Abstract
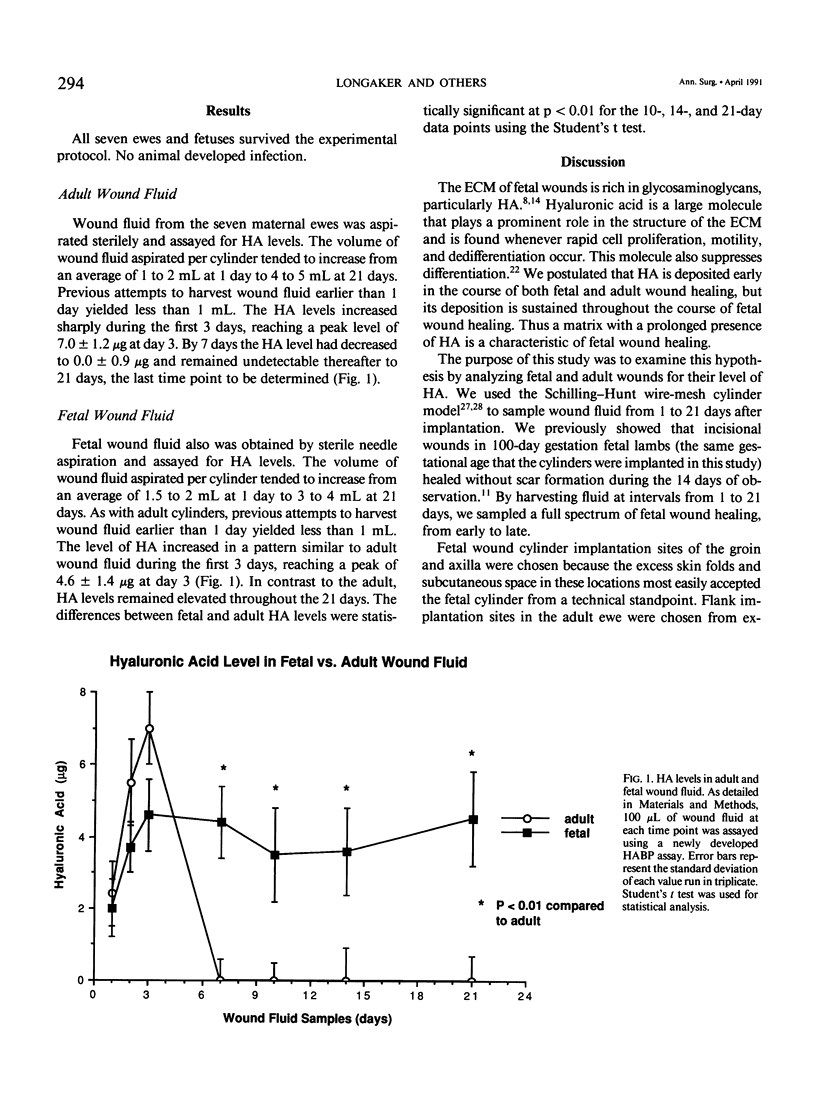
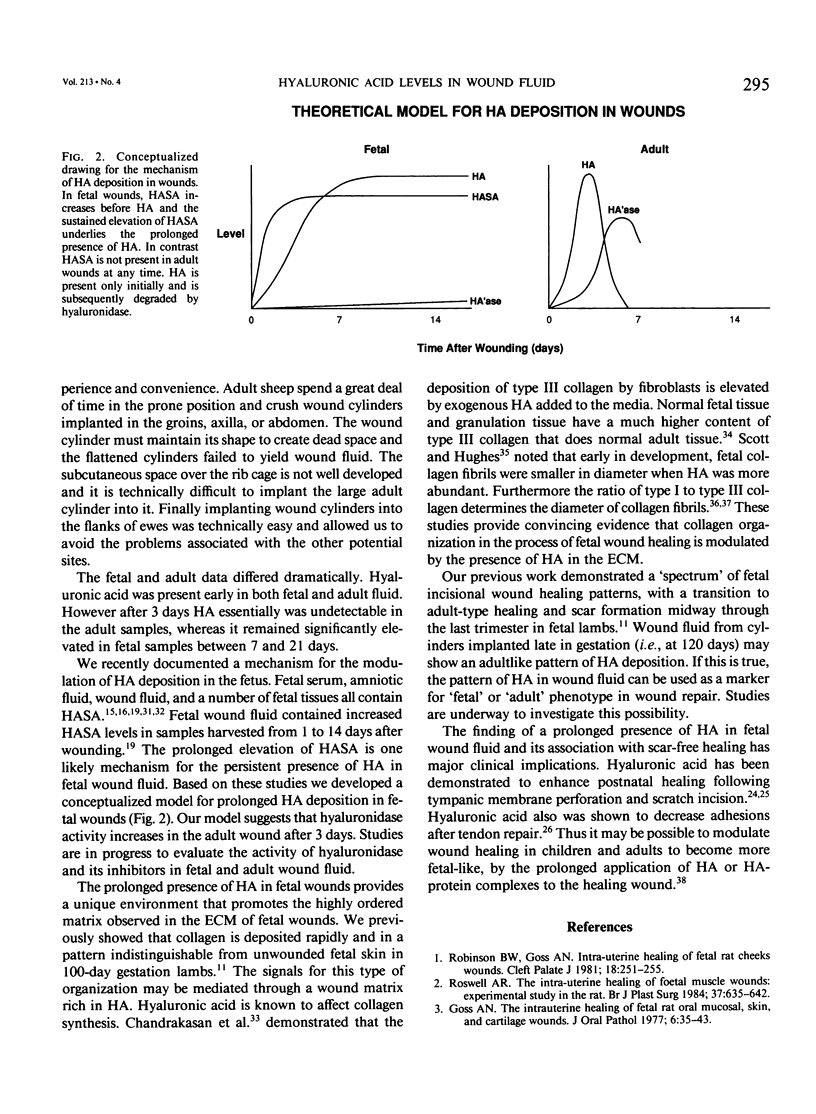
Midgestation fetal wound healing is characterized by healing without fibrosis or scar formation. The mechanisms that underlie this remarkable process are mediated in part through a fetal wound extracellular matrix rich in hyaluronic acid. In this study a newly developed assay was used to determine the hyaluronic acid levels in fetal and adult wound fluid. Adult wound fluid had a rapid increase in hyaluronic acid, which peaked at 3 days and decreased to 0 by 7 days. In contrast levels of hyaluronic acid in fetal wound fluid increased rapidly and remained significantly elevated for 3 weeks. This prolonged presence of hyaluronic acid in the matrix of fetal wounds creates a 'permissive' wound environment that promotes fetal fibroblast movement and proliferation and inhibits cytodifferentiation. Such a matrix environment promotes healing by regeneration rather than by scarring. This observation has therapeutic implications. The prolonged application of hyaluronic acid or hyaluronate protein complexes to wounds in children or adults may modulate healing in a manner that makes the wounds more fetal-like.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abatangelo G., Martelli M., Vecchia P. Healing of hyaluronic acid-enriched wounds: histological observations. J Surg Res. 1983 Nov;35(5):410–416. doi: 10.1016/0022-4804(83)90030-6. [DOI] [PubMed] [Google Scholar]
- Adzick N. S., Harrison M. R., Glick P. L., Beckstead J. H., Villa R. L., Scheuenstuhl H., Goodson W. H., 3rd Comparison of fetal, newborn, and adult wound healing by histologic, enzyme-histochemical, and hydroxyproline determinations. J Pediatr Surg. 1985 Aug;20(4):315–319. doi: 10.1016/s0022-3468(85)80210-4. [DOI] [PubMed] [Google Scholar]
- Burd D. A., Siebert J. W., Ehrlich H. P., Garg H. G. Human skin and post-burn scar hyaluronan: demonstration of the association with collagen and other proteins. Matrix. 1989 Aug;9(4):322–327. doi: 10.1016/s0934-8832(89)80008-3. [DOI] [PubMed] [Google Scholar]
- Crombleholme T. M., Harrison M. R., Langer J. C., Longaker M. T., Anderson R. L., Slotnick N. S., Filly R. A., Callen P. W., Goldstein R. B., Golbus M. S. Early experience with open fetal surgery for congenital hydronephrosis. J Pediatr Surg. 1988 Dec;23(12):1114–1121. doi: 10.1016/s0022-3468(88)80325-7. [DOI] [PubMed] [Google Scholar]
- DePalma R. L., Krummel T. M., Durham L. A., 3rd, Michna B. A., Thomas B. L., Nelson J. M., Diegelmann R. F. Characterization and quantitation of wound matrix in the fetal rabbit. Matrix. 1989 Jun;9(3):224–231. doi: 10.1016/s0934-8832(89)80054-x. [DOI] [PubMed] [Google Scholar]
- Decker M., Chiu E. S., Dollbaum C., Moiin A., Hall J., Spendlove R., Longaker M. T., Stern R. Hyaluronic acid-stimulating activity in sera from the bovine fetus and from breast cancer patients. Cancer Res. 1989 Jul 1;49(13):3499–3505. [PubMed] [Google Scholar]
- Glanville R. W. A comparison of models for the macromolecular structure of interstitial and basement membrane collagens. Arzneimittelforschung. 1982;32(10A):1353–1357. [PubMed] [Google Scholar]
- Goss A. N. Intra-uterine healing of fetal rat oral mucosal, skin and cartilage wounds. J Oral Pathol. 1977 Jan;6(1):35–43. doi: 10.1111/j.1600-0714.1977.tb01792.x. [DOI] [PubMed] [Google Scholar]
- Hallock G. G. In utero cleft lip repair in A/J mice. Plast Reconstr Surg. 1985 Jun;75(6):785–790. doi: 10.1097/00006534-198506000-00001. [DOI] [PubMed] [Google Scholar]
- Harrison M. R., Jester J. A., Ross N. A. Correction of congenital diaphragmatic hernia in utero. I. The model: intrathoracic balloon produces fatal pulmonary hypoplasia. Surgery. 1980 Jul;88(1):174–182. [PubMed] [Google Scholar]
- Harrison M. R., Langer J. C., Adzick N. S., Golbus M. S., Filly R. A., Anderson R. L., Rosen M. A., Callen P. W., Goldstein R. B., deLorimier A. A. Correction of congenital diaphragmatic hernia in utero, V. Initial clinical experience. J Pediatr Surg. 1990 Jan;25(1):47–57. doi: 10.1016/s0022-3468(05)80163-0. [DOI] [PubMed] [Google Scholar]
- Hellström S., Laurent C. Hyaluronan and healing of tympanic membrane perforations. An experimental study. Acta Otolaryngol Suppl. 1987;442:54–61. doi: 10.3109/00016488709102840. [DOI] [PubMed] [Google Scholar]
- Hunt T. K., Twomey P., Zederfeldt B., Dunphy J. E. Respiratory gas tensions and pH in healing wounds. Am J Surg. 1967 Aug;114(2):302–307. doi: 10.1016/0002-9610(67)90388-1. [DOI] [PubMed] [Google Scholar]
- Kistler A., Utsugi R., Ihara S. Wound healing in fetal limb organ culture. Ann Plast Surg. 1988 Oct;21(4):303–309. doi: 10.1097/00000637-198810000-00002. [DOI] [PubMed] [Google Scholar]
- Krummel T. M., Nelson J. M., Diegelmann R. F., Lindblad W. J., Salzberg A. M., Greenfield L. J., Cohen I. K. Fetal response to injury in the rabbit. J Pediatr Surg. 1987 Jul;22(7):640–644. doi: 10.1016/s0022-3468(87)80117-3. [DOI] [PubMed] [Google Scholar]
- Kujawa M. J., Tepperman K. Culturing chick muscle cells on glycosaminoglycan substrates: attachment and differentiation. Dev Biol. 1983 Oct;99(2):277–286. doi: 10.1016/0012-1606(83)90277-4. [DOI] [PubMed] [Google Scholar]
- Longaker M. T., Adzick N. S., Hall J. L., Stair S. E., Crombleholme T. M., Duncan B. W., Bradley S. M., Harrison M. R., Stern R. Studies in fetal wound healing, VII. Fetal wound healing may be modulated by hyaluronic acid stimulating activity in amniotic fluid. J Pediatr Surg. 1990 Apr;25(4):430–433. doi: 10.1016/0022-3468(90)90387-o. [DOI] [PubMed] [Google Scholar]
- Longaker M. T., Adzick N. S., Sadigh D., Hendin B., Stair S. E., Duncan B. W., Harrison M. R., Spendlove R., Stern R. Hyaluronic acid-stimulating activity in the pathophysiology of Wilms' tumors. J Natl Cancer Inst. 1990 Jan 17;82(2):135–139. doi: 10.1093/jnci/82.2.135. [DOI] [PubMed] [Google Scholar]
- Longaker M. T., Harrison M. R., Crombleholme T. M., Langer J. C., Decker M., Verrier E. D., Spendlove R., Stern R. Studies in fetal wound healing: I. A factor in fetal serum that stimulates deposition of hyaluronic acid. J Pediatr Surg. 1989 Aug;24(8):789–792. doi: 10.1016/s0022-3468(89)80538-x. [DOI] [PubMed] [Google Scholar]
- Longaker M. T., Harrison M. R., Langer J. C., Crombleholme T. M., Verrier E. D., Spendlove R., Stern R. Studies in fetal wound healing: II. A fetal environment accelerates fibroblast migration in vitro. J Pediatr Surg. 1989 Aug;24(8):793–798. doi: 10.1016/s0022-3468(89)80539-1. [DOI] [PubMed] [Google Scholar]
- Longaker M. T., Whitby D. J., Adzick N. S., Crombleholme T. M., Langer J. C., Duncan B. W., Bradley S. M., Stern R., Ferguson M. W., Harrison M. R. Studies in fetal wound healing, VI. Second and early third trimester fetal wounds demonstrate rapid collagen deposition without scar formation. J Pediatr Surg. 1990 Jan;25(1):63–69. doi: 10.1016/s0022-3468(05)80165-4. [DOI] [PubMed] [Google Scholar]
- Longaker M. T., Whitby D. J., Ferguson M. W., Harrison M. R., Crombleholme T. M., Langer J. C., Cochrum K. C., Verrier E. D., Stern R. Studies in fetal wound healing: III. Early deposition of fibronectin distinguishes fetal from adult wound healing. J Pediatr Surg. 1989 Aug;24(8):799–805. doi: 10.1016/s0022-3468(89)80540-8. [DOI] [PubMed] [Google Scholar]
- Merkel J. R., DiPaolo B. R., Hallock G. G., Rice D. C. Type I and type III collagen content of healing wounds in fetal and adult rats. Proc Soc Exp Biol Med. 1988 Apr;187(4):493–497. doi: 10.3181/00379727-187-42694. [DOI] [PubMed] [Google Scholar]
- Robinson B. W., Goss A. N. Intra-uterine healing of fetal rat cheek wounds. Cleft Palate J. 1981 Oct;18(4):251–255. [PubMed] [Google Scholar]
- Rowsell A. R. The intra-uterine healing of foetal muscle wounds: experimental study in the rat. Br J Plast Surg. 1984 Oct;37(4):635–642. doi: 10.1016/0007-1226(84)90166-8. [DOI] [PubMed] [Google Scholar]
- SCHILLING J. A., JOEL W., SHURLEY H. M. Wound healing: a comparative study of the histochemical changes in granulation tissue contained in stainless steel wire mesh and polyvinyl sponge cylinders. Surgery. 1959 Oct;46:702–710. [PubMed] [Google Scholar]
- Scott J. E., Hughes E. W. Proteoglycan-collagen relationships in developing chick and bovine tendons. Influence of the physiological environment. Connect Tissue Res. 1986;14(4):267–278. doi: 10.3109/03008208609017470. [DOI] [PubMed] [Google Scholar]
- Sullivan W. G. In utero cleft lip repair in the mouse without an incision. Plast Reconstr Surg. 1989 Nov;84(5):723–732. [PubMed] [Google Scholar]
- Tengblad A. Affinity chromatography on immobilized hyaluronate and its application to the isolation of hyaluronate binding properties from cartilage. Biochim Biophys Acta. 1979 Jun 19;578(2):281–289. doi: 10.1016/0005-2795(79)90158-2. [DOI] [PubMed] [Google Scholar]
- Thomas S. C., Jones L. C., Hungerford D. S. Hyaluronic acid and its effect on postoperative adhesions in the rabbit flexor tendon. A preliminary look. Clin Orthop Relat Res. 1986 May;(206):281–289. [PubMed] [Google Scholar]


