Abstract
Hormones and neurotransmitters utilize cyclic AMP (cAMP) as a second messenger in signal transduction pathways to regulate cell growth and division, differentiation, gene expression, and metabolism. Adeno-associated virus type 2 (AAV-2) nonstructural protein Rep78 inhibits members of the cAMP signal transduction pathway, the protein kinases PKA and PRKX. We mapped the kinase binding and inhibition domain of Rep78 for PRKX to amino acids (aa) 526 to 561 and that for PKA to aa 526 to 621. These polypeptides were as potent as full-length Rep78 in kinase inhibition, which suggests that the kinase-inhibitory domain is entirely contained in these Rep peptides. Steady-state kinetic analysis of Rep78-mediated inhibition of PKA and PRKX showed that Rep78 appears to increase the Km value of the peptide kinase substrate, while the maximal velocity of the reaction was unaffected. This indicates that Rep78 acts as a competitive inhibitor with respect to the peptide kinase substrate. We detected homology between a cellular pseudosubstrate inhibitor of PKA, the protein kinase inhibitor PKI, and the PRKX and PKA inhibition domains of Rep78. Due to this homology and the competitive inhibition mechanism of Rep78, we propose that Rep78 inhibits PKA and PRKX kinase activity by pseudosubstrate inhibition.
Adeno-associated virus type 2 (AAV-2) is a member of the Parvoviridae family and is assigned to the genus Dependovirus. Productive infection requires coinfection with a helper virus such as adenovirus or herpesvirus (2, 36). The AAV virion consists of a nonenveloped, icosahedral capsid harboring a linear, single-stranded DNA genome of ca. 4.7 kilonucleotides (knt). The coding regions containing the genes for the nonstructural proteins (rep) and capsid proteins (cap) are flanked by 145-nt inverted terminal repeats (ITR) that function in cis as the replication origin. Expression of the rep open reading frame (ORF) produces four proteins, Rep78, Rep68, Rep52, and Rep40, by translating alternatively spliced transcripts initiated from promoters at map units 5 and 19 (25, 29). Rep78 and Rep68 are essential for the production of infectious AAV-2 as well as for targeted integration. The large Rep proteins recognize a binding site within the ITR and possess single-strand DNA nicking, DNA ligase, ATPase, and 3′-to-5′ DNA helicase activities demonstrated in vitro (17, 18, 38, 51). Rep52 and Rep40 appear to be involved directly in the encapsidation of the viral genome into preformed capsids and have also been shown to possess ATPase and 3′-to-5′ DNA helicase activities (4, 10, 39). It has also been observed that Rep expressed in transfected cells causes pleiotropic effects. Rep78 disrupts cell cycle progression (32) and inhibits transformation by viral and cellular oncogenes (14, 21). Rep78 expression alone, or in combination with UV irradiation or incubation with cadmium, induces apoptosis, resulting in cell death (33, 49, 50). Previously, we have shown that several activities of Rep78, including its constitutive ATPase activity, interference with cellular gene expression, and protein interactions, contribute to its deleterious effects on the cell (33). Rep78 has been shown to bind to several cellular proteins, including transcription factors such as Sp1 (15), the transcription cofactor PC4 (44), high-mobility-group nonhistone protein 1 (HMG1) (8), and the oncosuppressor p53 (1). Rep78 also interacts with and inhibits the catalytic subunit of cyclic AMP (cAMP)-dependent protein kinase A (PKA) and its homolog PRKX (22). Thus, Rep78 affects cAMP signal transduction pathways, which play a central role in regulating cell growth and development (6, 9).
A variety of hormones and neurotransmitters utilize cAMP as a second messenger in signal transduction pathways to regulate cell growth and division, differentiation, gene expression, and metabolism (7). PKA is the major responder of cAMP in the mammalian cell. In the absence of cAMP, PKA forms an inactive heterotetramer consisting of two regulatory subunits (R) and two catalytic subunits (C). There are two classes of PKA, types I and II, which contain RI or RII regulatory subunits bound to a common C subunit (41). RI and RII differ in tissue specificity, subcellular localization, and affinity for cAMP (7). Multiple isoforms of the regulatory subunits (RIα, RIβ, RIIα, RIIβ) and catalytic subunits (Cα, Cβ, Cγ) are expressed and may contribute to the specificity of PKA (37). Upon binding of cAMP, the PKA holoenzyme dissociates into R2-cAMP4 and the active catalytic subunits. PKA affects the cell by transcriptional regulation as well as by controlling the activity of metabolic enzymes, such as glycogen synthase and pyruvate kinase, via phosphorylation (13). PKA activates gene expression via cAMP-responsive promoter elements (CRE). The active C subunit translocates into the nucleus, where it is able to phosphorylate, and thereby activate, transcription factors such as CREB, which when bound to a CRE site of cAMP-regulated promoters induce gene expression (27). Examples of CREB-regulated genes include c-fos and eNOS (31, 48).
PRKX has 53% identity and 75% homology to the catalytic subunit of PKA (Cα). PRKX has been shown to transactivate CREB-dependent expression via CREs (9) and phosphorylates a synthetic PKA peptide substrate, kemptide. These results suggest that PRKX is a member of the cAMP second messenger system pathway. One report describes the PRKX gene as specifically expressed in macrophages and granulocytes and as essential for myeloid differentiation (35).
In this study, we mapped the domain of Rep78 necessary to bind and inhibit the cAMP-dependent kinases PKA and PRKX. The kinetics and mechanism of this inhibition were analyzed. We show that Rep78 competes for the substrate binding and shares limited homology with a naturally occurring pseudosubstrate inhibitor of PKA—the protein kinase inhibitor PKI. Our results suggest that Rep7-mediated inhibition of PKA and PRKX occur through the same mechanism.
MATERIALS AND METHODS
Construction of plasmids.
A plasmid encoding a nuclear localized maltose binding protein (MBP) of Escherichia coli under the control of a T7 or cytomegalovirus promoter, pCI-Mal, was constructed by ligating the sequence coding for the simian virus 40 large T antigen nuclear localization signal PKKKRKV (19) to the MBP open reading frame (ORF) using PCR. The MBP ORF of pMAL-c2 (New England BioLabs) was amplified by PCR using primers 5′-TCTCTGCTAGCCACCATGGTTCCTAAGAAGAAGCGTAAGGTGAAAACTGAAGAAGGTAAACTG-3′ and 5′-CGCGCCCGGGTCCTTCCCTCGATCCCGAGGTT-3′, digested by NheI/XbaI, and cloned into pCI (Stratagene). Various fragments of the AAV-2 rep gene were cloned as carboxy-terminal fusions with MBP in pCI-Mal by PCR using the primers given in Table 1 with pAV2, a plasmid containing the AAV-2 genome (24), as a template. The rep PCR fragments were XbaI digested and inserted into XbaI/NotI-cut pCI-Mal to generate pCI-Mal-R1-5, pCI-Mal-RA1-5, and pCI-Mal-R3/RA3. Bacterial expression vectors encoding the MBP-Rep fusion proteins were cloned by ligating XbaI/BamHI fragments from pCI-Mal-R1-5, pCI-Mal-RA1-5, and pCI-Mal-R3/RA3 into pMal-c2, resulting in plasmids pMal-R1-5, pMal-RA1-5, and pMAl-R3/RA3. Single amino acid substitutions were introduced in pMal-R2 and pCI-Mal-R2 by PCR-based mutagenesis using the QuikChange Site-Directed Mutagenesis Kit (Stratagene) according to the manufacturer’s instructions. The primers used for this cloning are given in Table 1. The resulting plasmids, pMal-R2-M1-4 and pCI-Mal-R2-M1-4, have the following point mutations: R536A (M1), H537A (M2), G539S (M3), and R548A (M4). Amino acid changes given are relative to the wild-type Rep78 sequence. Mutations were confirmed by DNA sequencing. pET-41/PKA, a bacterial expression vector encoding the catalytic subunit of the PKA fused to the amino terminus of glutathione S-transferase (GST) and a His tag, was cloned by PCR. PKA sequences were amplified using primers 5′-GCGAAGCTTATGGGCAACGCCGCCGCCGCC-3′ and 5′-GTGCTCGAGCTAAAACTCAGTAAACTCCTT-3′ with pFC-PKA (Stratagene) as a template, digested with HindIII/XhoI, and inserted into similarly digested pET-41 (Novagen).
TABLE 1.
Primers used
| Name | Plus-strand primer | Minus-strand primer |
|---|---|---|
| R1 | CGCGCCCGGGAGCATGAATTCTACGTCAAAA | TTATTGTTCAAAGATGCAGTC |
| R2 | CGCGCCCGGGACGCGGAAGCTTCGATCAACT | TTATTGTTCAAAGATGCAGTC |
| R3 | GCGCCCGGGACTACGCAGACAGGTACCAA | TTATTGTTCAAAGATGCAGTC |
| R4 | GCGCCCGGGACGCAGACAGGTACCAAAAC | TTATTGTTCAAAGATGCAGTC |
| R5 | GCGCCCGGGACCAAAACAAATGTTCTCGT | TTATTGTTCAAAGATGCAGTC |
| RA1 | CGCGCCCGGGAGCATGAATTCTACGTCAAAA | GGCTATGGCACCTTTCCCATGATATG |
| RA2 | CGCGCCCGGGAGCATGAATTCTACGTCAAAA | TCAAGAAACGGGTTGAGATTCTGACA |
| RA3 | CGCGCCCGGGAGCATGAATTCTACGTCAAAA | GGTTAGAAGCAGATATTTGAATTCTG |
| RA4 | CGCGCCCGGGAGCATGAATTCTACGTCAAAA | GGTTAATTCATTCTCTCGCATTGTCT |
| RA5 | CGCGCCCGGGAGCATGAATTCTACGTCAAAA | GGTTATTGTCTGCAGGGAAACAGCAT |
| R3/RA3 | GCGCCCGGGACTACGCAGACAGGTACCAA | GGTTAGAAGCAGATATTTGAATTCTG |
| M1 | AGGTACCAAAACAAATGTTCTGCACACGTGGGCATGAATCTGATG | CATCAGATTCATGCCCACGTGTGCAGAACATTTGTTTTGGTACCT |
| M2 | TACCAAAACAAATGTTCTCGTGCAGTGGGCATGAATCTGATGCTG | CAGCATCAGATTCATGCCCACTGCACGAGAACATTTGTTTTGGTA |
| M3 | AACAAATGTTCTCGTCACGTGTCTATGAATCTGATGCTGTTTCCC | GGGAAACAGCATCAGATTCATAGACACGTGACGAGAACATTTGTT |
| M4 | AATCTGATGCTGTTTCCCTGCGCACAATGCGAGAGAATGAATCAG | CTGATTCATTCTCTCGCATTGTGCGCAGGGAAACAGCATCAGATT |
Protein expression and purification.
MBP fusion proteins expressed from pMal-c2, pMal-R1-5, pMal-RA1-5, pMal-R3/RA3, and pMal-R2-M1-4 in E. coli DH5α (Invitrogen) were purified by amylose affinity chromatography as previously described (39).
The GST- and His-tagged PKA fusion protein (GST-PKA) was produced from pET-41/PKA-transformed E. coli BL21(DE3) (Novagen) and purified using His-Bind Quick Columns (Novagen) according to the manufacturer’s instructions.
GST-PKA and GST-PRKX coprecipitation.
Radiolabeled recombinant proteins were generated by in vitro translation using the TNT Quick Coupled Transcription/Translation System (Promega) and [35S]methionine according to the manufacturer’s instructions. In vitro translation reaction mixtures were diluted 10-fold with phosphate-buffered saline (PBS) containing 1 mg of bovine serum albumin (BSA; Sigma)/ml and 0.5% NP-40 (Pierce). After addition of 1 μg of either GST-PKA or GST-PRKX (6), reaction mixtures were incubated for 30 min at 4°C before 40 μl of glutathione-conjugated resin (Cytosignal) was added. Following a 30-min incubation at 4°C, samples were washed three times with 0.5% NP-40 in PBS using the GSTcatcher system (Cytosignal). The precipitated protein was eluted from the resin with 10 mM glutathione in PBS. Eluates were analyzed by electrophoresis on sodium dodecyl sulfate (SDS)-Tris-glycine polyacrylamide gels. After drying under a vacuum, the gels were autoradiographically imaged with X-ray film (Kodak).
Kinase assay.
The kinase activities of GST-PRKX and PKA toward a dye-labeled synthetic PKA peptide substrate (kemptide) were determined using the PepTag cAMP-Dependent Protein Kinase Assay (Promega) under various conditions in a final volume of 25 μl of 1× reaction buffer (20 mM Tris [pH. 7.4], 10 mM MgCl2, 1 mM ATP). Reactions were stopped by boiling for 10 min. The degree of kemptide phosphorylation was quantified by fractionating the samples by agarose electrophoresis followed by quantitative analysis using a Storm 860 PhosphorImager (Molecular Dynamics). In an electric field, phosphorylated kemptide migrates toward the anode, while nonphosphorylated peptide migrates toward the cathode. Data were analyzed and plotted using Prism 3 for Macintosh (GraphPad Software).
RESULTS
Mapping of the PKA/PRKX binding region of Rep78.
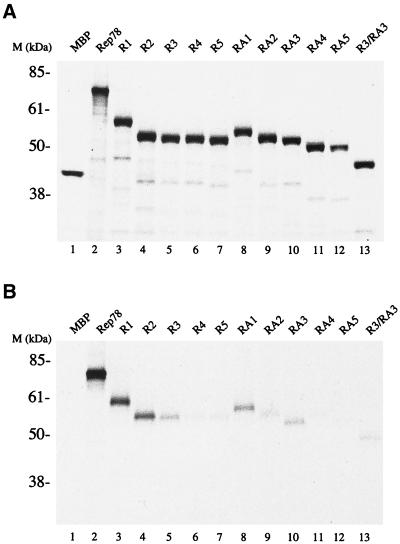
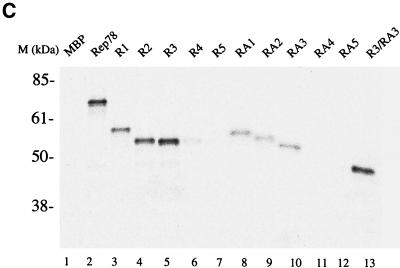
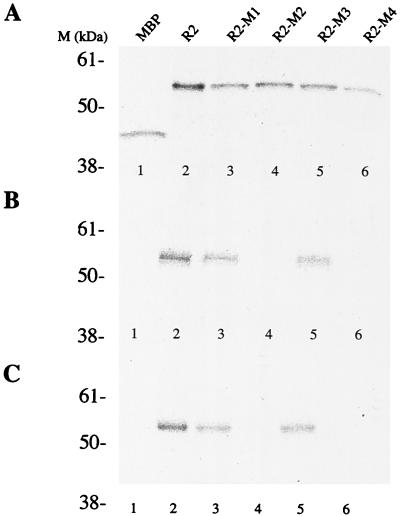
The interaction of Rep78 and cAMP-dependent protein kinase X (PRKX) was originally determined by two-hybrid screening studies (6, 9). Subsequent experiments demonstrated that Rep78 inhibits the kinase activities of PKA and PRKX. Rep68, which is translated from a spliced rep mRNA encoding the first 529 amino acids (aa) of Rep78 fused to an additional 7 aa unique for Rep68, was reported not to bind (9), or to bind only with low affinity (6), to PRKX. This suggests that at least part of the Rep-PKA/PRKX interaction region lies within the carboxy-terminal 92 aa of Rep78. To determine the Rep78 regions necessary to interact with PKA and PRKX, the carboxy-terminal 140 aa of Rep78 were expressed as a fusion protein with MBP (R1). R1, Rep78, and MPB were expressed and [35S]Met labeled by in vitro transcription and translation (Fig. 1A). Radiolabeled proteins were used in pulldown experiments with nonlabeled GST-PKA or GST-PRKX and glutathione resin. The carboxy-terminal 140 aa of Rep fused to MBP (R1) were found to be sufficient for stable interaction with PKA and PRKX (Fig. 1B and C) at levels comparable to those for full-length Rep78. There was no detectable interaction between MBP and either PRKX or PKA. A set of amino-terminal deletions of R1, R2, R3, R4, and R5, as well as carboxy-terminal deletions RA1, RA2, RA3, RA4, and RA5, was generated to delineate the residues involved in the interaction with PKA and PRKX. Recombinant proteins were expressed in vitro (Fig. 1A) and precipitated with glutathione resin in the presence of either GST-PKA or GST-PRKX (Fig. 1B and C). Polypeptides with amino-terminal deletions of Rep78 to aa 526 (R3) and those with carboxy-terminal deletions to aa 561 (RA3) retained affinity for PRKX and PKA. A Rep78 fragment consisting of residues 526 to 561 fused to MBP (R3/RA3) was found to be sufficient for stable interaction with PRKX (Fig. 1C), while only weak binding to PKA was observed (Fig. 1B).
FIG. 1.
Interactions of Rep78-derived peptides with PKA and PRKX. Radiolabeled proteins were produced by coupled in vitro transcription-translation in the presence of [35S]Met. (A) Autoradiograph of SDS-polyacrylamide gel electrophoresis showing aliquots of the in vitro translation reactions. (B and C) Soluble GST-kinase fusion proteins were precipitated from the reaction with glutathione-agarose resin. Recovery of radiolabeled protein is due to interaction between the in vitro translation products and GST-kinase. (B) Interaction between in vitro-translated proteins and PKA. (C) Interaction between in vitro translated proteins and PRKX. R1, R2, R3, R4, and R5 are amino-truncated Rep peptides; RA1, RA2, RA3, RA4, and RA5 are carboxyl-truncated Rep peptides; and R3/RA3 is truncated at both the amino and carboxy termini. All of the Rep peptides are fused to the carboxy terminus of MBP. The in vitro-translated protein(s) used in each reaction is given above the gel.
The recoveries of GST-PKA and GST-PRKX precipitation with glutathione resin were monitored by Coomassie blue staining of the polyacrylamide gels (data not shown). No significant differences between independent pulldown assays were found.
Mapping of the PKA/PRKX inhibition region of Rep78.
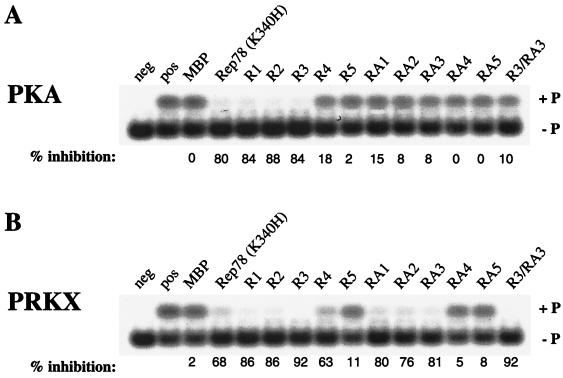
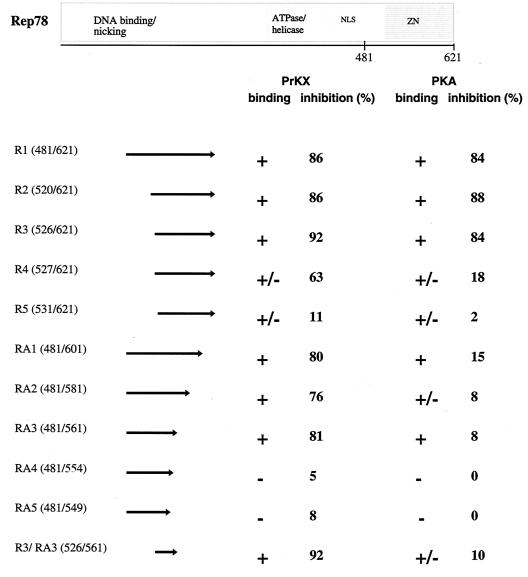
In order to map the residues of Rep78 necessary for PKA/PRKX inhibition, we studied the effects of bacterially expressed Rep polypeptides fused to MBP, R1, R2, R3, R4, R5, RA1, RA2, RA3, RA4, and RA5, on the kinase activity of PKA and PRKX toward the artificial heptapeptide substrate referred to as kemptide (LRRASLG) (Fig. 2). The ATPase-deficient Rep78 mutant Rep78 (K340H) (39) was used as a control instead of wild type Rep78, since the ATPase activity of Rep is constitutive and independent of a DNA substrate (51). The truncated Rep78 fusion protein R1 inhibited PKA- and PRK-mediated phosphorylation of kemptide at levels comparable to those with full-length Rep78 (K340H) fusion protein, while MBP by itself had little effect on the kinase activity of either PKA or PKRX. The R1 amino-terminal deletion products R2 and R3 efficiently inhibited cAMP-dependent kinase activity, while R4 and R5 were much less effective inhibitors of PKA and PRKX. All carboxy-terminal deletions of R1 showed little (RA1, RA2, and RA3) or no (RA4 and RA5) effect on PKA-mediated phosphorylation, while the carboxy-terminal 60 aa of Rep78 were not essential for PRKX inhibition (this sequence is deleted in RA3). Extending the carboxy-terminal deletions in RA4 and RA5 resulted in a profound loss of PRKX inhibition. R3/RA3, containing the Rep78 residues 526 to 561 fused to MBP, was sufficient to efficiently inhibit PRKX but showed little effect on PKA. The results of the mapping study are summarized in Fig. 3.
FIG. 2.
Effects of Rep78 (K340H) and Rep78 derivatives on PKA and PRKX phosphorylation of kemptide. Recombinant proteins (1 μg) were incubated in the presence of 2 μg of fluorescently labeled kemptide with either 8 ng of PKAC for 15 min (A) or 150 ng of PRKX for 30 min (B) at ambient temperatures. The phosphorylated form of kemptide (+ P) was resolved from the aphosphorylated form (− P) by agarose gel electrophoresis. The extent of phosphorylation could then be determined quantitatively. The lane marked “pos” is kinase without inhibitor and defines the 0% inhibition level. Rep78 (K340H), ATPase-deficient Rep78 mutant; R1, R2, R3, R4, and R5, amino-truncated Rep peptides; RA1, RA2, RA3, RA4, and RA5, carboxyl-truncated Rep peptides; R3/RA3 is truncated both at the amino and carboxy termini. All of the Rep peptides are fused to the carboxy terminus of MBP.
FIG. 3.
Schematic representation of Rep78 and Rep peptides. The Rep peptides used in the experiments are listed on the left, with the corresponding Rep amino acid numbers given in parentheses. Arrows represent the regions of Rep in each peptide. The extents of binding to PRKX and PKA are represented by plus signs (strong interaction), minus signs (little or no interaction), or plus-or-minus signs (weak interaction), and the extents of inhibition are shown as percentages. NLS, nuclear localization signal; ZN, putative zinc finger domain.
Efficiency of inhibition.
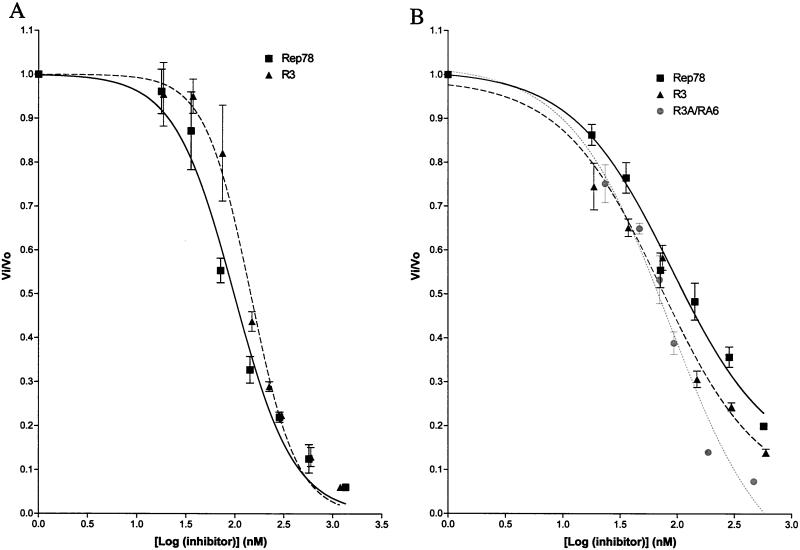
After identifying the regions within Rep78 which were necessary to bind and inhibit PKA and PRKX, we compared the inhibitor potencies of Rep78 (K340H), R3, and R3/RA3. The phosphotransferase activities of PKA and PRKX toward kemptide were measured in the presence of varying concentrations of Rep-derived MBP fusion proteins (Fig. 4). The dose-response plot was used to determine the IC50 (inhibitor concentration required to cause 50% inhibition) for each inhibitor by fitting the data to a sigmoidal function. R3 and Rep78 (K340H) displayed similar inhibitory effects on PKA, and only small differences in IC50s of 98 nM [Rep78 (K340H)] and 145 nM (R3) were observed. The kinase activity of PRKX was blocked by Rep78 (K340H) (IC50, 89 nM), R3 (IC50, 71 nM), and R3/RA3 (IC50, 85 nM). The similar PRKX IC50s obtained with full-length Rep78 (K340H) and the Rep-derived peptide fusion proteins R3 and R3/RA3 suggest that the kinase-inhibitory regions are entirely contained within these Rep peptides.
FIG. 4.
Inhibition of PKA and PRKX by Rep-derived peptides. Phosphorylation of fluorescently labeled kemptide was measured with increasing amounts of inhibitor. (A) PKA (10 nM) and kemptide (60 μM)were incubated for 15 min at ambient temperature with increasing amounts of MBP-Rep78 (K340H) or R3. (B) PRKX (88 nM) was incubated with kemptide (60 μM) for 15 min at ambient temperature with increasing amounts of either MBP-Rep78 (K340H), R3, or R3/RA3. Phosphorylated kemptide and unphosphorylated kemptide were resolved by agarose gel electrophoresis, and the relative amounts of each form of kemptide were determined on a Storm 860 PhosphorImager (Molecular Dynamics). Vo, reaction velocity in the absence of inhibitor; Vi, reaction velocity in the presence of inhibitor. Each data point is the arithmetic mean of three reactions. Error bars, sample standard deviations.
Kinetics and mechanism of Rep78 inhibition of PKA and PRKX.
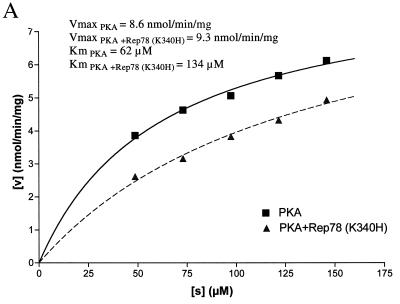
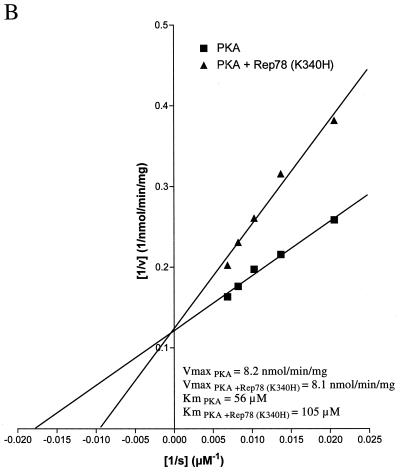
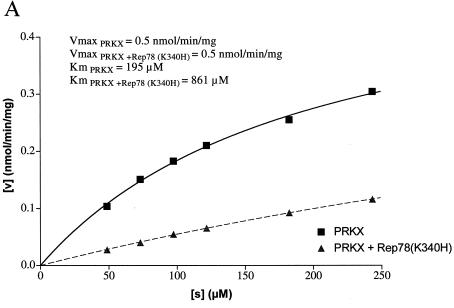
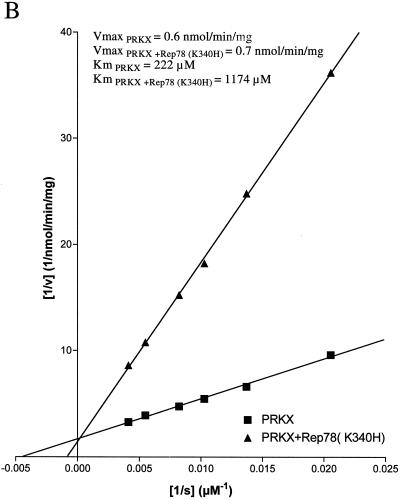
The ability of Rep78 (K340H) to inhibit PKA and PRKX was characterized by steady-state kinetic analysis. The kinase activity was measured at increasing concentrations of kemptide, while the concentration of Rep78 (K340H) was kept constant (Fig. 5A and 6A). As expected, the velocity of kemptide phosphorylation was reduced in the presence of Rep78 (K340H), but escalating kemptide concentrations resulted in increased kemptide phosphorylation in the presence or absence of Rep78. Values for Km and Vmax were obtained by fitting the data to the hyperbolic function Y = Vmax · X/(Km + X), where X is the kemptide concentration, Vmax is the maximal velocity at saturation, and Km is the substrate concentration required to reach half-maximal velocity. In addition, the data were displayed as a Lineweaver-Burk plot (Fig. 5B and 6B). The values for Vmax and Km are readily derived from this graph. The y intercept is defined as 1/Vmax, and the x intercept is defined as −1/Km. Data analysis by both methods demonstrated that the maximal velocities of PKA and PRKX were not significantly affected by the presence of Rep78 (K340H), while the apparent values for Km were significantly increased. The increase in Km, combined with unchanged Vmax in the presence of an inhibitor, is characteristic for competitive inhibition. We therefore propose that Rep78 acts as a competitive inhibitor for PKA and PRKX with respect to kemptide.
FIG. 5.
Steady-state kinetic analysis of PKA inhibition. PKA (8 ng) was incubated for 15 min at ambient temperature with various amounts of fluorescently labeled kemptide in the absence (squares) or presence (triangles) of MBP-Rep78 (K340H) (300 ng). Phosphorylated kemptide and unphosphorylated kemptide were resolved by agarose gel electrophoresis, and the amount of phosphorylated kemptide was determined by analysis on a Storm 860 PhosphorImager (Molecular Dynamics) in order to calculate the velocity of the reaction (v [expressed as nanomoles of phosphorylated kemptide per minute per milligram of enzyme]). Values for Km and Vmax were obtained by fitting the data to the hyperbolic function Y = Vmax · X/(Km + X), where X is the substrate concentration ([s]), Vmax is the maximal velocity at saturation, and Km (the Michaelis constant) is the substrate concentration required to reach half-maximal velocity. (B) The data obtained in panel A were analyzed by Lineweaver-Burk algorithm and displayed as a double-reciprocal graph. Maximum velocity, 1/Vmax, is obtained from the Y-intercept, and the X-intercept is −1/Km.
FIG. 6.
Steady-state kinetic analysis of PRKX inhibition. (A) PRKX (150 ng) was incubated for 30 min at ambient temperature with various amounts of fluorescently labeled kemptide in the absence (squares) or presence (triangles) of MBP-Rep78 (K340H) (500 ng). Phosphorylated kemptide and unphosphorylated kemptide were resolved by agarose gel electrophoresis, and the amount of phosphorylated kemptide was determined by analysis on a Storm 860 PhosphorImager (Molecular Dynamics) in order to determine the velocity of the reaction (v). Values for Km and Vmax were obtained by fitting the data to the hyperbolic function. (B) The data obtained in panel A were analyzed by Lineweaver-Burk algorithm and displayed as a double-reciprocal graph. Maximum velocity, 1/Vmax, is obtained from the Y-intercept, and the X-intercept is −1/Km.
Rep78 shares limited homology with PKI.
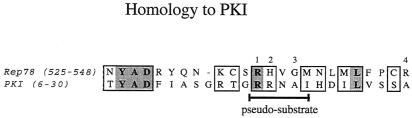
R3/RA3 and R3, the identified PRKX and PKA interaction elements of Rep78, share limited homology with the protein kinase inhibitor (PKI) (Fig. 7). PKI interacts with the free catalytic subunit of PKA (PKAC) and mediates active shuttling of the complex out of the nucleus (45). It inhibits PKAC by pseudosubstrate inhibition (43, 46). The PKI pseudosubstrate pentapeptide motif RRNAI resembles the PKA substrate consensus sequence RRXSU, where X is any residue and U is a hydrophobic amino acid (47), and occupies the catalytic site of PKAC to competitively inhibit substrate phosphorylation. The pseudosubstrate site of PKI alone is not sufficient for inhibition. Residues amino-terminal of the pseudosubstrate motif form an amphipathic helix which has been shown to be essential for high-affinity binding to the catalytic subunit of PKA (23). PKI also binds to PRKX, but the observed Kd is 30-fold higher than that for PKAC (52).
FIG. 7.
Alignment of PKI and Rep. Amino acid residues 6 to 30 of PKI and 525 to 548 of Rep78 are juxtaposed. Boxed residues are similar; dark shading indicates residues conserved between Rep78 and PKI. Alignments were performed with MacVector software (Oxford Molecular Group). The position of the pseudosubstrate element of PKI is shown. Numbers on top indicate residues substituted in M1 (R536A), M2 (H537A), M3 (G539S), and M4 (R548A).
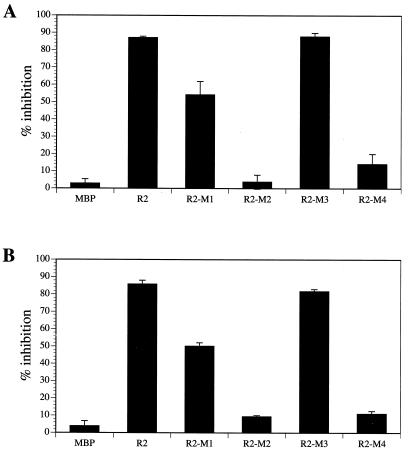
We identified residues 536-RHVGM of Rep78 as a putative pseudosubstrate site for PKA and PRKX by aligning Rep78 with PKI and by the similarity to a PKA substrate. We analyzed the importance of these residues for interaction with the serine/threonine kinases by introducing point mutations within this motif. In R2-M1 and R2-M2, basic amino acids were changed to alanine (R536A and H537A substitutions, respectively). We also analyzed a G539S mutation (R2-M3), which might convert the pseudosubstrate to a substrate. In addition, a peptide with the amino acid substitution R548A (peptide R2-M4) was produced. The Arg residue is outside of the homology region between Rep78 and PKI but within the element shown to be essential for PKA and PRKX inhibition. Recombinant proteins were expressed and [35S]Met labeled by in vitro transcription and translation (Fig. 8A). The affinities of the labeled proteins to PKA and PRKX were determined by precipitation with glutathione resin in the presence of either GST-PKA or GST-PRKX (Fig. 8B and C, respectively). R2-M1 and R2-M3 demonstrated relatively high affinities to PKA and PRKX, while the H537A (R2-M2) and R548A (R2-M4) substitutions resulted in loss of binding (Fig. 8B and C). We also analyzed the abilities of the recombinant proteins to interfere with the kinase activities of PKA and PRKX (Fig. 9). The G539S substitution in R2-M3 showed no effect on the potency as a PKA/PRKX inhibitor, while the R536A mutation (R2-M1) resulted in a ca. 40% reduced capability to block PKA- and PRKX-catalyzed kemptide phosphorylation. R2-M2 and R2-M4, which demonstrated no specific binding to either PKA or PRKX, did not significantly interfere with PKA and PRKX kinase activities. Thus, inhibition is dependent on binding.
FIG. 8.
Interactions between Rep-derived peptides and PKA or PRKX. Point mutations were introduced into the putative pseudosubstrate motif and expressed in a coupled in vitro transcription-translation reaction with [35S]Met. (A) Autoradiograph of in vitro-translated products following SDS-polyacrylamide gel electrophoresis. (B and C) The GST-kinase fusion proteins were precipitated from the reaction with glutathione-agarose resin and resolved by SDS-polyacrylamide gel electrophoresis. Recovery of radiolabeled protein is due to interaction between the in vitro translation products and GST-kinase. (B) Interaction between in vitro-translated proteins and PKA. (C) Interaction between in vitro-translated proteins and PRKX. Substitutions: R2-M1, R536A; R2-M2, H537A; R2-M3, G539S; R2-M4, R548A.
FIG. 9.
Inhibition of kinase activity in the presence of Rep peptide and mutated Rep peptide inhibitors. Rep pseudosubstrate site amino acids were expressed as MBP fusion proteins and incubated with 8 ng of PKA (A) or 150 ng of PRKX (B). The fluorescently labeled kemptide (2 μg) was used as a substrate, which can be resolved by agarose gel electrophoresis into phosphorylated and aphosphorylated forms. The amounts of each form of kemptide were determined by Storm 860 PhosphorImager analysis. Inhibition of phosphorylation was calculated as the percentage of kemptide phosphorylation in the presence of inhibitor compared to that for the untreated control. Data presented are means ± standard errors of values from three experiments. Substitutions: R2-M1, R536A; R2-M2, H537A; R2-M3, G539S; R2-M4, R548A.
DISCUSSION
Extracellular stimuli affect cell metabolism, growth, differentiation, and cell death in part by changing the intracellular concentration of cAMP, which serves as a second messenger for signal transduction (7, 40). The major responder in the cell to cAMP is PKA, a holoenzyme consisting of two regulatory subunits bound to the catalytic subunit (PKAC). PKAC is expressed as a fully active kinase and is maintained in an inactive state by forming a stable complex with an inhibitor (41). Two classes of cellular inhibitors of PKAC have been described—the regulatory subunits RI and RII and the heat-stable protein kinase inhibitor PKI. In addition to these cellular proteins, the AAV-2 Rep78 protein was shown to bind and inhibit PKAC as well as its homolog PRKX (6, 9). In this study, we mapped the PKA and PRKX interaction domains of Rep78 and analyzed the mechanism of inhibition. Protein kinases in general can be inhibited by a variety of mechanisms (reviewed in reference (42)). For example, a pseudosubstrate inhibitor resembling the physiological kinase substrate competes for binding to the kinase catalytic site with the peptide substrate (20). PKI and the regulatory subunits reduce PKA kinase activity by pseudosubstrate inhibition (41). An alternative mechanism is adenine mimetic inhibition. Here, a peptide fragment of the inhibitor is thought to resemble the adenine ring of ATP. This peptidic antagonist occupies the ATP binding pocket of the kinase, thereby preventing kinase-mediated phosphate transfer. One example is P27Kip1, which inhibits the cdk2-cyclin complex by adenine mimetic inhibition (30). Finally, allosteric changes in the protein conformation may also be utilized to control kinase activity (42).
We analyzed which region of Rep78 interacts with PRKX and PKA. Comparison of the sequence and structure of the Rep78 kinase inhibitory domain with those of other known kinase inhibitors might give some indication of the mechanism of Rep78-mediated kinase inhibition. Our initial experiments showed that the carboxy-terminal 140 aa (R1) bind and inhibit both PKA and PRKX at levels comparable to those for full-length Rep78. Amino- and carboxy-terminal deletion mutation analysis of this Rep78 polypeptide led to identification of the Rep78 domain, necessary for PRKX and PKA interaction. We observed that every deletion mutant of R1 that bound to PRKX was also capable of inhibiting PRKX. This suggests that the PRKX binding and inhibition domains of Rep78 coincide or are continuous. The Rep78 fragment comprising aa 526 to 561 (R3/RA3) fused to MBP was necessary and sufficient for stable interaction with PRKX and inhibition of PRKX kinase activity. R3/RA3 inhibited PRKX but demonstrated relatively low affinity to PKA and acted as a poor inhibitor of PKA. Rep78 residues 526 to 621 (R3) were needed for efficient blockage of PKA-mediated phosphorylation of kemptide, while aa 481 to 561 were sufficient to mediate PKA interaction but not inhibition. The PRKX and PKA interaction domains of Rep78 appear to be distinct but overlapping. These domains share the same amino-terminal region (aa 526 to 561), but a carboxy-terminal extension of aa 562 to 621 was needed to inhibit PKA. This result suggests that the interactions of Rep78 with PKA and PRKX are different. It might be that the Rep78/PKA interaction domain is noncontinuous with additional elements in the region between aa 562 to 621. This region might also act indirectly, by affecting the folding of aa 512 to 561. In addition, the carboxy-terminal region of Rep78 contains a putative zinc finger motif (9, 16) which might interact with other proteins to mediate PKA inhibition. Differences in binding to PRKX and PKAC were also observed with regulatory subunit types I and II (52). RI and RII bind to PKAC, but only type II interacts with PRKX. RI and RII have significant sequence diversity amino-terminal of the inhibition site and interact with different sites on the catalytic subunit of PKA (5). The interactions of the regulatory subunits RI and RII with PKAC are also described as bipartite. Distinct and nonoverlapping regions of RI and RII mediate binding and inhibition of PKAC (5, 41).
The potencies of Rep78 and Rep-derived peptides to inhibit the serine/threonine kinase activities of PRKX and PKA were compared by determining the respective IC50s. Deletion of the amino-terminal 525 aa of Rep78 in R3 had no effect on the efficiency of its function as a PKA inhibitor, since we observed PKA IC50s of about 100 nM for Rep78 (K340H) and 150 nM for R3. This finding demonstrates that the carboxy-terminal 96 aa (R3) are almost as effective as full-length Rep78 in interference with substrate phosphorylation, and it indicates that this region might contain the complete Rep78/PKA interaction domain. The comparable IC50s for Rep78 (K340H), R3, and R3/RA3 (all around 80 nM) define the Rep78/PRKX inhibition domain as residues 526 to 561 (R3/RA3).
The inhibition curves indicate that at a 1:1 stoichiometry of kinase and inhibitor, Rep78 (K340H) inhibits PRKX about 50%. Physiological kinase inhibitors, PKI and the regulatory subunit RIα, were shown to inhibit PRKX 60 and 80%, respectively, at a 1:1 molar ratio (52). Therefore, Rep78 (K340H) appears to be a potent inhibitor of PRKX. A 10-fold molar excess of Rep78 (K340H) versus PKA was required to inhibit PKA activity 50%. This result indicates that Rep78 inhibits PRKX more efficiently than it inhibits PKA. This result was unexpected, since our coprecipitation experiments indicate similar levels of interaction between Rep78 and PRKX or PKA. Also, the binding constants of Rep52 for PKA and PRKX were shown to be similar (6). These data suggest that the observed differences in the abilities to inhibit PKA and PRKX kinase activities are due not to a lower affinity of Rep78 with PKA than with PRKX but to other mechanisms not yet identified.
The 36-aa fragment of Rep78, R3/RA3, shares limited homology with the protein kinase inhibitor PKI. PKI is a competitive inhibitor of PKA with respect to the peptide substrate (43, 46). PKI contains an inhibitor sequence (18-RRNAI) which resembles the PKA substrate consensus sequence RRXSU, where X is any residue, U is a hydrophobic amino acid, and serine is the phosphate acceptor (47). Many natural PKA substrates contain only a single basic residue at position −2 or −3 relative to the site of phosphate transfer but have additional basic residues more amino-terminal at position −4 or −6 (28, 47). PKI mimics the natural PKA substrate and inhibits PKA by pseudosubstrate inhibition. The inhibitory sequence alone is not sufficient to mediate PKA inhibition. Residues that are amino-terminal of the pseudosubstrate element form an α-helix that interacts with PKA and are essential for efficient inhibition (23). The interaction between PKI and PKA is therefore bipartite, with distinct inhibition and binding elements which are continuous. The PKI derived peptide, aa 5 to 24, is sufficient for PKA inhibition (12). We observed homology between Rep78 and this PKI polypeptide. Replacement of the arginine residues in positions 18 and 19 of PKI caused a substantial decrease in inhibitory potency (34). Mutation of the homologous amino acids of R2 in R2-M1 (R536A) and R2-M2 (H537A) resulted in decreased efficiency in PKA and PRKX inhibition and, in the case of R2-M1, caused a loss of affinity to PRKX and PKA. These results demonstrate that the amino acids of Rep78, which are homologous to the PKI pseudosubstrate site, have a significant impact on the inhibitor potency of Rep78. In PKI, replacement of alanine-21 with serine converted the inhibitor into a substrate with a relatively low affinity (Km, 280 μM) for the enzyme (34). Substitution of the homologous amino acid in R2-M3 (G539S) resulted in no obvious change in inhibitor potency with regard to either PKA or PRKX. This might be explained in part by our observation that the binding affinities of R2-M2 and R2 for PKA and PRKX are the same. The inhibitory sites of the PKA regulatory subunits that occupy the peptide binding site of PKAC contain either a pseudosubstrate motif, RRGAI, as in RI, or a PKA substrate sequence, RRVSV, as in RII. That demonstrates that even a substrate can act as an inhibitor of PKA and might also explain the results obtained with R2-M2. The results so far indicate that Rep78 might inhibit PKA and PRKX by pseudosubstrate inhibition analogous to that of PKI and RI. This was further confirmed when we analyzed the steady-state kinetics of the Rep78-mediated inhibition of PKA and PRKX. Rep78 had no effect on the maximal velocity of the kinases but reduced the apparent affinity for the peptide substrate kemptide. These findings are characteristic of competitive inhibition, in this case with respect to the peptide substrate. The data presented here provide strong evidence that Rep78 inhibits PKA and PRKX by pseudosubstrate inhibition. The function of Rep78-mediated PRKX and PKA inhibition still remains unclear. AAV appears to inhibit adenovirus production during an AAV-adenovirus coinfection (3). The modulation of signal transduction pathways by Rep78 might be one of several mechanisms of AAV to interfere with helper virus replication. The adenovirus genome has been shown to contain several PKA-dependent CREs. Inhibition of PKA might therefore result in a selective reduction of adenovirus gene expression. On the other hand, adenovirus E1A protein also interferes with PKA-induced cellular gene expression by interaction with the cellular coactivator CBP, which binds to phosphorylated CREB. E1A can disturb PKA-induced expression by disruption of the CREB/CBP transcription factor-coactivator complex (11). E1A has also been shown to stimulate expression of some CRE-dependent genes, for example, PCNA (26). It might therefore be the case that Rep78 and E1A cooperate on different levels of the PKA pathways. One report has shown that PRKX is crucial during myeloid differentiation (35). The idea that AAV might interfere with the immune response by blocking the maturation of infiltrated monocytes to macrophages is intriguing and should be analyzed.
Acknowledgments
We thank Richard Smith for helpful discussions.
REFERENCES
- 1.Batchu, R. B., M. A. Shammas, J. Y. Wang, and N. C. Munshi. 1999. Interaction of adeno-associated virus Rep78 with p53: implications in growth inhibition. Cancer Res. 59:3592–3595. [PubMed] [Google Scholar]
- 2.Berns, K. I. 1990. Parvovirus replication. Microbiol. Rev. 54:316–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Casto, B. C., J. A. Armstrong, R. W. Atchison, and W. M. Hammon. 1967. Studies on the relationship between adeno-associated virus type 1 (AAV-1) and adenoviruses. II. Inhibition of adenovirus plaques by AAV; its nature and specificity. Virology 33:452–458. [DOI] [PubMed] [Google Scholar]
- 4.Chejanovsky, N., and B. J. Carter. 1989. Replication of a human parvovirus nonsense mutant in mammalian cells containing an inducible amber suppressor. Virology 171:239–247. [DOI] [PubMed] [Google Scholar]
- 5.Cheng, X., C. Phelps, and S. S. Taylor. 2000. Differential binding of cAMP-dependent protein kinase regulatory subunit isoforms Iα and IIβ to the catalytic subunit. J. Biol. Chem. 10:10. [DOI] [PubMed] [Google Scholar]
- 6.Chiorini, J. A., B. Zimmermann, L. Yang, R. H. Smith, A. Ahearn, F. Herberg, and R. M. Kotin. 1998. Inhibition of PrKX, a novel protein kinase, and the cyclic AMP-dependent protein kinase PKA by the regulatory proteins of adeno-associated virus type 2. Mol. Cell. Biol. 18:5921–5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cho-Chung, Y. S., S. Pepe, T. Clair, A. Budillon, and M. Nesterova. 1995. cAMP-dependent protein kinase: role in normal and malignant growth. Crit. Rev. Oncol. Hematol. 21:33–61. [DOI] [PubMed] [Google Scholar]
- 8.Costello, E., P. Saudan, E. Winocour, L. Pizer, and P. Beard. 1997. High mobility group chromosomal protein 1 binds to the adeno-associated virus replication protein (Rep) and promotes Rep-mediated site-specific cleavage of DNA, ATPase activity and transcriptional repression. EMBO J. 16:5943–5954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Di Pasquale, G., and S. N. Stacey. 1998. Adeno-associated virus Rep78 protein interacts with protein kinase A and its homolog PRKX and inhibits CREB-dependent transcriptional activation. J. Virol. 72:7916–7925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dubielzig, R., J. A. King, S. Weger, A. Kern, and J. A. Kleinschmidt. 1999. Adeno-associated virus type 2 protein interactions: formation of pre-encapsidation complexes. J. Virol. 73:8989–8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fax, P., K. S. Lipinski, H. Esche, and D. Brockmann. 2000. cAMP-independent activation of the adenovirus type 12 E2 promoter correlates with the recruitment of CREB-1/ATF-1, E1A(12S), and CBP to the E2-CRE. J. Biol. Chem. 275:8911–8920. [DOI] [PubMed] [Google Scholar]
- 12.Glass, D. B., H. C. Cheng, B. E. Kemp, and D. A. Walsh. 1986. Differential and common recognition of the catalytic sites of the cGMP-dependent and cAMP-dependent protein kinases by inhibitory peptides derived from the heat-stable inhibitor protein. J. Biol. Chem. 261:12166–12171. [PubMed] [Google Scholar]
- 13.Graves, J. D., and E. G. Krebs. 1999. Protein phosphorylation and signal transduction. Pharmacol. Ther. 82:111–121. [DOI] [PubMed] [Google Scholar]
- 14.Hermonat, P. L. 1989. The adeno-associated virus Rep78 gene inhibits cellular transformation induced by bovine papillomavirus. Virology 172:253–261. [DOI] [PubMed] [Google Scholar]
- 15.Hermonat, P. L., A. D. Santin, and R. B. Batchu. 1996. The adeno-associated virus Rep78 major regulatory/transformation suppressor protein binds cellular Sp1 in vitro and evidence of a biological effect. Cancer Res. 56:5299–5304. [PubMed] [Google Scholar]
- 16.Horer, M., S. Weger, K. Butz, F. Hoppe-Seyler, C. Geisen, and J. A. Kleinschmidt. 1995. Mutational analysis of adeno-associated virus Rep protein-mediated inhibition of heterologous and homologous promoters. J. Virol. 69:5485–5496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Im, D. S., and N. Muzyczka. 1990. The AAV origin binding protein Rep68 is an ATP-dependent site-specific endonuclease with DNA helicase activity. Cell 61:447–457. [DOI] [PubMed] [Google Scholar]
- 18.Im, D. S., and N. Muzyczka. 1992. Partial purification of adeno-associated virus Rep78, Rep52, and Rep40 and their biochemical characterization. J. Virol. 66:1119–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalderon, D., B. L. Roberts, W. D. Richardson, and A. E. Smith. 1984. A short amino acid sequence able to specify nuclear location. Cell 39:499–509. [DOI] [PubMed] [Google Scholar]
- 20.Kemp, B. E., and R. B. Pearson. 1991. Intrasteric regulation of protein kinases and phosphatases. Biochim. Biophys. Acta 1094:67–76. [DOI] [PubMed] [Google Scholar]
- 21.Khleif, S. N., T. Myers, B. J. Carter, and J. P. Trempe. 1991. Inhibition of cellular transformation by the adeno-associated virus rep gene. Virology 181:738–741. [DOI] [PubMed] [Google Scholar]
- 22.Klink, A., K. Schiebel, M. Winkelmann, E. Rao, B. Horsthemke, H. J. Ludecke, U. Claussen, G. Scherer, and G. Rappold. 1995. The human protein kinase gene PKX1 on Xp22.3 displays Xp/Yp homology and is a site of chromosomal instability. Hum. Mol. Genet. 4:869–878. [DOI] [PubMed] [Google Scholar]
- 23.Knighton, D. R., J. H. Zheng, L. F. Ten Eyck, N. H. Xuong, S. S. Taylor, and J. M. Sowadski. 1991. Structure of a peptide inhibitor bound to the catalytic subunit of cyclic adenosine monophosphate-dependent protein kinase. Science 253:414–420. [DOI] [PubMed] [Google Scholar]
- 24.Laughlin, C. A., J. D. Tratschin, H. Coon, and B. J. Carter. 1983. Cloning of infectious adeno-associated virus genomes in bacterial plasmids. Gene 23:65–73. [DOI] [PubMed] [Google Scholar]
- 25.Laughlin, C. A., H. Westphal, and B. J. Carter. 1979. Spliced adenovirus-associated virus RNA. Proc. Natl. Acad. Sci. USA 76:5567–5571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee, B. H., and M. B. Mathews. 1997. Transcriptional coactivator cAMP response element binding protein mediates induction of the human proliferating cell nuclear antigen promoter by the adenovirus E1A oncoprotein. Proc. Natl. Acad. Sci. USA 94:4481–4486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Montminy, M. 1997. Transcriptional regulation by cyclic AMP. Annu. Rev. Biochem. 66:807–822. [DOI] [PubMed] [Google Scholar]
- 28.Pearson, R. B., and B. E. Kemp. 1991. Protein kinase phosphorylation site sequences and consensus specificity motifs: tabulations. Methods Enzymol. 200:62–81. [DOI] [PubMed] [Google Scholar]
- 29.Redemann, B. E., E. Mendelson, and B. J. Carter. 1989. Adeno-associated virus Rep protein synthesis during productive infection. J. Virol. 63:873–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Russo, A. A., P. D. Jeffrey, A. K. Patten, J. Massague, and N. P. Pavletich. 1996. Crystal structure of the p27Kip1 cyclin-dependent-kinase inhibitor bound to the cyclin A-Cdk2 complex. Nature 382:325–331. [DOI] [PubMed] [Google Scholar]
- 31.Sassone-Corsi, P., J. Visvader, L. Ferland, P. L. Mellon, and I. M. Verma. 1988. Induction of proto-oncogene fos transcription through the adenylate cyclase pathway: characterization of a cAMP-responsive element. Genes Dev. 2:1529–1538. [DOI] [PubMed] [Google Scholar]
- 32.Saudan, P., J. Vlach, and P. Beard. 2000. Inhibition of S-phase progression by adeno-associated virus Rep78 protein is mediated by hypophosphorylated pRb. EMBO J. 19:4351–4361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmidt, M., S. Afione, and R. M. Kotin. 2000. Adeno-associated virus type 2 Rep78 induces apoptosis through caspase activation independently of p53. J. Virol. 74:9441–9450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scott, J. D., M. B. Glaccum, E. H. Fischer, and E. G. Krebs. 1986. Primary-structure requirements for inhibition by the heat-stable inhibitor of the cAMP-dependent protein kinase. Proc. Natl. Acad. Sci. USA 83:1613–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Semizarov, D., D. Glesne, A. Laouar, K. Schiebel, and E. Huberman. 1998. A lineage-specific protein kinase crucial for myeloid maturation. Proc. Natl. Acad. Sci. USA 95:15412–15417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Siegl, G., R. C. Bates, K. I. Berns, B. J. Carter, D. C. Kelly, E. Kurstak, and P. Tattersall. 1985. Characteristics and taxonomy of Parvoviridae. Intervirology 23:61–73. [DOI] [PubMed] [Google Scholar]
- 37.Skalhegg, B. S., and K. Tasken. 2000. Specificity in the cAMP/PKA signaling pathway. Differential expression, regulation, and subcellular localization of subunits of PKA. Front. Biosci. 5:D678–D693. [DOI] [PubMed] [Google Scholar]
- 38.Smith, R. H., and R. M. Kotin. 2000. An adeno-associated virus (AAV) initiator protein, Rep78, catalyzes the cleavage and ligation of single-stranded AAV ori DNA. J. Virol. 74:3122–3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith, R. H., and R. M. Kotin. 1998. The Rep52 gene product of adeno-associated virus is a DNA helicase with 3′-to-5′ polarity. J. Virol. 72:4874–4881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tasken, K., B. S. Skalhegg, K. A. Tasken, R. Solberg, H. K. Knutsen, F. O. Levy, M. Sandberg, S. Orstavik, T. Larsen, A. K. Johansen, T. Vang, H. P. Schrader, N. T. Reinton, K. M. Torgersen, V. Hansson, and T. Jahnsen. 1997. Structure, function, and regulation of human cAMP-dependent protein kinases. Adv. Second Messenger Phosphoprotein Res. 31:191–204. [DOI] [PubMed] [Google Scholar]
- 41.Taylor, S. S., and E. Radzio-Andzelm. 1994. Cyclic AMP-dependent protein kinase, p.1–29. In J. R. Woodgett (ed.), Protein kinases. IRL Press, Oxford, England.
- 42.Taylor, S. S., and E. Radzio-Andzelm. 1997. Protein kinase inhibition: natural and synthetic variations on a theme. Curr. Opin. Chem. Biol. 1:219–226. [DOI] [PubMed] [Google Scholar]
- 43.Van Patten, S. M., D. C. Ng, J. P. Th’ng, K. L. Angelos, A. J. Smith, and D. A. Walsh. 1991. Molecular cloning of a rat testis form of the inhibitor protein of cAMP-dependent protein kinase. Proc. Natl. Acad. Sci. USA 88:5383–5387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weger, S., M. Wendland, J. A. Kleinschmidt, and R. Heilbronn. 1999. The adeno-associated virus type 2 regulatory proteins Rep78 and Rep68 interact with the transcriptional coactivator PC4. J. Virol. 73:260–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wen, W., J. L. Meinkoth, R. Y. Tsien, and S. S. Taylor. 1995. Identification of a signal for rapid export of proteins from the nucleus. Cell 82:463–473. [DOI] [PubMed] [Google Scholar]
- 46.Whitehouse, S., J. R. Feramisco, J. E. Casnellie, E. G. Krebs, and D. A. Walsh. 1983. Studies on the kinetic mechanism of the catalytic subunit of the cAMP-dependent protein kinase. J. Biol. Chem. 258:3693–3701. [PubMed] [Google Scholar]
- 47.Zetterqvist, O., U. Ragnarsson, and L. Engstorm. 1990. Substrate specificity of cyclic AMP-dependent protein kinase, p.177–188. In B. E. Kemp (ed.), Peptides and protein phosphorylation. CRC Press, Orlando, Fla.
- 48.Zhang, R., W. Min, and W. C. Sessa. 1995. Functional analysis of the human endothelial nitric oxide synthase promoter. Sp1 and GATA factors are necessary for basal transcription in endothelial cells. J. Biol. Chem. 270:15320–15326. [DOI] [PubMed] [Google Scholar]
- 49.Zhou, C., and J. P. Trempe. 1999. Induction of apoptosis by cadmium and the adeno-associated virus Rep proteins. Virology 261:280–287. [DOI] [PubMed] [Google Scholar]
- 50.Zhou, C., Q. Yang, and J. P. Trempe. 1999. Enhancement of UV-induced cytotoxicity by the adeno-associated virus replication proteins. Biochim. Biophys. Acta 1444:371–383. [DOI] [PubMed] [Google Scholar]
- 51.Zhou, X., I. Zolotukhin, D. S. Im, and N. Muzyczka. 1999. Biochemical characterization of adeno-associated virus rep68 DNA helicase and ATPase activities. J. Virol. 73:1580–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zimmermann, B., J. A. Chiorini, Y. Ma, R. M. Kotin, and F. W. Herberg. 1999. PrKX is a novel catalytic subunit of the cAMP-dependent protein kinase regulated by the regulatory subunit type I. J. Biol. Chem. 274:5370–5378. [DOI] [PubMed] [Google Scholar]