Abstract
We have observed elevated NF-κB DNA-binding activity in nuclear extracts from human papillomavirus type 6- and 11-infected laryngeal papilloma tissues. The predominant DNA-binding species is the p50/p50 homodimer. The elevated NF-κB activity could be correlated with a reduced level of cytoplasmic IκBβ and could be associated with the overexpression of p21CIP1/WAF1 in papilloma cells. Increased NF-κB activity and cytoplasmic accumulation of p21CIP1/WAF1 might counteract death-promoting effects elicited by overexpressed PTEN and reduced activation of Akt and STAT3 previously noted in these tissues.
Laryngeal papillomas are caused by infection of the laryngeal epithelium with the low-risk human papillomavirus type 6 or 11 (HPV 6/11). The disease is characterized by thickening of the suprabasal layer of the epithelium (1). Epidermal growth factor receptor (EGFR) is overexpressed and becomes constitutively active in papilloma cells (14). As a result, activities of phosphatidylinositol 3-kinase and MAP kinase are high. However, Akt/PKB, a downstream target of phosphatidylinositol 3-kinase, is not activated (33). This is due, at least in part, to overexpression of the tumor suppressor PTEN (7, 16), a phosphatidylinositol 3,4,5-triphosphate (PIP3) phosphatase (18), in papilloma cells (33).
The prosurvival role of Akt is well established in many systems (8). Consequently, inhibition of Akt activation promotes apoptosis, as indicated by PTEN′s ability to promote cell death (17, 31). Activation of STAT3, another well-known prosurvival regulator (11, 12, 27), is also reduced in papilloma cells (Sun and Steinberg, submitted for publication). Reduction in STAT3 activation in papilloma cells involves PTEN′s protein phosphatase activity. It is not clear how papilloma cells manage to survive in the face of a drastically elevated PTEN.
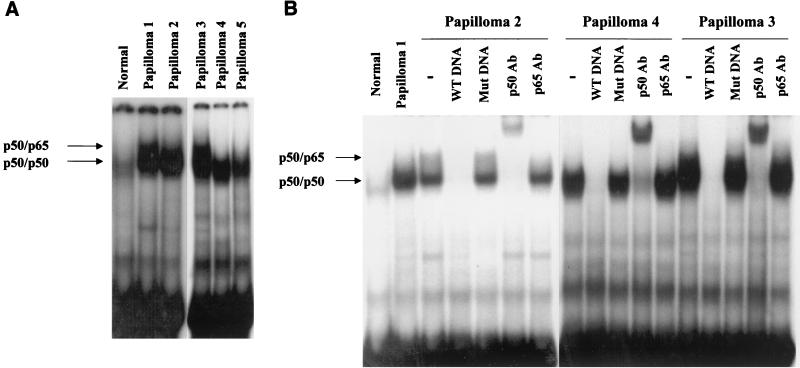
Elevated p50/p50 NF-κB DNA-binding activity in nuclear extracts of laryngeal papilloma cells.
Overexpression of PTEN results in reduction of both activated Akt and STAT3 in papilloma cells. We are puzzled by the fact that papilloma cells remain alive while two of the best characterized prosurvival molecules are downregulated. As a first step to gain insights into apoptosis/survival decision-making in laryngeal papilloma cells, we chose to examine activation of the transcription factor NF-κB.
NF-κB/Rel family transcription factors elicit a wide range of cellular effects, including immune and inflammatory responses, proliferation, and cell survival (19). NF-κB promotes survival by stimulating expression of TRAF2/6, caspase inhibitors IAP1 and IAP2 (30), IEX-1L (32), and X-IAP (25). The fact that NF-κB activates expression of nitrogen oxide synthase 2 (13, 28) and Cox-2 (2, 20) further attests to its potent antiapoptotic activity.
Cytoplasmic and nuclear extracts were prepared from surgical discards of normal and laryngeal papilloma tissues derived from patients undergoing laryngeal surgery. The use of human tissues was approved by the Institutional Review Board at Long Island Jewish Medical Center. Tissues were immediately frozen in liquid nitrogen. Normal laryngeal epithelial tissues from eight individuals were combined to prepare cytoplasmic and nuclear extracts. Frozen tissues were ground and reduced to a powder by using the Mikro-Dismembrator II (B. Braun). Powdered tissue was resuspended in ice-cold hypotonic buffer [100 mM HEPES (pH 7.6), 10 mM KCl, 3 mM NaCl, 3 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 2 mM dithiothreitol (DTT), and 10% (vol/vol) glycerol] in the presence of the protease inhibitor cocktail Complete (Roche Molecular Biochemicals, Indianapolis, Ind.) and phosphatase inhibitors (20 mM β-glycerophosphate, 1 mM sodium orthovanadate, and 30 mM sodium fluoride). After a 15-min incubation on ice, lysates were spun at 3,000 rpm for 10 min at 4°C in a microcentrifuge.
The supernatant was transferred to a new tube and designated the cytoplasmic extract. The pellet was washed once with hypotonic buffer and extracted on ice with four times the pellet size of nuclear extract buffer (20 mM HEPES, pH 7.6, 25% glycerol, 0.5 M NaCl, 1 mM MgCl2, 1% [vol/vol] Nonidet P40, 1 mM EDTA, 2 mM DTT) in the presence of protease and phosphatase inhibitors. After 30 min, the extraction mixture was spun at 12,000 rpm at 4°C in a microcentrifuge for 10 min. The resulting supernatant was designated the nuclear extract. The protein concentration of the extracts was determined by using the Micro BCA reagents (Pierce, Rockford, Ill.).
NF-κB DNA-binding activity in the nuclear extracts was measured by electrophoretic mobility shift assay (EMSA) using 32P-labeled NF-κB oligonucleotide (5′-TTGTTACAAGGGGACTTTCCGCTGGGGACTTTCCAGGGAGGC-3′ [concensus NF-κB binding sites are underlined and italicized]). As shown in Fig. 1A, whereas little activity was detected in an extract from pooled normal tissue, papilloma tissues exhibited significantly elevated NF-κB DNA-binding activity. These protein-DNA complexes were specific for NF-κB, since they could be competed out by a molar excess of the unlabeled NF-κB oligonucleotides (Fig. 1B), whereas a mutant oligonucleo-tide (5′-TTGTTACAATCTCACTTTCCGCTTCTCACTTTCCAGGGAGGC-3′ [mutated NF-κB binding sites are underlined and italicized]) had no effect.
FIG. 1.
Increased p50 NF-κB DNA-binding activity is observed in papilloma nuclear extracts. NF-κB DNA-binding activity was assessed by EMSA. (A) Normal extract was derived from a pool of eight normal tissues. Five papilloma nuclear extracts were analyzed. All papillomas were typed by PCR and by Southern blot analysis. Papilloma 1 is HPV 6 positive. Papillomas 2 to 5 are HPV 11 positive. Papillomas 3 to 5 were analyzed on a different gel. (B) In competition or supershift experiments, papilloma nuclear extract was incubated with a 30-fold molar excess of wild-type (WT) or mutant (Mut) oligonucleotides or 1 μg of anti-p50 or anti-p65 antibodies (Ab) before adding 32P-labeled NF-κB oligonucleotide. Papillomas 3 and 4 were analyzed on a different gel.
Supershift analysis revealed that the predominant species in these complexes was the p50/p50 homodimer. The migration of the p50/p50 homodimer-DNA complex differed slightly from one extract to another, and a slower migration correlated with a higher DNA-binding activity (compare papillomas 1 and 3 with papillomas 2, 4, and 5). The underlying mechanism(s) is currently unclear. The p50/p65 NF-κB was also activated in papillomas, although to a much lesser extent. It is important to note that in control laryngeal epithelial tissue, the p50/p50 homodimer was also the predominant DNA-binding species (Fig. 1A, lane 1).
The identification of the p50/p50 homodimer as the major DNA-binding species of NF-κB, even in normal laryngeal epithelial tissues, implies a tissue-specific function for the p50/p50 homodimer. While p50/p50 homodimer is usually considered an inhibitory NF-κB due to the lack of a transactivation domain in p50 (10, 22), it should be emphasized that transcription activation of an NF-κB reporter construct by overexpressing p50 in keratinocytes has been reported (24).
Increased NF-κB DNA-binding activity in papilloma extracts is associated with decreased IκBβ.
NF-κB can be activated by multiple signaling pathways, depending on the stimulus and the cell type. The point of convergence of these pathways is the recently identified IκB kinase (IKK) complex. Phosphorylation of IκB proteins by IKK leads to ubiquitination and degradation of IκB, thereby releasing and activating NF-κB (15).
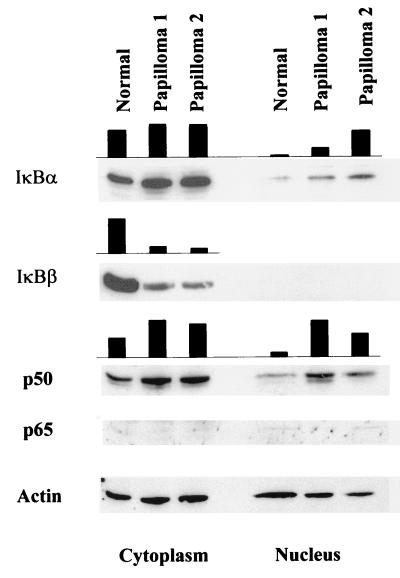
To investigate the mechanism(s) by which papilloma nuclear extracts displayed stronger NF-κB binding activity, Western blot analysis were performed on pooled normal extract and two separate papilloma cytoplasmic and nuclear extracts. Thirty micrograms of protein was electrophoresed, transferred to a nitrocellulose membrane, blocked, and probed with antibodies against the antigens indicated in Fig. 2. The amount of actin in extracts was determined and used as a loading control, as described (5).
FIG. 2.
Elevated nuclear p50 in papilloma extracts is associated with reduced cytoplasmic IκBβ. Thirty micrograms of cytoplasmic or nuclear extracts prepared from normal or papilloma tissues was fractionated on a denaturing sodium dodecyl sulfate-polyacrylamide gel. Separated polypeptides were transferred to a nitrocellulose membrane and probed for the indicated antigens. The amount of actin in the extract is indicated as a loading control. The solid bar stands for relative intensity of the indicated protein in the extract after normalization to actin.
Comparable levels of IκBα were detected in the cytoplasmic extracts of normal and papilloma tissues, but levels were increased in the nuclear extracts of papilloma tissues (Fig. 2). Significantly reduced levels of IκBβ were observed in the cytoplasmic extracts of papillomas compared to the normal tissue (Fig. 2). Whereas IκBα appears to be the primary regulator of rapid signal-induced activation of NF-κB in most cell types, IκBβ is associated with persistent activation of NF-κB (4, 6). In accord with the reported observation that IκBβ does not shuttle between the cytoplasm and the nucleus, no nuclear IκBβ was detected. The absence of nuclear IκBβ also suggests a reasonably clean separation of cytoplasmic and nuclear fractions. The reduction of IκBβ could account for the increased DNA-binding activity in the papilloma nuclear extracts if its loss resulted in increased translocation of NF-κB to the nucleus.
We therefore examined the level of p50 and p65 in the nuclear extracts of normal laryngeal epithelium and papillomas (Fig. 2). The level of p65 was at the lower limit of detection in all fractions, consistent with the observation that the p50/p65 heterodimer constituted the minor DNA-binding species of NF-κB in our system. In contrast, the level of p50 was higher in papilloma nuclear extracts than their normal counterpart. The increase in p50 is in agreement with the observed increase in p50/p50 NF-κB DNA-binding activity in papilloma nuclear extracts (see below) and is consistent with the reduction in IκBβ found in papillomas. Our observation thus suggests release of p50 from IκBβ-mediated cytoplasmic retention in papilloma cells.
Although reduction of IκBβ is in agreement with increased nuclear p50 level and elevated p50/p50 DNA-binding activity in papilloma extracts in a mechanistic sense, we did notice that the increase in p50/p50 DNA-binding activity exceeds the increase in p50 level. At present, we do not exclude the possibility that direct modulation of p50/p50 DNA-binding activity might also be involved. Further characterization of p50 in normal and papilloma cells should provide more detailed information.
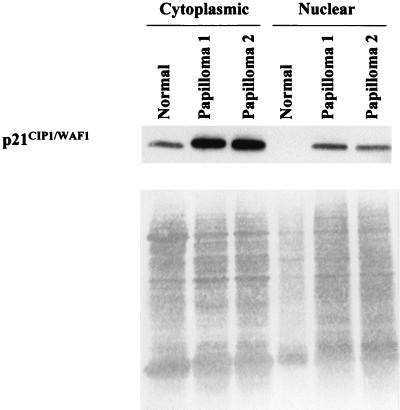
Expression of p21CIP1/WAF1 is enhanced in papilloma cells.
Expression of p50/p50 homodimer as well as p65/p65 homodimer and p50/p65 heterodimer has been shown to activate p21CIP1/WAF1expression, either directly or indirectly, in keratinocytes (23). We therefore asked whether p21CIP1/WAF1 was elevated in our papilloma tissues. As illustrated in Fig. 3, a fourfold increase in p21CIP1/WAF1 was observed in papilloma cytoplasmic extracts by Western blot analysis compared to normal extracts. While diminished nuclear p21CIP1/WAF1 was observed compared to cytoplasmic p21CIP1/WAF1 in papilloma extracts, it was undetectable in pooled normal nuclear extract. (Normal nuclear extract was underloaded in this particular experiment. In other experiments where equivalent normal nuclear extract was loaded, p21CIP1/WAF1 was still undetectable.) The increase in p21CIP1/WAF1 is consistent with the elevated p50/p50 NF-κB binding activity in papilloma nuclear extracts and strongly suggests active transcriptional function of the p50/p50 NF-κB in our system.
FIG. 3.
Augmented expression of p21CIP1/WAF1 in papillomas. Forty micrograms of cytoplasmic or nuclear extract prepared from normal or papilloma tissues was electrophoresed on a denaturing sodium dodecyl sulfate-polyacrylamide gel. Separated polypeptides were transferred to a nitrocellulose membrane and probed with an anti-p21CIP1/WAF1 antibody. Ponceau S staining of the membrane is shown for loading comparison.
We were surprised to learn that approximately 70% of p21CIP1/WAF1 accumulated in the cytoplasm in papilloma cells. While NF-κB itself exerts potent prosurvival activity, it is intriguing to learn that p21CIP1/WAF1 can display antiapoptotic activity as well. Cytoplasmically retained p21CIP1/WAF1 inactivates death-promoting kinase ASK1 in monocytes through direct interaction (3, 21). To this end, it will be interesting to examine whether p21CIP1/WAF1 in our system exhibits any prosurvival activity.
Papilloma cells proliferate more slowly than normal laryngeal epithelial cells (26), in accord with the elevated level of p21CIP1/WAF1 reported here. Overexpression of p21CIP1/WAF1 could also lead to disruption of normal epithelial differentiation programming previously observed in papilloma tissue (26, 29). This hypothesis is supported by the finding that expression of p21CIP1/WAF1 in keratinocytes inhibits expression of differentiation markers such as keratin 1, involucrin, and filaggrin (9). It is tempting to speculate on the potential contribution of p21CIP1/WAF1 during papilloma development from the angle of antiapoptosis. Further studies are in progress to determine its role in papilloma formation.
Acknowledgments
We thank Bettie M. Steinberg for her helpful advice and constructive criticism.
This study was supported by grant P50DC00203 from the National Institute on Deafness and Other Communication Disorders.
REFERENCES
- 1.Abramson, A. L., B. M. Steinberg, and B. Winkler. 1987. Laryngeal papillomatosis: clinical, histopathologic and molecular studies. Laryngoscope 97:678–685. [DOI] [PubMed] [Google Scholar]
- 2.Allport, V. C., D. M. Slater, R. Newton, and P. R. Bennett. 2000. NF-κB and AP-1 are required for cyclo-oxygenase 2 gene expression in amnion epithelial cell line (WISH). Mol. Hum. Reprod. 6:561–565. [DOI] [PubMed] [Google Scholar]
- 3.Asada, M., T. Yamada, H. Ichijo, D. Delia, K. Miyazono, K. Fukumuro, and S. Mizutani. 1999. Apoptosis inhibitory activity of cytoplasmic p21(Cip1/WAF1) in monocytic differentiation. EMBO J. 18:1223–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bourke, E., E. J. Kennedy, and P. N. Moynagh. 2000. Loss of IκBβ is associated with prolonged NF-κB activity in human glial cells. J. Biol. Chem. 275:39996–40002. [DOI] [PubMed] [Google Scholar]
- 5.Brasier, A. R., M. Lu, T. Hai, Y. Lu, and I. Boldogh. 2001. NF-κB inducible BCL-3 expression is an autoregulatory loop controlling nuclear p50/NF-κB1 residence. J. Biol. Chem. 31:31. [DOI] [PubMed] [Google Scholar]
- 6.Bureau, F., S. Delhalle, G. Bonizzi, L. Fievez, S. Dogne, N. Kirschvink, A. Vanderplasschen, M. P. Merville, V. Bours, and P. Lekeux. 2000. Mechanisms of persistent NF-κB activity in the bronchi of an animal model of asthma. J. Immunol. 165:5822–5830. [DOI] [PubMed] [Google Scholar]
- 7.Cairns, P., K. Okami, S. Halachmi, N. Halachmi, M. Esteller, J. G. Herman, J. Jen, W. B. Isaacs, G. S. Bova, and D. Sidransky. 1997. Frequent inactivation of PTEN/MMAC1 in primary prostate cancer. Cancer Res. 57:4997–5000. [PubMed] [Google Scholar]
- 8.Datta, S. R., A. Brunet, and M. E. Greenberg. 1999. Cellular survival: a play in three Akts. Genes Dev. 13:2905–2927. [DOI] [PubMed] [Google Scholar]
- 9.Di Cunto, F., G. Topley, E. Calautti, J. Hsiao, L. Ong, P. K. Seth, and G. P. Dotto. 1998. Inhibitory function of p21Cip1/WAF1 in differentiation of primary mouse keratinocytes independent of cell cycle control. Science 280:1069–1072. [DOI] [PubMed] [Google Scholar]
- 10.Franzoso, G., V. Bours, S. Park, M. Tomita-Yamaguchi, K. Kelly, and U. Siebenlist. 1992. The candidate oncoprotein Bcl-3 is an antagonist of p50/NF-κB-mediated inhibition. Nature 359:339–342. [DOI] [PubMed] [Google Scholar]
- 11.Fukada, T., M. Hibi, Y. Yamanaka, M. Takahashi-Tezuka, Y. Fujitani, T. Yamaguchi, K. Nakajima, and T. Hirano. 1996. Two signals are necessary for cell proliferation induced by a cytokine receptor gp130: involvement of STAT3 in anti-apoptosis. Immunity 5:449–460. [DOI] [PubMed] [Google Scholar]
- 12.Grandis, J. R., S. D. Drenning, Q. Zeng, S. C. Watkins, M. F. Melhem, S. Endo, D. E. Johnson, L. Huang, Y. He, and J. D. Kim. 2000. Constitutive activation of Stat3 signaling abrogates apoptosis in squamous cell carcinogenesis in vivo. Proc. Natl. Acad. Sci. USA 97:4227–4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hatano, E., B. L. Bennett, A. M. Manning, T. Qian, J. J. Lemasters, and D. A. Brenner. 2001. NF-κB stimulates inducible nitric oxide synthase to protect mouse hepatocytes from TNF-α- and Fas-mediated apoptosis. Gastroenterology 120:1251–1262. [DOI] [PubMed] [Google Scholar]
- 14.Johnston, D., H. Hall, T. P. DiLorenzo, and B. M. Steinberg. 1999. Elevation of the epidermal growth factor receptor and dependent signaling in human papillomavirus-infected laryngeal papillomas. Cancer Res. 59:968–974. [PubMed] [Google Scholar]
- 15.Karin, M., and Y. Ben-Neriah. 2000. Phosphorylation meets ubiquitination: the control of NF-κB activity. Annu. Rev. Immunol. 18:621–663. [DOI] [PubMed] [Google Scholar]
- 16.Li, D. M., and H. Sun. 1997. TEP1, encoded by a candidate tumor suppressor locus, is a novel protein tyrosine phosphatase regulated by transforming growth factor beta. Cancer Res. 57:2124–2129. [PubMed] [Google Scholar]
- 17.Lu, Y., Y. Z. Lin, R. LaPushin, B. Cuevas, X. Fang, S. X. Yu, M. A. Davies, H. Khan, T. Furui, M. Mao, R. Zinner, M. C. Hung, P. Steck, K. Siminovitch, and G. B. Mills. 1999. The PTEN/MMAC1/TEP tumor suppressor gene decreases cell growth and induces apoptosis and anoikis in breast cancer cells. Oncogene 18:7034–7045. [DOI] [PubMed] [Google Scholar]
- 18.Maehama, T., and J. E. Dixon. 1998. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J. Biol. Chem. 273:13375–13378. [DOI] [PubMed] [Google Scholar]
- 19.Mayo, M. W., and A. S. Baldwin. 2000. The transcription factor NF-κB: control of oncogenesis and cancer therapy resistance. Biochim. Biophys. Acta 1470:M55–M62. [DOI] [PubMed] [Google Scholar]
- 20.Nakao, S., Y. Ogata, E. Shimizu-Sasaki, M. Yamazaki, S. Furuyama, and H. Sugiya. 2000. Activation of NFκB is necessary for IL-1β-induced cyclooxygenase-2 (COX-2) expression in human gingival fibroblasts. Mol. Cell. Biochem. 209:113–118. [DOI] [PubMed] [Google Scholar]
- 21.Pennington, K. N., J. A. Taylor, G. D. Bren, and C. V. Paya. 2001. IκB kinase-dependent chronic activation of NF-κB is necessary for p21(WAF1/Cip1) inhibition of differentiation-induced apoptosis of monocytes. Mol. Cell. Biol. 21:1930–1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmitz, M. L., and P. A. Baeuerle. 1991. The p65 subunit is responsible for the strong transcription activating potential of NF-κB. EMBO J. 10:3805–3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seitz, C. S., H. Deng, K. Hinata, Q. Lin, and P. A. Khavari. 2000. Nuclear factor κB subunits induce epithelial cell growth arrest. Cancer Res. 60:4085–4092. [PubMed] [Google Scholar]
- 24.Seitz, C. S., Q. Lin, H. Deng, and P. A. Khavari. 1998. Alterations in NF-κB function in transgenic epithelial tissue demonstrate a growth inhibitory role for NF-κB. Proc. Natl. Acad. Sci. USA 95:2307–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stehlik, C., R. de Martin, I. Kumabashiri, J. A. Schmid, B. R. Binder, and J. Lipp. 1998. Nuclear factor (NF)-κB-regulated X-chromosome-linked iap gene expression protects endothelial cells from tumor necrosis factor alpha-induced apoptosis. J. Exp. Med. 188:211–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steinberg, B. M., R. Meade, S. Kalinowski, and A. L. Abramson. 1990. Abnormal differentiation of human papillomavirus-induced laryngeal papillomas. Arch. Otolaryngol. Head Neck Surg. 116:1167–1171. [DOI] [PubMed] [Google Scholar]
- 27.Takeda, K., T. Kaisho, N. Yoshida, J. Takeda, T. Kishimoto, and S. Akira. 1998. Stat3 activation is responsible for IL-6-dependent T cell proliferation through preventing apoptosis: generation and characterization of T cell-specific Stat3-deficient mice. J. Immunol. 161:4652–4660. [PubMed] [Google Scholar]
- 28.Taylor, B. S., M. E. de Vera, R. W. Ganster, Q. Wang, R. A. Shapiro, S. M. Morris, T. R. Billiar, and D. A. Geller. 1998. Multiple NF-κB enhancer elements regulate cytokine induction of the human inducible nitric oxide synthase gene. J. Biol. Chem. 273:15148–15156. [DOI] [PubMed] [Google Scholar]
- 29.Vambutas, A., T. P. Di Lorenzo, and B. M. Steinberg. 1993. Laryngeal papilloma cells have high levels of epidermal growth factor receptor and respond to epidermal growth factor by a decrease in epithelial differentiation. Cancer Res. 53:910–914. [PubMed] [Google Scholar]
- 30.Wang, C. Y., M. W. Mayo, R. G. Korneluk, D. V. Goeddel, and A. S. Baldwin. 1998. NF-κB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science 281:1680–1683. [DOI] [PubMed] [Google Scholar]
- 31.Weng, L., J. Brown, and C. Eng. 2001. PTEN induces apoptosis and cell cycle arrest through phosphoinositol-3-kinase/Akt-dependent and -independent pathways. Hum. Mol. Genet. 10:237–242. [DOI] [PubMed] [Google Scholar]
- 32.Wu, M. X., Z. Ao, K. V. Prasad, R. Wu, and S. F. Schlossman. 1998. IEX-1L, an apoptosis inhibitor involved in NF-κB-mediated cell survival. Science 281:998–1001. [DOI] [PubMed] [Google Scholar]
- 33.Zhang, P., and B. M. Steinberg. 2000. Overexpression of PTEN/MMAC1 and decreased activation of Akt in human papillomavirus-infected laryngeal papillomas. Cancer Res. 60:1457–1462. [PubMed] [Google Scholar]