Abstract
Human respiratory syncytial virus (HRSV) and bovine respiratory syncytial virus (BRSV) are major pathogens in infants and calves, respectively. Experimental BRSV infection of calves and lambs is associated with lymphopenia and a reduction in responsiveness of peripheral blood lymphocytes (PBLs) to mitogens ex vivo. In this report, we show that in vitro mitogen-induced proliferation of PBLs is inhibited after contact with RSV-infected and UV-inactivated cells or with cells expressing RSV envelope proteins on the cell surface. The protein responsible was identified as the RSV fusion protein (F), as cells infected with a recombinant RSV expressing F as the single envelope protein or cells transfected with a plasmid encoding F were able to induce this effect. Thus, direct contact with RSV F is necessary and sufficient to inhibit proliferation of PBLs. Interestingly, F derived from HRSV was more efficient in inhibiting human PBL proliferation, while F from BRSV was more efficient in inhibiting bovine PBLs. Since various T-cell activation markers were upregulated after presenter cell contact, T lymphocytes are viable and may still be activated by mitogen. However, a significant fraction of PBLs were delayed or defective in G0/G1 to S-phase transit.
Human respiratory syncytial virus (HRSV) and bovine respiratory syncytial virus (BRSV) are major causes of severe lower respiratory tract disease in humans and animals, respectively (5). HRSV is the prototype of the Pneumovirus genus of the Pneumoviridae family. Its 15-kb negative-stranded genome RNA is contained in a ribonucleoprotein (RNP) complex comprising 10 genes arranged in the order 3′-NS1-NS2-N-P-M-SH-G-F-M2-L-5′. At least five of the 11 encoded virus proteins are associated with the RNP: the nucleoprotein (N), the phosphoprotein (P), the RNA polymerase (L), and two regulatory proteins, M2-1 and M2-2. The two nonstructural proteins, NS1 and NS2, cooperatively mediate resistance of the virus to interferon (IFN)-induced cellular antiviral responses (35). The virus envelope contains an internal matrix protein (M) as well as three transmembrane glycoproteins, a small hydrophobic protein (SH), whose function is still unclear, the presumed attachment protein (G), and the fusion protein (F). G and F proteins represent the major targets for neutralizing antibodies, and F interacts with so far unidentified cellular receptors during virus entry (16, 45). BRSV is closely related to HRSV, and the clinical symptoms, immune response, and pathology caused by BRSV and HRSV are highly similar, making BRSV infection of calves a relevant animal model for the human disease.
Several observations indicate that RSV may actively interfere with early immune responses and prevent hosts from mounting a protective immunity. Multiple reinfections, even with members of the same virus subgroup, are a typical feature of HRSV and BRSV. Although the severity of disease decreases with repeated infection, adults remain susceptible (5). Moreover, mitogen-induced proliferation of peripheral blood lymphocytes (PBLs) isolated from experimentally BRSV-infected lambs was found to be reduced (19, 40, 41, 48), and lymphopenia (42, 48) as well as an increased susceptibility to opportunistic infections were observed (39, 46, 48). Most likely, RSV interferes with early immune mechanisms. Low gamma interferon (IFN-γ) and high interleukin-4 (IL-4) concentrations in the blood of HRSV-infected patients indicate an exaggerated T helper cell (THC) type 2 response (2, 31, 43). A delayed THC type 1 response seems also to be an important factor in measles virus (MeV)-mediated immune suppression and appears to be triggered by deteriorated cytokine secretion by infected macrophages and lymphocytes (14).
Inhibition of lymphocyte proliferation by RSV was previously attributed solely to effects caused by virus replication in lymphocytes and to cytokines secreted from infected cells. Infection of macrophages and lymphocytes with BRSV and HRSV has been demonstrated both in vitro and in vivo (6, 20, 22, 27, 38). In vitro, RSV infection of peripheral blood mononuclear cells (PBMCs) was reported to result in suppression or delay of proliferation of PBMCs after stimulation with phytohemagglutinin (PHA) or with Epstein-Barr virus antigen. Production of cytokines such as IFN-α and IL-1 inhibitor was considered responsible for the observed inhibition of PBMC proliferation (18, 30, 32).
Here, we present experimental evidence for another mechanism of RSV immune cell suppression, direct contact inhibition of PBL proliferation, which is independent of soluble factors or of virus infection of cells. Using a previously described cocultivation assay (33, 34, 36), we could show that contact with cells presenting RSV antigen (presenter cells [PCs]) causes a strong inhibition of the proliferation of mitogen-stimulated PBLs (responder cells [RCs]). Neither viability nor activation of PCs was affected; rather, activated PBLs were accumulated in the G0/G1-phase. The responsible RSV contact antigen was identified as the RSV fusion protein F. Expression of F alone was able to cause contact inhibition of RCs. Interestingly, although HRSV and BRSV F proteins were able to arrest both human and bovine RCs, a much stronger effect was observed with cells of the appropriate host.
MATERIALS AND METHODS
Cell culture and proliferation assays.
Human or bovine PBMCs serving as RCs were isolated from buffy coats of healthy adult donors or from heparinized blood from a cow and a calf by using Ficoll (Lymphoflot; Biotech, Dreieich, Germany) gradient centrifugation at 1,500 rpm in a Heraeus Varifuge. PBLs were separated from monocytes by plastic adherence and were incubated in RPMI (Gibco) with 10% fetal calf serum (FCS) at 37°C and 5% CO2. HL60 and Vero RCs were maintained in RPMI with 10% FCS and Dulbecco’s modified Eagle’s medium (DMEM) (Gibco) with 5% FCS, respectively.
PCs were generated by transfecting baby hamster kidney (BHK)-derived BSR-T7/5 cells expressing phage T7 RNA polymerase (4) as described below or by virus infection of different cell lines. Infections of HL60 cells, a human macrophage-derived cell line, was done at a multiplicity of infection (MOI) of 0.1, except for recombinant viruses lacking the G gene (see below), for which an MOI of 1 was used. Vero PCs were generated by infection with HRSV (strain Long) at an MOI of 1.
Surface expression of viral antigen was determined by fluorescence-activated cell sorting (FACS). Only populations of greater than 80% RSV protein-positive cells were used for inhibition experiments (Fig. 1C) and the total cell count (100%) was used for calculation of the PC/RC ratio. Control presenter cells (CPCs) were generated by transfecting BSR-T7/5 cells with a vector plasmid or by mock infection of Vero or HL60 cells. PC or CPC proliferation and virus replication in PCs were inactivated by 10 min of UV (254 nm) irradiation.
FIG. 1.
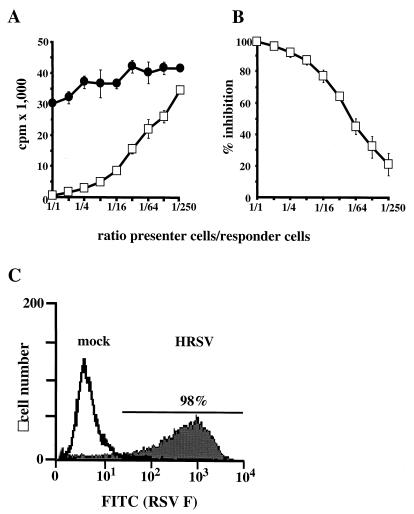
Cells presenting HRSV antigens inhibit proliferation of PHA-stimulated human PBLs. (A) HRSV-infected, inactivated Vero cells (PC; open squares) or mock-infected CPCs (solid circles) were cocultivated with human PBLs (RCs) in the ratios indicated. Proliferation of RCs was determined by [3H]dT incorporation. (B) Inhibition of proliferation compared to mock-infected control PCs and expressed as a percent. Values in A and B represent the result of four experiments using PBLs from four different donors. Error bars indicate standard deviation. (C) Surface expression of HRSV F protein in PCs and mock-infected control cells.
Inactivated PCs or CPCs were seeded in a volume of 100 μl per well into 96-cluster plates in concentrations as indicated. In the same volume, 5 × 105 human or bovine PBLs were added which had been stimulated with PHA (2.5 μg/ml) immediately before. After 72 h of incubation at 37°C and 5% CO2, cells were labeled for 24 h with [3H]dT (0.5 mCi/ml [185 Gbq/mmol]; Amersham). [3H]dT incorporation was determined after washing and harvesting (Tomtec) in a β-plate reader (Wallac). All assays were performed at least in duplicate. No significant differences were noted between individual human or bovine donors of RCs.
Assays in which inhibition of spontaneous proliferation of cell lines of different origin was analyzed were done by using these cell lines (see Results and Discussion) as RCs and by cocultivation of 5 × 104 cells with inactivated HRSV-infected PCs in ratios of 1:1 to 1:250 in the same volume and buffer as described for PBLs. Incorporation of [3H]dT was determined after 72 h of cocultivation. For physical separation of PCs from RCs, cell culture membrane inlays with a pore size of 0.2 μm were used, and 5 × 104 HRSV-infected and UV-irradiated HL60 PCs or CPCs were seeded with 2.5 μg of PHA per ml in the membrane inlays, which were then inserted into culture wells containing 5 × 105 allogeneic PBLs serving as RCs.
HRSV strain Long and recombinant BRSVs derived from strain ATue-51908 (4) as well as BRSV gene deletion mutants were grown on Vero cells. For preparation of virus stocks, 70 to 80% confluent Vero cell layers were infected at a multiplicity of 0.1 in DMEM in the absence of FCS. The inoculum was removed after 1 h, and cells were incubated in DMEM supplemented with 2.5% FCS at 37°C and in a 5% CO2 atmosphere. Virus was released by freezing and thawing the cell monolayer upon development of extensive cytopathic effect. Infectious virus titers were determined on Vero cells by endpoint dilution and counting of infected cell foci stained for indirect immunofluorescence with RSV F-specific monoclonal antibody F56, kindly provided by J. A. Melero, Madrid, or RSV-F (Serotec).
Immunohistochemistry and cell cycle analysis.
For cell surface staining of viral envelope proteins or lymphocyte surface markers, cells were fixed for 5 min with 3% paraformaldehyde at room temperature. After washing with FACS buffer (PBS containing 0.4% FCS and 0.02% NaN3), staining for 30 min on ice was performed with mouse monoclonal antibodies specific for RSV F (F56) or G (021-1G; provided by J. A. Melero) or specific for CD25, CD122, CD40L (Pharmingen), and CD45R0 (Immunotech). For negative controls, isotype-specific antibodies provided by the suppliers were used. After washing, cells were incubated for 30 min with fluorescein isothiocyanate (FITC)-labeled anti-mouse immunoglobulin (Ig) antibody (Dianova), followed by washing and FACS.
For cell activation studies and cell cycle analysis, 106 human PBLs were stimulated with 2.5 μg of PHA/ml and cocultivated with HRSV-infected HL60 PCs or with mock-treated CPCs in a ratio of 5:1 for 48 h followed by immunostaining or DNA staining. For DNA staining, cells were fixed for 5 min with 3% paraformaldehyde, washed, and permeabilized with 0.1% Triton for 2 min. After washing, cells were resuspended in 100 μl of RNase solution (100 μg of RNase A per ml in 1% trisodium citrate) and incubated at 37°C for 30 min. After washing, cells were resuspended in 0.5 ml of propidium iodide (50 μg/ml in 1% trisodium citrate) and incubated for 15 min in the dark prior to FACS analysis.
Cloning of HRSV glycoprotein expression plasmids.
HRSV (strain Long) F and G cDNAs were generated by reverse transcription (RT)-PCR (Titan; Roche) from infected HeLa cells with primers olFLs (5′-GGGCCATGGAGTTGCCAATCCTCAAAGC-3′), and olGLsSGHS/A (5′-GGGAAGCTTACATGTCCAAAAACAAGGACCAACGC-3′), containing an NcoI or AflIII recognition site (underlined) located at the translation initiation codons for insertion of the amplified DNA immediately downstream of the encephalomyocarditis virus internal ribosome entry site (IRES) of the T7 promoter-controlled expression plasmid pTIT (4). The reverse primers olFLr (5′-AGGAATTCTCGAGTTTTTATATAACTATAAACTAGGAATCC-3′) and olGLrRSGEI (5′-GGGGAATTCAATAACTACTGGCGTGTTGTG-3′) each contained an EcoRI site (underlined) downstream of the stop codon which were used for cloning in pTIT, giving rise to pTIT-Fh and pTIT-Gh, respectively.
PCs expressing either RSV G or RSV F were generated by transfecting 106 BSR-T7/5 cells expressing phage T7 RNA polymerase (4) with 5 μg of the respective pTIT plasmids. PCs coexpressing G and F were generated by cotransfecting BSR-T7/5 cells with 3 μg each of pTIT-Fh and pTIT-Gh.
Construction of recombinant viruses.
The BRSV full-length cDNA clone pBRSV (4) was cleaved with PflMI, resulting in removal of a 2.8-kbp fragment containing the SH, G, and most of the F gene but retaining the SH transcriptional start signal. Digestion with NheI resulted in further removal of a 1.3-kbp fragment spanning the reminder of the F gene and the M2 gene. The 0.9-kbp SspI-NheI part of this fragment containing the M2 gene part was reintroduced into the plasmid together with a short DNA generated by annealing of two synthetic oligomers, olMBRSJF (5′-CTCGAGGTCGACTATAGTTATTTAAAAAGATATGACGCGT-3′) and olMBRSAJF (5′-ACGCGTCATATCTTTTTAAATAACTATAGTCGACCTCGSGCTG-3′), containing a transcription stop signal and a unique SalI recognition site (underlined). This gave rise to a BRSV cDNA lacking the SH, G, and F open reading frames (pBRSV ΔO). pBRSV ΔO was digested with SalI, the recognition site for which is located between the extra transcription start and stop signals and was treated with Klenow polymerase to fill in the 5′ overhang. BRSV F cDNA (1.9 kbp) containing the complete F open reading frame was excised from pTIT-Fb with NcoI and BamHI, also treated with Klenow, and ligated into pBRSV ΔO, resulting in pBRSV ΔSH-G/Fb.
For generation of a chimeric virus containing the F gene from HRSV instead of BRSV, pBRSV ΔO was cleaved with XhoI followed by Klenow fill-in and digestion with SalI. A fragment of 1.7 kbp containing the HRSV F ORF was removed from pTIT-Fh (see above) with NcoI/Klenow and XhoI and ligated into the cleaved pBRSV ΔO, giving rise to pBRSV ΔSH-G/Fh.
For generation of a chimeric virus containing both G and F from HRSV (pBRSV ΔSH/GhFh), the F gene was isolated from pTIT-Fh and the G gene was generated by RT-PCR from HRSV strain Long-infected HeLa cells as described above by using primers GHRSVSalI (5′-GGGGGTCGACATGTCCAAAAACAGG-3′) and FGHRSVNcoI (5′-GGGGGATTGGCAACTCCATGGTTA-3′). The resulting product was cleaved by SalI and NcoI. pBRSV ΔO was cleaved by SalI and ligated with the G PCR product and the F fragment from pTIT-hF. All viruses were recovered from cDNA as described previously (35).
RESULTS
HRSV inhibits mitogen-induced lymphocyte proliferation.
To determine whether contact-mediated mechanisms may contribute to RSV-mediated inhibition of lymphocyte proliferation, we cocultivated cells presenting RSV antigens on their surface (PCs) with mitogen-stimulated human PBLs serving as RCs. First, Vero cells infected with HRSV strain Long were used as PCs. Cultures were UV irradiated to abolish virus replication, release of infectious virus, and proliferation of PCs. Noninfected control cells (CPCs) were treated in the same way. RCs were generated from freshly isolated human PBLs from different donors and were stimulated with 2.5 μg of PHA per ml immediately before coincubation. PCs or CPCs were mixed with RCs at ratios in the range of 1:1 to 1:250, and the mixed cells were coincubated for 72 h. Proliferation of RCs was monitored by [3H]dT incorporation for a further 24 h.
Compared to CPCs, cocultivation with PCs resulted in a dose-dependent inhibition of RC proliferation (Fig. 1A). Inhibition was almost complete when equal numbers of PCs and RCs were mixed. A 50% reduction of RC proliferation was observed at a ratio of 1:50, and even at 1:250 a distinct 20% inhibition of lymphocyte proliferation was determined (Fig. 1B). Not only mitogen-stimulated proliferation of primary lymphocytes but also spontaneous proliferation of the human T-cell line Jurkat, the human B-cell line BJAB, and the macrophage-derived cell line HL60 was reduced in a comparable degree by cocultivation with HRSV-infected PCs (not shown).
HRSV-mediated inhibition of RC proliferation depends on direct contact.
To exclude the possibility that infectious virus contributed to the observed inhibition of RC proliferation, we determined whether infectious virus was released from the UV-irradiated PCs. Confluent Vero cell layers in six-well plates were incubated for 2 h with HRSV-infected and UV-irradiated HL60 PCs or with infected but nonirradiated cells. After washing, the monolayers were incubated for 3 days and analyzed for HRSV-specific immunofluorescence. Whereas nonirradiated cells caused extensive infection, UV-irradiated PCs did not result in detectable infection (Fig. 2A). Thus, infectious virus particles released from PCs could not be responsible for the observed inhibition of RC proliferation.
FIG. 2.
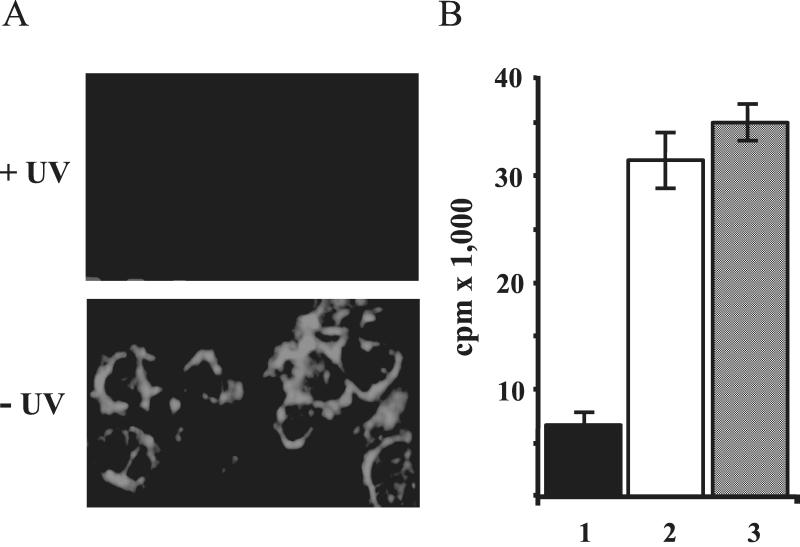
Direct contact with PCs is responsible for inhibition of RC proliferation. (A) UV-irradiated PCs do not produce infectious virus. Vero cell layers were exposed to UV-irradiated HL60 PCs (+UV) or to nonirradiated controls (−UV) and infection was visualized by immunostaining with an RSV F antibody. (B) Soluble factors produced by PCs or PC-contacted PBLs do not inhibit RC proliferation. A mixture of PBLs and PCs (column 2) or CPCs (column 3) was separated from RCs by membranes allowing passage of soluble factors. Aliquots of the PCs efficiently inhibited proliferation of RCs in standard contact assays (column 1). Error bars indicate standard deviation.
To further exclude a contribution of soluble factors released by HRSV-infected PCs, HL60 PCs were physically separated from RCs by membranes allowing passage of soluble factors such as PHA. The inhibitory effect on RC proliferation was completely abolished by the separation of RCs from PCs (not shown). Another experiment was performed to exclude that active autocrine factors are secreted by RCs upon contact with PCs. A mixture of HL60 PCs and PBLs was separated by a membrane from PBLs serving as RCs (Fig. 2B). In contrast to the controls where PCs and RCs were directly mixed (Fig. 2B, column 1), no inhibition was observed when direct contact between the PC-PBL or CPC-PBL mixtures with RCs was prevented by the membranes (Fig. 2B, columns 2 and 3, respectively). Thus, direct contact of PCs and RCs is required and necessary for the observed suppression of RC proliferation, and soluble factors do not contribute in the present in vitro system.
HRSV F protein contact inhibits proliferation of lymphocytes.
The above results suggested the involvement of viral structures on the surface of PCs in delivering an inhibitory signal to RCs by contact. To identify the proteins responsible, individual HRSV envelope proteins were expressed in PCs. BSR-T7/5 cells expressing T7 RNA polymerase (11) were transfected, either alone or in combination with plasmids containing the HRSV F or G open reading frames (ORFs) under the control of a T7 RNA polymerase promoter (pTIT-Fh and pTIT-Gh, respectively). Three days posttransfection, the majority of cells expressed considerable levels of the proteins, as determined by FACS (Fig. 3). After UV irradiation, cells were used as PCs and were mixed at ratios of 1:5 to 1:100 with PHA-stimulated PBL RCs.
FIG. 3.
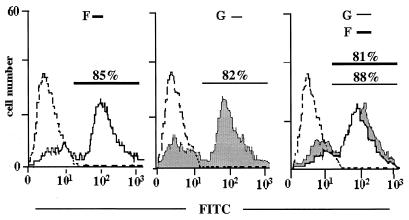
Cells expressing RSV F from cDNA inhibit proliferation of RCs. Surface expression of HRSV F, HRSV G, or both proteins on BSR-T7/5 cells after transfection of plasmids. Expression levels were determined by FACS using F- or G-specific monoclonal antibodies. The dotted line represents cells transfected with the vector plasmid.
Cocultivation of RCs with PCs expressing only HRSV F protein resulted in a significant and dose-dependent inhibition of RC proliferation, with more than 50% inhibition at a 1:5 PC/RC ratio (Table 1). To the contrary, PCs presenting HRSV G protein did not lead to detectable inhibition at any concentration. Most interestingly, however, expression of F protein in combination with G was found to clearly augment the inhibitory capacity of F (Table 1). At a PC/RC ratio of 1:5, an approximately 90% inhibition was observed, which is comparable to that caused by virus-infected cells. Also at higher dilutions, cells coexpressing F and G led to increased inhibition. Notably, the level of F surface expression in cells coexpressing F and G was not higher than in cells expressing F alone (Fig. 3), suggesting that G may influence or stabilize the structure of F required for signaling inhibition. Yet these experiments demonstrated that contact with F protein alone at the surface of PCs is sufficient to inhibit PBL proliferation. Neither virus infection nor cellular structures induced by virus infection of PCs are required for the inhibitory effect on PBL proliferation.
TABLE 1.
RSV F inhibits proliferation of RCs
| PC/RC ratio | % Inhibitiona by PCs expressing HRSV:
|
||
|---|---|---|---|
| F | G | F + G | |
| 1:5 | 57 ± 5.7 | 0 | 88 ± 2.6 |
| 1:10 | 23 ± 7.3 | 0 | 54 ± 9.0 |
| 1:50 | 10 ± 6.0 | 2 ± 4.3 | 16 ± 9.5 |
| 1:100 | 0 | 3 ± 5.7 | 0 |
Values are means ± standard deviation for three independent experiments with PBLs from three individual donors in triplicate assays.
Differential inhibition of bovine and human lymphocytes by HRSV and BRSV F proteins.
RSVs have a narrow host tropism in vivo, with HRSV infecting humans and BRSV infecting cattle. Thus, it was of interest to assess whether PCs displaying proteins from human or bovine RSV are able to also suppress proliferation of lymphocytes of the heterologous host. Here, we used HL60 suspension cells as PCs, because these are equally well infected by HRSV and BRSV. Upon comparable envelope protein expression on the cell surface as determined by FACS analysis (data not shown), cells were UV irradiated and used in proliferation assays with PHA-stimulated PBLs isolated from either human or bovine blood.
Proliferation of both human and bovine RCs was affected by contact with all of the infected PCs compared to controls with CPCs. However, HRSV was clearly superior to BRSV in inhibiting proliferation of human PBLs. In the contrary, BRSV caused a more pronounced inhibition of bovine PBLs at all dilutions (Fig. 4A and B). In both cases, a PC/RC ratio of 1:16 resulted in an approximately 80% reduction in proliferation of RCs from the adequate host, whereas an approximately 40% reduction was reached with PBLs from the inadequate host. This suggested that HRSV and BRSV F proteins are adapted to signaling growth arrest to PBLs of the appropriate host.
FIG. 4.
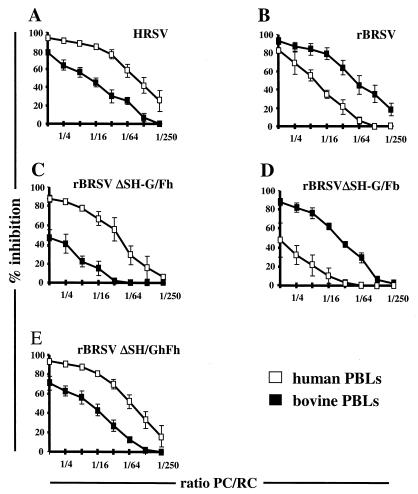
Inhibition of lymphocyte proliferation is more pronounced with PBLs of the specific host. Bovine RCs (solid squares) or human RCs (open squares) were coincubated with HL60 PCs infected with HRSV (A), BRSV (B), or recombinant BRSV-derived viruses possessing either HRSV F (C) or BRSV F (D) as the only viral surface protein, or with a virus having both F and G from HRSV (E). Mock-infected CPCs were used for calculating percent inhibition. Results are from three independent experiments using PBLs from four individual donors and two individual animals.
To verify that only the sequence of HRSV or BRSV F protein is responsible for the differential inhibition of RCs from different origin and that other virus factors do not contribute, recombinant RSVs were generated by using recently established reverse genetics protocols (4, 35) and used for preparation of PCs. First, a BRSV was constructed lacking the SH and G genes while retaining the F gene (rBRSV ΔSH-G/Fb). In addition, a corresponding virus carrying the F gene from HRSV in place of the BRSV F gene (rBRSV ΔSH-G/Fh) was recovered from cDNA. Both viruses carrying either the BRSV or HRSV F as the single envelope protein grew only slightly more slowely than wild-type BRSV in Vero or HL60 cell cultures, confirming again that SH and G are not required for RSV propagation in cell culture (16, 17, 45).
HL60 PCs infected with rBRSV ΔSH-G/Fb carrying the BRSV F gene induced a much greater inhibition of bovine PBL RCs than the counterpart virus with the HRSV F protein (approximately 80 and 40%, respectively) at high PC/RC ratios (Fig. 4D and C). In contrast, rBRSV ΔSH-G/Fh induced a greater inhibition in human PBLs than in bovine PBLs (80 and 30%, respectively; Fig. 4C and D). As the two viruses differ only in the F protein, the observed specificity in suppressing mitogenic proliferation of RCs from different hosts is determined solely by the sequence of the F protein.
Notably, and supporting the previous results with cDNA-expressed surface proteins, the recombinant viruses lacking both the G and SH genes were somewhat less effective in signaling proliferation inhibition than wild-type HRSV or wild-type BRSV (compare Fig. 4A and C or B and D). To determine whether the above observed supportive effect of G also applies to virus-infected cells, an additional recombinant was generated by introducing the HRSV G gene into rBRSV ΔSH-G/Fh, resulting in rBRSV ΔSH/GhFh. As shown in Fig. 4E, PCs infected with this virus were virtually equal in RC proliferation inhibition to PCs infected with wild-type HRSV, with values of greater than 90% for inhibition of human PBLs and greater than 70% for inhibition of bovine PBLs. These results also suggest that the third envelope protein of RSV, SH, is not involved in signaling PBL arrest.
RSV F contact blocks the cell cycle but not mitogen-mediated activation of PBLs.
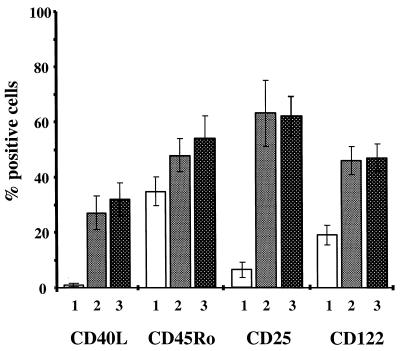
To examine in more detail the nature of PBL proliferation inhibition, cellular activation markers of nonstimulated or PHA-stimulated human PBLs were monitored. Irrespective of whether PHA-stimulated PBLs were cocultured with PCs or with CPCs, surface expression of CD40-L, CD25 (IL-2 α-chain), and CD122 (IL-2 β-chain) was greatly increased, and a slight increase was also observed for CD45Ro (Fig. 5). In parallel experiments, aliquots of the PCs used were shown to inhibit RC proliferation in a range of 65 to 82% (not shown). Thus, proliferation inhibition does not interfere with activation of RCs by PHA but rather appears to affect the cell cycle.
FIG. 5.
PBL activation by mitogen is not affected after contact with PCs. Expression of surface activation markers is induced by PHA in the presence of HRSV-infected HL60 PCs (column 2) or mock-infected HL60 PCs (column 3). Nonactivated PBLs from the same donor are shown in column 1. Error bars indicate standard deviation of four experiments performed with PBLs from four individual donors.
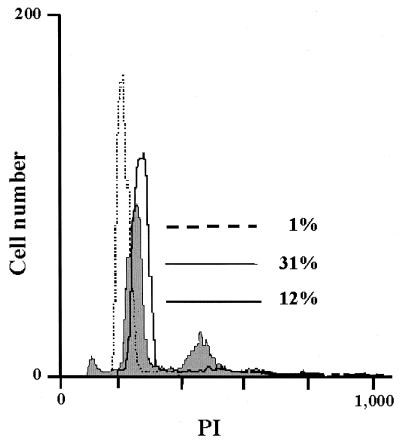
The DNA content of PBLs was therefore determined by FACS using propidium iodide staining. In nonstimulated PBL cultures, almost all cells were resting in the G0/G1 phase as indicated by a single DNA content (Fig. 6, dotted line). After PHA stimulation for 48 h, 31% of PBLs contained a doubled DNA content (Fig. 6, continuous line), indicative of actively proliferating cells. However, after contact with PCs, which in parallel experiments gave a 75% proliferation inhibition, significantly fewer cells (12%) with double DNA content were observed (Fig. 6, bold line). This indicates that proliferation inhibition is due to a defect of contacted PBLs in reaching the S phase.
FIG. 6.
Mitogen-stimulated PBLs accumulate in G0/G1 phase after PC contact. The DNA content of PBLs was determined by propidium iodide (PI) staining. Nonstimulated PBLs represented by the dotted line have a single DNA content. Compared to control PC-contacted cultures (continuous line), PC-contacted cultures (bold line) contain a lower number of proliferating cells with doubled DNA content (12 versus 31%).
DISCUSSION
Distortion and suppression of host immune cell responses after HRSV and BRSV infection is a well-recognized feature that may have an important role in RSV pathogenesis (12, 13, 15, 31, 40, 43, 48) and is also observed in different in vitro systems (1, 28, 29). However, the mechanisms involved have not been satisfactorily dissected. In this in vitro study, we could show that RSV F protein is able to mediate inhibition of mitogen-induced proliferation of T cells by contact with the target cell. Active virus replication in presenter cells is not necessary, as UV-irradiated PCs not able to produce virus were effective. Moreover, cells expressing RSV F protein from transfected cDNA could substitute for virus-infected presenter cells.
Previous reports on inhibition of RSV immune cells were based on the analysis of mixed populations of infected and noninfected lymphocytes and macrophages and therefore have not been able to distinguish the effects caused by virus, virus proteins, or cellular factors released by either virus-infected or noninfected cells. Here, we have used an in vitro system which has previously proven successful for investigation of MeV immune suppression mechanisms (36). Production of infectious virus in PCs is abolished by UV irradiation, and only noninfected PBLs were used as RCs. A contribution of soluble factors produced by RSV-infected PCs or by PC-contacted RCs could also be excluded, as physical separation of PCs and RCs by large-pored membranes allowing passage of soluble factors such as PHA completely abolished the inhibitory effect. In particular, a contribution of IFN-α, which has been described as a potent RSV-induced inhibitor of leukocyte proliferation (30), was excluded by using Vero PCs, which lack IFN-α genes (8) and also do not produce active IFN-β (35).
In the present assays, we thus exclusively monitored effects caused by direct contact of PCs and RCs. The situation is thus highly reminiscent of the findings with MeV, where direct contact inhibition by viral surface proteins (36) has been shown to arrest leukocytes in vitro (9, 37). Moreover, in the case of MeV, this mechanism contributes considerably to the pronounced systemic immune suppression following measles infection in an animal model (24–26). Typically, such a systemic immune suppression is not observed after RSV infection, although a reduction of responsiveness of peripheral leukocytes has been described in animals experimentally infected with BRSV (40, 42).
In vitro, however, the effects of contact inhibition by RSV and MeV are highly comparable and seem to be due to similarly effective mechanisms. A ratio of 1 PC presenting RSV proteins cocultivated with 50 RCs resulted in an approximately 50% inhibition of PHA-stimulated lymphocyte proliferation. Significant effects were observed at ratios of up to 1:250. This is somewhat lower than with MeV, where ratios of up to 1:1,000 were shown to be effective in vitro (36). Also similar to MeV (9, 37), contact with RSV PCs did not inhibit the PHA mitogen-mediated activation of RCs, as shown by successful upregulation of important activation markers and unimpaired viability of cells, arguing against apoptosis of RCs upon PC contact.
As can be concluded from the analysis of DNA content, proliferation inhibition is due rather to a defect or delay in the transit from G0/G1 to S-phase. In the absence of PCs, activation of PBLs by PHA lead to an enhanced DNA content in approximately one third of PBLs after 2 days, indicating that these have reached S phase. Contact with PCs, however, reduced the percentage of cells reaching S phase at least 2.5-fold. It was reported that unresponsiveness of lymphocytes can also result from infection with RSV; however, this appears to be correlated with a loss of cell surface activation markers, including IL-2 receptor chains (32).
Expression from cDNA of individual RSV surface proteins as well as analysis of genetically engineered RSV lacking surface protein genes clearly identified the RSV F protein as the factor responsible for contact inhibition. This is in striking contrast to the situation with MeV, where a functional complex of F and H is required and the single proteins F and H do not have any effect. RSV F expressed in the absence of other virus proteins was able to inhibit PHA-stimulated lymphocyte proliferation, whereas expression of comparable amounts of cell surface G protein did not lead to inhibition. Moreover, F alone was responsible for the observed species-specific response pattern in human and bovine PBLs, as shown by transfection experiments as well as by chimeric BRSVs in which BRSV and HRSV F genes were exchanged.
Most remarkable, however, although F alone was sufficient for inhibition, coexpression of F along with G markedly enhanced the capacity of F in signaling unresponsiveness. This was again reflected by the behavior of recombinant viruses. RSVs containing either HRSV F or BRSV F as the sole virus surface protein were able to cause inhibition of human and bovine PBLs, yet less efficiently than wild-type virus containing all three virus surface proteins, F, G, and SH. Upon reintroduction of the HRSV G gene into the BRSV-derived construct carrying HRSV F, the inhibiting capacity of wild-type HRSV was fully restored (Fig. 4). These results suggest that RSV G has distinct auxiliary functions in signaling inhibition, while SH protein appears not to contribute.
The role of RSV G in the virus life cycle is much less clear than that of the corresponding type II glycoproteins of other paramyxoviruses, such as MeV hemagglutinin (H). As previously indicated by the analysis of RSV mutants in which the SH and G genes were incomplete or missing (16, 17, 45) and as described here, RSV SH and G are nonessential virus proteins, at least in vitro. This again is in striking contrast to the situation with MeV, where a functional H protein, or rather a functional F/H complex, is an absolute requirement not only for attachment to the cellular MeV receptors CD46 and SLAM and entry into target cells (7, 23, 44), but also for contact-mediated suppression of T cells in vitro and in vivo (24, 36).
Another requirement for MeV contact inhibition is proteolytic cleavage of the fusion protein, whereas membrane fusion is not necessary (47). From these data it appears that only the fusion-competent MeV F/H complex may acquire a structure effective in signaling inhibition. However, it has not been clarified which part of the protein makes the contact to cell surface signaling receptors. This is also true for other members of the Morbillivirus genus recently identified to cause inhibition of T-cell proliferation, such as mumps virus and canine distemper virus (34), rinderpest virus, and peste des petits ruminants virus (J. Heaney, T. Barrett, and S. L. Cosby, Abstr. 11th International Conference on Negative Strand Viruses, abstr. 225, 2000).
RSV G, however, is not required for signaling and is thus probably not involved in direct contact with responsive molecules on the cell surface of PCs. Nevertheless, it considerably supports F-mediated contact inhibition. As the level of F surface expression was not altered in the presence of G, a more efficient transport of F to the cell surface is excluded. It is rather suggested that G may facilitate access to the cell surface by binding to, e.g., heparin-like surface structures (3, 10, 21) or is able to directly or indirectly chaperone surface F into a conformation that allows more efficient interaction with the target ligands on RCs.
The identity of cell surface molecules signaling nonresponsiveness to contacted lymphocytes is not known, nor is it known which mediate membrane fusion and RSV entry into cells. Our experiments showed a differential ability of RSV F proteins from different origins in inhibiting PBLs from different host species. This is also in contrast to MeV-induced contact suppression, which affects PBLs from different species equally (36). Proliferation inhibition of human PBLs was more pronounced when HRSV F protein was used as an effector, whereas PBLs of bovine origin were more sensitive to BRSV F protein. Apparently, this correlates with the permissivity of cells for virus infection, as human and bovine PBLs are only poorly permissive for the heterologous virus, BRSV and HRSV, respectively (unpublished data).
In addition to mitogen-induced proliferation of primary PBLs, spontaneous proliferation of the human T-cell-derived Jurkat line and the B-cell-derived BJAB line could be inhibited more efficiently by HRSV F than by BRSV F (not shown), which also correlates with permissivity of the cells to HRSV and BRSV. However, in all combinations proliferation inhibition was significant. Even proliferation of murine PBLs, which in vitro are nonpermissive for HRSV or BRSV, was slightly reduced after contact with HRSV- or BRSV-infected PCs (data not shown).
This apparent correlation of infectibility and the degree of contact inhibition on first sight prompts the speculation of a common receptor for entry and for signaling unresponsiveness. The degree of the inhibitory signal in RCs after PC contact could reflect the affinity of the different RSV F proteins to this putative receptor. However, only cells of hematopoietic origin are susceptible to proliferation inhibition by RSV. A large variety of cells, such as Hep2, Vero, and BSR, can be readily infected by RSV, while their spontaneous proliferation is not inhibited when they are used as RCs in standard cocultivation experiments with HRSV-infected PCs.
Future experiments should help to clarify whether different receptors are used for entry into hematopoietic or nonhematopoietic cell types. In the case of common receptors, these might be able to signal downstream unresponsiveness only in hematopoietic cells. In the case of MeV-mediated contact inhibition, virus receptors for entry, CD46 and SLAM, are most probably not involved in mediating contact inhibition. This suggests that other leukocyte-specific molecules are responsible for responding to contact. Further experiments towards identification of responder cell structures should be facilitated by the fact that RSV F alone is effective and might reveal whether common mechanisms are involved in morbillivirus and pneumovirus-mediated T-cell suppression or whether individual strategies have been evolved by these diverse viruses.
Acknowledgments
This work was supported by the European Commission (5th Framework Programme, QLK2-CT-1999-00443).
We thank J. A. Melero, Madrid, for providing RSV monoclonal antibodies F6 and G7 and Stefan Finke for valuable comments on the manuscript.
REFERENCES
- 1.Alwan, W. H., W. J. Kozlowska, and P. J. Openshaw. 1994. Distinct types of lung disease caused by functional subsets of antiviral T cells. J. Exp. Med. 179:81–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bembridge, G. P., N. Rodriguez, R. Garcia-Beato, C. Nicolson, J. A. Melero, and G. Taylor. 2000. DNA encoding the attachment (G) or fusion (F) protein of respiratory syncytial virus induces protection in the absence of pulmonary inflammation. J. Gen. Virol. 81:2519–2523. [DOI] [PubMed] [Google Scholar]
- 3.Bourgeois, C., J. B. Bour, K. Lidholt, C. Gauthray, and P. Pothier. 1998. Heparin-like structures on respiratory syncytial virus are involved in its infectivity in vitro. J. Virol. 72:7221–7227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buchholz, U. J., S. Finke, and K. K. Conzelmann. 1999. Generation of bovine respiratory syncytial virus (BRSV) from cDNA: BRSV NS2 is not essential for virus replication in tissue culture, and the human RSV leader region acts as a functional BRSV genome promoter. J. Virol. 73:251–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collins, P. L., K. McIntosh, and R. M. Chanock. 1996. Respiratory syncytial virus, p.1313–1352. In B. N. Fields, D. M. Knipe, P. M. Howley, R. M. Chanock, J. L. Melnik, T. P. Monath, B. Roizman, and S. E. Straus (ed.), Fields virology, vol. 2. Lippincott-Raven, Philadelphia, Pa.
- 6.Domurat, F., N. J. J. Roberts, E. E. Walsh, and R. Dagan. 1985. Respiratory syncytial virus infection of human mononuclear leukocytes in vitro and in vivo. J. Infect. Dis. 152:895–902. [DOI] [PubMed] [Google Scholar]
- 7.Dorig, R. E., A. Marcil, A. Chopra, and C. D. Richardson. 1993. The human CD46 molecule is a receptor for measles virus (Edmonston strain). Cell 75:295–305. [DOI] [PubMed] [Google Scholar]
- 8.Emeny, J. M., and M. J. Morgan. 1979. Regulation of the interferon system: evidence that Vero cells have a genetic defect in interferon production. J. Gen. Virol. 43:247–252. [DOI] [PubMed] [Google Scholar]
- 9.Engelking, O., L. M. Fedorov, R. Lilischkis, V. ter Meulen, and S. Schneider-Schaulies. 1999. Measles virus-induced immunosuppression in vitro is associated with deregulation of G1 cell cycle control proteins. J. Gen. Virol. 80:1599–1608. [DOI] [PubMed] [Google Scholar]
- 10.Feldman, S. A., R. M. Hendry, and J. A. Beeler. 1999. Identification of a linear heparin binding domain for human respiratory syncytial virus attachment glycoprotein G. J. Virol. 73:6610–6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Finke, S., and K. K. Conzelmann. 1999. Virus promoters determine interference by defective RNAs: selective amplification of mini-RNA vectors and rescue from cDNA by a 3′ copy-back ambisense rabies virus. J. Virol. 73:3818–3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graham, B. S. 1996. Immunological determinants of disease caused by respiratory syncytial virus. Trends Microbiol. 4:290–293. [DOI] [PubMed] [Google Scholar]
- 13.Graham, B. S., T. R. Johnson, and R. S. Peebles. 2000. Immune-mediated disease pathogenesis in respiratory syncytial virus infection. Immunopharmacology 48:237–247. [DOI] [PubMed] [Google Scholar]
- 14.Griffin, D. E. 1995. Immune responses during measles virus infection. Curr. Top. Microbiol. Immunol. 191:117–134. [DOI] [PubMed] [Google Scholar]
- 15.Johnson, T. R., J. E. Johnson, S. R. Roberts, G. W. Wertz, R. A. Parker, and B. S. Graham. 1998. Priming with secreted glycoprotein G of respiratory syncytial virus (RSV) augments interleukin-5 production and tissue eosinophilia after RSV challenge. J. Virol. 72:2871–2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kahn, J. S., M. J. Schnell, L. Buonocore, and J. K. Rose. 1999. Recombinant vesicular stomatitis virus expressing respiratory syncytial virus (RSV) glycoproteins: RSV fusion protein can mediate infection and cell fusion. Virology 254:81–91. [DOI] [PubMed] [Google Scholar]
- 17.Karron, R. A., D. A. Buonagurio, A. F. Georgiu, S. S. Whitehead, J. E. Adamus, M. M. Clements, D. O. Harris, V. B. Randolph, S. A. Udem, B. R. Murphy, and M. S. Sidhu. 1997. Respiratory syncytial virus (RSV) SH and G proteins are not essential for viral replication in vitro: clinical evaluation and molecular characterization of a cold-passaged, attenuated RSV subgroup B mutant. Proc. Natl. Acad. Sci. USA 94:13961–13966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keles, I., A. K. Sharma, Z. Woldehiwet, and R. D. Murray. 1999. The effects of bovine respiratory syncytial on normal ovine lymphocyte responses to mitogens or antigens in vitro. Comp. Immunol. Microbiol. Infect. Dis. 22:1–13. [DOI] [PubMed] [Google Scholar]
- 19.Keles, I., Z. Woldehiwet, and R. D. Murray. 1998. In-vitro studies on mechanisms of immunosuppression associated with bovine respiratory syncytial virus. J. Comp. Pathol. 118:337–345. [DOI] [PubMed] [Google Scholar]
- 20.Krilov, L. R., T. W. McCloskey, S. H. Harkness, L. Pontrelli, and S. Pahwa. 2000. Alterations in apoptosis of cord and adult peripheral blood mononuclear cells induced by in vitro infection with respiratory syncytial virus. J. Infect. Dis. 181:349–353. [DOI] [PubMed] [Google Scholar]
- 21.Krusat, T., and H. J. Streckert. 1997. Heparin-dependent attachment of respiratory syncytial virus (RSV) to host cells. Arch. Virol. 142:1247–1254. [DOI] [PubMed] [Google Scholar]
- 22.Meehan, J. T., R. C. Cutlip, H. D. Lehmkuhl, J. P. Kluge, and M. R. Ackermann. 1994. Infected cell types in ovine lung following exposure to bovine respiratory syncytial virus. Vet. Pathol. 31:229–236. [DOI] [PubMed] [Google Scholar]
- 23.Naniche, D., G. Varior-Krishnan, F. Cervoni, T. F. Wild, B. Rossi, C. Rabourdin-Combe, and D. Gerlier. 1993. Human membrane cofactor protein (CD46) acts as a cellular receptor for measles virus. J. Virol. 67:6025–6032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Niewiesk, S., I. Eisenhuth, A. Fooks, J. C. Clegg, J. J. Schnorr, S. Schneider-Schaulies, and V. ter Meulen. 1997. Measles virus-induced immune suppression in the cotton rat (Sigmodon hispidus) model depends on viral glycoproteins. J. Virol. 71:7214–7219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Niewiesk, S., M. Gotzelmann, and V. ter Meulen. 2000. Selective in vivo suppression of T lymphocyte responses in experimental measles virus infection. Proc. Natl. Acad. Sci. USA 97:4251–4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niewiesk, S., H. Ohnimus, J. J. Schnorr, M. Gotzelmann, S. Schneider-Schaulies, C. Jassoy, and V. ter Meulen. 1999. Measles virus-induced immunosuppression in cotton rats is associated with cell cycle retardation in uninfected lymphocytes. J. Gen. Virol. 80:2023–2029. [DOI] [PubMed] [Google Scholar]
- 27.Olchowy, T. W., T. R. Ames, and T. W. Molitor. 1994. Interaction of bovine respiratory syncytial virus with bovine alveolar macrophages in vivo: effects of virus infection upon selected cell functions. Can. J. Vet. Res. 58:42–48. [PMC free article] [PubMed] [Google Scholar]
- 28.Openshaw, P. J. 1995. Immunopathological mechanisms in respiratory syncytial virus disease. Springer Semin. Immunopathol. 17:187–201. [DOI] [PubMed] [Google Scholar]
- 29.Paton, A. W., and P. N. Goldwater. 1990. Respiratory syncytial virus modulation of adult and neonatal lymphocyte mitogenic responses and the role of interferon-gamma. Microb. Pathog. 9:235–241. [DOI] [PubMed] [Google Scholar]
- 30.Preston, F. M., P. L. Beier, and J. H. Pope. 1995. Identification of the respiratory syncytial virus-induced immunosuppressive factor produced by human peripheral blood mononuclear cells in vitro as interferon-alpha. J. Infect. Dis. 172:919–926. [DOI] [PubMed] [Google Scholar]
- 31.Roman, M., W. J. Calhoun, K. L. Hinton, L. F. Avendano, V. Simon, A. M. Escobar, A. Gaggero, and P. V. Diaz. 1997. Respiratory syncytial virus infection in infants is associated with predominant Th-2-like response. Am. J. Respir. Crit. Care Med. 156:190–195. [DOI] [PubMed] [Google Scholar]
- 32.Salkind, A. R., D. O. McCarthy, J. E. Nichols, F. M. Domurat, E. E. Walsh, and N. J. J. Roberts. 1991. Interleukin-1-inhibitor activity induced by respiratory syncytial virus: abrogation of virus-specific and alternate human lymphocyte proliferative responses. J. Infect. Dis. 163:71–77. [DOI] [PubMed] [Google Scholar]
- 33.Sanchez-Lanier, M., P. Guerin, L. C. McLaren, and A. D. Bankhurst. 1988. Measles virus-induced suppression of lymphocyte proliferation. Cell. Immunol. 116:367–381. [DOI] [PubMed] [Google Scholar]
- 34.Schlender, J. 1998. Molekulare Mechanismen der Masernvirus-induzierten Immunsuppression. Ph.D. thesis. Medizinische Fakultät, Universität Würzburg, Würzburg, Germany.
- 35.Schlender, J., B. Bossert, U. Buchholz, and K. K. Conzelmann. 2000. Bovine respiratory syncytial virus nonstructural proteins NS1 and NS2 cooperatively antagonize alpha/beta interferon-induced antiviral response. J. Virol. 74:8234–8242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schlender, J., J. J. Schnorr, P. Spielhoffer, T. Cathomen, R. Cattaneo, M. A. Billeter, V. ter Meulen, and S. Schneider-Schaulies. 1996. Interaction of measles virus glycoproteins with the surface of uninfected peripheral blood lymphocytes induces immunosuppression in vitro. Proc. Natl. Acad. Sci. USA 93:13194–13199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schnorr, J. J., M. Seufert, J. Schlender, J. Borst, I. C. Johnston, V. ter Meulen, and S. Schneider-Schaulies. 1997. Cell cycle arrest rather than apoptosis is associated with measles virus contact-mediated immunosuppression in vitro. J. Gen. Virol. 78:3217–3226. [DOI] [PubMed] [Google Scholar]
- 38.Sharma, A. K., and Z. Woldehiwet. 1996. Replication of bovine respiratory syncytial virus in ovine peripheral blood lymphocytes and monocytes in vitro. Vet. Microbiol. 48:125–134. [DOI] [PubMed] [Google Scholar]
- 39.Sharma, R., and Z. Woldehiwet. 1990. Increased susceptibility to Pasteurella haemolytica in lambs infected with bovine respiratory syncytial virus. J. Comp. Pathol. 103:411–420. [DOI] [PubMed] [Google Scholar]
- 40.Sharma, R., and Z. Woldehiwet. 1991. Depression of lymphocyte responses to phytohaemagglutinin in lambs experimentally infected with bovine respiratory syncytial virus. Res. Vet. Sci. 50:152–156. [DOI] [PubMed] [Google Scholar]
- 41.Sharma, R., and Z. Woldehiwet. 1992. Reinfection of lambs with bovine respiratory syncytial virus. Res. Vet. Sci. 52:72–77. [DOI] [PubMed] [Google Scholar]
- 42.Sharma, R., Z. Woldehiwet, D. G. Spiller, and H. M. Warenius. 1990. Lymphocyte subpopulations in peripheral blood of lambs experimentally infected with bovine respiratory syncytial virus. Vet. Immunol. Immunopathol. 24:383–391. [DOI] [PubMed] [Google Scholar]
- 43.Srikiatkhachorn, A., and T. J. Braciale. 1997. Virus-specific CD8+ T lymphocytes downregulate T helper cell type 2 cytokine secretion and pulmonary eosinophilia during experimental murine respiratory syncytial virus infection. J. Exp. Med. 186:421–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tatsuo, H., N. Ono, K. Tanaka, and Y. Yanagi. 2000. SLAM (CDw150) is a cellular receptor for measles virus. Nature 406:893–897. [DOI] [PubMed] [Google Scholar]
- 45.Techaarpornkul, S., N. Barretto, and M. E. Peeples. 2001. Functional analysis of recombinant respiratory syncytial virus deletion mutants lacking the small hydrophobic and/or attachment glycoprotein gene. J. Virol. 75:6825–6834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Trigo, F. J., R. G. Breeze, H. D. Liggitt, J. F. Evermann, and E. Trigo. 1984. Interaction of bovine respiratory syncytial virus and Pasteurella haemolytica in the ovine lung. Am. J. Vet. Res. 45:1671–1678. [PubMed] [Google Scholar]
- 47.Weidmann, A., C. Fischer, S. Ohgimoto, C. Ruth, V. ter Meulen, and S. Schneider-Schaulies. 2000. Measles virus-induced immunosuppression in vitro is independent of complex glycosylation of viral glycoproteins and of hemifusion. J. Virol. 74:7548–7553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Woldehiwet, Z., and R. Sharma. 1992. Evidence of immunosuppression by bovine respiratory syncytial virus. Scand J. Immunol. Suppl. 11:75–80. [DOI] [PubMed] [Google Scholar]