Abstract
To achieve stable gene transfer into human hematopoietic cells, we constructed a new vector, ΔAd5/35.AAV. This vector has a chimeric capsid containing adenovirus type 35 fibers, which conferred efficient infection of human hematopoietic cells. The ΔAd5/35.AAV vector genome is deleted for all viral genes, allowing for infection without virus-associated toxicity. To generate high-capacity ΔAd5/35.AAV vectors, we employed a new technique based on recombination between two first-generation adenovirus vectors. The resultant vector genome contained an 11.6-kb expression cassette including the human γ-globin gene and the HS2 and HS3 elements of the β-globin locus control region. The expression cassette was flanked by adeno-associated virus (AAV) inverted terminal repeats (ITRs). Infection with ΔAd5/35.AAV allowed for stable transgene expression in a hematopoietic cell line after integration into the host genome through the AAV ITR(s). This new vector exhibits advantages over existing integrating vectors, including an increased insert capacity and tropism for hematopoietic cells. It has the potential for stable ex vivo transduction of hematopoietic stem cells in order to treat sickle cell disease.
Gene therapy of inherited blood disorders requires efficient gene transfer into human hematopoietic stem cells (HSC) with stable integration of the therapeutic gene into the host genome. To achieve stable gene expression at therapeutic levels, large endogenous regulatory sequences or locus control regions are thought to be needed to control transgene expression (7). Current gene transfer vectors do not adequately fulfill these requirements. Integrating viral vectors based on recombinant adeno-associated virus (AAV) or oncoretroviruses are inefficient at transducing human HSC (17). Lentivirus vectors show promise in transducing human hematopoietic progenitor cells (18). However, the use of vectors based on human immunodeficiency virus raises a number of issues, including the complexity of production, safety, and transgene expression level.
Adenovirus (Ad) vectors can be prepared at high titer and purity and are remarkably efficient at the cell and nucleus entry process leading to high-level gene expression. However, existing Ad vectors have a number of limitations. (i) Infection with Ad vectors based on serotype 5 requires the presence of coxsackie-adenovirus receptors (CAR) and αv integrins on cells. Both receptors are expressed only at low levels on human bone marrow cells (19). We have generated capsid-modified Ad vectors which contain the short-shafted Ad35 fiber incorporated into an Ad5 capsid (Ad5/35) (26). This chimeric vector attaches to a receptor different from CAR and enters cells by an αv integrin-independent pathway. This feature allows for efficient transduction of human CD34+ cells, particularly subsets with potential stem cell activity (17, 26, 33). (ii) Infection with Ad vectors from which E1 and E3 have been deleted is associated with cytotoxicity caused by the expression of viral proteins in transduced cells (12, 27). To produce Ad vectors devoid of all viral genes (ΔAd.IR), we have recently employed a new technique based on recombination between inverted homology regions (30). ΔAd.IR vectors allow for transduction of human hematopoietic cells without toxic side effects (29). (iii) Ad vectors do not efficiently integrate into the host genome, which limits their persistence in proliferating cells. To address this problem, we have tested a specific modification of ΔAd.IR vectors containing AAV inverted terminal repeats (ITRs) as inverted repeats and demonstrated that these ΔAd.AAV hybrid vectors stably transduce cultured cells with efficiencies comparable to that of rAAV (14).
Here, we have combined these improvements to create a capsid-modified, high-capacity Ad.AAV vector devoid of all viral genes. We analyzed the transduction properties of this vector in human hematopoietic cell lines.
MATERIALS AND METHODS
Cell lines.
MO7e (3), HEL (16), and K562 (ATCC 45506) cells were maintained in RPMI 1640 medium containing 10% fetal calf serum, 2 mM l-glutamine, 100 U of penicillin per ml, and 100 μg of streptomycin per ml. For culturing of MO7e cells, 500 U of granulocyte-macrophage colony-stimulating factor (Immunex, Seattle, Wash.) per ml was added to the medium.
Analysis of GFP expression and FACS.
The percentage of green fluorescent protein (GFP)-expressing cells was analyzed by flow cytometry as described earlier (26). For long-term transduction studies, 2 × 105 MO7e cells were infected with viral vectors at indicated multiplicities of infection (MOIs) for 6 h. Twenty-four hours postinfection, single GFP-positive cells were sorted and seeded onto 96-well plates. Two to 3 weeks later, when the number of cells in the colonies reached 2 × 103 to 3 × 103 cells, the wells containing GFP-positive cells were counted and GFP-positive cells were sorted from GFP-negative cells by fluorescence-activated cell sorter (FACS). To obtain enough cells for subsequent analyses, both sorted fractions were allowed to grow for 2 to 3 weeks. At this time, we noticed that GFP-positive fractions of selected clones contained different amounts of GFP-negative cells, probably due to technical limitations of FACS. However, this did not affect the outcome of our integration and long-term expression studies.
Adenovirus vectors.
To generate pAdAAV-a, the SpeI-BglII (GenBank coordinates 3837 to 9218) fragment containing the core elements HS3 and HS2 of the human globin locus control region (LCR) (11) and the DraI-EcoRV fragment containing AAV ITR (derived from pALAPSN [1]) were cloned into pΔE1sp1A shuttle plasmid (31). The resultant plasmid pAdAAV-a contained AAV ITR and a 5.3-kb fragment of human globin LCR within the Ad E1 region deletion (positions 345 to 3523). To generate pAdAAV-b, fragments containing AAV ITR, the GFP expression cassette (pEGFP-1; Clontech, Palo Alto, Calif.) under the control of the murine stem cell virus (MSCV) promoter (pMSCVneoEB), the 1.4-kb modified human γ-globin gene deleted for the second intron under the control of the β-globin promoter (11), and the 3-kb HindIII-BglII fragment containing core element HS2 (as a homology element for pAdAAV-a) were cloned into pΔE1sp1A. The Ad5/35.AAV-a and -b viruses (Fig. 1A) were rescued in 293 cells after cotransfection of pAdAAV-a and pAdAAV-b with pAdΔΨF35 as described earlier (13). pAdΔΨF35 is based on pBHG-10 (Microbix, Toronto, Canada), in which the Ad5 fiber gene was replaced with the Ad35 fiber gene, as described in reference 26. To produce ΔAd5/35.AAV, 2 × 108 293 cells were infected with a 1:1 mixture of Ad5/35.AAV-a and Ad5/35.AAV-b at an MOI of 25 PFU per cell of each virus. Forty-eight hours later, cells were harvested and 5 ml of the cleared cell lysates was layered on a step gradient consisting of 3.5 ml of 1.32-g/ml CsCl and 3.5 ml of 1.25-g/ml CsCl and spun at 35,000 rpm (Beckman SW41 rotor) for 4 h. Then, the ΔAd5/35.AAV band was collected, mixed with the 1.32-g/ml CsCl solution, and subjected to ultracentrifugation for 24 h at 35,000 rpm. Virus was dialyzed against 10 mM Tris-HCl (pH 7.5)−10 mM MgCl2−10% glycerol (virus dilution buffer) overnight and stored at −80°C. Contamination of the ΔAd5/35.AAV preparation with Ad5/35.AAV-a or -b was measured by quantitative Southern blotting and plaque titering on 293 cells with serial dilutions of ΔAd5/35.AAV preparations, whereby the Ad5/35.AAV-a or -b derived plaques were counted 14 days after infection.
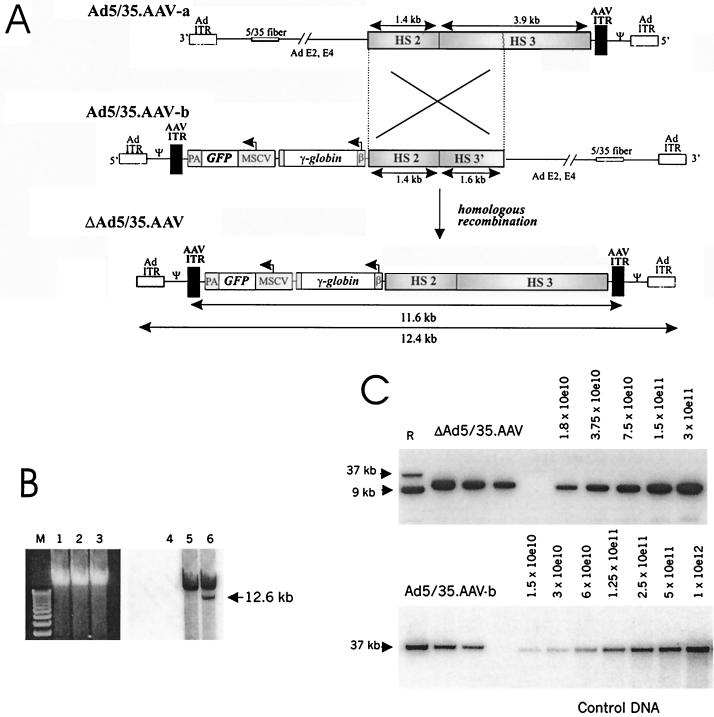
FIG. 1.
Generation of capsid-modified ΔAd.AAV vectors containing a 11.6-kb transgene γ-globin cassette. (A) Scheme for formation of ΔAd5/35.AAV genomes. The expression cassette for human γ-globin included the β promoter from −127 to the β initiation codon, which was connected in frame with the γ coding region partially deleted for intron 2 (11). Transcription of the γ-globin gene is terminated by the endogenous globin polyadenylation signal. The β promoter is linked to the 5.3-kb LCR fragment composed of HS2 and HS3 regions (6). The vector also contained the eGFP gene under the control of the MSCV promoter, which has been shown to be active in human HSC after retroviral gene transfer (21). To generate ΔAd5/35.AAV, two first-generation vectors (Ad5/35.AAV-a and Ad5/35.AAV-b) were constructed. Each of the vectors contained a common region of 3 kb encompassing the HS2 core element and part of the HS3 element and only one AAV ITR. Both Ad5/35.AAV-a and -b vectors contained a chimeric fiber gene composed of the coding sequences of the Ad5 fiber tail, the short Ad35 shaft, and the Ad35 knob (26). ΔAd.AAV is generated by homologous recombination in 293 cells upon coinfection of Ad5/35.AAV-a and -b. The structure of ΔAd5/35.AAV genomes was confirmed by restriction analysis and partial sequencing of viral DNA isolated from purified particles. Ad ITR, adenoviral inverted terminal repeats; Ψ, Ad packaging signal; β, β-globin promoter; PA, bovine growth hormone gene polyadenylation signal. (B) Formation of ΔAd5/35.AAV genomes after coinfection of two first-generation vectors. 293 cells were infected with Ad5/35.AAV-a (lanes 1 and 4), Ad5/35.AAV-b (lanes 2 and 5), or a combination of both (lanes 3 and 6) (at an MOI of 25 each). Forty-eight hours after infection, total DNA was extracted and analyzed by electrophoresis in a 0.8% agarose gel (lanes 1 to 3) followed by Southern blotting with a GFP gene-specific probe (lanes 4 to 6) The ΔAd5/35.AAV genome that forms after coinfection of both parental vectors has the expected size of 12.6 kb. M, 1-kb ladder. (C) Titering of ΔAd5/35.AAV genomes (upper panel) and Ad5/35.AAV-b genomes (lower panel) by quantitative Southern blot analysis. Twenty-five microliters of purified ΔAd5/35.AAV or Ad5/35.AAV-b particles was mixed with 2 × 105 MO7e cells as a carrier. Total DNA was then extracted, and twofold serial dilutions were run on an agarose gel to be analyzed by Southern blotting with a GFP-specific probe. The ΔAd5/35.AAV genomes and Ad5/35.AAV-b genomes are 12.4 and 35 kb, respectively. To calculate the genome titer, the signal intensity of viral genomes was quantitated by a phosphorimager and compared to serial dilutions of control DNA. The concentrations of the standard are shown as numbers of DNA molecules loaded per lane. The genome titer of the Ad5/35.AAV-b preparation shown was 3 × 1012 genomes/ml. R, reference DNA (9- and 37-kb plasmid DNA containing the GFP gene).
Southern blot and PCR analyses.
Isolation of cellular DNA and Southern analysis were performed as described elsewhere (13). The 32P-labeled DNA probes used for hybridization are described in the figure legends. For PCR amplification, the following pairs of primers were used: left, 5′-CGATTTAGAGCTTGACGGGGAAAGCCGGCGAAC and 3′-AACGAGAAGCGCGATCACATGGTCCTGCTG, allowing to amplify the sequence containing bovine growth hormone poly(A) and part of the GFP gene, located directly downstream of the left AAV ITR in the vector DNA (Fig. 1A); and right, 5′-CCGTGAGGGTCTTGTTGTTTGCTGAGTCAAAATTC and3′-GATCAAGGTCAGGAACAGATGGAACAGCTGAATATGGGC, allowing to amplify the sequence containing parts of the HS3 element and AAV located directly upstream of the right AAV ITR in the vector DNA. For amplification the following conditions were used: 94°C for 1 min, 62°C for 1 min, 72°C for 1 min. DNA was amplified for 30 cycles using Taq DNA polymerase (GIBCO) in 50 μl. Ten microliters of reaction mixture was run into a 0.8% agarose gel containing ethidium bromide.
Isolation of vector-cellular DNA junctions.
Five micrograms of total cellular DNA isolated from GFP-expressing clones was digested with HindIII and religated under conditions that promote intramolecular reaction. The ligation mixture was subjected to nested PCR (30 cycles each) using the Expand Long Template PCR system (Roche, Mannheim, Germany) based on the manufacturer’s protocol. The sequences of the primers were as follows: for the first pair, P1, 5′-GGCCCATATTCAGCTGTTCCATCTGTTCCTG-3′, and P2, 5′-CCAGCTAGGGGCAAGTGCCTTGACTCCTATGT-3′; and for the second pair, P3, 5′-CTGTTCCTGACCTTGATCTGAACTTCTCTATTCTC-3′, and P4, 5′-CACACTCTCTGGACCAGTGGCCTAACAGTT-3′. The PCR fragments obtained from different MO7e clones were cloned into a plasmid vector, pCR2.1-TA (Invitrogen, Carlsbad, Calif.), and sequenced using optimized Big Dye Terminators Ready Reaction Kit (Applied Biosystems, Foster City, Calif.).
RNase protection assay.
Total cellular RNA was purified from GFP-positive fractions of individual MO7e clones on weeks 5 and 12 postinfection. Five micrograms of RNA from each clone was used to analyze the level of the human γ-globin gene by the RNase protection assay as described earlier (11). The 5′-untranslated regions of the recombinant and the wild-type γ-globin genes differ in length, and corresponding transcripts produced fragments of distinctive sizes.
RESULTS
Production of ΔAd5/35.AAV.
To achieve high-level stable γ-globin expression, we constructed a capsid-modified ΔAd.AAV vector containing the human γ-globin gene under the control of the β-globin promoter and the HS2 and HS3 elements of the β-globin LCR (6) flanked by two AAV ITRs (Fig. 1A). To more easily assess the transduction properties of these vectors, a GFP gene expression cassette was also incorporated into the viral genome. To produce ΔAd.AAV vectors, we employed homologous recombination between two first-generation, parental vectors. Using two parental vectors instead of one (as previously published [14]) allowed us to avoid having in the same vector backbone two AAV ITRs, which are prone to undesired rearrangements during multiple vector passages (5, 14). Also, our designed expression cassette would not fit in one first-generation Ad vector (transgene capacity limited to 8 kb [4]). Therefore, we generated two first-generation vectors (Ad5/35.AAV-a and Ad5/35.AAV-b) containing chimeric Ad5/35 fiber genes (Fig. 1A). Both parental vectors were produced separately at high yields (in average, 3 × 1012 genomes/ml, with a ratio of genome to PFU titer of ≈20). Viral DNA analysis from purified Ad5/35.AAV-a/-b particles demonstrated intact genomes, indicating that the presence of only one AAV ITR did not affect vector stability (data not shown). To produce ΔAd5/35.AAV, both parental vectors were coinfected onto 293 cells. ΔAd5/35.AAV genomes were formed during replication of the parental vectors through homologous recombination (Fig. 1B). ΔAd5/35.AAV genomes consisted of the 11.6-kb γ-globin/GFP cassette flanked on both sides by AAV ITRs and are devoid of all viral genes. These genomes are packaged, and corresponding ΔAd5/35.AAV particles can be purified from full-length genome-containing particles (Ad5/35.AAV-a/-b) based on their lighter buoyant density in CsCl gradients. The average yield of ΔAd5/35.AAV preparations was >103 packaged genomes per cell, and the average final concentration was 2 × 1011 genomes per ml (Fig. 1C). The contamination with parental first-generation vectors in ΔAd5/35.AAV preparations was less than 0.01% or equivalent to less than 1 PFU per 105 genomes.
Analysis of ΔAd5/35.AAV transduction properties in human hematopoietic cell lines.
Preliminary transduction studies were performed with the human hematopoietic cell lines K562, HEL, and MO7e (9). Similarly to primary human hematopoietic cells, these cell lines are relatively resistant to infection with Ad5-based vectors; however, they can be efficiently infected with Ad vectors containing 5/35 chimeric capsids (Fig. 2A). The level of transduction correlated with that of virus attachment and internalization (data not shown).
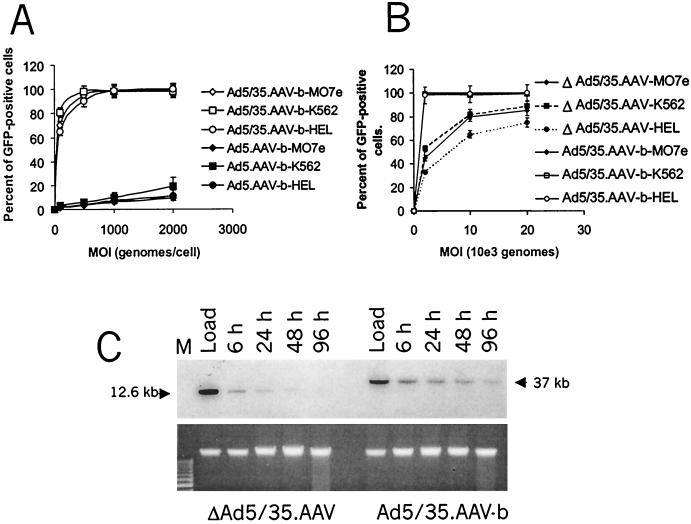
FIG. 2.
Transduction of human hematopoietic cell lines. (A) Transduction of MO7e, K562, and HEL cells with Ad5.AAV-b and chimeric Ad5/35.AAV-b. Ad5.AAV-b and Ad5/35.AAV-b contain the same GFP expression cassette (Fig. 1A) but differ in their fiber molecules (Ad5 versus Ad35). Two hundred thousand cells were infected with different MOIs (genomes per cell) of viruses in 200 μl of media for 6 h at 37°C. Virus-containing media were then removed, and the cells were resuspended in fresh media followed by incubation for 18 h at 37°C. Then, the percentage of GFP-expressing cells was determined by flow cytometry. N ≥ 3 (N, number of experiments). (B) Dose-dependent GFP expression after infection with ΔAd5/35.AAV or Ad5/35.AAV-b. Two hundred thousand MO7e, K562, or HEL cells were infected with ΔAd5/35.AAV or Ad5/35.AAV-b at different MOIs (genomes per cell) in 200 μl of growth medium as described above. The percentage of GFP-positive cells was determined 24 h postinfection by flow cytometry. N = 4. (C) Stability of vector DNA in transduced MO7e cells. Two hundred thousand MO7e cells were infected with an MOI of 2,000 genomes per cell of ΔAd5/35.AAV or Ad5/35.AAV-b for 6 h. One set of cells was harvested immediately after virus addition. This sample contained both the free and the adsorbed viruses (Load). The other sets of infected cells were incubated for 6, 24, 48, or 96 h. One-tenth of the purified total genomic DNA from infected cells was analyzed by Southern blotting (upper panel) using a GFP-specific probe. To ensure equivalent gel loading of cellular DNA, the lower panel demonstrates the ethidium bromide-stained agarose gel. Note that for the later time points, the amount of cellular DNA proportionally increases due to cell divisions, which might account for the slight decrease in the amount of Ad5/35.AAV-b vector DNA at 96 h. The sizes for ΔAd5/35.AAV and Ad5/35.AAV-b genomes are 12.4 and 37 kb, respectively.
Infection of the cell lines with ΔAd5/35.AAV was less efficient than with the parental first-generation vectors containing the same GFP expression cassette (Ad5/35.AAV-b) (Fig. 2B). At an MOI of 2,000 genomes per cell, 100% of the cells were transduced with the Ad5/35.AAV-b vector, whereas only 30 to 55% of cells were GFP positive 24 h after infection with ΔAd5/35.AAV. While the uptakes of the two vector genomes were similarly efficient (Fig. 2C; compare samples harvested at 6 h postinfection), ΔAd5/35.AAV genomes were almost completely degraded by 96 h after infection as shown for MO7e cells (Fig. 2C). Correspondingly, the number of GFP-expressing cells decreased over time after ΔAd5/35.AAV infection (data not shown).
Stable transduction with ΔAd5/35.AAV was investigated in MO7e cells (9). MO7e cells display early markers of megakaryocytic and erythroid differentiation and are CD34 and c-kit positive. MO7e cells can be maintained in the presence of growth factors, which allows for long-term expression studies, expansion of transduced cells as clones, and detailed integration studies. Previous studies have demonstrated that the presence of two AAV ITRs in ΔAd.AAV vectors can mediate vector integration and stable transgene (neomycin phosphotransferase) expression as shown based on the formation of G418-resistant colonies (14). In this study, stable gene transfer was analyzed without selection pressure after expansion of single transduced cells in 96-well plates (Table 1). Infection with the first-generation Ad5/35.AAV-b vector under similar conditions was associated with strong cytotoxicity, which prevented cell proliferation and colony formation (data not shown). In contrast, the ΔAd5/35.AAV vector could be applied at high MOIs without toxic side effects. At an MOI of 104 ΔAd5/35.AAV genomes per cell, 27.5% of seeded (GFP-positive) cells gave rise to clones that stably expressed GFP.
TABLE 1.
Stable integration of ΔAd5/35.AAV into MO7e cellsa
| Vector | MOI (genomes/cell) | % Transduced cells | % Surviving cells | Total no. of colonies analyzed | Total no. of stable GFP+ clones obtained | % of stable transduction |
|---|---|---|---|---|---|---|
| Mock | 0 | >98 | 300 | 0 | 0 | |
| ΔAd5/35.AAV | 2,000 | 45 | >98 | 298 | 9 | 3.1 |
| ΔAd5/35.AAV | 10,000 | 75 | >98 | 102 | 29 | 27.5 |
MO7e cells were infected with ΔAd5/35.AAV at MOIs of 2,000 and 10,000 genomes per cell or incubated with a corresponding volume of virus dilution buffer only (Mock). Twenty-four hours after infection, GFP-positive cells were sorted and plated onto 96-well plates at a density of one cell per well. Two weeks later, plated cells were analyzed for GFP expression. The total number of colonies and the number of colonies containing GFP expressing cells were determined.
Although single GFP-positive cells were initially seeded, about 85% of the colonies obtained after 2 weeks had less than 25% GFP-positive cells. Interestingly, in 2 of 37 analyzed colonies exactly one-half of the colony expressed GFP (Fig. 3A), indicating that ΔAd5/35.AAV had stably integrated into the host chromosome after the first mitosis.
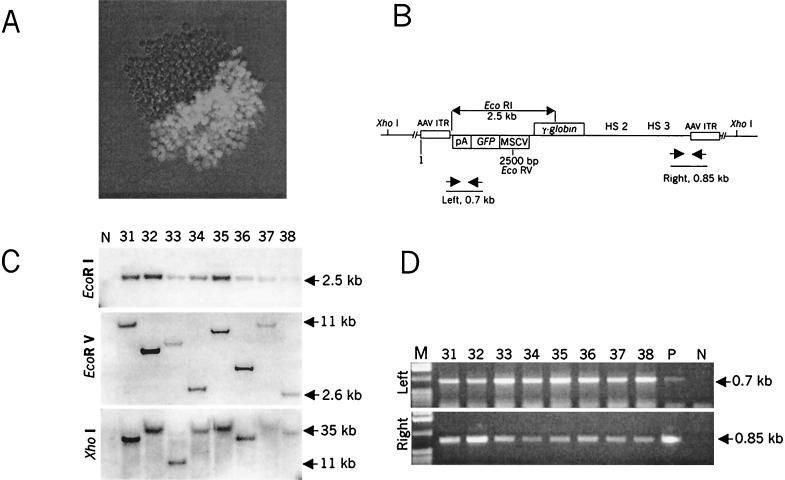
FIG. 3.
Analysis of GFP-positive clones transduced with ΔAd5/35.AAV. (A) GFP expression in a sample MO7e colony expanded for 14 days after infection with ΔAd5/35.AAV as described for Table 1. (B) Schematic representation of the localization of restriction sites and PCR primers in the integrated vector DNA. (C) Southern blot analysis of genomic DNA isolated from representative stable, GFP-positive, MO7e clones. Genomic DNA (10 μg) was digested with EcoRI, EcoRV, and XhoI with 2, 1, or 0 recognition sites, respectively, within the transgene cassette. Blots were hybridized with a GFP-specific probe. Note that at the time of analysis, clones contained different ratios of GFP-positive to GFP-negative cells (see Materials and Methods). N, negative control (DNA isolated from uninfected MO7e cells). (D) PCR analysis of the integrated transgene cassette. Genomic DNAs purified from representative GFP-expressing MO7e clones (1 μg) were subjected to PCR amplification to demonstrate the presence of intact left and right sequences of the vector in the same clone. Amplification was done as described in Materials and Methods.
Analysis of integrated vector DNA.
Genomic DNA obtained 2 weeks postinfection from representative GFP-negative clones as well as from GFP-positive and GFP-negative fractions of clones contained GFP-positive cells was analyzed for the presence of vector DNA by Southern blotting with a GFP-specific probe. Genomic DNA was digested with EcoRI, which cuts twice within the vector, resulting in a transgene fragment of 2.5 kb (Fig. 3B). No vector DNA was found in GFP-negative clones (n = 7) or in GFP-negative fractions of clones that contained GFP expressing cells (n = 16) (data not shown). In contrast, after EcoRI digestion, vector-specific signals were present in genomic DNA from GFP-positive fractions (Fig. 3C, EcoRI). Digestion with EcoRV, which cuts only once within the vector genome, resulted in single bands that varied in size among the clones. This indicated the absence of vector concatemers and suggested random integration. Random vector integration was corroborated by the restriction pattern obtained with XhoI, which has no recognition sites within the ΔAd5/35.AAV genome, demonstrating signals in the range of 11 to 35 kb. Analysis of undigested genomic DNA revealed signals in the range of 40 to 50 kb, which indicated the absence of episomal vector forms (data not shown). The restriction pattern obtained with the single cutter as well as the quantification of vector-specific signals in comparison to signals from an endogenous human gene allowed the conclusion that one vector copy per cell was present in the genomic DNA of analyzed clones. The absence of integrated Ad genes was confirmed by hybridization of the blot with a probe specific to Ad sequences (3523 to 10589), located directly downstream of the GFP/γ-globin cassette (data not shown). The intactness of the integrated transgene cassette was confirmed by PCR, which demonstrated the presence of vector sequences directly adjacent to the left and right AAV ITRs within the genomic DNA of analyzed clones (Fig. 3D). Taken together, Southern and PCR analyses demonstrated random integration of single vector copies with intact transgene cassettes.
To ultimately prove vector integration and to investigate the involvement of AAV ITRs in the integration process, the junctions between vector and chromosomal DNAs were isolated by nested inverted PCR (Fig. 4A). Detailed restriction analysis and sequencing of cloned PCR fragments revealed that chromosomal-vector DNA junctions are localized within A and B-B′ regions of AAV ITRs (Fig. 4B). The presumptive human genomic DNA sequences were screened for homology with an advanced BLAST search, and several different loci in the human genome were identified as integration sites with an overall homology of more than 99% for the analyzed fragments of 500 bp or longer.
FIG. 4.
Analysis of ΔAd5/35.AAV vector-cellular DNA junctions. (A) Schematic representation of junction between chromosomal DNA and the 3′ end of the vector. P1 to P4, primers used for nested inverted PCR amplifications. Dots below the AAV ITR indicate the actual locations of DNA breaks and junctions between vector and cellular DNA. (B) Sequence of junctions. AAV ITR sequences are in bold. The actual locations of junctions between vector and chromosomal DNAs are indicated by arrows. Sites of integration and National Center for Biotechnology Information accession numbers for identified loci are indicated on the right of the corresponding sequence. The URL for the BLAST search is http://www.ncbi.nlm.nih.gov/BLAST/. WT, partial sequence of the parental (wild-type) AAV ITR; NI, locus is not identified
Persistence of transgene expression over time in clones.
In hematopoietic cells, transgenes transferred with viral vectors are frequently exposed to silencing (20). Therefore, we monitored the level of transgene expression in clones transduced with ΔAd5/35.AAV over time (Fig. 5). The percentage of GFP-expressing cells was stable between weeks 5 and 12 after infection in all clones but one, clone 35 (Fig. 5A). The intensity of GFP fluorescence in GFP-positive cells differed among the clones (Fig. 5, compare localizations of peaks along the abscissas), indicating different levels of transgene expression. A detailed analysis of GFP expression in clone 35 demonstrated that the declining percentage of GFP-positive cells was caused by the outgrowth of GFP-negative cells compared to GFP-positive cells during multiple passages and was not due to the gene silencing (data not shown).
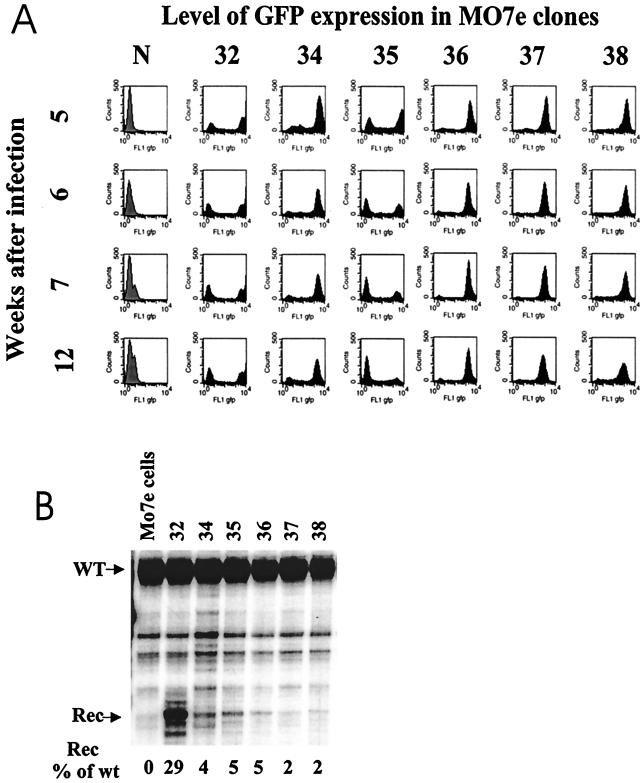
FIG. 5.
Stability of GFP expression (A) and γ-globin gene expression (B) over time. (A) GFP-positive fractions of individual MO7e clones were sorted 4 weeks postinfection. GFP-positive cells were plated, and the GFP expression profile was monitored by flow cytometry once a week for 12 weeks postinfection. The histograms show GFP expression in stable MO7e clones from week 5 to week 12. (B) RNase protection analysis of recombinant human γ-globin gene expression in GFP-positive MO7e clones 12 weeks postinfection. This assay allowed a direct comparison between the levels of γ-globin mRNA transcribed from ΔAd5/35.AAV (Rec) and wild-type human γ-globin mRNA (WT). Quantitation of globin gene expression levels was done by PhosphorImager analysis. The percentages of recombinant γ-globin compared to endogenous γ-globin expression are indicated below the corresponding lanes.
The analysis of γ-globin expression in GFP-positive clones by FACS using fluorescent anti-γ-globin antibodies was complicated by the fact that MO7e cells displayed a high level of endogenous γ-globin expression (data not shown). Therefore, the expression of γ-globin was assessed on the RNA level by RNase protection assay 5 and 12 weeks after infection (Fig. 5B). Recombinant γ-globin mRNA was detected in all clones in the range of 2 to 29% of endogenous human γ-globin mRNA. The level of globin mRNA expression in individual clones did not significantly change from week 5 to week 12, except for clone 35 (data not shown). Moreover, the level of γ-globin mRNA correlated with the intensity of GFP expression in individual clones. The concurrent expression of γ-globin-mRNA and GFP from two different promoters suggests that the two expression cassettes were subjected to the same epigenetical regulatory mechanism.
In conclusion, transduction of MO7e cells with ΔAd5/35.AAV resulted in stable gene expression over the period of analysis. However, the level of GFP as well as γ-globin expression levels varied among the analyzed clones.
DISCUSSION
Although some success has been achieved in transducing mouse HSCs, efficient gene transfer into human HSCs is still problematic. We have developed a hybrid Ad-AAV vector with a chimeric adenoviral capsid for efficient gene transfer into human hematopoietic cells. This vector is devoid of all viral genes, is nontoxic for cells at high MOIs, and provides stable integration and stable transgene expression. It can accommodate larger transgene cassettes than the other existing integrating vectors, whose insert size is limited to 5 kb (murine leukemia virus and rAAV vectors) or 8 kb (lentiviral vectors). This allowed us to utilize large endogenous fragments of the human β-globin LCR to control γ-globin expression. So far, most of the γ-globin expression cassettes for sickle cell gene therapy have been designed for retroviral vectors. These cassettes contained only “micro” or “nano” LCRs, were devoid of internal splicing sites (28), and were sensitive to silencing (22). We hypothesized that the larger portion of the β-globin LCR containing the HS2 and HS3 elements used in ΔAd5/35.AAV would confer position-independent and stable expression in HSCs.
ΔAd5/35.AAV was produced in 293 cells, whereby the full-length vector genome coreplicated in the same cell and provided all the necessary viral proteins in trans. Previous studies by members of our group (14, 30), as well as a recent report (24), found that ΔAd.AAV genomes were packaged efficiently into stable virus particles. Using a modified purification protocol, we were able to produce ΔAd.AAV vectors with less than 0.01% of contaminating first-generation vectors. For comparison, optimized techniques for production of gutless, helper-dependent Ad vectors resulted in vector preparations contaminated with more than 0.2% of helper virus (25). The higher purity of our vector preparations may be attributed in part to the lighter buoyant density of ΔAd5/35.AAV particles, which allowed a better separation from full-length genome-containing particles in CsCl gradients.
A notable problem was the low infectivity of the ΔAd5/35.AAV vector in hematopoietic cell lines compared to that of the corresponding first-generation vector. We have excluded that the relatively low infectivity of ΔAd.5/35AAV vectors is due to less-efficient attachment to or internalization into cells (Fig. 2C). Infection of ΔAd5/35.AAV may be affected at other steps, including endosomal release, intracellular transport of viral particles, nuclear import, or stability of viral genomes. We hypothesize that the low infectivity of ΔAd5/35.AAV vectors could be specific to hematopoietic cells or 5/35 capsid-modified ΔAd vectors, because in an earlier study, a serotype 5-based ΔAd vector infected a liver-derived, human endothelial cell line with an efficiency comparable to that of a first-generation vector (30).
Southern and PCR analyses as well as sequencing of vector-cellular DNA junctions demonstrated random integration of single vector copies with intact transgene cassettes. In the absence of AAV Rep proteins, site-specific integration of ΔAd5/35.AAV was not expected (32). Integration involved the AAV ITRs, demonstrating that the AAV ITRs are the active substrates for integration. This suggests that their sequence or structure within the context of double-stranded DNA can be recognized by cellular factors capable of mediating vector integration. In this context, structure-specific cellular recombination enzymes have been described as being associated with recombination processes in prokaryotes and eukaryotes (15). In this study, an MOI of 10,000 ΔAd5/35.AAV genomes per cell allowed for stable transduction of 27.5% of infected cells, which is in the range of the integration frequency seen previously with neomycin phosphotransferase-expressing ΔAd.AAV (14) or rAAV vectors (23). Because infection with ΔAd5/35.AAV is not associated with cytotoxicity, its integration frequency may potentially be scaled up by applying higher MOIs.
ΔAd5/35.AAV genomes were short-lived in transduced cells. This implies that transgene integration occurred shortly after infection. In agreement with this, most of the clones had 10 to 25% GFP-positive cells, suggesting that the transgene integrated after two or three rounds of cell division. It remains to be investigated whether ΔAd5/35.AAV allows for transduction of cell cycle-arrested cells.
Both GFP and γ-globin expression levels varied concurrently in individual clones. Considering that ΔAd5/35.AAV integrated into different chromosomal sites as single copies, we hypothesize that transgene expression was subjected to position effects. This clonal analysis suggests that the HS2/HS3 elements did not function as an insulator as described for the complete (22-kb) β-globin LCR in transgenic mice (8). The level of γ-globin expression in MO7e cells was relatively low in most of the analyzed clones. This could be due to the fact that the γ-globin gene was deleted for the second intron, which is thought to confer globin mRNA stability (2). Clearly, transduction studies in human CD34+ cells will ultimately clarify whether the HS2/HS3-containing fragment of the β-globin LCR can overcome position effects and provide optimal globin gene expression.
The concept of combining elements from different viruses is not new (for a review, see reference 10). Viral vector chimeras were generated, for example, between adenovirus and retrovirus, adenovirus and Epstein-Barr virus, adenovirus and simian virus 40, adenovirus and AAV, and hepatitis B virus and AAV. This study underscores the utility of combining different viral elements into one vector to increase its therapeutic potential. These new ΔAd5/35.AAV vectors have the potential for stable ex vivo transduction of hematopoietic stem cells in order to treat sickle cell disease.
Acknowledgments
We thank Thalia Papayannopoulou, David Russell, and David Emery for helpful discussions.
This work was supported by NIH grants P01 HL53750, R21 DK55590, and P30 DK 47754.
REFERENCES
- 1.Alexander, I. E., D. W. Russell, A. M. Spence, and A. D. Miller. 1996. Effects of gamma irradiation on the transduction of dividing and nondividing cells in brain and muscle of rats by adeno-associated virus vectors. Hum. Gene Ther. 7:841–850. [DOI] [PubMed] [Google Scholar]
- 2.Antoniou, M., F. Geraghty, J. Hurst, and F. Grosveld. 1998. Efficient 3′-end formation of the human beta-globin mRNA in vivo requires sequences within the last intron but occurs independently of the splicing reaction. Nucleic Acids Res. 26:721–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Avanzi, G. C., P. Lista, B. Giovinazzo, R. Miniero, G. Saglio, G. Benetton, R. Coda, G. Cattoretti, and L. Pegoraro. 1988. Selective growth response to IL-3 of a human leukaemic cell line with megakaryoblastic features. Br. J. Haematol. 69:359–366. [DOI] [PubMed] [Google Scholar]
- 4.Bett, A. J., W. Haddara, L. Prevec, and F. L. Graham. 1994. An efficient and flexible system for construction of adenovirus vectors with insertions in early regions 1 and 3. Proc. Natl. Acad. Sci. USA 91:8802–8806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carlson, C. A., D. Steinwaerder, A. Lieber. 2002. Rearrangements in adenoviral genomes mediated by inverted repeats: application for vector production and tumor-specific gene expression. Methods Enzymol. 346:277–292. [DOI] [PubMed] [Google Scholar]
- 6.Ellis, J., K. C. Tan-Un, A. Harper, D. Michalovich, N. Yannoutsos, S. Philipsen, and F. Grosveld. 1996. A dominant-opening activity in 5′ hypersensitive site 3 of the human beta-globin LCR. EMBO J. 15:562–568. [PMC free article] [PubMed] [Google Scholar]
- 7.Emery, D. W., and G. Stamatoyannopoulos. 1999. Stem cell gene therapy for the beta-chain hemoglobinopathies. Problems and progress. Ann. N. Y. Acad. Sci. 872:94–107. [DOI] [PubMed] [Google Scholar]
- 8.Grosveld, F., G. B. van Assendelft, D. R. Greaves, and G. Kollias. 1987. Position-independent, high-level expression of the human beta-globin gene in transgenic mice. Cell 51:975–985. [DOI] [PubMed] [Google Scholar]
- 9.Horie, M., and H. E. Broxmeyer. 1995. The combination of Steel factor and GM-CSF blocks apoptosis induced by retinoic acid and upregulates AP-1 in a human growth factor-dependent cell line. Exp. Hematol. 23:168–173. [PubMed] [Google Scholar]
- 10.Lam, P. Y., and X. O. Breakefield. 2000. Hybrid vector designs to control the delivery, fate and expression of transgenes. J. Gene Med. 2:395–408. [DOI] [PubMed] [Google Scholar]
- 11.Li, Q., and J. A. Stamatoyannopoulos. 1994. Position independence and proper developmental control of gamma-globin gene expression require both a 5′ locus control region and a downstream sequence element. Mol. Cell. Biol. 14:6087–6096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lieber, A., C.-Y. He, L. Meuse, C. Himeda, C. Wilson, M. A. Kay. 1998. Inhibition of NF-κB activation in combination with Bcl-2 expression allows for persistence of first-generation adenovirus vectors in mouse livers. J. Virol. 72:9267–9277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lieber, A., C.-Y. He, I. Kirillova, and M. A. Kay. 1996. Recombinant adenoviruses with large deletions generated by Cre-mediated excision exhibit different biological properties compared with first-generation vectors in vitro and in vivo. J. Virol. 70:8944–8960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lieber, A., D. S. Steinwaerder, C. A. Carlson, and M. A. Kay. 1999. Integrating adenovirus-adeno-associated virus hybrid vectors devoid of all viral genes. J. Virol. 73:9314–9324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lyu, Y. L., C.-T. Lin, and L. F. Liu. 1999. Inversion/dimerization of plasmids mediated by inverted repeats. J. Mol. Biol. 285:1485–1501. [DOI] [PubMed] [Google Scholar]
- 16.Martin, P., and T. Papayannopoulou. 1982. HEL cells: a new human erythroleukemia cell line with spontaneous and induced globin expression. Science 216:1233–1235. [DOI] [PubMed] [Google Scholar]
- 17.Medin, J. A., and S. Karlsson. 1997. Viral vectors for gene therapy of hematopoietic cells. Immunotechnology 3:3–19. [DOI] [PubMed] [Google Scholar]
- 18.Miyoshi, H., K. A. Smith, D. E. Mosier, I. M. Verma, and B. E. Torbett. 1999. Transduction of human CD34+ cells that mediate long-term engraftment of NOD/SCID mice by HIV vectors. Science 283:682–686. [DOI] [PubMed] [Google Scholar]
- 19.Neering, S. J., S. F. Hardy, D. Minamoto, S. K. Spratt, and C. T. Jordan. 1996. Transduction of primitive human hematopoietic cells with recombinant adenovirus vectors. Blood 88:1147–1155. [PubMed] [Google Scholar]
- 20.Osborne, C. S., P. Pasceri, R. Singal, T. Sukonnik, G. D. Ginder, and J. Ellis. 1999. Amelioration of retroviral vector silencing in locus control region beta-globin-transgenic mice and transduced F9 embryonic cells. J. Virol. 73:5490–5496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramezani, A., T. S. Hawley, and R. G. Hawley. 2000. Lentiviral vectors for enhanced gene expression in human hematopoietic cells. Mol. Ther. 2:458–469. [DOI] [PubMed] [Google Scholar]
- 22.Rivella, S., and M. Sadelain. 1998. Genetic treatment of severe hemoglobinopathies: the combat against transgene variegation and transgene silencing. Semin. Hematol. 35:112–125. [PubMed] [Google Scholar]
- 23.Rutledge, E. A., and D. W. Russell. 1997. Adeno-associated virus vector integration junctions. J. Virol. 71:8429–8436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sandalon, Z., D. V. Gnatenko, W. F. Bahou, and P. Hearing. 2000. Adeno-associated virus (AAV) Rep protein enhances the generation of a recombinant mini-adenovirus (Ad) utilizing an Ad/AAV hybrid virus. J. Virol. 74:10381–10389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sandig, V., R. Youil, A. J. Bett, L. L. Franlin, M. Oshima, D. Maione, F. Wang, M. L. Metzker, R. Savino, and C. T. Caskey. 2000. Optimization of the helper-dependent adenovirus system for production and potency in vivo. Proc. Natl. Acad. Sci. USA 97:1002–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shayakhmetov, D. M., T. Papayannopoulou, G. Stamatoyannopoulos, and A. Lieber. 2000. Efficient gene transfer into human CD34(+) cells by a retargeted adenovirus vector. J. Virol. 74:2567–2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shtrichman, R., and T. Kleinberger. 1998. Adenovirus type 5 E4 open reading frame 4 protein induces apoptosis in transformed cells. J. Virol. 72:2975–2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stamatoyannopoulos, J. A., C. H. Clegg, and Q. Li. 1997. Sheltering of gamma-globin expression from position effects requires both an upstream locus control region and a regulatory element 3′ to the A gamma-globin gene. Mol. Cell. Biol. 17:240–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stecher, H., D. M. Shayakhmetov, G. Stamatoyannopoulos, and A. Lieber. 2001. A capsid-modified adenovirus vector devoid of all viral genes: assessment of transduction and toxicity in human hematopoietic cells. Mol. Ther. 4:36–44. [DOI] [PubMed] [Google Scholar]
- 30.Steinwaerder, D. S., C. A. Carlson, and A. Lieber. 1999. Generation of adenovirus vectors devoid of all viral genes by recombination between inverted repeats. J. Virol. 73:9303–9313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steinwaerder, D. S., C. A. Carlson, D. L. Otto, Z. Y. Li, S. Ni, and A. Lieber. 2001. Tumor-specific gene expression in hepatic metastases by a replication-activated adenovirus vector. Nat. Med. 7:240–243. [DOI] [PubMed] [Google Scholar]
- 32.Weitzman, M. D., S. R. Kyostio, R. M. Kotin, and R. A. Owens. 1994. Adeno-associated virus (AAV) Rep proteins mediate complex formation between AAV DNA and its integration site in human DNA. Proc. Natl. Acad. Sci. USA 91:5808–5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yotnda, P., H. Onishi, H. E. Heslop, D. Shayakhmetov, A. Lieber, M. Brenner, and A. Davis. 2001. Efficient infection of primitive hematopoietic stem cells by modified adenovirus. Gene Ther. 8:930–937 [DOI] [PubMed] [Google Scholar]