/6/60 cold-adapted virus (H2N2) on the A/Korea/82 (H3N2) reassortant virus results from a gene constellation effect. Virus Res. 25:37–50. [DOI] [PubMed] [Google Scholar]
- 54.Subbarao, K., R. G. Webster, Y. Kawaoka, and B. R. Murphy. 1995. Are there alternative avian influenza viruses for generation of stable attenuated avian-human influenza A reassortant viruses? Virus Res. 39:105–118. [DOI] [PubMed] [Google Scholar]
- 55.Tao, T., F. Davoodi, C. J. Cho, M. H. Skiadopoulos, A. P. Durbin, P. L. Collins, and B. R. Murphy. 2000. A live attenuated recombinant chimeric parainfluenza virus (PIV) candidate vaccine containing the hemagglutinin-neuraminidase and fusion glycoproteins of PIV1 and the remaining proteins from PIV3 induces resistance to PIV1 even in animals immune to PIV3. Vaccine 18:1359–1366. [DOI] [PubMed] [Google Scholar]
- 56.Tapparel, C., D. Maurice, and L. Roux. 1998. The activity of Sendai virus genomic and antigenomic promoters requires a second element past the leader template regions: a motif (GNNNNN)3 is essential for replication. J. Virol. 72:3117–3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Teng, M. N., and P. L. Collins. 1998. Altered growth characteristics of recombinant respiratory syncytial viruses which do not produce the NS2 protein. J. Virol. 73:466–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Teng, M. N., S. S. Whitehead, A. Bermingham, M. St. Claire, W. R. Elkins, B. R. Murphy, and P. L. Collins. 2000. Recombinant respiratory syncytial virus that does not express the NS1 or M2-2 protein is highly attenuated and immunogenic in chimpanzees. J. Virol. 74:9317–9321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weber, M. W., E. K. Mulholland, and B. M. Greenwood. 1998. Respiratory syncytial virus infection in tropical and developing countries. Trop. Med. Int. Health 3:268–280. [DOI] [PubMed] [Google Scholar]
- 60.Whitehead, S. S., A. Bukreyev, M. N. Teng, C. Y. Firestone, M. St. Clair, W. R. Elkins, P. L. Collins, and B. R. Murphy. 1999. Recombinant respiratory syncytial virus (RSV) bearing a deletion of either the NS2 or SH gene is attenuated in chimpanzees. J. Virol. 73:3438–3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Whitehead, S. S., C.-Y. Firestone, R. A. Karron, J. E. Crowe, Jr., W. R. Elkins, P. L. Collins, and B. R. Murphy. 1999. Addition of a missense mutation present in the L gene of respiratory syncytial virus (RSV) cpts530/1030 to RSV vaccine candidate cpts248/404 increases its attenuation and temperature sensitivity. J. Virol. 73:871–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Whitehead, S. S., M. G. Hill, C.-Y. Firestone, M. St. Claire, W. R. Elkins, B. R. Murphy, and P. L. Collins. 1999. Replacement of the F and G proteins of respiratory syncytial virus (RSV) subgroup A with those of subgroup B generates chimeric live attenuated RSV subgroup B vaccine candidates. J. Virol. 73:9773–9780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.World Health Organization. 2000. World health report 2000. World Health Organization, Geneva, Switzerland.
- 64.Wright, P. F., R. B. Belshe, H. W. Kim, L. P. Van Voris, and R. M. Chanock. 1982. Administration of a highly attenuated, live respiratory syncytial virus vaccine to adults and children. Infect. Immun. 37:397–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wright, P. F., R. A. Karron, R. B. Belshe, J. Thompson, J. E. Crowe, Jr., T. G. Boyce, L. L. Halburnt, G. W. Reed, S. S. Whitehead, E. L. Anderson, A. E. Wittek, R. Casey, M. Eichelberger, B. Thumar, V. B. Randolph, S. A. Udem, R. M. Chanock, and B. R. Murphy. 2000. Evaluation of a live, cold-passaged, temperature-sensitive, respiratory syncytial virus vaccine candidate in infancy. J. Infect. Dis. 182:1331–1342. [DOI] [PubMed] [Google Scholar]
- 66.Wyatt, L. S., B. Moss, and S. Rozenblatt. 1995. Replication-deficient vaccinia virus encoding bacteriophage T7 RNA polymerase for transient gene expression in mammalian cells. Virology 210:202–205. [DOI] [PubMed] [Google Scholar]