Abstract
Intraperitoneal (IP) abscesses frequently are composed of aerobes and anaerobes, and, in experimental models, a particulate adjuvant. The environmental changes effected by these components, either singularly or in combination, have not been well defined. The IP pO2, pH, and recoverable bacteria from the peritoneum of rats were quantified over 6 hours during simple aerobic and anaerobic infections and during mixed peritonitis with and without a sterile feces-barium sulfate adjuvant (SFA). Fourteen groups were studied, receiving intraperitoneally, at time of oxygen probe placement, 1 mL normal saline (control), Escherichia coli (EC), Bacteroides fragilis (BF), SFA alone, or a mixture of EC and BF, EC and SFA, BF and SFA, or EC, BF, and SFA. Control animals exhibited a stable IP pO2 and pH during 6 hours. In monomicrobial EC peritonitis, inocula well below the LD50 produced an increased IP pO2 and reduced arterial-peritoneal gradient (APG), with a stable IP pH. By 6 hours lethal doses of EC produced a dramatic decline in IP pO2, with no change in arterial pO2 as well as acidic IP and arterial pHs. Simple BF peritonitis caused no or minor elevations in IP and arterial pO2 with no change in pH. During mixed infections a significant decline in the IP pO2 and pH at 6 hours in those groups infected with both SFA and EC of a moderate, normally sublethal inoculation was observed, while arterial pO2 was unchanged and arterial pH was decreased only slightly. Concomitantly there was a significant increased number of aerobic bacteria in those groups with SFA as adjuvant compared to similar inocula without SFA. This study demonstrates the complex interactions of bacteria, sterile particulate adjuvant (SFA), and the host peritoneum. It suggests that the combination of SFA and aerobic bacteria alter the peritoneal environment to one permitting anaerobic growth and promoting abscess formation.
Full text
PDF
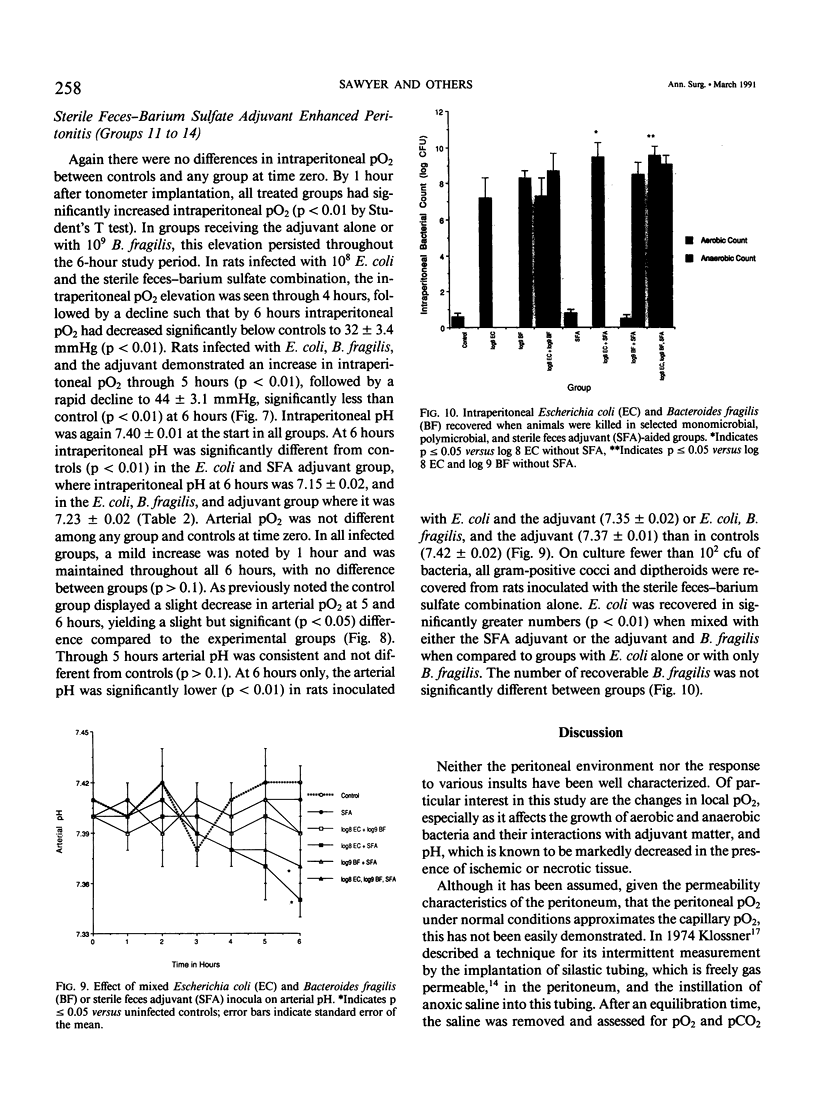


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altemeier W. A., Culbertson W. R., Fullen W. D., Shook C. D. Intra-abdominal abscesses. Am J Surg. 1973 Jan;125(1):70–79. doi: 10.1016/0002-9610(73)90010-x. [DOI] [PubMed] [Google Scholar]
- Altemeier W. A. THE BACTERIAL FLORA OF ACUTE PERFORATED APPENDICITIS WITH PERITONITIS: A BACTERIOLOGIC STUDY BASED UPON ONE HUNDRED CASES. Ann Surg. 1938 Apr;107(4):517–528. doi: 10.1097/00000658-193804000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn D. L., Barke R. A., Ewald D. C., Simmons R. L. Macrophages and translymphatic absorption represent the first line of host defense of the peritoneal cavity. Arch Surg. 1987 Jan;122(1):105–110. doi: 10.1001/archsurg.1987.01400130111017. [DOI] [PubMed] [Google Scholar]
- Dunn D. L., Barke R. A., Knight N. B., Humphrey E. W., Simmons R. L. Role of resident macrophages, peripheral neutrophils, and translymphatic absorption in bacterial clearance from the peritoneal cavity. Infect Immun. 1985 Aug;49(2):257–264. doi: 10.1128/iai.49.2.257-264.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn D. L., Rotstein O. D., Simmons R. L. Fibrin in peritonitis. IV. Synergistic intraperitoneal infection caused by Escherichia coli and Bacteroides fragilis within fibrin clots. Arch Surg. 1984 Feb;119(2):139–144. doi: 10.1001/archsurg.1984.01390140005001. [DOI] [PubMed] [Google Scholar]
- Gottrup F., Firmin R., Chang N., Goodson W. H., 3rd, Hunt T. K. Continuous direct tissue oxygen tension measurement by a new method using an implantable silastic tonometer and oxygen polarography. Am J Surg. 1983 Sep;146(3):399–403. doi: 10.1016/0002-9610(83)90427-0. [DOI] [PubMed] [Google Scholar]
- Klossner J., Kivisaari J., Niinikoski J. Oxygen and carbon dioxide tensions in the abdominal cavity and colonic wall of the rabbit. Am J Surg. 1974 Jun;127(6):711–715. doi: 10.1016/0002-9610(74)90354-7. [DOI] [PubMed] [Google Scholar]
- Lorber B., Swenson R. M. The bacteriology of intra-abdominal infections. Surg Clin North Am. 1975 Dec;55(6):1349–1354. doi: 10.1016/s0039-6109(16)40792-9. [DOI] [PubMed] [Google Scholar]
- Mackowiak P. A. Microbial synergism in human infections (second of two parts). N Engl J Med. 1978 Jan 12;298(2):83–87. doi: 10.1056/NEJM197801122980206. [DOI] [PubMed] [Google Scholar]
- Ninikoski J., Hunt T. K. Measurement of wound oxygen with implanted Silastic tube. Surgery. 1972 Jan;71(1):22–26. [PubMed] [Google Scholar]
- Nulsen M. F., Finlay-Jones J. J., Skinner J. M., McDonald P. J. Intra-abdominal abscess formation in mice: quantitative studies on bacteria and abscess-potentiating agents. Br J Exp Pathol. 1983 Aug;64(4):345–353. [PMC free article] [PubMed] [Google Scholar]
- Onderdonk A. B., Bartlett J. G., Louie T., Sullivan-Seigler N., Gorbach S. L. Microbial synergy in experimental intra-abdominal abscess. Infect Immun. 1976 Jan;13(1):22–26. doi: 10.1128/iai.13.1.22-26.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onderdonk A. B., Kasper D. L., Cisneros R. L., Bartlett J. G. The capsular polysaccharide of Bacteroides fragilis as a virulence factor: comparison of the pathogenic potential of encapsulated and unencapsulated strains. J Infect Dis. 1977 Jul;136(1):82–89. doi: 10.1093/infdis/136.1.82. [DOI] [PubMed] [Google Scholar]
- Renvall S., Niinikoski J. Intraperitoneal oxygen and carbon dioxide tensions in experimental adhesion disease and peritonitis. Am J Surg. 1975 Sep;130(3):286–292. doi: 10.1016/0002-9610(75)90387-6. [DOI] [PubMed] [Google Scholar]
- Stone H. H., Kolb L. D., Geheber C. E. Incidence and significance of intraperitoneal anaerobic bacteria. Ann Surg. 1975 May;181(5):705–715. doi: 10.1097/00000658-197505000-00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker C. B., Wilkins T. D. Use of semisolid agar from initiation of pure Bacteroides fragilis infection in mice. Infect Immun. 1976 Sep;14(3):721–725. doi: 10.1128/iai.14.3.721-725.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein W. M., Onderdonk A. B., Bartlett J. G., Gorbach S. L. Experimental intra-abdominal abscesses in rats: development of an experimental model. Infect Immun. 1974 Dec;10(6):1250–1255. doi: 10.1128/iai.10.6.1250-1255.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein W. M., Onderdonk A. B., Bartlett J. G., Louie T. J., Gorbach S. L. Antimicrobial therapy of experimental intraabdominal sepsis. J Infect Dis. 1975 Sep;132(3):282–286. doi: 10.1093/infdis/132.3.282. [DOI] [PubMed] [Google Scholar]
- Wichterman K. A., Baue A. E., Chaudry I. H. Sepsis and septic shock--a review of laboratory models and a proposal. J Surg Res. 1980 Aug;29(2):189–201. doi: 10.1016/0022-4804(80)90037-2. [DOI] [PubMed] [Google Scholar]



