Abstract
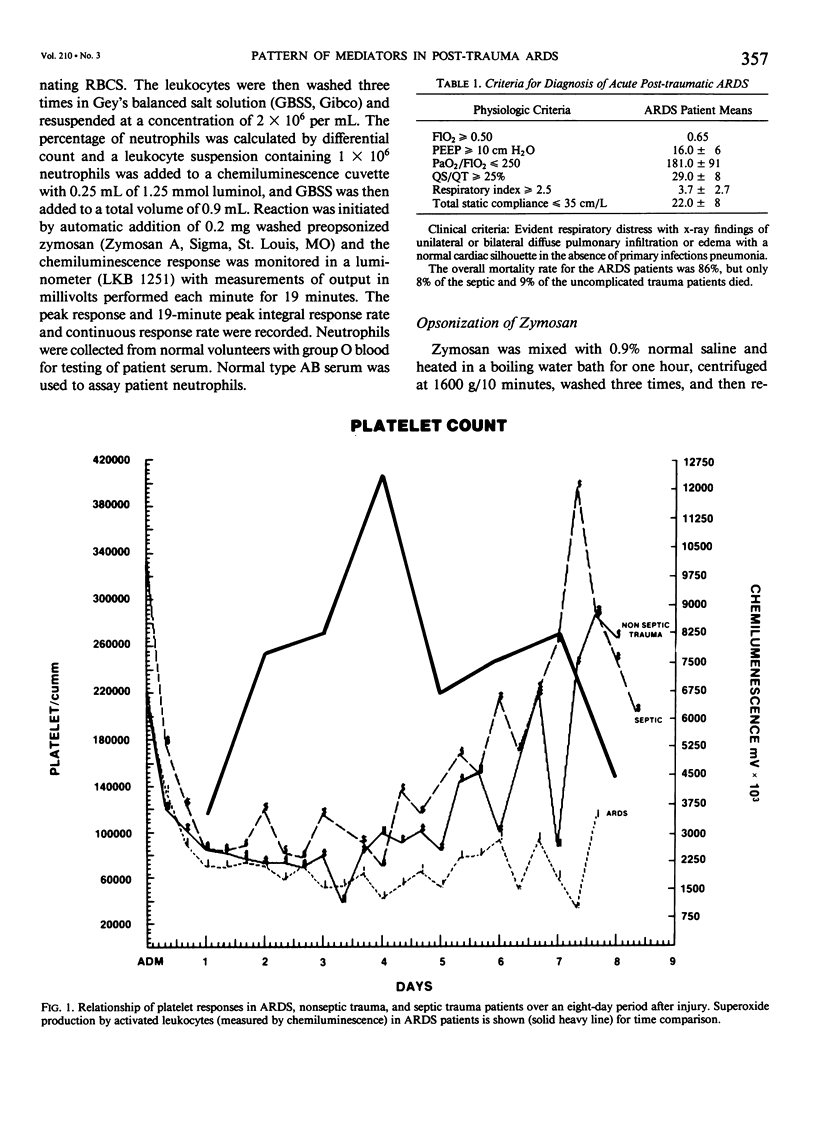
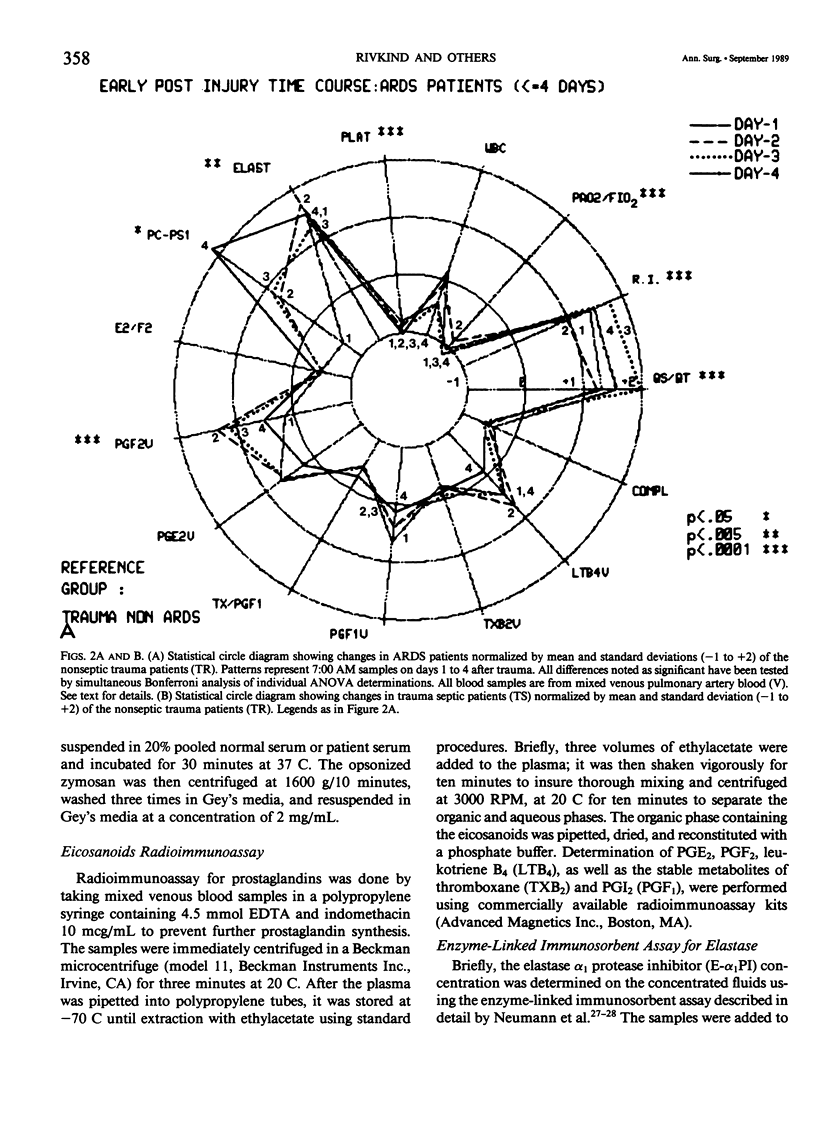
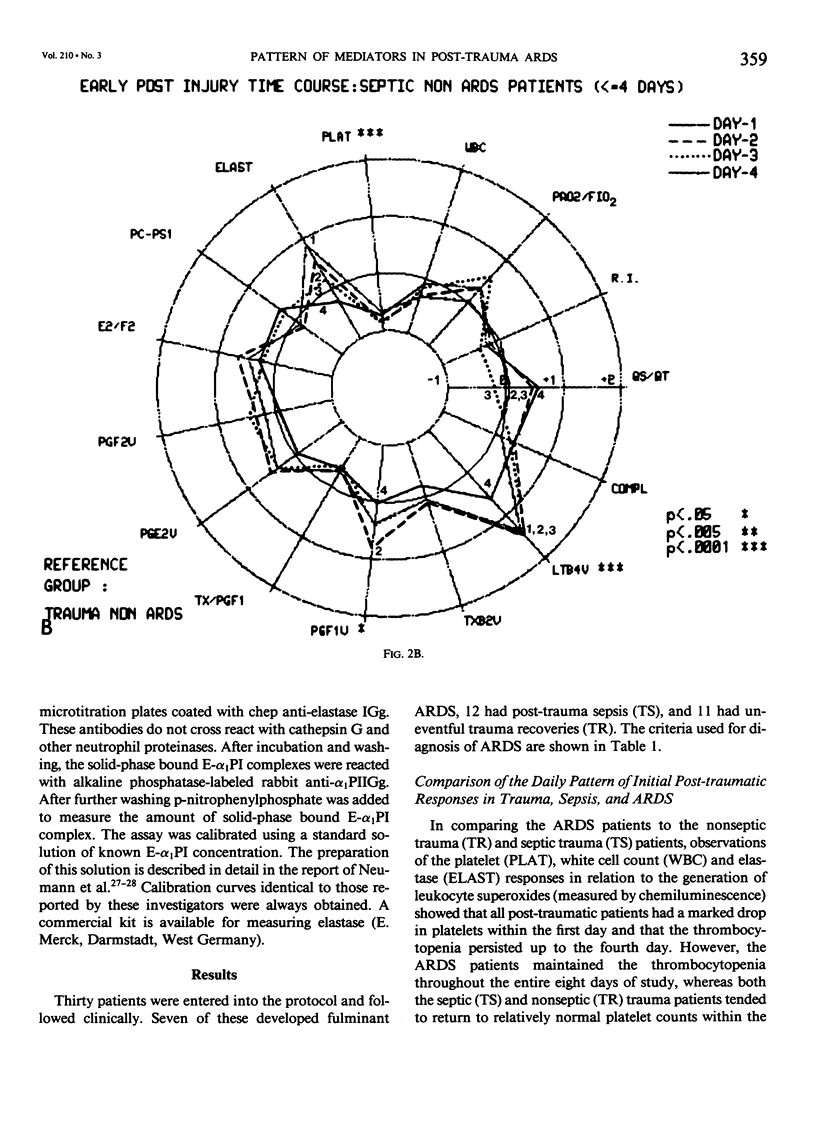
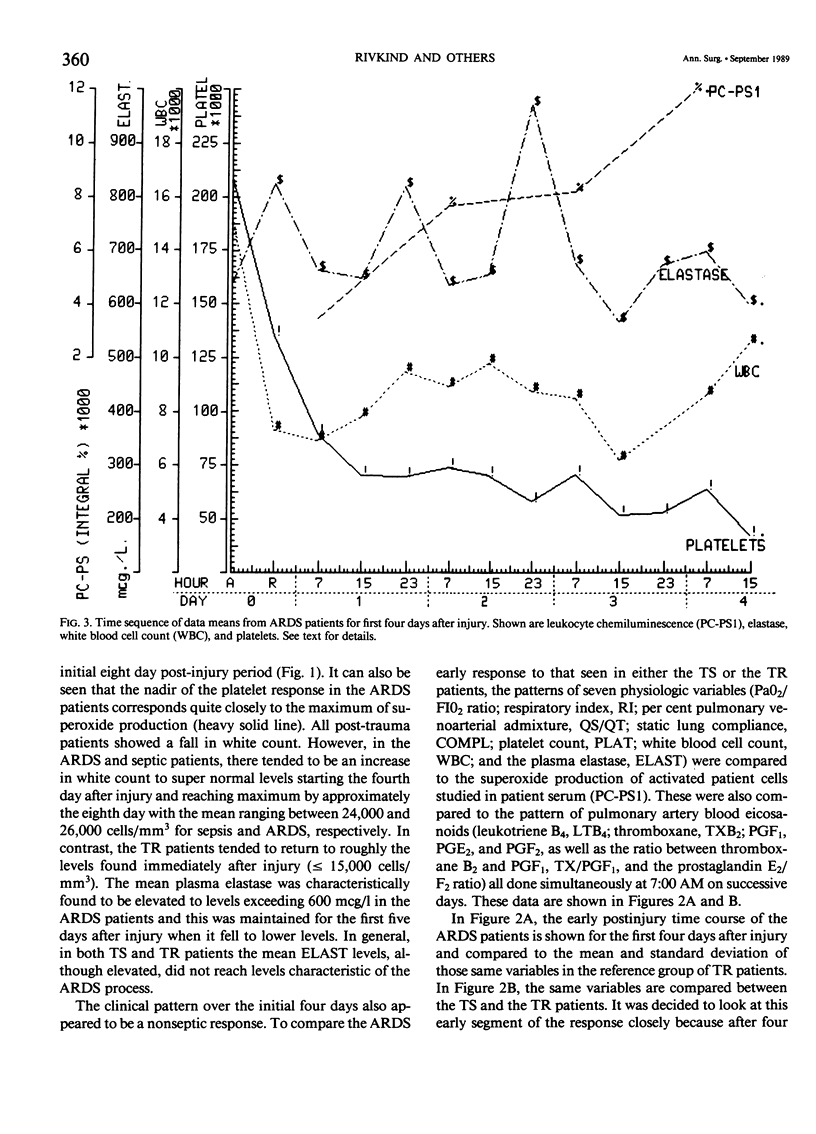
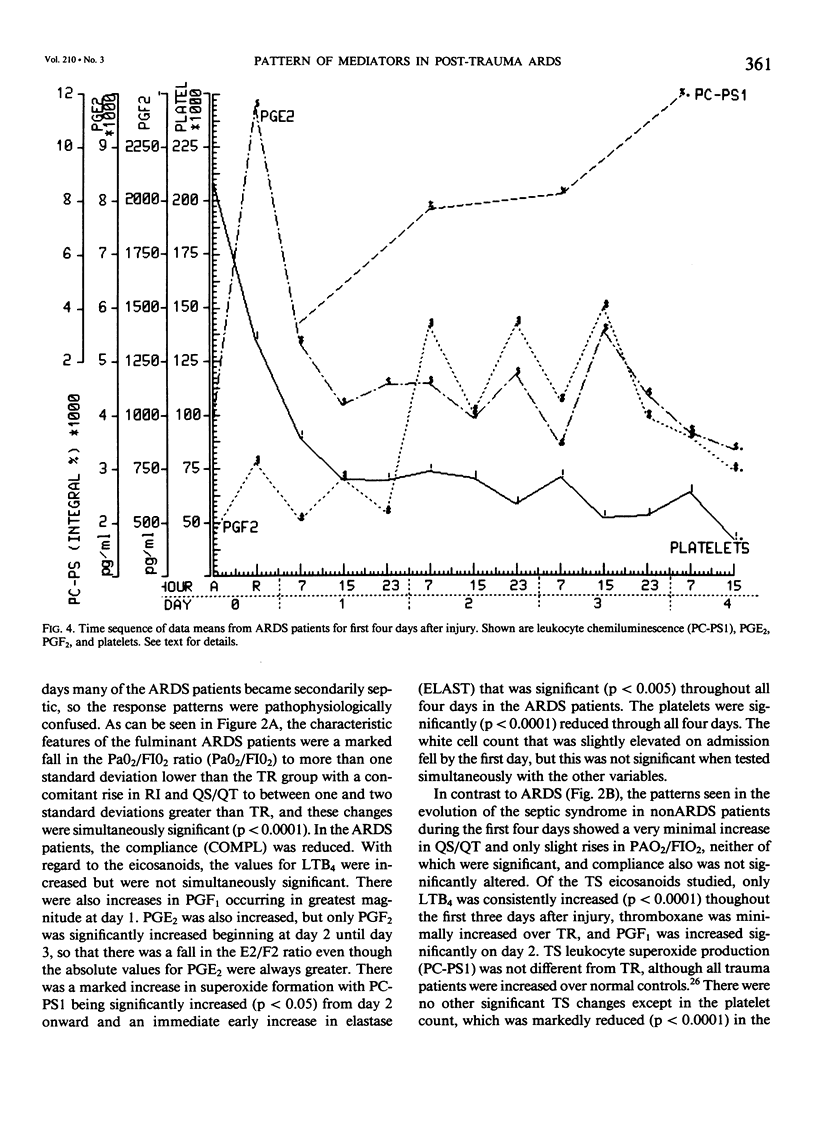
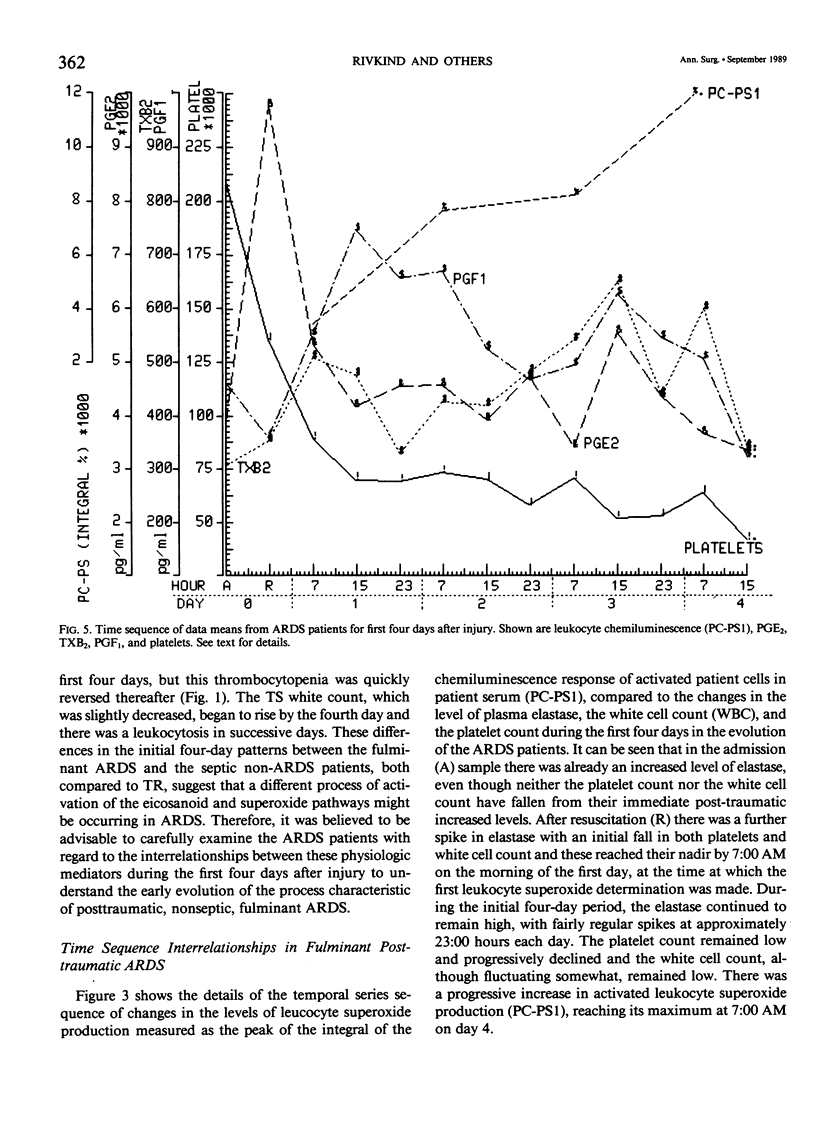
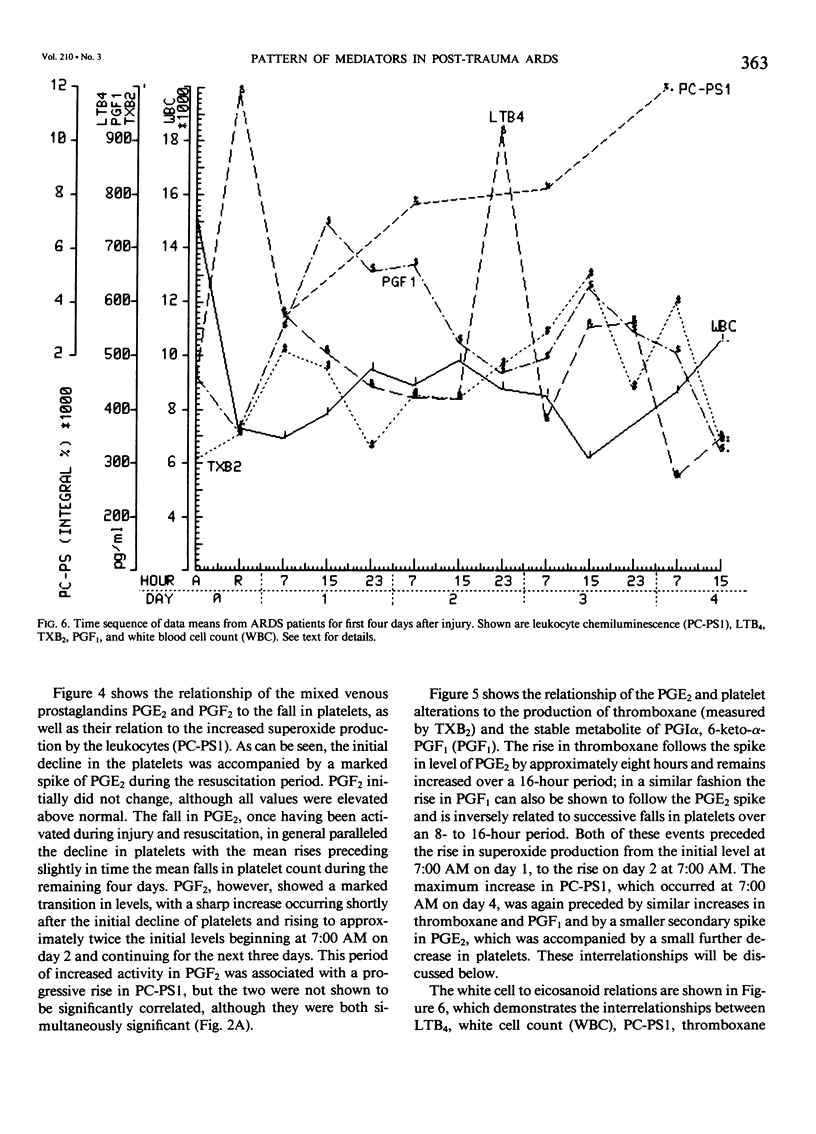
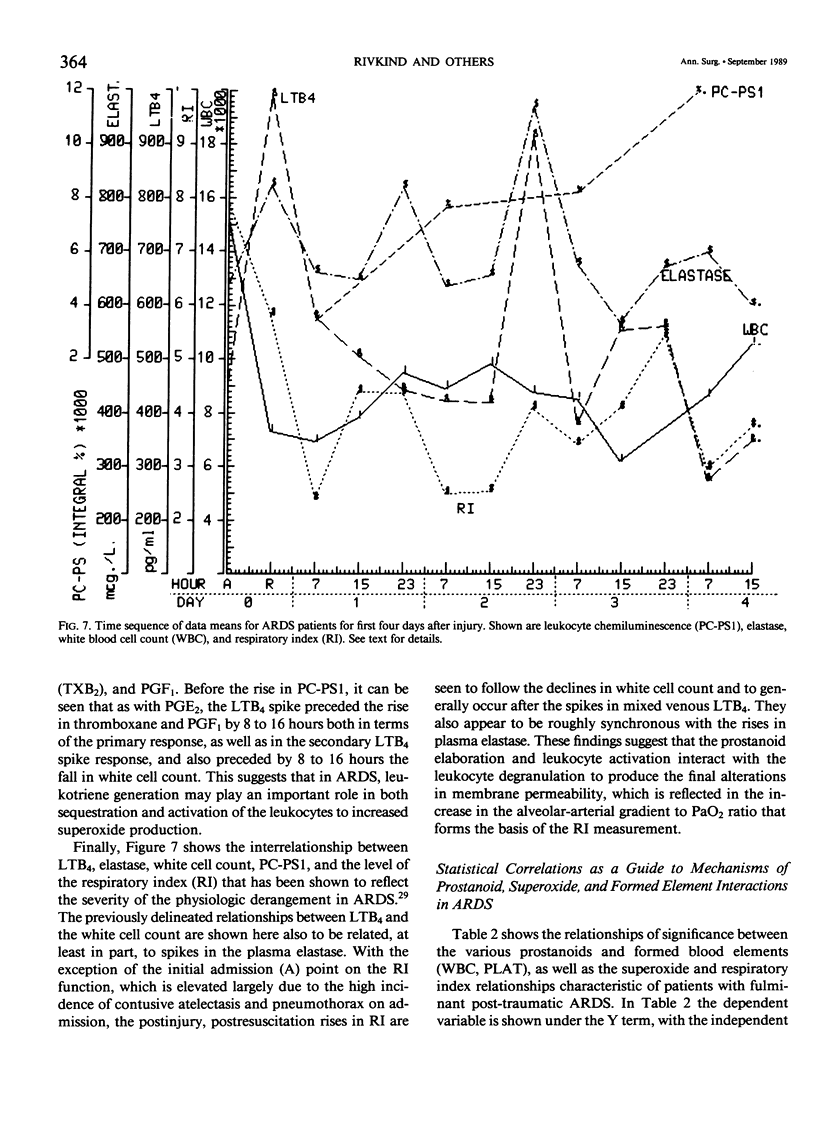
Thirty multiply injured blunt-trauma patients at high risk for development of ARDS (multisystem trauma including more than one organ or extremity, Injury Severity Score of 26 or more, hypotension and need for 1500 mL or more blood within the first hour after admission, and PaO2 less than or equal to 70 torr) were studied sequentially with blood and physiologic evaluations beginning immediately after injury and every eight hours for eight days, or until death, to study the evolution of the ARDS process. Mixed venous blood samples were obtained for eicosanoids PGE2, PGF2 alpha, thromboxane B2, PGI2 (6-KetoPGF1 alpha) and leukotriene B4 (LTB4). Platelet (PLAT), and neutrophil (WBC) counts were also done and plasma elastase was measured. At 7:00 AM each day patient neutrophils were obtained for a study of zymosan-activated superoxide production using a chemiluminescence assay. These data were correlated with physiologic measurements of the Respiratory Index (RI), per cent pulmonary shunt (QS/QT), and respiratory compliance measures. Seven patients developed a fulminant post-traumatic ARDS syndrome within 96 hours after injury. Twelve patients without ARDS developed sepsis (TS) four or more days after injury, and 11 had uncomplicated postinjury courses (TR). Compared to both TR and TS, ARDS had a significant (p less than 0.01) rise in neutrophil superoxide production beginning on day 2 through day 4 after injury. This was preceded by rises in PGE2 and LTB4, which were significantly correlated with subsequent falls in PLAT and WBC and rises in TXB2, PGF1, and superoxide production and followed by increases in RI, QS/QT, and a fall in compliance. The significant difference in the pattern and sequence of events in ARDS compared to TR and TS patients suggests that in ARDS the earliest event may be related to peripheral release of PGE2 and LTB4 due to platelet activation and lung sequestration with release of PGF2 alpha, and by aggregation and leukocyte adherence with release of elastase. However, fulminant ARDS mortality appears to be related to the subsequent amplification of the LTB4 leukocyte activation with superoxide production that does not achieve significance before the second day after injury and rises to a maximum by day 4 after injury. These data suggest that post-trauma ARDS follows a different evolutionary pattern than that reported in animal models and is also different from that seen in human TS or TR patients.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF



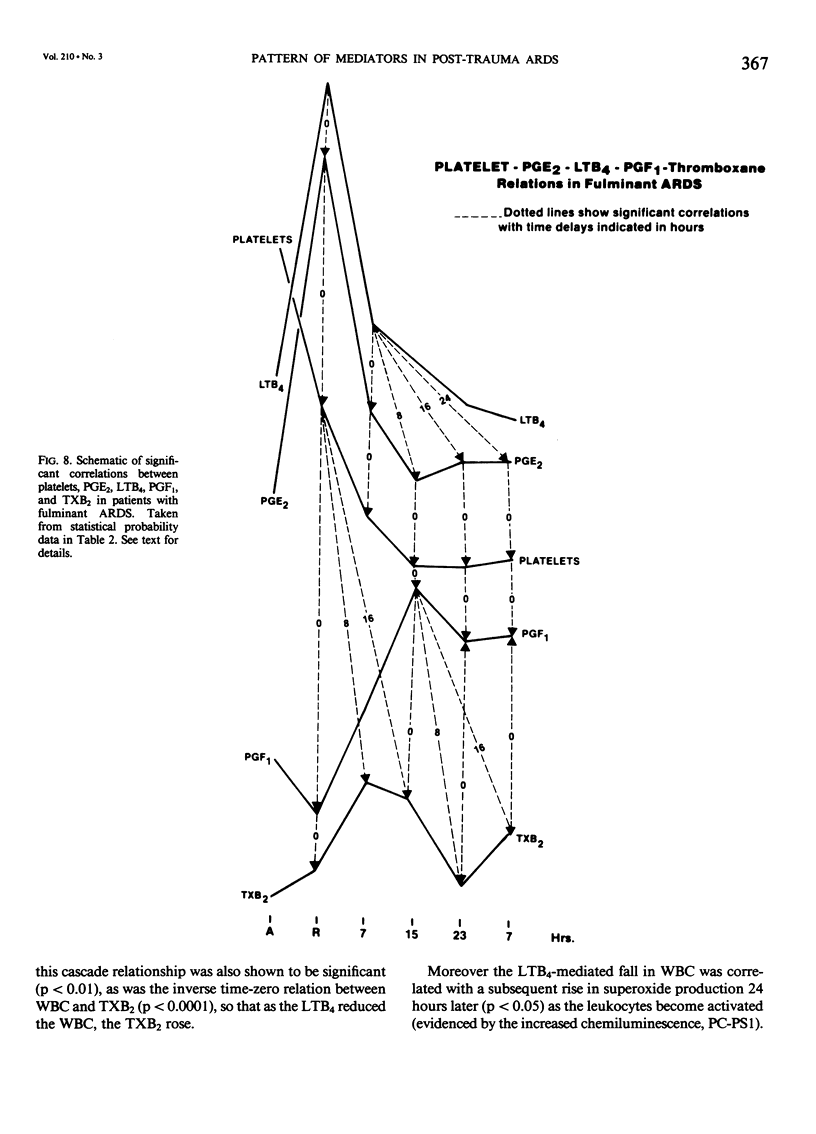
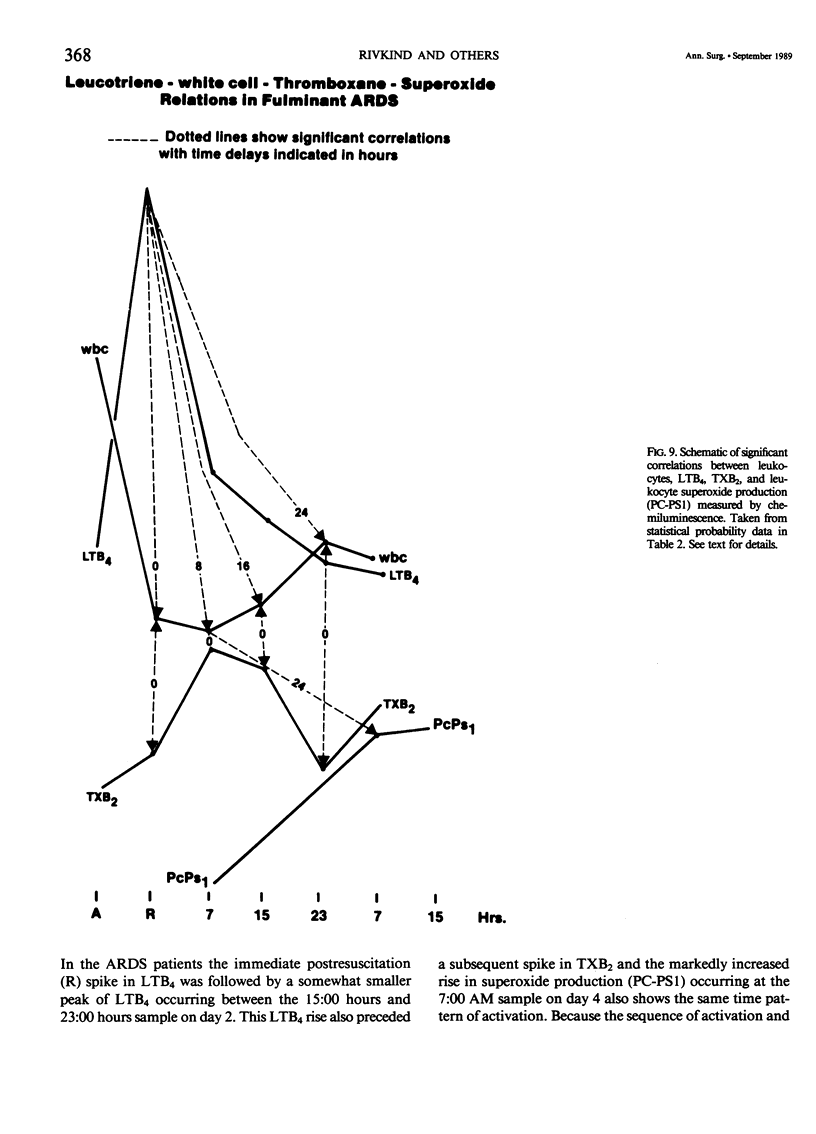
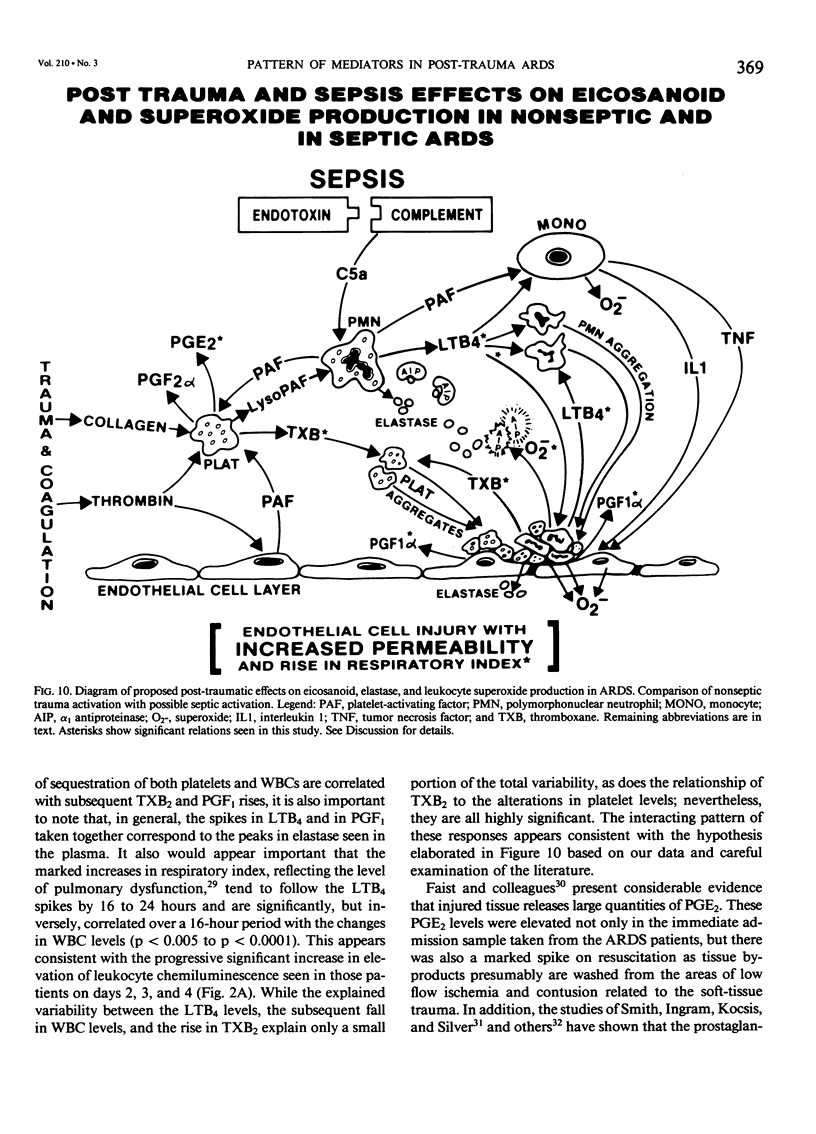



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. C., Loose L. D. Phagocytic activation of a luminol-dependent chemiluminescence in rabbit alveolar and peritoneal macrophages. Biochem Biophys Res Commun. 1976 Mar 8;69(1):245–252. doi: 10.1016/s0006-291x(76)80299-9. [DOI] [PubMed] [Google Scholar]
- Anner H., Kaufman R. P., Jr, Kobzik L., Valeri C. R., Shepro D., Hechtman H. B. Pulmonary leukosequestration induced by hind limb ischemia. Ann Surg. 1987 Aug;206(2):162–167. doi: 10.1097/00000658-198708000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior B. M. Oxidants from phagocytes: agents of defense and destruction. Blood. 1984 Nov;64(5):959–966. [PubMed] [Google Scholar]
- Braquet P., Touqui L., Shen T. Y., Vargaftig B. B. Perspectives in platelet-activating factor research. Pharmacol Rev. 1987 Jun;39(2):97–145. [PubMed] [Google Scholar]
- Brewer L. A., Burbank B., Samson P. C., Schiff C. A. The "Wet Lung" in War Casualties. Ann Surg. 1946 Mar;123(3):343–362. [PMC free article] [PubMed] [Google Scholar]
- Bruch M., Bieth J. G. Influence of elastin on the inhibition of leucocyte elastase by alpha 1-proteinase inhibitor and bronchial inhibitor. Potent inhibition of elastin-bound elastase by bronchial inhibitor. Biochem J. 1986 Aug 15;238(1):269–273. doi: 10.1042/bj2380269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bult H., Beetens J., Herman A. G. Blood levels of 6-oxo-prostaglandin F 1 alpha during endotoxin-induced hypotension in rabbits. Eur J Pharmacol. 1980 Apr 11;63(1):47–56. doi: 10.1016/0014-2999(80)90115-6. [DOI] [PubMed] [Google Scholar]
- Butler R. R., Jr, Wise W. C., Halushka P. V., Cook J. A. Thromboxane and prostacyclin production during septic shock. Adv Shock Res. 1982;7:133–145. [PubMed] [Google Scholar]
- Campbell E. J., Campbell M. A. Pericellular proteolysis by neutrophils in the presence of proteinase inhibitors: effects of substrate opsonization. J Cell Biol. 1988 Mar;106(3):667–676. doi: 10.1083/jcb.106.3.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochrane C. G., Spragg R., Revak S. D. Pathogenesis of the adult respiratory distress syndrome. Evidence of oxidant activity in bronchoalveolar lavage fluid. J Clin Invest. 1983 Mar;71(3):754–761. doi: 10.1172/JCI110823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craddock P. R., Fehr J., Brigham K. L., Kronenberg R. S., Jacob H. S. Complement and leukocyte-mediated pulmonary dysfunction in hemodialysis. N Engl J Med. 1977 Apr 7;296(14):769–774. doi: 10.1056/NEJM197704072961401. [DOI] [PubMed] [Google Scholar]
- Cybulsky M. I., Chan M. K., Movat H. Z. Acute inflammation and microthrombosis induced by endotoxin, interleukin-1, and tumor necrosis factor and their implication in gram-negative infection. Lab Invest. 1988 Apr;58(4):365–378. [PubMed] [Google Scholar]
- Dahlén S. E., Björk J., Hedqvist P., Arfors K. E., Hammarström S., Lindgren J. A., Samuelsson B. Leukotrienes promote plasma leakage and leukocyte adhesion in postcapillary venules: in vivo effects with relevance to the acute inflammatory response. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3887–3891. doi: 10.1073/pnas.78.6.3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deby-Dupont G., Braun M., Lamy M., Deby C., Pincemail J., Faymonville M. E., Damas P., Bodson L., Lecart M. P., Goutier R. Thromboxane and prostacyclin release in adult respiratory distress syndrome. Intensive Care Med. 1987;13(3):167–174. doi: 10.1007/BF00254700. [DOI] [PubMed] [Google Scholar]
- Del Maschio A., Maclouf J., Corvazier E., Grange M. J., Borgeat P. Activated platelets stimulate human neutrophils functions. Nouv Rev Fr Hematol. 1985;27(4):275–278. [PubMed] [Google Scholar]
- Dunham B., Shepro D., Hechtman H. B. Leukotriene induction of TxB2 in cultured bovine aortic endothelial cells. Inflammation. 1984 Sep;8(3):313–321. doi: 10.1007/BF00916419. [DOI] [PubMed] [Google Scholar]
- Faist E., Mewes A., Baker C. C., Strasser T., Alkan S. S., Rieber P., Heberer G. Prostaglandin E2 (PGE2)-dependent suppression of interleukin alpha (IL-2) production in patients with major trauma. J Trauma. 1987 Aug;27(8):837–848. doi: 10.1097/00005373-198708000-00001. [DOI] [PubMed] [Google Scholar]
- Garcia J. G., Noonan T. C., Jubiz W., Malik A. B. Leukotrienes and the pulmonary microcirculation. Am Rev Respir Dis. 1987 Jul;136(1):161–169. doi: 10.1164/ajrccm/136.1.161. [DOI] [PubMed] [Google Scholar]
- Halushka P. V., Reines H. D., Barrow S. E., Blair I. A., Dollery C. T., Rambo W., Cook J. A., Wise W. C. Elevated plasma 6-keto-prostaglandin F1 alpha in patients in septic shock. Crit Care Med. 1985 Jun;13(6):451–453. doi: 10.1097/00003246-198506000-00001. [DOI] [PubMed] [Google Scholar]
- Hamberg M., Samuelsson B. Prostaglandin endoperoxides. Novel transformations of arachidonic acid in human platelets. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3400–3404. doi: 10.1073/pnas.71.9.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerschmidt D. E., Harris P. D., Wayland J. H., Craddock P. R., Jacob H. S. Complement-induced granulocyte aggregation in vivo. Am J Pathol. 1981 Feb;102(2):146–150. [PMC free article] [PubMed] [Google Scholar]
- Harlan J. M. Leukocyte-endothelial interactions. Blood. 1985 Mar;65(3):513–525. [PubMed] [Google Scholar]
- Hoover R. L., Karnovsky M. J., Austen K. F., Corey E. J., Lewis R. A. Leukotriene B4 action on endothelium mediates augmented neutrophil/endothelial adhesion. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2191–2193. doi: 10.1073/pnas.81.7.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hällgren R., Borg T., Venge P., Modig J. Signs of neutrophil and eosinophil activation in adult respiratory distress syndrome. Crit Care Med. 1984 Jan;12(1):14–18. doi: 10.1097/00003246-198401000-00004. [DOI] [PubMed] [Google Scholar]
- Jose P., Niederhauser U., Piper P. J., Robinson C., Smith A. P. Degradation of porstaglandin F2alpha in the human pulmonary circulation. Thorax. 1976 Dec;31(6):713–719. doi: 10.1136/thx.31.6.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. T., Fein A. M., Lippmann M., Holtzman H., Kimbel P., Weinbaum G. Elastolytic activity in pulmonary lavage fluid from patients with adult respiratory-distress syndrome. N Engl J Med. 1981 Jan 22;304(4):192–196. doi: 10.1056/NEJM198101223040402. [DOI] [PubMed] [Google Scholar]
- Maclouf J., de Laclos B. F., Borgeat P. Stimulation of leukotriene biosynthesis in human blood leukocytes by platelet-derived 12-hydroperoxy-icosatetraenoic acid. Proc Natl Acad Sci U S A. 1982 Oct;79(19):6042–6046. doi: 10.1073/pnas.79.19.6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire W. W., Spragg R. G., Cohen A. B., Cochrane C. G. Studies on the pathogenesis of the adult respiratory distress syndrome. J Clin Invest. 1982 Mar;69(3):543–553. doi: 10.1172/JCI110480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann S., Hennrich N., Gunzer G., Lang H. Enzyme-linked immunoassay for human granulocyte elastase in complex with alpha 1-proteinase inhibitor. Adv Exp Med Biol. 1984;167:379–390. doi: 10.1007/978-1-4615-9355-3_33. [DOI] [PubMed] [Google Scholar]
- Piper P. J., Vane J. R., Wyllie J. H. Inactivation of prostaglandins by the lungs. Nature. 1970 Feb 14;225(5233):600–604. doi: 10.1038/225600a0. [DOI] [PubMed] [Google Scholar]
- Powe J. E., Short A., Sibbald W. J., Driedger A. A. Pulmonary accumulation of polymorphonuclear leukocytes in the adult respiratory distress syndrome. Crit Care Med. 1982 Nov;10(11):712–718. doi: 10.1097/00003246-198211000-00003. [DOI] [PubMed] [Google Scholar]
- Rampart M., Bult H., Herman A. G. Effect of complement activation on the biosynthesis of prostacyclin (PGI2) by rabbit peritoneum in vitro. Arch Int Pharmacodyn Ther. 1981 Oct;253(2):327–329. [PubMed] [Google Scholar]
- Reines H. D., Halushka P. V., Cook J. A., Wise W. C., Rambo W. Plasma thromboxane concentrations are raised in patients dying with septic shock. Lancet. 1982 Jul 24;2(8291):174–175. doi: 10.1016/s0140-6736(82)91027-3. [DOI] [PubMed] [Google Scholar]
- Sacks T., Moldow C. F., Craddock P. R., Bowers T. K., Jacob H. S. Oxygen radicals mediate endothelial cell damage by complement-stimulated granulocytes. An in vitro model of immune vascular damage. J Clin Invest. 1978 May;61(5):1161–1167. doi: 10.1172/JCI109031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotman G. J., Burchard K. W., Gann D. S. Thromboxane and prostacyclin in clinical acute respiratory failure. J Surg Res. 1985 Jul;39(1):1–7. doi: 10.1016/0022-4804(85)90154-4. [DOI] [PubMed] [Google Scholar]
- Smith J. B., Ingerman C., Kocsis J. J., Silver M. J. Formation of prostaglandins during the aggregation of human blood platelets. J Clin Invest. 1973 Apr;52(4):965–969. doi: 10.1172/JCI107262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens K. E., Ishizaka A., Larrick J. W., Raffin T. A. Tumor necrosis factor causes increased pulmonary permeability and edema. Comparison to septic acute lung injury. Am Rev Respir Dis. 1988 Jun;137(6):1364–1370. doi: 10.1164/ajrccm/137.6.1364. [DOI] [PubMed] [Google Scholar]
- Tate R. M., Repine J. E. Neutrophils and the adult respiratory distress syndrome. Am Rev Respir Dis. 1983 Sep;128(3):552–559. doi: 10.1164/arrd.1983.128.3.552. [DOI] [PubMed] [Google Scholar]
- Weiss S. J. Tissue destruction by neutrophils. N Engl J Med. 1989 Feb 9;320(6):365–376. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- Wise W. C., Cook J. A., Eller T., Halushka P. V. Ibuprofen improves survival from endotoxic shock in the rat. J Pharmacol Exp Ther. 1980 Oct;215(1):160–164. [PubMed] [Google Scholar]
- Zimmerman G. A., Renzetti A. D., Hill H. R. Functional and metabolic activity of granulocytes from patients with adult respiratory distress syndrome. Evidence for activated neutrophils in the pulmonary circulation. Am Rev Respir Dis. 1983 Mar;127(3):290–300. doi: 10.1164/arrd.1983.127.3.290. [DOI] [PubMed] [Google Scholar]


