Abstract
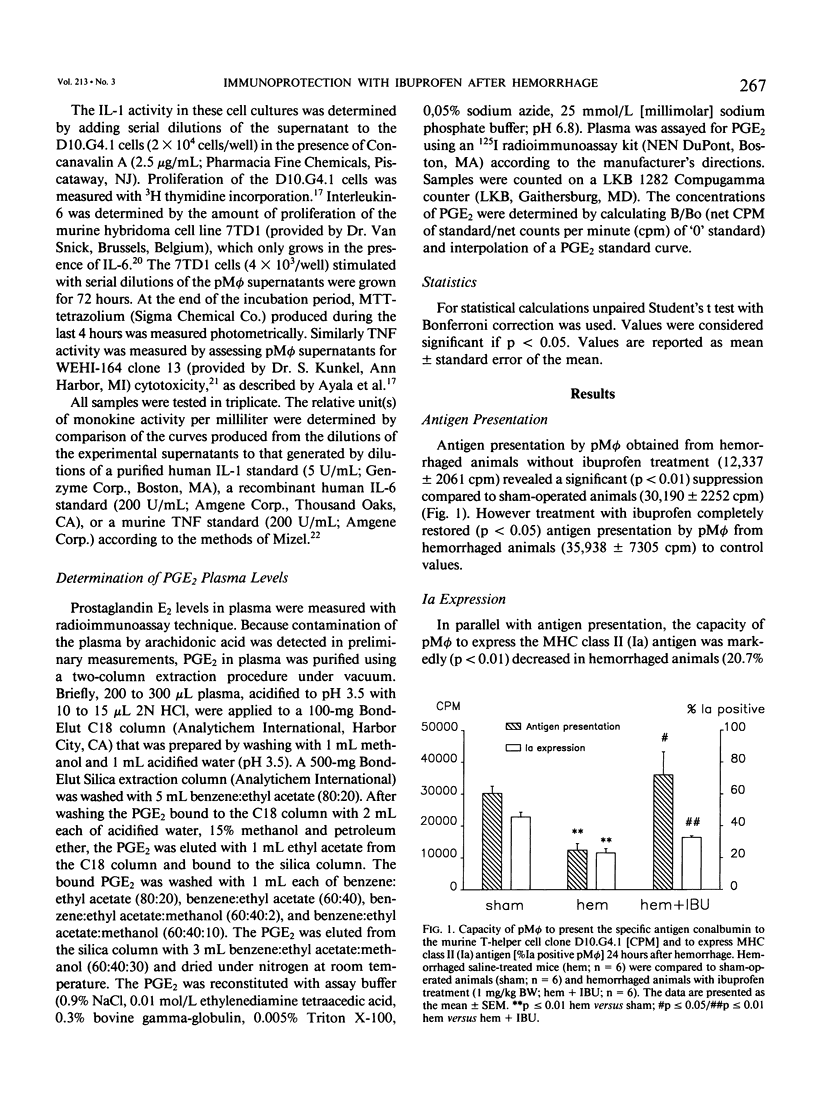
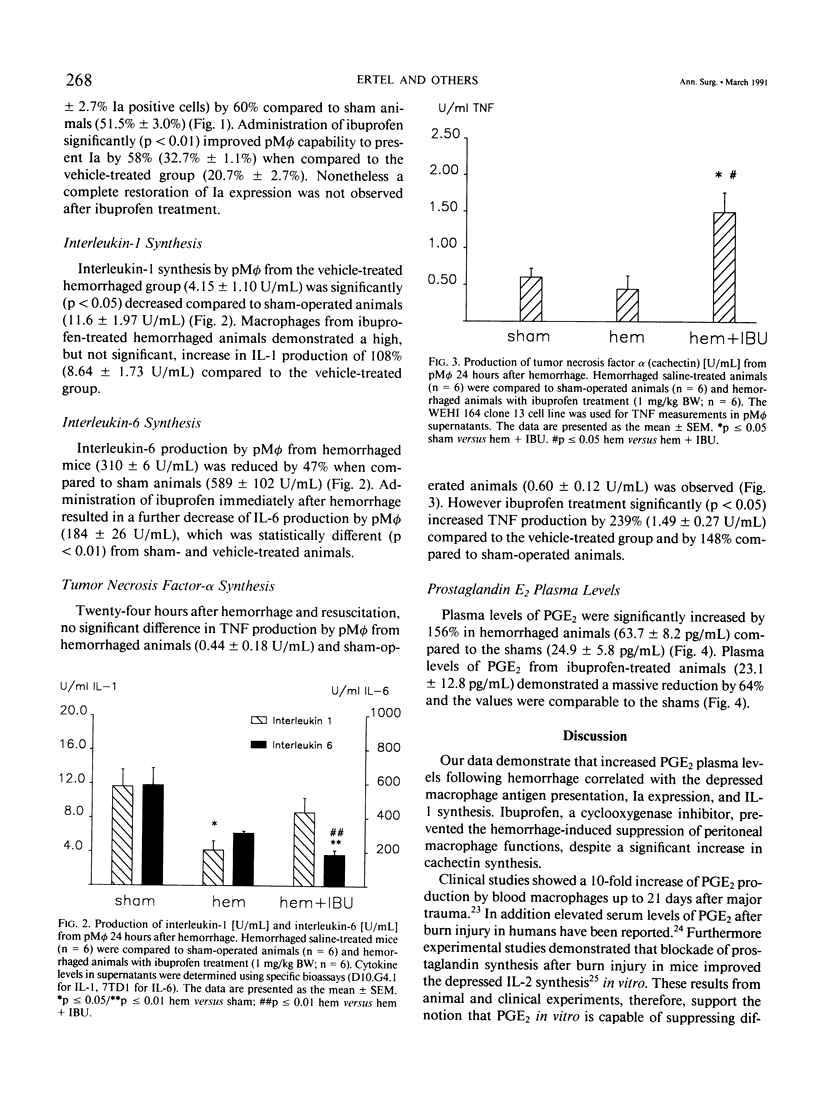
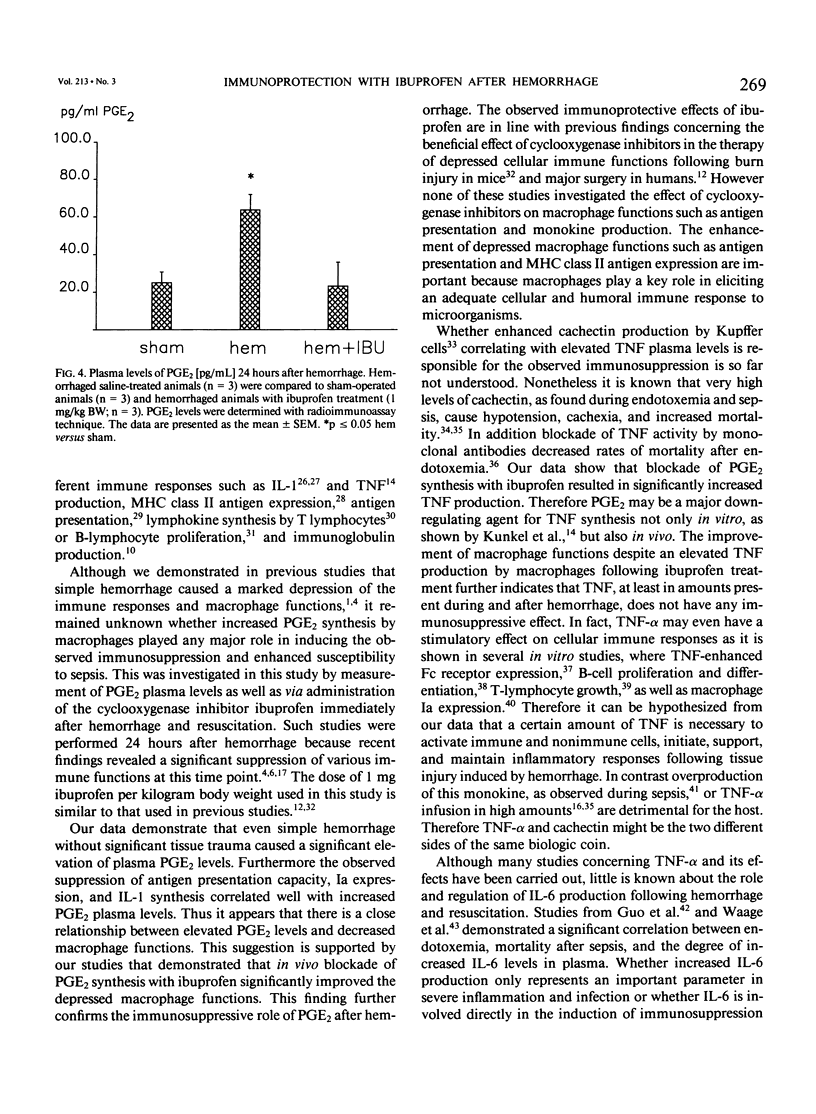
Although hemorrhage severely depresses macrophage functions, it is not known whether the increased TNF-alpha or PGE2 production is responsible for it. To study this C3H/HeN mice were bled to mean blood pressure of 35 mmHg for 60 minutes, resuscitated, and treated with either ibuprofen (1.0 mg/kg body weight) or vehicle (saline). Hemorrhage increased plasma prostaglandin E2 (PGE2) levels by 151.7% +/- 40.0% (p less than 0.05) and significantly decreased peritoneal macrophage (pM phi) antigen presentation (AP) by 60.5% +/- 7.3%, Ia expression by 52.3% +/- 7.6%, and interleukin-1 (IL-1) synthesis by 60.5% +/- 12.3% compared to shams. However ibuprofen treatment reduced PGE2 plasma levels by 61.3% +/- 12.1% and significantly increased AP (+237.0% +/- 95.3%), Ia expression (+72.8% +/- 27.5%), IL-1 synthesis (+235.7% +/- 134.7%), and cachectin synthesis (+485.8% +/- 209.0%) compared to vehicle-treated animals. These results indicate that prostaglandins but not cachectin are involved in the suppression of pM phi functions following hemorrhage because blockade of prostaglandin synthesis improved depressed macrophage functions despite enhanced cachectin synthesis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham E., Freitas A. A. Hemorrhage in mice induces alterations in immunoglobulin-secreting B cells. Crit Care Med. 1989 Oct;17(10):1015–1019. doi: 10.1097/00003246-198910000-00010. [DOI] [PubMed] [Google Scholar]
- Ayala A., Kierszenbaum F. The effects of p-chloromercuriphenylsulfonic acid on Trypanosoma cruzi infection of mammalian host cells in vitro. Mol Biochem Parasitol. 1987 Feb;23(1):63–69. doi: 10.1016/0166-6851(87)90188-5. [DOI] [PubMed] [Google Scholar]
- Ayala A., Perrin M. M., Chaudry I. H. Defective macrophage antigen presentation following haemorrhage is associated with the loss of MHC class II (Ia) antigens. Immunology. 1990 May;70(1):33–39. [PMC free article] [PubMed] [Google Scholar]
- Ayala A., Perrin M. M., Meldrum D. R., Ertel W., Chaudry I. H. Hemorrhage induces an increase in serum TNF which is not associated with elevated levels of endotoxin. Cytokine. 1990 May;2(3):170–174. doi: 10.1016/1043-4666(90)90012-i. [DOI] [PubMed] [Google Scholar]
- Ayala A., Perrin M. M., Wagner M. A., Chaudry I. H. Enhanced susceptibility to sepsis after simple hemorrhage. Depression of Fc and C3b receptor-mediated phagocytosis. Arch Surg. 1990 Jan;125(1):70–75. doi: 10.1001/archsurg.1990.01410130076010. [DOI] [PubMed] [Google Scholar]
- Beutler B., Cerami A. The biology of cachectin/TNF--a primary mediator of the host response. Annu Rev Immunol. 1989;7:625–655. doi: 10.1146/annurev.iy.07.040189.003205. [DOI] [PubMed] [Google Scholar]
- Beutler B., Milsark I. W., Cerami A. C. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science. 1985 Aug 30;229(4716):869–871. doi: 10.1126/science.3895437. [DOI] [PubMed] [Google Scholar]
- Chang R. J., Lee S. H. Effects of interferon-gamma and tumor necrosis factor-alpha on the expression of an Ia antigen on a murine macrophage cell line. J Immunol. 1986 Nov 1;137(9):2853–2856. [PubMed] [Google Scholar]
- Espevik T., Nissen-Meyer J. A highly sensitive cell line, WEHI 164 clone 13, for measuring cytotoxic factor/tumor necrosis factor from human monocytes. J Immunol Methods. 1986 Dec 4;95(1):99–105. doi: 10.1016/0022-1759(86)90322-4. [DOI] [PubMed] [Google Scholar]
- Faist E., Ertel W., Cohnert T., Huber P., Inthorn D., Heberer G. Immunoprotective effects of cyclooxygenase inhibition in patients with major surgical trauma. J Trauma. 1990 Jan;30(1):8–18. doi: 10.1097/00005373-199001000-00002. [DOI] [PubMed] [Google Scholar]
- Faist E., Mewes A., Baker C. C., Strasser T., Alkan S. S., Rieber P., Heberer G. Prostaglandin E2 (PGE2)-dependent suppression of interleukin alpha (IL-2) production in patients with major trauma. J Trauma. 1987 Aug;27(8):837–848. doi: 10.1097/00005373-198708000-00001. [DOI] [PubMed] [Google Scholar]
- Faist E., Mewes A., Strasser T., Walz A., Alkan S., Baker C., Ertel W., Heberer G. Alteration of monocyte function following major injury. Arch Surg. 1988 Mar;123(3):287–292. doi: 10.1001/archsurg.1988.01400270021002. [DOI] [PubMed] [Google Scholar]
- Hansbrough J., Peterson V., Zapata-Sirvent R., Claman H. N. Postburn immunosuppression in an animal model. II. Restoration of cell-mediated immunity by immunomodulating drugs. Surgery. 1984 Mar;95(3):290–296. [PubMed] [Google Scholar]
- Hoffman M., Weinberg J. B. Tumor necrosis factor-alpha induces increased hydrogen peroxide production and Fc receptor expression, but not increased Ia antigen expression by peritoneal macrophages. J Leukoc Biol. 1987 Dec;42(6):704–707. doi: 10.1002/jlb.42.6.704. [DOI] [PubMed] [Google Scholar]
- Jelinek D. F., Lipsky P. E. Enhancement of human B cell proliferation and differentiation by tumor necrosis factor-alpha and interleukin 1. J Immunol. 1987 Nov 1;139(9):2970–2976. [PubMed] [Google Scholar]
- Kaye J., Porcelli S., Tite J., Jones B., Janeway C. A., Jr Both a monoclonal antibody and antisera specific for determinants unique to individual cloned helper T cell lines can substitute for antigen and antigen-presenting cells in the activation of T cells. J Exp Med. 1983 Sep 1;158(3):836–856. doi: 10.1084/jem.158.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp W., Baumgartner G. Monocyte-mediated suppression of human B lymphocyte differentiation in vitro. J Immunol. 1978 Sep;121(3):1177–1183. [PubMed] [Google Scholar]
- Knudsen P. J., Dinarello C. A., Strom T. B. Prostaglandins posttranscriptionally inhibit monocyte expression of interleukin 1 activity by increasing intracellular cyclic adenosine monophosphate. J Immunol. 1986 Nov 15;137(10):3189–3194. [PubMed] [Google Scholar]
- Kunkel S. L., Chensue S. W., Phan S. H. Prostaglandins as endogenous mediators of interleukin 1 production. J Immunol. 1986 Jan;136(1):186–192. [PubMed] [Google Scholar]
- Kunkel S. L., Spengler M., May M. A., Spengler R., Larrick J., Remick D. Prostaglandin E2 regulates macrophage-derived tumor necrosis factor gene expression. J Biol Chem. 1988 Apr 15;263(11):5380–5384. [PubMed] [Google Scholar]
- Miller-Graziano C. L., Fink M., Wu J. Y., Szabo G., Kodys K. Mechanisms of altered monocyte prostaglandin E2 production in severely injured patients. Arch Surg. 1988 Mar;123(3):293–299. doi: 10.1001/archsurg.1988.01400270027003. [DOI] [PubMed] [Google Scholar]
- Ninnemann J. L., Stockland A. E. Participation of prostaglandin E in immunosuppression following thermal injury. J Trauma. 1984 Mar;24(3):201–207. doi: 10.1097/00005373-198403000-00003. [DOI] [PubMed] [Google Scholar]
- Rappaport R. S., Dodge G. R. Prostaglandin E inhibits the production of human interleukin 2. J Exp Med. 1982 Mar 1;155(3):943–948. doi: 10.1084/jem.155.3.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler R., Mancilla J., Endres S., Ghorbani R., Clark S. C., Dinarello C. A. Correlations and interactions in the production of interleukin-6 (IL-6), IL-1, and tumor necrosis factor (TNF) in human blood mononuclear cells: IL-6 suppresses IL-1 and TNF. Blood. 1990 Jan 1;75(1):40–47. [PubMed] [Google Scholar]
- Sheppard B. C., Fraker D. L., Norton J. A. Prevention and treatment of endotoxin and sepsis lethality with recombinant human tumor necrosis factor. Surgery. 1989 Aug;106(2):156–162. [PubMed] [Google Scholar]
- Simkin N. J., Jelinek D. F., Lipsky P. E. Inhibition of human B cell responsiveness by prostaglandin E2. J Immunol. 1987 Feb 15;138(4):1074–1081. [PubMed] [Google Scholar]
- Stephan R. N., Ayala A., Harkema J. M., Dean R. E., Border J. R., Chaudry I. H. Mechanism of immunosuppression following hemorrhage: defective antigen presentation by macrophages. J Surg Res. 1989 Jun;46(6):553–556. doi: 10.1016/0022-4804(89)90019-x. [DOI] [PubMed] [Google Scholar]
- Stephan R. N., Conrad P. J., Saizawa M., Dean R. E., Chaudry I. H. Prostaglandin E2 depresses antigen-presenting cell function of peritoneal macrophages. J Surg Res. 1988 Jun;44(6):733–739. doi: 10.1016/0022-4804(88)90108-4. [DOI] [PubMed] [Google Scholar]
- Stephan R. N., Kupper T. S., Geha A. S., Baue A. E., Chaudry I. H. Hemorrhage without tissue trauma produces immunosuppression and enhances susceptibility to sepsis. Arch Surg. 1987 Jan;122(1):62–68. doi: 10.1001/archsurg.1987.01400130068010. [DOI] [PubMed] [Google Scholar]
- Tracey K. J., Beutler B., Lowry S. F., Merryweather J., Wolpe S., Milsark I. W., Hariri R. J., Fahey T. J., 3rd, Zentella A., Albert J. D. Shock and tissue injury induced by recombinant human cachectin. Science. 1986 Oct 24;234(4775):470–474. doi: 10.1126/science.3764421. [DOI] [PubMed] [Google Scholar]
- Tracey K. J., Lowry S. F., Fahey T. J., 3rd, Albert J. D., Fong Y., Hesse D., Beutler B., Manogue K. R., Calvano S., Wei H. Cachectin/tumor necrosis factor induces lethal shock and stress hormone responses in the dog. Surg Gynecol Obstet. 1987 May;164(5):415–422. [PubMed] [Google Scholar]
- Van Snick J., Cayphas S., Vink A., Uyttenhove C., Coulie P. G., Rubira M. R., Simpson R. J. Purification and NH2-terminal amino acid sequence of a T-cell-derived lymphokine with growth factor activity for B-cell hybridomas. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9679–9683. doi: 10.1073/pnas.83.24.9679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waage A., Brandtzaeg P., Halstensen A., Kierulf P., Espevik T. The complex pattern of cytokines in serum from patients with meningococcal septic shock. Association between interleukin 6, interleukin 1, and fatal outcome. J Exp Med. 1989 Jan 1;169(1):333–338. doi: 10.1084/jem.169.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waage A., Halstensen A., Espevik T. Association between tumour necrosis factor in serum and fatal outcome in patients with meningococcal disease. Lancet. 1987 Feb 14;1(8529):355–357. doi: 10.1016/s0140-6736(87)91728-4. [DOI] [PubMed] [Google Scholar]
- Walker C., Kristensen F., Bettens F., deWeck A. L. Lymphokine regulation of activated (G1) lymphocytes. I. Prostaglandin E2-induced inhibition of interleukin 2 production. J Immunol. 1983 Apr;130(4):1770–1773. [PubMed] [Google Scholar]
- Wood J. J., Grbic J. T., Rodrick M. L., Jordan A., Mannick J. A. Suppression of interleukin 2 production in an animal model of thermal injury is related to prostaglandin synthesis. Arch Surg. 1987 Feb;122(2):179–184. doi: 10.1001/archsurg.1987.01400140061007. [DOI] [PubMed] [Google Scholar]
- Zlotnik A., Shimonkevitz R., Kappler J., Marrack P. Effect of prostaglandin E2 on the gamma-interferon induction of antigen-presenting ability in P388D1 cells and on IL-2 production by T-cell hybridomas. Cell Immunol. 1985 Jan;90(1):154–166. doi: 10.1016/0008-8749(85)90177-7. [DOI] [PubMed] [Google Scholar]
- Zucali J. R., Elfenbein G. J., Barth K. C., Dinarello C. A. Effects of human interleukin 1 and human tumor necrosis factor on human T lymphocyte colony formation. J Clin Invest. 1987 Sep;80(3):772–777. doi: 10.1172/JCI113133. [DOI] [PMC free article] [PubMed] [Google Scholar]


