Abstract
Measles has a host range restricted to humans and monkeys in captivity. Fresh measles virus (MV) isolates replicate readily in several human and simian B-cell lines but need a period of adaptation to other types of cells. The identification of CD46 and CD150 (SLAM) as cellular receptors for MV has helped to clarify certain aspects of the immunobiology of MV infections. We have examined the properties of an MV wild-type strain grown in the epithelial cell line Vero. After adaptation, this virus expressed high levels of both the viral glycoproteins (hemagglutinin and fusion protein) but did not induce fusion (syncytia). No changes in the amino acid sequence were found in either of the viral glycoproteins. Using several approaches, the Vero-adapted virus could not be shown to interact with CD46 either in the initiation or during the course of infection. The presence of human SLAM expressed in the Vero cells rapidly gave rise to fusion and lower yields of infectious virus.
Despite the availability of an efficient vaccine, measles remains one of the major causes of infant mortality. Molecular epidemiological studies have established that despite the monotypic status, measles viruses (MVs) from geographically different regions can be differentiated by nucleic acid sequence analysis (20); however, the biological significance of these observations, if any, remains to be established. MV was first isolated in 1953 and was initially passaged in primary embryo human kidney cells and then serially passaged in chicken embryo fibroblasts until it had an attenuated phenotype when reinoculated into children. During this adaptation, there were a number of mutations introduced into the MV genome (14). Among the properties associated with the vaccine virus was the ability to replicate in human and monkey epithelial and fibroblastic cell lines. This has been shown to be due in part to the ability of the vaccine virus to use the molecule CD46 as a cell receptor (1, 10). During MV replication the expression of the MV hemagglutinin (HA) at the surface of the cells leads to the down-regulation of the CD46 (11), which is one of the proteins responsible for protecting the cell from complement lysis, and this leads to an increased susceptibility to lysis by complement factors (16). It has been proposed that this may be an attenuating property.
Whereas the virus can be rapidly isolated from clinical specimens in both human and monkey B-cell lines (6), isolation on epithelial cells such as Vero cells can take weeks and several blind passages. Recently it was shown that freshly isolated MV could attach to the cellular receptor CD150 (SLAM) and the vaccine strain could use either this molecule or CD46 (5, 12, 19).
After adaptation to Vero cells, the virus may induce syncytia similar to those in cells infected by the vaccine strain. This is usually correlated to the introduction of certain mutations in the HA, especially at position 481, where the wild-type amino acid, which is normally Asn, may be mutated to Tyr in order to become syncytial (4, 7). Our studies have established that the amino acid at 481 and to a lesser extent that at amino acid 451 govern the ability of the virus to efficiently use CD46 as a receptor and also its ability to down-regulate this molecule from the cell surface (8). In monkey models, MVs isolated in B-cell lines retain their virulent pathogenic phenotype, in contrast to viruses isolated in Vero cells, which become attenuated (17). Sequence studies have identified differences in the HA, P/V/C, M, and L genes between the virulent and attenuated strains (17, 18).
In the present study we have examined the properties of an MV isolate during its adaptation to B95a (monkey B-cell line) or Vero (monkey epithelial) cell lines. We show that after adaptation to Vero cells, this virus is unable to induce fusion. The amino acid sequences of the HA and fusion (F) proteins are unchanged, and the F protein precursor, F0, is cleaved into the potentially biologically active subunits F1 and F2. Further, we show that the Vero-adapted virus does not enter the cell by CD46. As Vero cells do not express SLAM, this poses the question of how the virus infects Vero cells.
MV adaptation to B95a and Vero cells.
An MV isolate, G954, which had been maintained by infection of phytohemagglutinin-stimulated peripheral blood lymphocyte (PBL) cultures was used to infect B95a and Vero cells. In B95a cells syncytia were observed within 24 h, rapidly extending to the majority of the cells. In contrast, a cytopathic effect or fusion was not observed in the Vero cells. These cells were passaged twice at 5-day intervals before detecting MV antigen-positive cells. In this case the cells became enlarged but did not display classical fusion. This virus (freeze-thawed cells) was serially passaged 10 times in Vero cells, and at this point the adapted virus, G954.V10, was further characterized.
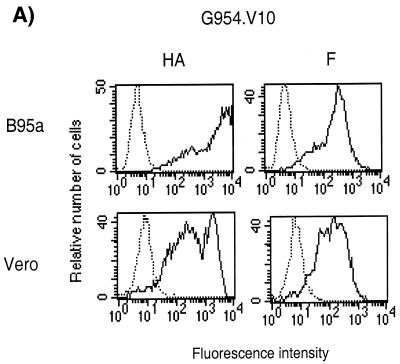
Infection of either B95a or Vero cells with G954.V10 showed that although there was a high expression of both viral glycoproteins at the cell surface as shown by FACScan analysis (Fig. 1A), no fusion was observed. In control cultures, infection of Vero or B95a cells with Hallé (vaccine-like) or B95a cells with G954-PBL induced fusion (Fig. 1B).
FIG. 1.
MV infection of Vero and B95a cells. (A) Flow cytometry analysis of MV G954.V10 infected cells. The cells were stained with anti-HA, monoclonal antibody 55 (2), or anti-F monoclonal antibody 263 (9). Continuous lines represent infected cells, and the dotted lines represent the uninfected control cells. (B) Morphology (fusion) of MV-infected Vero and B95a cells. Cells were infected at 1 PFU/cell for G954.V10 and Hallé and 0.1 PFU/cell for G954-PBL. Photographs were taken 48 h after infection.
To study whether the lack of fusion in the Vero-adapted G954 virus was due to modifications in the structure of either of the glycoproteins, the mRNA from PBLs and B95a and Vero cells infected with the corresponding virus were subjected to reverse transcription-PCR and the DNA was sequenced with the Big Dye terminator system (accession no. for H and F, AY059391 and AY059392, respectively). A single nucleotide change in the HA was found at position 7278 in the genome (A→G) but did not lead to a change in the amino acid sequence. The same change was observed when the virus was adapted to either Vero or B95a cells.
MV-F protein is synthesized as the precursor F0, which is cleaved into the biologically active form F1 plus F2. Although the sequence is unaltered, we examined the possibility that the F expressed in Vero cells by G954.V10 was not cleaved. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting of the virus-infected cell extracts showed that F was cleaved to the same extent in both viruses (results not shown).
CD46 and SLAM as cell receptors.
The interaction of MV HA with its cellular receptors initiates the infection. Later during the virus cycle when this viral glycoprotein is expressed at the surface of the cell, it can interact with the receptors on neighboring cells. It has been reported that MV lymphotropic strains use SLAM whereas vaccine strains can also use CD46. To investigate the mechanism(s) by which G954.V10 enters cells, we infected B95a and Vero cells and compared the modulations of CD46 and/or SLAM at the cell surface. In B95a cells, CD46 lacks SCR-1 and so is nonfunctional as an MV receptor (3). Infection by either Hallé or G954.V10 down-regulated SLAM, whereas the nonfunctional CD46 in B95a cells was not modified (Fig. 2). Vero cells express only CD46 and not SLAM. Infection with G954.V10 did not alter the levels of CD46 even though high levels of the HA were expressed. In contrast, after infection with Hallé the presence of CD46 was undetectable with an anti-CD46 monoclonal antibody directed to the SCR-1 (13/42,) but when an antibody to SCR-4 (10/88) was used the level of CD46 was only slightly reduced. This may suggest that the lack of detection with 13/42 is due to the masking of the site caused by the interaction with the HA. Thus, either G954.V10 HA does not interact with CD46 or its interaction is of an affinity too low to be detected by this method.
FIG. 2.
Flow cytometry analysis of the cell surface expression of CD46 and SLAM in MV infection. B95a and Vero cells infected with either Hallé or G954.V10 were stained either with anti-CD46 monoclonal antibodies 13/42 and 10/88 (J. Schneider-Schaulies, Würzburg, Germany) or anti-SLAM IPO3 (Biodesign International). Continuous lines represent MV-infected cells. Dotted lines represent control uninfected cells.
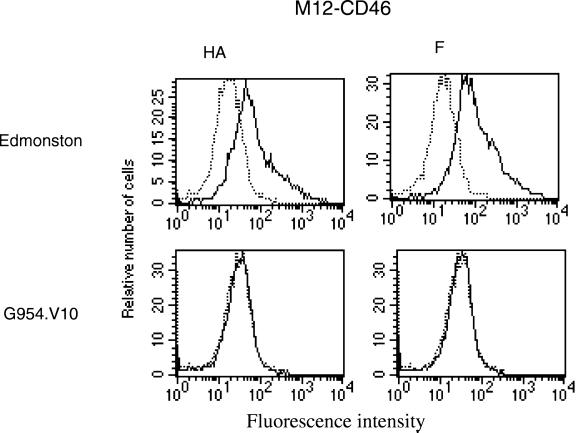
To compare the ability of Edmonston and G954.V10 to use CD46 as a receptor we infected a murine cell line (M12-CD46) which was transfected with the human CD46 (10). As measured by the expression of the HA at the cell surface, only the Edmonston strain could infect these cells (Fig. 3). We confirmed that infection of the cells was via CD46, as preincubation with the monoclonal anti-CD46 antibody 13/42 blocked infection (results not shown). These studies suggest that MV G954 in adapting to Vero cells does not use CD46, although when SLAM is available it can still use it.
FIG. 3.
Flow cytometry analysis of MV-infected M12-CD46 cells. M12-CD46 cells were infected with Edmonston or G954.V10 (1 PFU/cell). At 72 h postinfection, cells were analyzed for HA (monoclonal antibody 55) and F (monoclonal antibody 263) expression. Continuous lines represent the infected cells. Dotted lines represent the uninfected control cells.
G954 adapted to grow in Vero retains its ability to interact (down-regulation) with simian SLAM but not to induce fusion. In contrast, infection of Vero cells expressing human SLAM induced syncytia. To study the contribution of SLAM to the biology of the infections, Vero and Vero-SLAM cells were infected with G954.V10 virus and the virus synthesis and fusion properties were examined. In the Vero-SLAM cultures, fusion was initiated within 24 h, leading to the destruction of the cell monolayer by 5 days. In contrast, infection of Vero cells initially yielded lower levels of virus, but in the absence of fusion, the infectious virus yield eventually rose to more than 10 times that of the SLAM expressing cells (7 days) (Fig. 4).
FIG. 4.
Comparison of MV G954.V10 growth cycles in Vero and Vero-SLAM cells. Vero and Vero-SLAM cells were infected with G954.V10 (0.1 PFU/cell). The yield of virus (cells plus supernatant) was titrated on Vero-SLAM cells.
In our studies, we examined the adaptation of a wild-type MV to replicate in Vero cells. After a period of adaptation the virus grew to high titers without inducing fusion. As the predicted amino acid sequence of the two viral glycoproteins was unchanged, this suggests that the ability to replicate in Vero cells was not at the receptor level. This was substantiated by showing that the virus did not use CD46 as a receptor, which is in agreement with a lack of Tyr at position 481 for CD46 usage (7). However, as Vero cells do not express SLAM, the mechanism by which the virus infects the cell remains to be determined. In human immunodeficiency virus infections, the virus can incorporate cellular proteins which can attach to their corresponding ligands on host cells (reviewed in references 13 and 15). However, in our system, anti-Vero sera failed to neutralize G954.V10 infection of Vero cells (T. F. Wild, unpublished results). In the present study, we have analyzed a number of the parameters displayed by wild-type MV when adapted to tissue culture. MV can enter such cells and following a period grows to high titers without giving classical fusion. These observations may be relevant to the in vivo situation in which viral antigen is found in cells which are not known to express the MV wild-type receptor SLAM. It remains to be established if these cells become infected by a mechanism similar to the present studies or via a third MV cell receptor.
Acknowledgments
We thank Bernadette Maret for editorial assistance and H. Whittle (Gambia) for lymphocytes from measles patients.
This work was supported by a grant (no. HHC02F) from the Rhône-Alpes Région. D. Waku Kouomou was supported by a scholarship from the Ministry of Foreign Affairs and Cooperation.
REFERENCES
- 1.Dorig, R. E., A. Marcil, A. Chopra, and C. D. Richardson. 1993. The human CD46 molecule is a receptor for measles virus (Edmonston strain). Cell 75:295–305. [DOI] [PubMed] [Google Scholar]
- 2.Giraudon, P., and T. F. Wild. 1985. Correlation between epitopes on hemagglutinin of measles virus and biological activities: passive protection by monoclonal antibodies is related to their hemagglutination inhibiting activity. Virology 144:46–58. [DOI] [PubMed] [Google Scholar]
- 3.Hsu, E. C., R. E. Dörig, F. Sarangi, A. Marcil, C. Iorio, and C. D. Richardson. 1997. Artificial mutations and natural variations in the CD46 molecules from human and monkey cells define regions important for measles virus binding. J. Virol. 71:6144–6154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hsu, E. C., F. Sarangi, C. Iorio, M. S. Sidhu, S. A. Udem., D. L. Dillehay, W. Xu, P. A. Rota, W. J. Bellini, and C. D. Richardson. 1998. A single amino acid change in the hemagglutinin protein of measles virus determines its ability to bind CD46 and reveals another receptor on marmoset B cells. J. Virol. 72:2905–2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hsu, E. C., C. Iorio, F. Sarangi, A. A. Khine, and C. D. Richardson. 2001. CDw150 (SLAM) is a receptor for a lymphotropic strain of measles virus and may account for the immunosuppressive properties of this virus. Virology 279:9–21. [DOI] [PubMed] [Google Scholar]
- 6.Kobune, F., H. Sakata, and A. Sugiura. 1990. Marmoset lymphoblast cells as a sensitive host for isolation of measles virus. J. Virol. 64:700–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lecouturier, V., J. Fayolle, M. Caballero, J. Carabana, M. L. Celma, R. Fernandez-Munoz, T. F. Wild, and R. Buckland. 1996. Identification of two amino acids in the hemagglutinin glycoproteins of measles virus (MV) that govern hemadsorption, HeLa cell fusion and C46 downregulation: phenotypic markers that differentiate vaccine and wild-type MV strains. J. Virol. 70:4200–4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lecouturier, V., A. Rizzitelli, J. Fayolle, L. Daviet, T. F. Wild, and R. Buckland. 1999. Interaction of measles virus (Hallé strain) with CD46: evidence that a common binding site on CD46 facilitates both CD46 downregulation and MV infection. Biochem. Biophys. Res. Commun. 264:268–275. [DOI] [PubMed] [Google Scholar]
- 9.Malvoisin, E., and T. F. Wild. 1990. Contribution of measles virus fusion protein in protective immunity: anti-F monoclonal antibodies neutralize virus infectivity and protect mice against challenge. J. Virol. 64:5160–5162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naniche, D., G. Varior-Krishnan, F. Cervoni, T. F. Wild, B. Rossi, C. Rabourdin-Combe, and D. Gerlier. 1993. Human membrane cofactor protein (CD46) acts as a cellular receptor for measles virus. J. Virol. 67:6025–6032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naniche, D., T. F. Wild, C. Rabourdin-Combe, and D. Gerlier. 1993. Measles virus haemagglutinin induces down regulation of gp57/67, a molecule involved in virus binding. J. Gen. Virol. 74:1073–1079. [DOI] [PubMed] [Google Scholar]
- 12.Ono, N., H. Tatsuo, Y. Hidaka, T. Aoki, H. Minagawa, and Y. Yanagi. 2001. Measles virus on throat swabs from measles patients use signaling lymphocytic activation molecule (CDw150) but not CD46 as a cellular receptor. J. Virol. 75:4399–4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ott, D. E. 1997. Cellular proteins in HIV virions. Rev. Med. Virol. 7:167–180. [DOI] [PubMed] [Google Scholar]
- 14.Parks, C. L., R. A. Lerch, P. Walpita, H. P. Wang, M. S. Sidhu, and S. A. Udem. 2001. Comparison of predicted amino acid sequences of measles virus strains in the Edmonston vaccine lineage. J. Virol. 75:910–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roberts, B. D., and S. T. Butera. 1999. Host protein incorporation is conserved among diverse HIV-1 subtypes. AIDS 13:425–427. [DOI] [PubMed] [Google Scholar]
- 16.Schnorr, J. J., L. M. Dunster, R. Nanan, J. Schneider-Schaulies, S. Schneider-Schaulies, and V. ter Meulen. 1995. Measles virus-induced down-regulation of CD46 is associated with enhanced sensitivity to complement-mediated lysis of infected cells. Eur. J. Immunol. 25:976–984. [DOI] [PubMed] [Google Scholar]
- 17.Takeda, M., A. Kato, F. Kobune, H. Sakata, Y. Li, T. Shioda, Y. Sakai, M. Asakawa, and Y. Nagai. 1998. Measles virus attenuation associated with transcriptional impediment and a few amino acid changes in the polymerase and accessory proteins. J. Virol. 72:8690–8696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takeuchi, K., N. Miyajima, F. Kobune, and M. Tashiro. 2000. Comparative nucleotide sequence analyses of the entire genomes of B95a cell-isolated and vero cell-isolated measles viruses from the same patient. Virus Genes 20:253–257. [DOI] [PubMed] [Google Scholar]
- 19.Tatsuo, H., N. Ono, K. Tanaka, and Y. Yanagi. 2000. SLAM (CDw150) is a cellular receptor for measles virus. Nature 406:893–897. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization. 1998. Expanded Programme on Immunization (EPI). Standardization of the nomenclature for describing the genetic characteristics of wild-type measles viruses. Wkly. Epidemiol. Rec. 73:265–272. [PubMed] [Google Scholar]