Abstract
Herpes simplex virus type 1 (HSV-1) and HSV-2 trigger or counteract apoptosis by a cell-specific mechanism. Our studies are based on previous findings that the protein kinase (PK) domain of the large subunit of HSV-2 ribonucleotide reductase (ICP10) activates the Ras/MEK/MAPK pathway (Smith et al., J. Virol. 74:10417, 2000). Because survival pathways can modulate apoptosis, we used cells that are stably or transiently transfected with ICP10 PK, an HSV-2 mutant deleted in ICP10 PK (ICP10ΔPK) and the MEK-specific inhibitor U0126 to examine the role of ICP10 PK in apoptosis. Apoptosis was induced by staurosporine or d-mannitol in human (HEK293) cells or HEK293 cells stably transfected with the ICP10 PK-negative mutant p139 (JHL15), as determined by morphology, DNA fragmentation, terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL), caspase-3 activation, and poly(ADP-ribose) polymerase (PARP) cleavage. HEK293 cells stably transfected with ICP10 (JHLa1) were protected from apoptosis. ICP10 but not p139 protected neuronally differentiated PC12 cells from death due to nerve growth factor withdrawal, and apoptosis (determined by TUNEL) and caspase-3 activation were seen in primary hippocampal cultures infected with ICP10ΔPK but not with HSV-2 or a revertant virus [HSV-2(R)]. The data indicate that ICP10 has antiapoptotic activity under both paradigms and that it requires a functional PK activity. The apoptotic cells in primary hippocampal cultures were neurons, as determined by double immunofluorescence with fluorescein-labeled dUTP (TUNEL) and phycoerythrin-labeled antibodies specific for neuronal proteins (TuJ1 and NF-160). Protection from apoptosis was associated with MEK/MAPK activation, as evidenced by (i) increased levels of activated (phosphorylated) MAPK in HSV-2- but not ICP10ΔPK-infected cultures and (ii) inhibition of MAPK activation by the MEK-specific inhibitor U0126. MEK and MAPK were activated by infection with UV-inactivated but not antibody-neutralized HSV-2, suggesting that activation requires cellular penetration but is independent of de novo viral protein synthesis.
Signaling pathways, the ultimate targets of which are nuclear transcription factors, determine the cell’s ability to respond to external stimuli. Transduced signals can be interpreted as mitogenic/proliferative, differentiating, or apoptotic, depending on the cell type and the nature and duration of the stimulus.
Apoptosis is an irreversible process that results in cell death in the absence of inflammation. It is primarily mediated by caspases, which are cysteine proteases with aspartate specificity that are activated by the cleavage of inactive zymogens (procaspases). Caspase-3 is one of the key executioners of apoptosis. Its activation requires proteolytic cleavage of the inactive pro-caspase-3 into activated 17- to 20-kDa and 12-kDa subunits. Activated caspase-3 is, in turn, responsible, either partially or totally, for the proteolytic cleavage of many key proteins, such as the nuclear poly(ADP-ribose) polymerase (PARP) that is involved in DNA repair. PARP cleavage is a crucial event in the commitment to undergo apoptosis (reviewed in reference 52).
Cell homeostasis depends on the balance between apoptotic and survival/proliferation processes. Survival stimuli cause the membrane-bound G protein Ras to adopt an active, GTP-bound state, and it, in turn, coordinates the activation of a multitude of downstream effectors. The mitogenic/survival Ras/MEK/MAPK pathway begins with the activation of Raf kinase and is followed by the activation of MAP kinase kinase (MEK) and mitogen-activated protein kinase (MAPK). A variety of genes, including those required for cell cycle progression, are targets for MAPK (reviewed in reference 58). The Ras/MEK/MAPK pathway is also involved in the control of apoptosis, presumably by upregulating antiapoptotic proteins such as bcl-2 and mcl-1 (19, 57).
Viruses depend on cells for their replication, and they can differentially affect various signaling pathways. Herpes simplex virus type 1 (HSV-1) and HSV-2 can trigger or counteract apoptosis in a cell-specific manner (6, 7, 22, 35). Antiapoptotic activity was ascribed to the HSV-1 and HSV-2 gene US3 (42, 50) and to the HSV-1 genes γ134.5, US5, ICP27, and LAT (6, 22, 50, 72). However, their exact mechanism of action and their activity in hippocampal neurons, if any, are still poorly understood.
The large subunits of HSV-1 and HSV-2 ribonucleotide reductase (R1) differ from their counterparts in eukaryotic and prokaryotic cells and in other viruses in that they have an intrinsic protein kinase (PK) activity (1, 9, 23, 25, 26, 61, 62, 69, 71). It was originally concluded that R1 is expressed with apparently biphasic kinetics that consist of immediate-early (IE; also known as α) and early components (3, 45, 88). However, studies from our and other laboratories indicated that the R1 promoter has an octamer/TAATGARAT sequence which responds to the VP16/oct1 complex, and its expression is independent of the regulatory IE protein ICP4, suggesting that R1 is an IE gene (29, 85, 89, 90, 94). This conclusion is generally accepted at present (59). Basal expression of the HSV-2 R1 (also known as ICP10) requires AP-1 cognate promoter sites (89, 90, 94), and it may be involved in the reactivation of latent virus (9, 10, 12). ICP10 PK is required for IE gene expression and HSV-2 growth (78, 81).
These studies were stimulated by our previous finding that ICP10 PK activates the Ras/MEK/MAPK pathway in nonneuronal cells (47, 69, 79, 82) and independent observations that, in neurons, apoptosis is blocked by activation of the MEK/MAPK pathway, thereby promoting their survival (reviewed in reference 53). Here, we report that ICP10 PK blocks apoptosis due to nerve growth factor (NGF) withdrawal or virus infection of primary hippocampal neurons, a function that involves MEK/MAPK activation.
(A preliminary account of these findings was presented at the 30th Annual Meeting of the Society for Neuroscience, abstract no. 398.9, 2000.)
MATERIALS AND METHODS
Viruses and plasmids.
HSV-2 (G strain), HSV-2 mutants ICP10ΔPK and ICP10ΔRR, which carry deletions in the PK and RR domains of ICP10, respectively, and the revertant virus HSV-2(R) have been described previously (12, 81, 82). For UV inactivation, virus (100 μl; 107 PFU) was UV irradiated for 45 min using a Sylvania G15 T8 bulb at a distance of 17 cm with occasional agitation (10, 74). The construction of eukaryotic expression vectors for ICP10 (pJW17) and the transmembrane (TM)-deleted PK-negative mutant p139 (pJHL15) has been described elsewhere (23, 62, 79).
Cells, virus infection, and plasmid transfection.
Vero (African green monkey kidney) cells were grown in minimal essential medium (MEM) with 10% fetal bovine serum (FBS) and 100 U of penicillin-streptomycin (Gibco-BRL, Gaithersburg, Md) per ml. Cell lines JHLa1 and JHL15, which express ICP10 and p139, respectively (47, 62, 79), were established from human embryonic kidney cells (HEK293). JHLa1, JHL15, and HEK293 cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) with 10% FBS, 1 mM sodium pyruvate, 0.1 mM nonessential amino acids, and 100 U of penicillin-streptomycin (Gibco-BRL). Rat pheochromocytoma (PC12) cells were cultured in DMEM/F12 (Gibco-BRL) with 10% FBS, 0.36% d-glucose (Sigma, St. Louis, Mo.), 0.21% sodium bicarbonate (Sigma), and 0.009% gentamicin (Sigma).
To establish primary hippocampal cultures, cells were dissociated from the hippocampi of 16- to 19-day-old fetuses of Sprague-Dawley rats and plated at a density of approximately 750,000/2 ml on collagen-coated 35-mm dishes (Nunc, Rochester, N.Y.) or glass coverslips coated with poly-l-lysine (Sigma), as described (2). Six-day-old cultures, which consist primarily of nondividing cells (>85%) as determined with the 5-bromo-2′-deoxyuridine labeling and detection kit (Roche Molecular Biochemicals, Indianapolis, Ind.), were used. Infection was with HSV-2, HSV-2(R), ICP10ΔPK, or ICP10ΔRR in medium containing 10% FBS, a condition that allows ICP10ΔPK and ICP10ΔRR replication in nonneuronal cells (12, 79, 81, 82).
In some experiments, cells were pretreated (1 h, 37°C) with various concentrations of the MEK-specific inhibitor U0126 (32) or the phosphatidylinositol 3-kinase (PI3-K)-specific inhibitor LY294002 (87) and infected in medium containing the same drug concentration. Transient transfection was done with expression vector pJW17 or pJHL15 using FuGene 6 transfection reagent (Roche Molecular Biochemicals) according to the manufacturer’s instructions.
Antibodies.
The hyperimmune HSV-2 serum was generated in mice injected with HSV-2 (106 PFU) as described (10). The ICP10 antibody was raised in rabbits using a synthetic peptide consisting of residues 13 to 26 within the PK domain (8). It recognizes ICP10 and the p95 and p175 proteins, expressed by ICP10ΔPK and ICP10ΔRR, respectively (12). Antibodies to neuronal proteins included TuJ1 (class III β-tubulin) and NF-160 (160-kDa neurofilament) purchased from Roche Molecular Biochemicals. Their staining patterns and specificity for neuronal cells were described previously (20).
Monoclonal antibodies (MAbs) to glial fibrillary acidic protein (GFAP) and galactocerebroside (GalC) that are specific for astrocytes and oligodendrocytes, respectively, were part of the neural cell typing set for identification and typing of neural cells (Roche Molecular Biochemicals). Polyclonal antibodies specific for MAPK (recognizes MAPK1 and -2 [MAPK1/2]) (Oncogene, Cambridge, Mass.), the dually phosphorylated active forms of MAPK1/2 (P-MAPK1/2) (Promega Corporation, Madison, Wis.), PARP (Roche Molecular Biochemicals), activated caspase-3 (D175; recognizes the large 17- to 20-kDa fragment) (Cell Signaling Technology, Beverly, Mass.), pro-caspase-3 (recognizes the uncleaved species) (Santa Cruz Biotechnology, Santa Cruz, Calif.), and the neutralizing immunoglobulin G (IgG) fraction of a MAb to HSV-1/2 glycoprotein D (gD) (Advanced Biotechnologies, Columbia, Md.) were used as per the manufacturers’ instructions. Their respective specificities are established.
Chemicals.
The PI3-K-specific inhibitor LY294002 (87) and the MEK-specific inhibitor U0126 (32) were purchased from Calbiochem (San Diego, Calif.) and Promega, respectively. Apoptosis inducers staurosporine (STS) and d-mannitol (d-Mann) were purchased from Calbiochem and Sigma, respectively.
TUNEL.
The in situ cell death detection kit (Roche Molecular Biochemicals) was used according to the manufacturer’s instructions for terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL). Briefly, cells were fixed with 4% paraformaldehyde (PFA) in phosphate-buffered saline (PBS, pH 7.4) for 30 min at room temperature, followed by permeabilization in 0.1% Triton X-100 (in 0.1% sodium citrate) for 2 min on ice. DNA breaks were labeled by addition of terminal deoxynucleotidyltransferase (TdT) and nucleotide mixture containing fluorescein isothiocyanate (FITC)-conjugated dUTP and incubation for 60 min at 37°C. Cover slips were mounted in PBS-glycerol, and cells were analyzed by fluorescence microscopy. After extensive washes in PBS, cells were incubated for 30 min at 37°C with an anti-FITC antibody conjugated with alkaline phosphatase (AP). Chromogenic reaction was carried out by adding AP substrate solution (0.4 mg of nitro blue tetrazolium chloride and 0.2 mg of 5-bromo-4-chloro-3-indolylphosphate toluidine salt [Roche Molecular Biochemicals] in 0.1 M Tris-HCl [pH 9.5], 0.05 M MgCl2, 0.1 M NaCl, and 1 mM levamisole) for 10 min at room temperature. Cover slips were mounted in PBS-glycerol and analyzed by light microscopy. Apoptotic cells (characterized by a dark nuclear precipitate) and nonapoptotic cells (unstained or displaying a diffuse, light, and uneven cytoplasmic staining) were counted in five randomly chosen microscopic fields (containing at least 250 cells). The percentage of apoptotic mock-infected cells was subtracted from each average. Results are expressed as percent apoptotic cells ± standard error of the mean (SEM).
Viability assay.
Cell viability was determined by the CellTiter 96 Aqueous One solution cell proliferation assay (Promega) used according to the manufacturer’s instructions on cells grown in 96-well plates. This assay determines the levels of cellular 3-[4,5-dimethylthiazol-2-yl-5-(3-carboxymethoxyphenyl)-2-(4-sulfo-phenyl)-2H-tetrazolium, inner salt] (MTS) reduction to formazan, a measure of mitochondrial function. Briefly, the Cell Titer 96 Aqueous One solution was added to the wells (1:5, vol/vol), and the plates were incubated for 1 h at 37°C. Absorbance was read at 490 nm using an enzyme-linked immunosorbent assay (ELISA) plate reader. Values are the means ± SEM of three independent experiments (each done in quadruplicate), and results are expressed as percent survival (MTS reduction).
Immunofluorescent and immunoperoxidase staining.
The identity of the TUNEL-positive cells in primary hippocampal cultures was determined by double immunofluorescence. The cultures were incubated with TdT and the nucleotide mixture (containing FITC-conjugated dUTP) for 1 h at 37°C and stained (1 h, room temperature) with antibodies NF-160, TuJ1, GFAP, or GalC followed by phycoerythrin (PE)-conjugated anti-mouse IgG (30 min, room temperature). Stained cells were visualized with an epifluorescence confocal microscope fitted with an argon ion laser (Zeiss LSM 410) as described (11, 13).
The Dako LSAB 2 kit horseradish peroxidase (Dako Corporation, Carpinteria, Calif.) was used for immunoperoxidase staining. Cells were exposed overnight (4°C) to the primary antibodies (specific for ICP10 or activated caspase-3), and the immunolabeled cells were detected by the streptavidin-biotin method according to the manufacturer’s instructions. The cultures were counterstained with Mayer’s hematoxylin (Sigma) (11, 13, 54), and the cells staining with primary antibodies were counted in five randomly chosen microscopic fields (containing at least 250 cells). The average percentage of staining cells was calculated, and the percentage of staining mock-infected or mock-treated cells was subtracted from each average. The results are expressed as percent positive cells ± SEM.
Hoechst staining.
Cells were fixed in 4% PFA (pH 7.4), permeabilized with 0.2% Triton X-100, and stained with the fluorescent DNA-binding dye Hoechst 32258 (40).
DNA fragmentation.
DNA fragmentation (ladder formation) was assayed as described by Hata et al. (42). Cells were collected by trypsinization, and DNA was extracted as described by Hirt (44). It was quantitated by spectrophotometry, and 5 to 10 μg was separated on 1.5% agarose gels. Gels were stained with 0.1 μg of ethidium bromide per ml and visualized by exposure to UV light.
Immunoblotting.
Immunoblotting was done as previously described (47, 62, 69, 79–82). Briefly, cells were lysed with radioimmunoprecipitation assay buffer (30 mM Tris-HCl [pH 7.4], 0.15 mM NaCl, 1% Nonidet P-40, 0.1% sodium dodecyl sulfate [SDS], 0.5% sodium deoxycholate, 1 mM EDTA, 1 mM dithiothreitol [DTT], 2 mM MgCl2, 0.5 mM phenylmethylsulfonyl fluoride [PMSF]) supplemented with phosphatase and protease inhibitor cocktails (Sigma) and sonicated for 30 s at 25% output power using a Sonicator ultrasonic processor (Misonix, Inc., Farmingdale, N.Y.). Total protein was determined by the bicinchoninic acid assay (Pierce, Rockford, Ill.), and proteins were resolved by SDS-polyacrylamide gel electrophoresis (PAGE) and transferred to nitrocellulose membranes. The blots were incubated (1 h, 37°C) in TN-T buffer (0.01 M Tris-HCl [pH 7.4], 0.15 M NaCl, 0.05% Tween 20) containing 1% bovine serum albumin (BSA) to block nonspecific binding and exposed (2 h, room temperature) to the appropriate antibodies (diluted in TN-T buffer with 0.1% BSA).
In some experiments, exposure to primary antibodies was done using a miniblotter (model 25; Immunetics, Cambridge, Mass.). After three washes with TN-T buffer, the blots were incubated with protein A-peroxidase for 1 h at room temperature. Detection was done with ECL reagents (Amersham Life Science, Arlington Heights, Ill.) and exposure to high-performance chemiluminescence film (Hyperfilm ECL; Amersham). Quantitation was done by densitometric scanning using the Bio-Rad GS-700 imaging densitometer.
Virus neutralization.
For neutralization with the anti-HSV-2 or preimmune serum, HSV-2 (107 PFU) was incubated (1 h, 37°C) with an equal volume of the respective serum. For neutralization with the gD MAb, cells were incubated for 30 min with 2.5 μg of the IgG fraction per ml and exposed (1 h, 37°C) to a virus-antibody mixture consisting of HSV-2 (107 PFU) and 2.5 μg of of gD IgG per ml (1 h, 37°C). Virus surviving neutralization was determined by plaque assay as described elsewhere (11).
Single-step virus growth assays.
Single-step growth assays were done as previously described (11, 81, 82). Primary cultures of hippocampal neurons were infected with HSV-2, HSV-2(R), ICP10ΔPK, or ICP10ΔRR (5 PFU/cell). Adsorption was for 1 h (0 h in growth curve). Cells and supernatants were harvested at 2 to 48 h after adsorption, frozen and thawed, and assayed for virus titers by plaque assay (11, 81, 82). Results are expressed as mean PFU ± SEM.
Statistical analyses.
One-way analysis of variance (ANOVA) with Tukey-Kramer posttest was done using GraphPad InStat version 3.01 for Windows 95/NT (GraphPad Software, San Diego, Calif.).
RESULTS
Cells stably transfected with ICP10 PK are protected from apoptosis induced by STS or d-Mann.
We have previously shown that the Ras/MEK/MAPK pathway is activated in cells that express ICP10 PK (JHLa1) but not its PK-negative mutant p139 (JHL15) (79). Because this pathway was implicated in the control of apoptosis (53), we examined the response of JHLa1, JHL15, and parental (HEK293) cells to the known apoptosis inducers STS and d-Mann (49, 64). Cells were treated (24 h) with STS (250 nM) or d-Mann (300 mM) in medium containing 1% FBS and examined by TUNEL, an assay that is widely considered to be specific for apoptosis (36, 37).
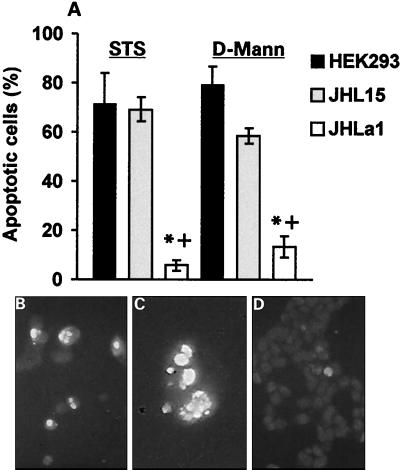
As shown in Fig. 1A, the proportion of TUNEL-positive (apoptotic) cells was significantly higher in STS-treated HEK293 (71% ± 12.4%) and JHL15 (69% ± 4.9%) than JHLa1 (5.8% ± 2.1%) cells (P < 0.01 by ANOVA), and similar results were obtained for d-Mann (79% ± 7.4%, 58% ± 7%, and 13% ± 8% for HEK293, JHL15, and JHLa1 cells, respectively). TUNEL-positive STS-treated HEK293 and JHL15 cells evidenced condensed chromatin and nuclear fragmentation, which are hallmark morphologic features of apoptosis (Fig. 1B and C), but these features were essentially absent in STS-treated JHLa1 cells (Fig. 1D). We interpret the data to indicate that ICP10 negatively regulates apoptosis induced by STS or d-Mann, a function that requires PK activity.
FIG. 1.
ICP10 PK inhibits STS- and d-Mann-induced apoptosis in constitutively expressing cells. Parental HEK293 cells and HEK293 cells stably transfected with ICP10 (JHLa1) or the PK-negative ICP10 mutant p139 (JHL15) were treated (24 h) with 250 nM STS or 300 mM d-Mann and assayed for apoptosis by TUNEL. (A) TUNEL-positive and -negative cells were counted in five randomly chosen microscopic fields, and the mean percent apoptotic cells ± SEM was calculated as described in Materials and Methods. *, P < 0.01 versus HEK293; +, P < 0.01 versus JHL15 by ANOVA. (B and C) Apoptotic nuclei were seen for STS-treated HEK293 (B) and JHL15 (C) cells as determined by TUNEL with FITC-conjugated dUTP. (D) Apoptotic nuclei were not seen for STS-treated JHLa1 cells examined by TUNEL with FITC-conjugated dUTP.
ICP10 PK blocks STS- or d-Mann-induced DNA fragmentation.
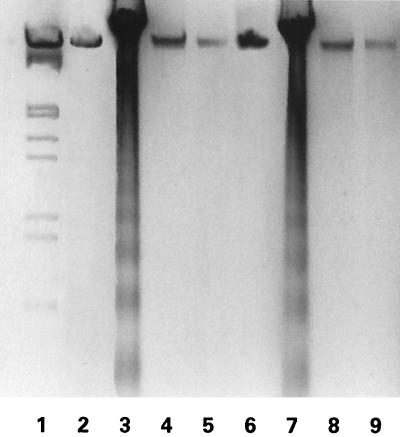
Because DNA fragmentation (ladder formation) is a hallmark of most apoptotic conditions (5), we wanted to know whether ICP10 PK can block ladder formation induced by apoptotic stimuli. HEK293, JHL15, and JHLa1 cells were treated (24 h) with STS (250 nM) or d-Mann (300 mM), and genomic DNA was examined for fragmentation as described in Materials and Methods. Cells mock-treated with dimethyl sulfoxide (DMSO) (1:4,000 [vol/vol], diluent for STS) or MEM (diluent for d-Mann) were studied in parallel. DNA fragmentation was seen in HEK293 cells treated with STS (Fig. 2, lane 3) or d-Mann (Fig. 2, lane 7), but not in mock-treated HEK293 cells (Fig. 2, lanes 2 and 6). Similar results were obtained for JHL15 cells (data not shown). DNA fragmentation was not seen in JHLa1 cells treated with STS (Fig. 2, lane 4) or d-Mann (Fig. 2, lane 8) or mock-treated with DMSO (Fig. 2, lane 5) or MEM (Fig. 2, lane 9). The data indicate that ICP10 PK blocks DNA fragmentation induced by the apoptotic stimuli studied in these series of experiments.
FIG. 2.
STS-induced DNA fragmentation is inhibited in JHLa1 cells. HEK293 (lanes 2, 3, 6, and 7) and JHLa1 (lanes 4, 5, 8, and 9) cells were treated with 250 nM STS (lanes 3 and 4) or 300 mM d-Mann (lanes 7 and 8) or mock treated with DMSO (control for STS) (lanes 2 and 5) or MEM (control for d-Mann) (lanes 6 and 9). Genomic DNA was extracted at 24 h postinfection and separated on agarose gels as described in Materials and Methods.
ICP10 PK blocks STS-induced caspase-3 activation and PARP cleavage.
Two series of experiments were done in order to further examine the ability of ICP10 PK to block apoptosis induced by STS. In the first series, we asked whether ICP10 PK interferes with cleavage (and, thereby, activation) of pro-caspase-3, a central determinant of many apoptotic processes (52). Duplicate cultures of HEK293, JHL15, and JHLa1 cells were treated with STS, and cell extracts were immunoblotted with an antibody specific for the inactive (uncleaved) pro-caspase-3 or stained with an antibody (D175) that is specific for the large fragment of activated caspase-3 (33, 70) and recognizes an 18-kDa band in STS-treated but not untreated HEK293 cells (data not shown). Cells treated with DMSO (diluent for STS) served as the control.
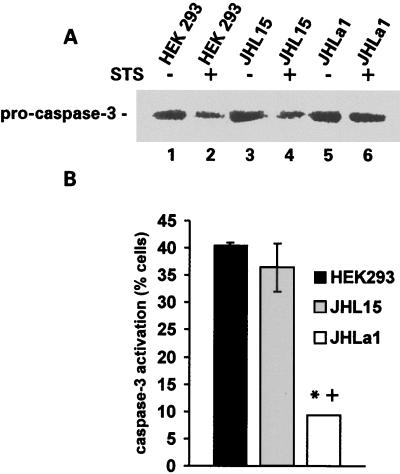
A 32-kDa band consistent with pro-caspase-3 was seen in all cell extracts, but its levels were lower in HEK293 (Fig. 3A, lane 2) and JHL15 (Fig. 3A, lane 4) cells treated with STS than in those treated with DMSO (Fig. 3A, lanes 1 and 3). By contrast, the levels of the 32-kDa band were similar in JHLa1 cells treated with STS (Fig. 3A, lane 6) or DMSO (Fig. 3A, lane 5), suggesting that ICP10 PK blocks the cleavage (activation) of pro-caspase-3. Consistent with this interpretation, the mean percentage of cells staining with the antibody to activated caspase-3 was significantly (P < 0.01 by ANOVA) higher in STS-treated HEK293 (40.5% ± 0.9%) and JHL15 (36.4% ± 7.6%) than JHLa1 (9.3% ± 0.1%) cells (Fig. 3B).
FIG. 3.
Caspase-3 activation is inhibited in JHLa1 cells. (A) Extracts of HEK293 (lanes 1 and 2), JHL15 (lanes 3 and 4) and JHLa1 (lanes 5 and 6) cells, mock treated (with DMSO) (lanes 1, 3, and 5) or treated (24 h) with 250 nM of STS (lanes 2, 4, and 6) were resolved by SDS-PAGE (7% acrylamide gels), transferred to nitrocellulose membranes, and immunoblotted with antibody specific for the inactive pro-caspase-3. (B) Duplicates of the cultures shown in A were stained with antibody specific for the large fragment of activated caspase-3, and cells in five randomly chosen microscopic fields were counted. Results are expressed as mean percent positive cells ± SEM. *, P < 0.01 versus HEK293; +, P < 0.01 versus JHL15 by ANOVA. The cultures did not stain with normal rabbit serum, and the proportion of untreated staining cells was minimal (<5%).
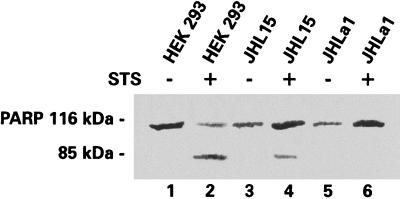
Because PARP cleavage by caspase-3 is a hallmark of the commitment to undergo apoptosis (86), the second series of experiments asked whether ICP10 PK blocks PARP cleavage. HEK293, JHL15, and JHLa1 cells were treated with STS (or DMSO control) as above, and the cell extracts were immunoblotted with antibody specific for PARP. A 116-kDa band consistent with uncleaved PARP was seen in all cell extracts (Fig. 4, lanes 1 to 6). By contrast, an 85-kDa band that is consistent with the PARP cleavage product was seen only in STS-treated HEK293 (Fig. 4, lane 2) and JHL15 (Fig. 4, lane 4) cells. It was not seen in STS-treated JHLa1 cells (Fig. 4, lane 6) or in DMSO-treated HEK293, JHL15, or JHLa1 cells (Fig. 4, lanes 1, 3, and 5). The exact interpretation for the apparently higher levels of uncleaved (116 kDa) PARP in STS-treated JHL15 and JHLa1 cells is still unclear. Nonetheless, the data indicate that ICP10 PK blocks PARP cleavage.
FIG. 4.
PARP is not cleaved in JHLa1 cells. The blot in Fig. 3A was stripped and immunoblotted with anti-PARP antibody. The 85-kDa band consistent with the PARP cleavage product was seen in STS-treated HEK293 (lane 2) and JHL15 (lane 4) but not JHLa1 (lane 6) cells.
ICP10 PK but not p139TM rescues neuronally differentiated PC12 cells from death due to NGF withdrawal.
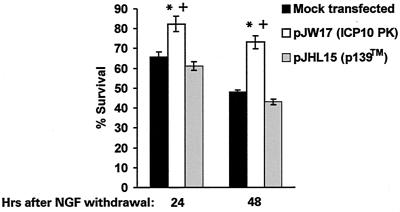
To examine whether ICP10 PK has antiapoptotic activity under paradigms other than those studied in JHLa1 cells, we took advantage of previous findings that PC12 cells grown in NGF-containing medium acquire properties of sympathetic neurons (neurite outgrowth, electrical excitability, and expression of specific neuronal markers) and die by apoptosis upon NGF withdrawal (39). Specifically, PC12 cells were neuronally differentiated by growth (at least 12 days) in serum-free DMEM/F12 supplemented with 0.36% d-glucose, 0.21% sodium bicarbonate, 0.009% gentamicin, and 100 ng of nerve growth factor (NGF) (Roche Molecular Biochemicals) per ml. They were transfected with pJW17 or pJHL15 (which express ICP10 and p139, respectively) using the FuGene 6 transfection reagent (Roche Molecular Biochemicals) and cultured (24 h) in NGF-containing medium to allow transgene expression. At this time (0 h post-NGF withdrawal), the medium was replaced with NGF-free medium, and the cells were cultured for an additional 48 h in the absence of NGF. They were examined daily for viability using the MTS assay (15, 75). Results are expressed as percent survival relative to 0 h post-NGF withdrawal ± SEM.
Consistent with previous reports for nonneuronal cells transfected with the pJW17 (ICP10) or pJHL15 (p139) expression vectors (23, 62, 94), approximately 25 to 35% of the transfected PC12 cells stained with ICP10 antibody at 24 h posttransfection. Staining was not seen in mock-transfected PC12 cells or with normal serum. Similar results were obtained in three independent experiments, suggesting that ICP10 and p139 are expressed equally well in transfected cells (23, 62, 94). By contrast, the percent survival was significantly (P < 0.05 by ANOVA) higher for pJW17-transfected cultures (82.3% ± 3.9% and 73.2% ± 3.3% at 24 and 48 h, respectively) than for mock-transfected cultures (65.7% ± 2.7% and 48.1% ± 1% at 24 and 48 h, respectively). Increased survival in pJW17-transfected cultures is consistent with the estimated proportion of cells expressing ICP10. We conclude that survival requires a functional ICP10 PK activity, because the percentage of pJHL15-transfected cells surviving NGF withdrawal (61.2% ± 1% and 43.1% ± 1.4% at 24 and 48 h, respectively) was similar to that of mock-transfected cultures and significantly lower (P < 0.05 by ANOVA) than that seen for pJW17 (Fig. 5).
FIG. 5.
ICP10 PK protects neuronally differentiated PC12 cells from death due to NGF withdrawal. PC12 cells were differentiated by growth (12 to 14 days) in serum-free medium supplemented with 100 ng of NGF per ml. They were transfected with pJW17 (expresses ICP10 PK) or pJHL15 (p139) or mock transfected with FuGene and examined for cell survival by the MTS reduction assay using the Cell Titer 96 Aqueous One solution cell proliferation assay. Results are expressed as percent survival relative to 0 h post-NGF withdrawal ± SEM. *, P < 0.05 versus control; +, P < 0.05 versus pJHL15-transfected cells, by ANOVA.
ICP10 PK inhibits apoptosis and caspase-3 activation in virus-infected hippocampal cultures.
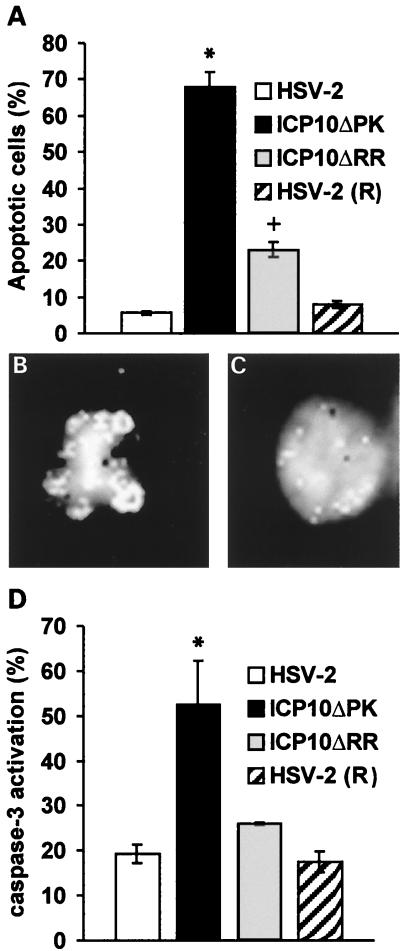
Having seen that ICP10 PK has antiapoptotic activity that encompasses neuronally differentiated PC12 cells, we wanted to know whether this activity extends to virus-induced apoptosis in primary hippocampal cultures. Cells were infected with HSV-2 or ICP10ΔPK (10 PFU/cell) and examined by TUNEL at 24 h postinfection The revertant virus [HSV-2(R)] (81, 82) and the HSV-2 mutant ICP10ΔRR, which is deleted in the RR domain of ICP10 (12), were studied in parallel and served as controls. The data shown in Fig. 6A represent the results of three independent experiments.
FIG. 6.
ICP10 PK has antiapoptotic activity and inhibits caspase-3 activation in virus-infected hippocampal cultures. (A) Primary hippocampal cultures were infected (24 h) with HSV-2, HSV-2(R), ICP10ΔPK, or ICP10ΔRR (10 PFU/cell) and analyzed by TUNEL. The results are expressed as the mean percent apoptotic cells ± SEM estimated by counting cells in five randomly chosen microscopic fields and subtracting the percent positive cells in mock-infected cultures, as described in Materials and Methods. *, P < 0.01 versus HSV-2 and HSV-2(R) or ICP10ΔRR; +, P = 0.046 versus HSV-2 or HSV-2(R) by ANOVA. (B) Hoechst staining of representative nuclei from TUNEL-positive ICP10ΔPK-infected cells. (C) Hoechst staining of representative nuclei from TUNEL-negative HSV-2-infected cells. (D) Primary hippocampal cultures infected with HSV-2, HSV-2(R), ICP10ΔPK, or ICP10ΔRR as in A were stained with antibody to the activated caspase-3. The results are expressed as the mean percent staining cells ± SEM estimated by counting cells in five randomly chosen microscopic fields as in A. *, P < 0.05 versus HSV-2, HSV-2(R), or ICP10ΔRR by ANOVA.
The percent apoptotic cells was significantly higher (P < 0.01 by ANOVA) in cultures infected with ICP10ΔPK (68% ± 3.9%) than HSV-2 (5.7% ± 0.5%) or HSV-2(R) (7.9% ± 1%), suggesting that ICP10 PK has antiapoptotic activity also in this paradigm. TUNEL-positive ICP10ΔPK-infected cells evidenced nuclear fragmentation characteristic of apoptosis (Fig. 6B) that was not seen in TUNEL-negative HSV-2- (Fig. 6C) or HSV-2(R)- (data not shown) infected cells, and similar results were obtained at 16 h postinfection (data not shown). The mean percent apoptotic cells in cultures infected with ICP10ΔRR (23% ± 2.1%) was significantly (P < 0.01 by ANOVA) lower than that seen for ICP10ΔPK, but higher than that seen for HSV-2 or HSV-2(R) (P = 0.046 by ANOVA), suggesting that ICP10 RR contributes to the ability of HSV-2 to negatively regulate apoptosis (Fig. 6A).
To examine whether antiapoptotic activity involves blocking of caspase-3 activation, primary hippocampal cultures were infected with HSV-2 or ICP10ΔPK (10 PFU/cell, 24 h) and stained with the D175 antibody (specific for activated caspase-3) or normal rabbit serum (control). ICP10ΔRR and HSV-2(R) were studied in parallel and served as controls. The percentage of cells staining with the activated caspase-3 antibody was significantly higher (P < 0.05 by ANOVA) for ICP10ΔPK (52.6% ± 9.6%) than HSV-2 (19.3% ± 2%), and similar results were obtained in three independent experiments (Fig. 6D). However, the proportion of staining cells was similar for ICP10ΔRR (26% ± 0.2%), HSV-2 (19.3% ± 2%), and HSV-2(R) (17.5% ± 2.3%) (P > 0.05 by ANOVA), suggesting that the contribution of ICP10 RR to the antiapoptotic activity of HSV-2 is likely to be downstream of caspase-3 activation. Normal rabbit serum was negative.
TUNEL-positive ICP10ΔPK-infected hippocampal cells are neurons.
Because primary hippocampal cultures consist of various cell populations, two series of experiments were done in order to determine the identity of the apoptotic cells in ICP10ΔPK-infected cultures. In the first series, we used staining with antibodies to neuronal proteins NF-160 and TuJ1 (specific for postmitotic neurons [34]) in order to estimate the proportion of neurons in our hippocampal cultures. Antibodies to GFAP and GalC, which are specific for astrocytes and oligodendrocytes, respectively, served as controls. Consistent with previous conclusions that the majority of cells in such cultures are neurons (14), approximately 85 to 95% of the cells stained with NF-160 or TuJ1 antibodies, while only 5 to 8% stained with the GFAP antibody. Staining was not seen with the GalC antibody, consistent with previous reports that such cultures are free of oligodendrocytes (18).
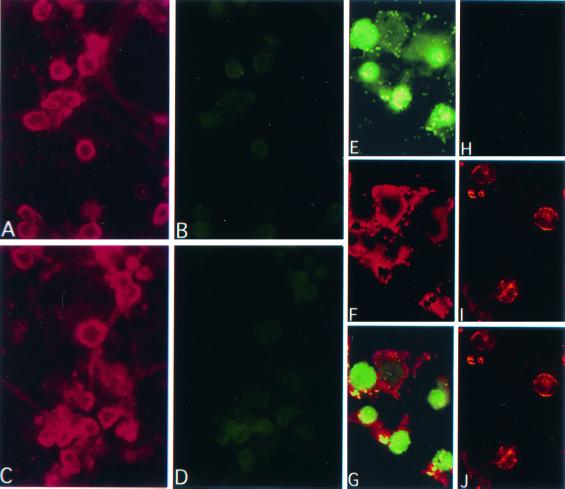
The second series of experiments used double immunofluorescent staining in order to examine the identity of the TUNEL-positive cells. Cultures infected (24 h) with HSV-2 or ICP10ΔPK (10 PFU/cell) were examined for TUNEL using FITC-labeled dUTP and stained with PE-labeled antibodies to NF-160, TuJ1, or GFAP, as described in Materials and Methods. HSV-2-infected cultures stained with NF-160 or TuJ1 antibody (Fig. 7A), but they were mostly TUNEL negative (Fig. 7B). Uninfected cultures also stained with these antibodies (Fig. 7C) and were TUNEL negative (Fig. 7D). By contrast, cultures infected with ICP10ΔPK had TUNEL-positive cells (Fig. 7E), and these cells stained with TuJ1 (Fig. 7F and G) or NF-160 (data not shown) antibodies. As described previously (20), the TUJ1 and NF-160 staining (PE) localized in the cell bodies and projections (Fig. 7F), while the FITC staining (TUNEL) was primarily nuclear (Fig. 7G). Some cells showed TUNEL staining in the cytoplasm (Fig. 7E and G), presumably representing leakage of DNA fragments from the nucleus in late-stage apoptotic cells (56). GFAP-staining cells in the HSV-2-infected cultures (Fig. 7I) were TUNEL negative (Fig. 7H and J). We interpret the data to indicate that TUNEL-positive cells in virus-infected hippocampal cultures are neurons.
FIG. 7.
TUNEL-positive cells in virus-infected hippocampal cultures are neurons. Primary hippocampal cultures infected with HSV-2 (10 PFU/cell; 24 h) (A and B) or mock infected (C and D) were stained with NF-160 antibody (A and C) and assayed by TUNEL (B and D). Similar results were obtained with TuJ1 antibody (data not shown). ICP10ΔPK-infected primary hippocampal cultures were assayed by TUNEL (E and H) and stained with TuJ1 (F) or GFAP (I) antibody. TUNEL colocalized with TuJ1 (G) but not GFAP (J) staining.
Growth defects of the ICP10 mutants are independent of their ability to induce apoptosis.
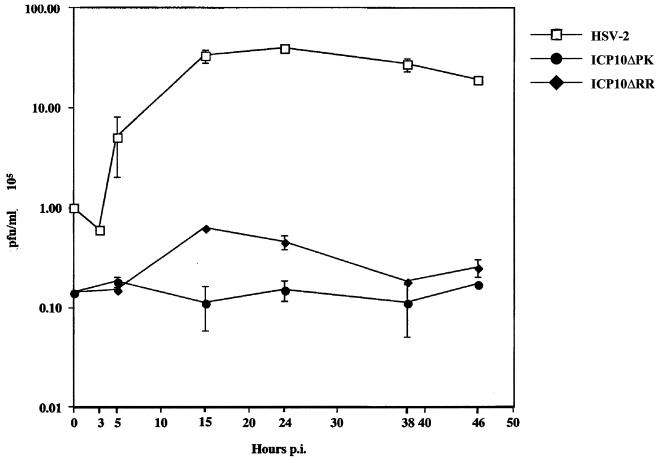
Viruses have acquired various strategies to escape apoptosis, including completing the replicative cycle before onset of apoptosis (55). To determine whether apoptosis in infected hippocampal neurons is related to virus replication, single-step growth curves were done as previously described (81, 82). The data summarized in Fig. 8 indicate that HSV-2 replication began at 3 h postinfection, with maximal titers reached at 15 to 24 h postinfection and remaining relatively stable until 48 h postinfection These growth kinetics are similar to those reported previously for nonneuronal cells (81, 82) and identical to those seen for HSV-2(R) (data not shown).
FIG. 8.
ICP10ΔPK and ICP10ΔRR are growth defective in primary hippocampal cultures. Single-step growth assays were done in hippocampal cultures infected with HSV-2, ICP10ΔPK, or ICP10ΔRR (5 PFU/cell). Virus titers were determined at 0 to 48 h postinfection, and results are expressed as mean 105 PFU per milliliter ± SEM.
In nondividing cells that are not neuronal (grown in 1% serum), both ICP10ΔPK and ICP10ΔRR are growth compromised, but the ICP10ΔPK mutant is much more affected, suggesting that PK activity may be more significant for virus growth under these conditions (12). However, in hippocampal neurons, both ICP10ΔPK, which causes high levels of apoptosis, and ICP10ΔRR, which causes relatively little apoptosis, were similarly growth defective. We interpret the data to suggest that the growth defects seen in these mutants are independent of their ability (or inability) to cause apoptosis in hippocampal neurons.
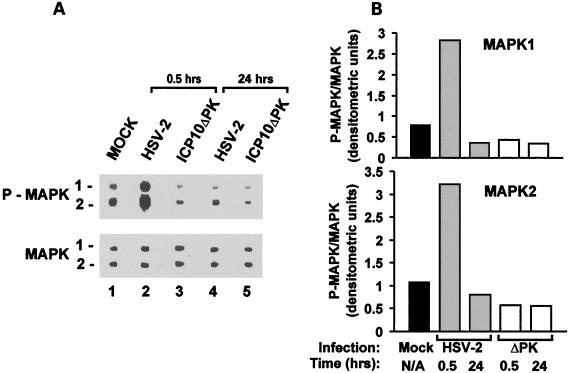
MEK/MAPK are activated in HSV-2- but not ICP10ΔPK-infected hippocampal cultures.
Having shown that ICP10 PK has antiapoptotic activity in hippocampal neurons, we wanted to know whether this is related to its ability to activate MEK/MAPK (79, 82). Cultures of hippocampal neurons were infected with HSV-2 or ICP10ΔPK (10 PFU/cell) and analyzed for MAPK activation at 30 min and 24 h postinfection (0 h postinfection is at the end of adsorption) by immunoblotting with antibody specific for the phosphorylated (activated) MAPK1/2 species (P-MAPK1/2). Antibody to the unphosphorylated MAPK1/2 species served as the control for improper gel loading or other technical artifacts.
Two bands consistent with P-MAPK1/2 were seen in mock-infected cultures (Fig. 9A, lane 1). At 30 min postinfection, their levels were significantly higher in cultures infected with HSV-2 (Fig. 9A, lane 2) than ICP10ΔPK (Fig. 9A, lane 3). The levels of MAPK1/2 were similar in all cultures. We conclude that MAPK activation is a relatively rapid (30 min postinfection) and transient event, because the levels of P-MAPK1/2 were not increased in cells infected with HSV-2 (Fig. 9A, lane 4) or ICP10ΔPK (Fig. 9A, lane 5) for 24 h. The conclusion that MAPK1/2 are activated within 30 min postinfection with HSV-2 but not ICP10ΔPK is supported by densitometric scanning and data expression as P-MAPK/MAPK ratios (Fig. 9B).
FIG. 9.
MAPK is activated in HSV-2- but not ICP10ΔPK-infected hippocampal cultures. (A) Cells were infected with HSV-2 (lanes 2 and 4) or ICP10ΔPK (lanes 3 and 5) (10 PFU/cell) or mock infected with growth medium (lane 1) and harvested at 0.5 and 24 h postinfection. Proteins were resolved by SDS-PAGE (8.5% acrylamide gels), transferred to nitrocellulose membranes, and immunoblotted with antibody specific for P-MAPK1/2. Blots were stripped and reblotted with antibody to MAPK1/2. (B) Protein levels were quantitated by densitometric scanning, and the results are expressed as P-MAPK/MAPK ratios for both isoforms.
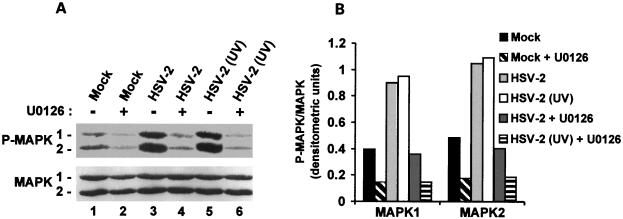
To examine the contribution of upstream components of the Ras survival pathway to MAPK activation, cultures of hippocampal neurons were infected with HSV-2 in the presence or absence of the MEK-specific inhibitor U0126 (20 μM) (32), and cell extracts were examined for MAPK activation by immunoblotting with antibody specific for P-MAPK1/2 (or the MAPK1/2 control), as described above. As expected, the levels of P-MAPK1/2 were higher in HSV-2- (Fig. 10A, lane 3) than mock-infected (Fig. 10A, lane 1) cultures. However, P-MAPK1/2 levels were decreased by U0126 treatment in both mock-infected (Fig. 10, lane 2) and HSV-2-infected (Fig. 10A, lane 4) cultures. The levels of MAPK1/2 were similar in all cultures. Densitometric scanning of the blots and data expression as P-MAPK/MAPK ratios (Fig. 10B) support the conclusion that MAPK activation is MEK dependent.
FIG. 10.
MAPK activation in HSV-2-infected hippocampal cultures is MEK dependent and does not require de novo viral protein synthesis. (A) Extracts of cells mock infected (lanes 1 and 2) or infected with HSV-2 (lanes 3 and 4) or UV-inactivated HSV-2 (lanes 5 and 6) in the absence (lanes 1, 3, and 5) or presence of 20 μM U0126 (lanes 2, 4, and 6) were obtained at 30 min postinfection and immunoblotted with antibody specific for P-MAPK1/2, followed by MAPK1/2 as in Fig. 9. (B) The bands in panel A were analyzed by densitometric scanning, and the results are expressed as P-MAPK/MAPK ratios for both isoforms.
MEK/MAPK activation does not require de novo viral protein synthesis.
The finding that MEK and MAPK are not activated by ICP10ΔPK, which does not replicate in hippocampal cultures, suggests that activation may require de novo viral protein synthesis. However, activation is seen at 30 min postinfection, before onset of viral protein synthesis (2 to 3 h postinfection). To examine whether viral protein synthesis is required for MEK/MAPK activation, hippocampal cultures were infected with HSV-2 or UV-inactivated HSV-2 (10 PFU/cell), which penetrates the cells but is defective in protein synthesis (74). Infection was done in the absence or presence of U0126 (20 μM). Cell extracts collected at 30 min postinfection were examined for MAPK activation by immunoblotting with P-MAPK1/2 antibody and MAPK1/2 antibody (control).
P-MAPK1/2 levels were increased in cultures infected with HSV-2 (Fig. 10A, lane 3) or UV-inactivated HSV-2 (Fig. 10A, lane 5) relative to mock-infected cells (Fig. 10A, lane 1), and the increase was virtually identical for both HSV-2 and UV-inactivated HSV-2. P-MAPK1/2 levels were not increased when infection was done in the presence of U0126 (Fig. 10A, lanes 4 and 6), and MAPK1/2 levels were similar in all samples. Densitometric scanning and data analysis as P-MAPK/MAPK ratios (Fig. 10B) confirmed the conclusion that MAPK is activated equally well by HSV-2 and UV-inactivated HSV-2, suggesting that MEK/MAPK activation is independent of de novo viral protein synthesis.
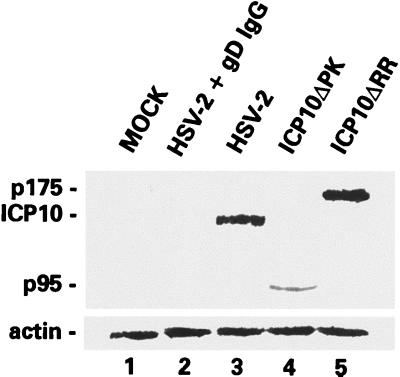
We considered the possibility that the virion ICP10 protein causes MEK/MAPK activation, an interpretation that carries the implicit conclusion that ICP10ΔPK (and ICP10ΔRR) cause apoptosis (albeit at different levels) because their respective ICP10 proteins (p95 and p175) are not released upon virion uncoating (p95 and p175 are also incorporated into the virion tegument [80; unpublished data]). To test this interpretation, hippocampal cultures were infected with HSV-2, ICP10ΔPK, ICP10ΔRR, or antibody-neutralized viruses (10 PFU/cell), and cell extracts obtained at 30 min postinfection were immunoblotted with ICP10 antibody, which recognizes ICP10, p95, and p175 (12).
ICP10 (Fig. 11, lane 3), p95 (Fig. 11, lane 4), and p175 (Fig. 11, lane 5) were seen in the respective cell extracts, but they were not seen in extracts of cells infected with antibody-neutralized virus, as shown for HSV-2 in Fig. 11, lane 2. This is consistent with the failure of neutralized virus to penetrate the cells (43, 51) and suggests that cell penetration occurs within 30 min postinfection Taken together with the finding that MEK/MAPK activation does not require de novo viral protein synthesis, the presence of ICP10 in extracts of HSV-2-infected cells at 30 min postinfection suggests that ICP10 is released into the cell upon virion uncoating. However, the exact reason for the lower levels of p95 relative to ICP10 and p175 in hippocampal cultures is still unclear.
FIG. 11.
ICP10 PK and its mutants are present in 30-min-infected primary hippocampal cultures. Extracts from hippocampal cultures mock infected (lane 1) or infected (10 PFU/cell) with HSV-2 neutralized with gD MAb (lane 2), HSV-2 (lane 3), ICP10ΔPK (lane 4), or ICP10ΔRR (lane 5) were immunoblotted with ICP10 antibody. Blots were stripped and reprobed with actin antibody control.
MEK/MAPK activation is required for HSV-2 antiapoptotic activity in hippocampal neurons.
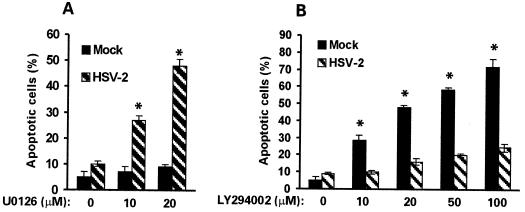
Having shown that MEK and MAPK are activated in HSV-2-infected hippocampal cultures, we wanted to know whether activation is required for the antiapoptotic activity of HSV-2 in these cells. Hippocampal cultures were mock infected with growth medium or infected with HSV-2 (10 PFU/cell) in the absence or presence of 10 or 20 μM U0126 and examined for apoptosis by TUNEL at 24 h postinfection. A dose-dependent increase in the percentage of TUNEL-positive (apoptotic) cells was seen in HSV-2-infected cultures treated with U0126 (27% ± 1.6% and 48.3% ± 2.8% for 10 and 20 μM, respectively) compared to similarly infected but untreated cells (10% ± 0.7%) (P < 0.01 by ANOVA), suggesting that the antiapoptotic activity of HSV-2 requires MEK/MAPK activation (Fig. 12A). Significantly, however, U0126 treatment had no effect on the survival of mock-infected cultures (Fig. 12A), although U0126 inhibits MEK/MAPK activation. The data suggest that MEK/MAPK activation is not required for the survival of uninfected hippocampal neurons, at least under these experimental conditions.
FIG. 12.
MEK/MAPK and PI3-K/Akt pathways are involved in survival of HSV-2-infected and uninfected hippocampal cultures, respectively. (A) Hippocampal cultures were infected with HSV-2 (10 PFU/cell, 24 h) or mock infected with MEM in the absence or presence (10 to 20 μM) of U0126 and analyzed by TUNEL. Cells in five randomly chosen microscopic fields were counted, the percent positive cells in mock-infected cultures was subtracted, and the results are expressed as mean percent TUNEL-positive (apoptotic) cells ± SEM. *, P < 0.01 versus untreated HSV-2-infected cells by ANOVA. (B) Hippocampal cultures were infected with HSV-2 or mock infected in the absence or presence of LY294002 (10 to 100 mM) and analyzed by TUNEL as in A. *, P < 0.01 versus untreated mock-infected cells by ANOVA.
Because the PI3-K/Akt pathway was implicated in the protection of neurons from apoptosis due to growth factor withdrawal (27), we considered the possibility that this pathway is also involved in the survival of HSV-2-infected hippocampal cultures. Cells were infected with HSV-2 (10 PFU/cell) or mock infected with growth medium in the absence or presence of increasing concentrations (10 to 100 μM) of the PI3-K-specific inhibitor LY294002 (87) and assayed by TUNEL at 24 h postinfection As shown in Fig. 12B, LY294002 had a minimal effect on the percentage of TUNEL-positive cells in HSV-2-infected cultures (9.1% ± 1% and 24.8% ± 2.3% at 0 and 100 μM, respectively), suggesting that this pathway has little contribution to the survival of HSV-2-infected hippocampal neurons. By contrast, LY294002 had a major effect on the survival of mock-infected cells, with 5% ± 3% apoptotic cells in the untreated cultures compared to 72% ± 4.5% at 100 μM LY294002 (Fig. 12B) (P < 0.01 by ANOVA), suggesting that the PI3-K pathway is required for basal maintenance of hippocampal neurons. Presumably, HSV-2 infection uncouples cell survival from PI3-K activation by activating the MEK/MAPK pathway. However, the possibility cannot be excluded that both pathways converge upon similar downstream effectors.
Cell penetration is required for MEK/MAPK activation.
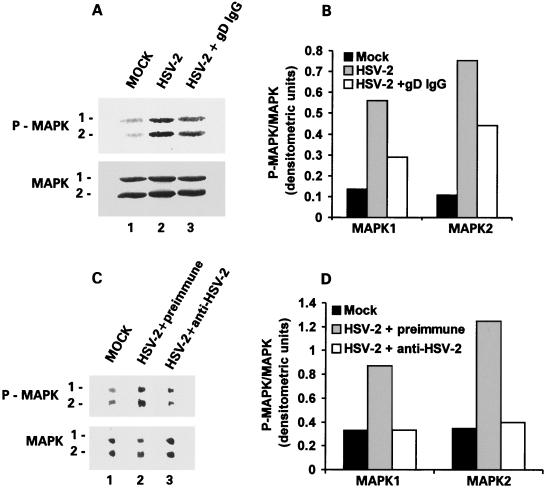
The finding that MEK and MAPK are activated at 30 min postinfection of hippocampal cultures is amenable to two potential interpretations. According to the first interpretation, activation is mediated by the ICP10 PK located in the tegument of the incoming virion (80). Implicit in this interpretation is the conclusion that cellular penetration and virion uncoating are required for MEK/MAPK activation. An alternative interpretation is that MEK and MAPK are activated by virus binding to receptors on the cell surface, and both it and the resulting antiapoptotic effect are independent of cellular penetration. This possibility is particularly significant because an HSV receptor can generate a signal that regulates the transcription factor AP-1 upon ligand binding (65).
To examine whether MEK/MAPK activation is dependent on cellular penetration, hippocampal cultures were infected with antibody-neutralized HSV-2, which can attach to but does not penetrate the cells (43, 51), and examined for MAPK activation at 30 min postinfection by immunoblotting with antibody specific for P-MAPK1/2 (and antibody to MAPK1/2 as a control). The levels of P-MAPK1/2 were significantly lower in cells exposed to virus neutralized with gD MAb (Fig. 13A, lane 3) or HSV-2 antiserum (Fig. 13C, lane 3) than nonneutralized virus (Fig. 13A, lane 2) or virus treated with preimmune serum (Fig. 13C, lane 2). MAPK1/2 levels were similar in all cultures (Fig. 13A and C). Densitometric scanning and data analysis as P-MAPK/MAPK ratios (Fig. 13B and D) indicated that MAPK is not activated in cells infected with antibody-neutralized virus, suggesting that cell penetration is required for MEK/MAPK activation. The superior effect of the anti-HSV-2 serum relative to the gD MAb presumably reflects its broader antigenic specificity.
FIG. 13.
Cell penetration is required for HSV-2-mediated activation of MEK/MAPK in primary hippocampal cultures. (A) Extracts from cells mock infected with MEM (lane 1) or infected (24 h) with 10 PFU of HSV-2 (lane 2) or HSV-2 neutralized with the IgG fraction from a gD MAb (lane 3) were immunoblotted with antibody specific for P-MAPK1/2 (upper bands) followed by MAPK1/2 (bottom bands). (B) Densitometric scanning of the bands in panel A expressed as P-MAPK/MAPK for both isoforms. (C) Extracts from cells mock infected (lane 1) or infected with HSV-2 neutralized with preimmune (lane 2) or HSV-2 hyperimmune (lane 3) serum were immunoblotted with antibody specific for P-MAPK1/2 (upper bands) followed by MAPK (bottom bands). (D) Densitometric scanning of bands in panel C expressed as P-MAPK/MAPK ratios for both isoforms.
DISCUSSION
Viruses have evolved various strategies to prevent apoptosis, including expression of bcl-2 homologues, inhibition of caspases, and repression of p53 activity (reviewed in reference 41). HSV-1 and HSV-2 have antiapoptotic activity that is cell type specific and has been attributed to the HSV-1 and HSV-2 gene US3 and to the HSV-1 genes γ134.5, US5, ICP27, and LAT, which function by a still poorly understood mechanism (6, 7, 22, 35, 42, 50, 72). However, to the extent of our knowledge, viral genes that inhibit apoptosis by activating survival pathways have not been described previously. Starting with our previous observation that it activates the Ras/MEK/MAPK pathway in nonneuronal cells (47, 79, 82), we showed that ICP10 PK prevents apoptosis in a constitutively expressing human cell line (JHLa1) and protects both neuronally differentiated PC12 cells and primary hippocampal neurons from apoptosis due to growth factor withdrawal and virus infection, respectively. In hippocampal neurons, antiapoptotic activity involves activation of the MEK/MAPK pathway and appears to be mediated by ICP10 PK in the virion tegument (80). The following comments seem pertinent to these findings.
Cell death can be due to apoptosis, necrosis, or a combination of both (4, 73), and the suitability of various assays for the detection of apoptosis has recently come under scrutiny (21, 24). Our definition of apoptosis is based on a multiplicity of criteria (cell survival, nuclear morphology, TUNEL, DNA fragmentation, caspase-3 activation, a crucial factor in the commitment to undergo apoptosis in response to certain stimuli [52], and PARP cleavage). According to these criteria, STS and d-Mann induced apoptosis in HEK293 and JHL15 cells, which express the PK-negative ICP10 mutant p139, but not in JHLa1 cells, which express ICP10 (62, 79). Protection requires a functional ICP10 PK activity, because (i) the JHLa1 and JHL15 cell lines were similarly established from HEK293 cells, and (ii) p139 is expressed as well as ICP10 (62, 79).
The antiapoptotic activity of ICP10 PK is relatively broad, as evidenced by the finding that it also protected neuronally differentiated PC12 cells and primary hippocampal neurons from apoptosis caused by growth factor withdrawal and virus infection, respectively. Indeed, 73% of differentiated PC12 cells transiently transfected with ICP10 survived for 48 h after NGF withdrawal, as determined by the MTS reduction assay, which is widely used to determine cell viability (15, 75). This compares to a 48% survival in untransfected cells and 43% survival in cells transfected with the ICP10 PK-negative mutant p139. Because p139 is expressed as well as ICP10 in transiently transfected cells, the data suggest that PK activity is required for protection. The approximately 30% increase in the survival of ICP10-transfected cells is consistent with the estimated proportion of cells positive for ICP10 expression (25 to 35%) and with previous reports of transfection efficiencies and gene expression using these vectors (23, 62, 94).
To examine the ability of ICP10 PK to protect hippocampal neurons from virus-induced apoptosis, we used ICP10ΔPK, an HSV-2 mutant that is deleted in the PK domain of ICP10 and expresses the PK-negative p95 protein, and ICP10ΔRR, an HSV-2 mutant in which the ICP10 RR domain is replaced with lacZ and expresses the PK-positive p175 protein (12, 79, 81, 82). Primary hippocampal cultures were infected in medium containing 10% FCS, a condition which allows the replication of ICP10ΔPK and ICP10ΔRR in nonneuronal cells (12, 79, 81, 82). Similar conditions were used for infection with HSV-2 and the revertant virus HSV-2(R) in order to allow direct comparison and control for the potential contribution of growth factors in the serum.
Significantly, HSV-2 [and HSV-2(R)] replicated in these cells with growth kinetics similar to those reported previously for nonneuronal cells (81, 82). However, both ICP10ΔPK and ICP10ΔRR were growth defective. The defect is not due to improper adsorption or penetration, since immunoblotting with ICP10 antibody revealed the presence of both p95 and p175 in extracts of cells infected with ICP10ΔPK or ICP10ΔRR, respectively, at 30 min postinfection, but the proteins were not seen in extracts of cells similarly infected with antibody-neutralized viruses, which can adsorb to but do not penetrate the cells (43, 51). These findings are consistent with previous reports that mutants deleted in the RR or PK domain of R1 are growth compromised in neuronal cell lines (10, 38). However, the growth defect seen in our cultures is significantly more pronounced, and it may be due to the use of primary cultures and/or hippocampal neurons.
Apparently unrelated to virus replication, high percentages of TUNEL-positive (apoptotic) cells were seen in cultures infected with ICP10ΔPK but not HSV-2 or HSV-2(R) (12, 81). Apoptotic cells were neurons, as evidenced by double immunofluorescent staining using FITC-labeled dUTP (TUNEL) and PE-labeled TUJ1 or NF-160 antibodies, both of which are specific for neuronal proteins (34). Although 5 to 8% of the cells in these cultures stained with GFAP antibody, they were TUNEL negative, suggesting that astrocytes do not become apoptotic under the experimental conditions used in these studies. We conclude that antiapoptotic activity depends on a functional ICP10 PK because (i) ICP10ΔPK does not have genetic alterations other than this deletion (81), (ii) US3, the only HSV-2 gene previously shown to have antiapoptotic activity in nonneuronal cells (42), is functional in ICP10ΔPK (unpublished), and (iii) the revertant virus HSV-2(R) had antiapoptotic activity similar to that of HSV-2. Antiapoptotic activity was also not due to infection in the presence of 10% serum, because ICP10ΔPK caused apoptosis under the same conditions.
It is particularly interesting that the percentage of TUNEL-positive (apoptotic) cells was also higher in cultures infected with ICP10ΔRR than with HSV-2. Although this percentage was significantly lower than that seen in ICP10ΔPK-infected cultures, the observation suggests that the ICP10 RR domain also contributes to the antiapoptotic activity of HSV-2. The exact mechanism responsible for this contribution is still unclear. We assume that the ICP10 RR domain functions downstream of caspase-3 activation because the proportion of cells positive for activated caspase-3 was similar for ICP10ΔRR and HSV-2. If RR enzymatic activity is involved in the contribution of the ICP10 RR domain to the antiapoptotic activity of HSV-2, it occurs after MEK/MAPK activation (30 min postinfection) because (i) the small RR subunit (R2) is synthesized with classic β kinetics (peaks at 6 to 8 h postinfection) and it imparts β kinetics to RR activity (88) and (ii) MEK/MAPK activation is independent of de novo viral protein synthesis. However, we cannot exclude the possible contribution of protein modification resulting from lacZ insertion.
The mechanism responsible for the ability of ICP10 PK to block apoptosis is still unclear. Antiapoptotic activity involves inhibition of caspase-3 activation, because (i) caspase-3 activation, PARP cleavage, and oligonucleosomal DNA fragmentation were inhibited in the constitutively expressing JHLa1 cells, (ii) ICP10 PK increased the survival of growth factor-deprived neuronally differentiated PC12 cells, the apoptosis of which involves caspase activation (16, 83), and (iii) the percentages of cells positive for TUNEL (apoptotic) and caspase-3 activation were significantly higher for ICP10ΔPK than HSV-2 or HSV-2(R). The antibodies used in the immunoblotting and immunohistochemistry studies on which these conclusions are based have established specificities (confirmed in our laboratory), and normal rabbit serum was negative in all assays.
The antiapoptotic activity of ICP10 PK involves activation of the MEK/MAPK survival pathway. Indeed, the levels of activated (phosphorylated) MAPK1/2 (P-MAPK1/2) were significantly higher in hippocampal cultures infected with HSV-2 than in mock-infected cultures, but this increase was not seen in cells infected with ICP10ΔPK. MAPK activation was inhibited by U0126, which is a MEK-specific inhibitor (32), and U0126 treatment of HSV-2-infected cultures caused a dose-dependent increase in the proportion of apoptotic cells, suggesting that MEK/MAPK activation is involved in the antiapoptotic activity of HSV-2. Since U0126 did not increase the proportion of apoptotic cells in uninfected cultures, pathway activation appears to be required for the antiapoptotic activity of HSV-2. Presumably, activation occurs upstream of caspase-3 and it involves the activation of Ras, since (i) ICP10 PK activates Ras, and thereby MEK/MAPK, in nonneuronal cells (82) and (ii) activation of Ras effector pathways protects neurons from apoptosis induced by various stimuli, including growth factor withdrawal (31, 60, 91). Ongoing studies are using dominant negative Raf and Ras mutants in order to further document the validity of this interpretation.
Consistent with previous reports which implicate the PI3-K/Akt pathway in the survival of neurons after growth factor withdrawal (30, 53, 66, 71, 87, 92), we found that the proportion of apoptotic cells in uninfected cultures was increased by treatment with the PI3-K-specific inhibitor LY294002. Similar results were also obtained with wortmannin (100 to 200 nM), which is another PI3-K inhibitor (92) (data not shown). However, these inhibitors had a minimal, if any, effect on apoptosis in HSV-2-infected hippocampal cultures, suggesting that infection uncouples the survival of these cells from PI3-K activity. Presumably, uncoupling is due to the activation of the MEK/MAPK pathway. However, we do not exclude the possibility that both pathways activate the same downstream effectors that are ultimately responsible for survival. Our studies also do not exclude the possibility that the antiapoptotic activity of ICP10 PK also involves inhibition of the JNK/p38 stress pathway, which is activated by NGF withdrawal and triggers apoptosis of neuronal cells even in the presence of NGF (91).
We assume that activation of the MEK/MAPK pathway results in increased transcription of antiapoptotic proteins such as bcl-2 family members (63). However, the identity of the survival functions involved in the antiapoptotic activity of ICP10 PK and the identity of the pathways that connect Ras/MEK/MAPK to caspase-3 and its downstream effectors are still unknown. We also do not know the exact kinetics of MEK/MAPK activation as they relate to inhibition of apoptosis. Indeed, activation was observed as early as 30 min postinfection, while apoptosis was only studied at 24 h postinfection, when MEK/MAPK were no longer activated. Because the levels of p95 in 30 min ICP10ΔPK-infected hippocampal cultures are low, we cannot exclude the possibility that the apoptotic activity of this virus is due to low levels of RR enzymatic activity. However, this interpretation seems unlikely, because the percentage of apoptotic cells in ICP10ΔRR-infected cultures was only marginally higher than that seen for HSV-2 (and significantly lower than that seen for ICP10ΔPK), suggesting that the contribution of the RR activity to HSV-2 antiapoptotic activity is minimal at best.
Ongoing studies are designed to identify upstream and downstream effectors of the MEK/MAPK pathway activated by ICP10 PK and the survival functions that it induces, define the mechanism responsible for the decreased significance of the PI3-K/Akt pathway in the survival of HSV-2-infected hippocampal neurons, examine the contribution, if any, of the stress p38/JNK pathway, and define the contribution of the ICP10 RR domain to the HSV-2 antiapoptotic activity.
In nonneuronal cells the MEK/MAPK pathway is activated at 2 to 3 h postinfection by newly synthesized ICP10 PK (82). By contrast, in HSV-2-infected hippocampal neurons, activation occurred as early as 30 min postinfection and did not require de novo viral protein synthesis, suggesting that it may be mediated by the virion ICP10 PK (80), which is released into the cytoplasm upon cellular penetration and virion uncoating. Alternatively, MEK/MAPK activation could be a nonspecific response to the mechanics of infection or due to growth factors or cytokines present in the inoculum. This is particularly significant, since an HSV receptor is a member of the tumor necrosis factor receptor (TNFR) family, which, upon ligand binding, can generate a signal that regulates NF-κB and AP-1 activation (65).
We favor the former interpretation because (i) MEK/MAPK were not activated by ICP10ΔPK, the adsorption and penetration of which are identical to those of the wild-type HSV-2 (81), (ii) for both viruses, infection was done in 10% serum, in which ICP10ΔPK ultimately replicates (81), and (iii) MEK and MAPK are activated within 30 min postinfection, prior to detectable de novo viral protein synthesis. This assumption is also supported by the observations that (i) ICP10 was not seen in cells infected with antibody-neutralized HSV-2, which can attach to but does not penetrate the cells (43, 51), and the levels of P-MAPK1/2 were not increased in these cells, and (ii) MEK and MAPK were activated equally well by HSV-2 and UV-inactivated HSV-2, which can penetrate the cells but is defective in protein synthesis (74). However, because anti-HSV-2 antibody blocks MAPK1/2 activation, the possibility cannot be excluded that virus binding per se also contributes to MAPK activation.
Final conclusions about the relative contribution of the tegument protein compared to newly synthesized ICP10 must await the results of further studies that identify the viral apoptotic genes and can, therefore, differentiate between the contribution of de novo protein synthesis to apoptosis versus antiapoptosis. Moreover, it remains to be determined whether and how binding per se contributes to MAPK activation and whether the virion ICP10 PK is involved in MEK/MAPK activation in any nonreplicating cells or only in hippocampal neurons.
What is the biological or clinical relevance of the ICP10 PK antiapoptotic activity in hippocampal neurons? Because hippocampal neurons are involved in virus-induced encephalitis (17, 28, 68), a tantalizing hypothesis is that the relative paucity of adult HSV-2 encephalitis is due to the ability of ICP10 PK to block apoptosis in these cells. Implicit in this interpretation is the conclusion that HSV-1, the major cause of HSV-induced adult encephalitis (84), causes apoptosis in hippocampal neurons. This conclusion is consistent with previous findings that (i) the HSV-1 counterpart of ICP10 PK is structurally and functionally distinct (23, 25, 26), (ii) the antiapoptotic activity of HSV-1 is cell type specific (35), and (iii) HSV-1 activates the p38/JNK stress pathway by a Ras-independent process (67, 93).
Another biologically and clinically relevant aspect of the finding that ICP10 PK has antiapoptotic activity in hippocampal neurons is that it could be used in gene therapy for neurodegenerative diseases such as Alzheimer’s disease and Down syndrome, the pathogenesis of which includes apoptosis of hippocampal neurons (46, 76, 77). Indeed, the only genes presently known to block apoptosis in these cells, the viral serpin crmA and bcl-2, have a relatively narrow spectrum of activity and do not activate survival pathways (48). ICP10 PK is a particularly promising candidate for gene therapy of neurodegenerative diseases, because activation of the MEK/MAPK pathway has been implicated in long-term potentiation, which improves cognitive functions (30). Ongoing studies are designed to test the validity of these interpretations.
Acknowledgments
We thank Cynthia Smith for helpful discussions and critical review of the manuscript and Paul H. Lackey for graphics assistance.
These studies were supported by Public Health Service grants AI40105 to L. Aurelian and NS25296 to E. X. Albuquerque and E. F. R. Pereira.
REFERENCES
- 1.Ali, M. A., S. S. Prakash, and R. J. Jariwalla. 1992. Localization of the antigenic sites and intrinsic protein kinase domain within a 300 amino acid segment of the ribonucleotide reductase large subunit from herpes simplex virus type 2. Virology 187:360–367. [DOI] [PubMed] [Google Scholar]
- 2.Alkondon, M., and E. X. Albuquerque. 1993. Diversity of nicotinic acetylcholine receptors in rat hippocampal neurons. I. Pharmacological and functional evidence for distinct structural subtypes. J. Pharmacol. Exp. Ther. 265:1455–1473. [PubMed] [Google Scholar]
- 3.Anderson, K. P., R. J. Frink, G. B. Devi, B. H. Gaylord, and E. K. Wagner. 1981. Detailed characterization of the mRNA mapping in the HindIII fragment K region of the herpes simplex virus type 1 genome. J. Virol. 37:1011–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ankarcrona, M., J. M. Dypbukt, E. Bonfoco, B. Zhivotovsky, S. Orrenius, S. A. Lipton, and P. Nicoterra. 1995. Glutamate-induced neuronal death: a succession of necrosis or apoptosis depending on mitochondrial function. Neuron 15:961–973. [DOI] [PubMed] [Google Scholar]
- 5.Arends, M. J., R. G. Morris, and A. H. Wyllie. 1990. Apoptosis: the role of the endonuclease. Am. J. Pathol. 136:593–608. [PMC free article] [PubMed] [Google Scholar]
- 6.Aubert, M., and J. A. Blaho. 1999. The herpes simplex virus type 1 regulatory protein ICP27 is required for the prevention of apoptosis in infected human cells. J. Virol. 73:2803–2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aubert, M., J. O’Toole, and J. A. Blaho. 1999. Induction and prevention of apoptosis in human Hep-2 cells by herpes simplex virus type 1. J. Virol. 73:10359–10370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aurelian, L., P. Terzano, C. C. Smith, T. D. Chung, A. Shamsuddin, S. Costa, and C. Orlandi. 1989. Amino-terminal epitope of herpes simplex virus type 2 ICP10 protein as a molecular diagnostic marker for cervical intraepithelial neoplasia. Cancer Cells 7:187–191. [Google Scholar]
- 9.Aurelian, L. 1998. Herpes simplex virus type 2: unique biological properties include neoplastic potential mediated by the PK domain of the large subunit of ribonucleotide reductase. Front. Biosci. 3:d237–d249. [DOI] [PubMed] [Google Scholar]
- 10.Aurelian, L., H. Kokuba, C. C. Smith. 1999. Vaccine potential of a herpes simplex virus type 2 mutant deleted in the PK domain of the large subunit of ribonucleotide reductase (ICP10). Vaccine 17:1951–1963. [DOI] [PubMed] [Google Scholar]
- 11.Aurelian, L. 2000. Herpes simplex viruses, p. 384–409. In S. Specter, R. Hodinka, and A. Young (ed.), Clinical virology manual, 3rd ed. ASM Press, Washington, D.C.
- 12.Aurelian, L., and C. C. Smith. 2000. Herpes simplex type 2 growth and latency reactivation by co cultivation are inhibited with antisense oligonucleotides complementary to the translation initiation site of the large subunit of ribonucleotide reductase (RR1). Antisense Nucleic Acid Drug Dev. 10:77–85. [DOI] [PubMed] [Google Scholar]
- 13.Aurelian, L., C. C. Smith, R. Winchurch, M. Kulka, L. Zaccaro, T. Gyotoku, F. J. Chrest, and J. W. Burnett. 2001. A novel gene expressed in human keratinocytes with long term growth potential is required for cell growth. J. Investig. Dermatol. 116:286–295. [DOI] [PubMed] [Google Scholar]
- 14.Banker, G. A., and W. M. Cowan. 1977. Rat hippocampal neurons in dispersed cell culture. Brain Res. 126:397–402. [DOI] [PubMed] [Google Scholar]
- 15.Barltrop, J. A., T. C. Owen, A. H. Cory, and J. G. Cory. 1991. 5-(3-Carboxymethoxyphenyl)-2-(4,5-dimethylthiazoly)-3-(4-sulphophenyl)tetrazolium, inner salt (MTS) and related analogs of 3-(4,5-dimethylthiazolyl)-2,5-diphenyltetrazolium bromide (MTT) reducing to purple water-soluble formazans as cell viability indicators. Bioorg. Med. Chem. Lett. 1:611–614. [Google Scholar]
- 16.Batistatou, A., and L. A. Greene. 1991. Aurintricarboxylic acid rescues PC12 cells and sympathetic neurons from cell death caused by nerve growth factor deprivation: correlation with suppression of endonuclease activity. J. Cell Biol. 115:461–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bergstrom, T., B. Svennerholm, N. Conradi, P. Horal, and A. Vahlne. 1991. Discrimination of herpes simplex types 1 and 2 cerebral infections in a rat model. Acta Neuropathol. 82:395–401. [DOI] [PubMed] [Google Scholar]
- 18.Bertollini, L., M. T. Ciotti, E. Cherubini, and A. Cattaneo. 1997. Neurotrophin-3 promotes the survival of oligodendrocyte precursors in embryonic hippocampal cultures under chemically defined conditions. Brain Res. 746:19–24. [DOI] [PubMed] [Google Scholar]
- 19.Bonni, A., A. Brunet, A. E. West, S. R. Datta, M. A. Takasu, and M. E. Greenberg. 1999. Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and independent mechanisms. Science 286:1358–1362. [DOI] [PubMed] [Google Scholar]
- 20.Brazelton, T. R., F. M. V. Rossi, G. I. Keshet, and H. M. Blau. 2000. From marrow to brain: Expression of neuronal phenotypes in adult mice. Science 20:1775–1779. [DOI] [PubMed] [Google Scholar]
- 21.Charriaut-Marlangue, C., and Y. Ben-Ari. 1995. A cautionary note on the use of the TUNEL stain to determine apoptosis. Neuroreport 7:61–64. [PubMed] [Google Scholar]
- 22.Chou, J., and B. Roizman. 1992. The γ134.5 gene of herpes simplex virus 1 precludes neuroblastoma cells from triggering total shutoff of protein synthesis characteristic of programmed cell death in neuronal cells. Proc. Natl. Acad. Sci. USA 89:3266–3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chung, T. D., J. P. Wymer, C. C. Smith, M. Kulka, and L. Aurelian. 1989. Protein kinase activity associated with the large subunit of herpes simplex virus type 2 ribonucleotide reductase (ICP10). J. Virol. 63:3389–3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cohen, G. M., X.-M. Sun, R. T. Snowden, D. Dinsdale, and D. N. Skilleter. 1992. Key morphological features of apoptosis may occur in the absence of internucleosomal DNA fragmentation. Biochem. J. 286:331–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Conner, J., J. Cooper, J. Furlong, and J. B. Clements. 1992. An autophosphorylating but not transphosphorylating activity is associated with the unique N terminus of the herpes simplex virus type 1 ribonucleotide reductase large subunit. J. Virol. 66:7511–7516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cooper, J., J. Conner, and J. B. Clements. 1995. Characterization of the novel protein kinase activity present in the R1 subunit of herpes simplex virus ribonucleotide reductase. J. Virol. 69:4979–4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crowder, R. J., and R. S. Freeman. 1998. Phosphatidylinositol 3-kinase and Akt protein kinase are necessary and sufficient for the survival of nerve growth factor-dependent sympathetic neurons. J. Neurosci. 18:2933–2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Damasio, A. R., and G. W. Van Hoesen. 1985. The limbic system and the localization of herpes simplex encephalitis. J. Neurol. Neurosurg. Psychiatry 48:297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Desai, P., R. Ramakrishnan, Z. W. Lin, B. Osak, J. C. Glorioso, and M. Levine. 1993. The RR1 gene of herpes simplex virus type 1 is uniquely trans-activated by ICP0 during infection. J. Virol. 67:6125–6135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Di Cristo, G., N. Berardi, L. Cancedda, T. Pizzorusso, E. Putigano, G. M. Ratto, and L. Maffei. 2001. Requirement of ERK activation for visual cortical plasticity. Science 292:2337–2340. [DOI] [PubMed] [Google Scholar]
- 31.Erhardt, P., E. J. Schremser, and G. M. Cooper. 1999. B-Raf inhibits programmed cell death downstream of cytochrome c release from mitochondria by activating the MEK/ERK pathway. Mol. Cell. Biol. 19:5308–5315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Favata, M. F., K. Y. Horiuchi, E. J. Manos, A. J. Daulerio, D. A. Stradley, W. S. Feeser, D. E. Van Dyk, W. J. Pitts, R. A. Earl, F. Hobbs, R. A. Copeland, R. L. Magolda, P. A. Scherle, and J. M. Trzaskos. 1998. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J. Biol. Chem. 273:18623–18632. [DOI] [PubMed] [Google Scholar]
- 33.Fernandes-Alnemri, T., G. Litwack, and E. Alnemri. 1994. CPP32, a novel human apoptotic protein with homology to Caenorhabditis elegans cell death protein Ced-3 and mammalian interleukin-1 beta-converting enzyme. J. Biol. Chem. 269:30761–30764. [PubMed] [Google Scholar]
- 34.Ferreira, A., and A. Caceres. 1992. Expression of the class III beta-tubulin isotype in developing neurons in culture. J. Neurosci. Res. 32:516–529. [DOI] [PubMed] [Google Scholar]
- 35.Galvan, V., and B. Roizman. 1998. Herpes simplex virus 1 induces and blocks apoptosis at multiple steps during infection and protects cells from exogenous inducers in a cell-type-dependent manner. Proc. Natl. Acad. Sci. USA 95:3931–3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gavrieli, Y., Y. Sherman, and S. A. Ben-Sasson. 1992. Identification of programmed cell death via specific labeling of nuclear DNA fragmentation. J. Cell Biol. 119:493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gold, R., M. Schmied, G. Giegerich, H. Beitschopf, H. P. Hartung, K. V. Toyka, and H. Lassman. 1994. Differentiation between cellular apoptosis and necrosis by the combined use of in situ tailing and nick translation techniques. Lab. Investig. 71:219–225. [PubMed] [Google Scholar]
- 38.Goldstein, D. J., and S. K. Weller. 1988. Herpes simplex virus type 1-induced ribonucleotide reductase activity is dispensible for virus growth and DNA synthesis: isolation and characterization of an ICP6lacZ insertion mutant. J. Virol. 62:196–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Greene, L. A., and A. S. Tischler. 1982. PC12 pheochromocytoma cells on neurobiological research. Adv. Cell. Neurobiol. 3:373–414. [Google Scholar]
- 40.Guo, Q., B. L. Sopher, K. Furukawa, D. G. Pham, N. Robinson, G. M. Martin, and M. P. Mattson. 1997. Alzheimer’s presenilin mutation sensitizes neural cells to apoptosis induced by trophic factor withdrawal and amyloid beta-peptide: involvement of calcium and oxyradicals. J. Neurosci. 17:4212–4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hardwick, J. M., G. Ketner, and R. J. Clem. 1998. Viral genes that modulate apoptosis, p.243–279. In J. W. Wilson, C. Booth, and C. S. Potten (ed.), Apoptosis genes. Kluwer Academic Publishers, New York, N.Y.
- 42.Hata, S., A. H. Koyama, H. Shiota, A. Adachi, F. Goshima, and Y. Nishiyama. 1999. Antiapoptotic activity of herpes simplex virus type 2: the role of US3 protein kinase gene. Microbes Infect. 1:601–607. [DOI] [PubMed] [Google Scholar]
- 43.Highlander, S. L., S. L. Sutherland, P. J. Gage, D. C. Johnsons, M. Levine, and J. C. Glorioso. 1987. Neutralizing monoclonal antibodies specific for herpes simplex virus glycoprotein D inhibit virus penetration. J. Virol. 61:3356–3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hirt, B. 1967. Selective extraction of polyoma DNA from infected mouse cell culture. J. Mol. Biol. 26:365–369. [DOI] [PubMed] [Google Scholar]
- 45.Honess, R. W., and B. Roizman. 1974. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J. Virol. 14:8–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Honig, L., and R. N. Rosenberg. 2000. Apoptosis and neurologic disease. Am. J. Med. 108:317–330. [DOI] [PubMed] [Google Scholar]
- 47.Hunter, J. C. R., C. C. Smith, D. Bose, M. Kulka, R. Broderick, and L. Aurelian. 1995. Intracellular internalization and signaling pathways triggered by the large subunit of HSV-2 ribonucleotide reductase (ICP10). Virology 210:345–360. [DOI] [PubMed] [Google Scholar]
- 48.Ivins, K. J., J. K. Ivins, J. P. Sharp, and C. W. Cotman. 1999. Multiple pathways of apoptosis in PC12 cells. CrmA inhibits apoptosis induced by β-amyloid. J. Biol. Chem. 274:2107–2112. [DOI] [PubMed] [Google Scholar]
- 49.Jacobson, M. D., M. Weil, and M. C. Raff. 1996. Role of Ced-3/ICE-family proteases in staurosporine-induced programmed cell death. J. Cell Biol. 133:1041–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jerome, K. R., R. Fox, Z. Chen, A. E. Sears, H.-Y. Lee, and L. Corey. 1999. Herpes simplex virus inhibits apoptosis through the action of two genes, US5 and US3. J. Virol. 73:8950–8957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Johnson, D. C., R. L. Burke, and T. Gregory. 1990. Soluble forms of herpes simplex virus glycoprotein D bind to a limited number of cell surface receptors and inhibit virus entry into cells. J. Virol. 64:2564–2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Johnson Webb, S., D. J. Harrison, and A. H. Wyllie. 1997. Apoptosis: an overview of the process and its relevance in disease. Adv. Pharmacol. 41:1–31. [DOI] [PubMed] [Google Scholar]
- 53.Kaplan, D., and F. Miller. 2000. Neurotrophin signal transduction in the nervous system. Curr. Opin. Neurobiol. 10:381–391. [DOI] [PubMed] [Google Scholar]
- 54.Kokuba, H., L. Aurelian, and J. W. Burnett. 1999. Herpes simplex virus associated erythema multiforme (HAEM) is mechanistically distinct from drug-induced erythema multiforme: interferon-gamma is expressed in HAEM lesions and tumor necrosis factor-alpha in drug-induced erythema multiforme lesions. J. Investig. Dermatol. 113:808–815. [DOI] [PubMed] [Google Scholar]
- 55.Koyama, A. H., T. Fukumori, M. Fujita, H. Irie, and A. Adachi. 2000. Physiological significance of apoptosis in animal virus infection. Microbes Infect. 2:1111–1117. [DOI] [PubMed] [Google Scholar]
- 56.LaFerla, F. M., and J. Gilbert. 1997. β-Amyloid induced neuronal cell death in transgenic mice and Alzheimer’s disease, p.183–195. In J. Pirier (ed.), Neuromethods, vol. 29. Apoptosis techniques and protocols. Humana Press, New York, N.Y.
- 57.Leu, C. M., C. Chang, and C. Hu. 2000. Epidermal growth factor (EGF) suppresses staurosporine-induced apoptosis by inducing mcl-1 via the mitogen-activated protein kinase pathway. Oncogene 19:1665–1675. [DOI] [PubMed] [Google Scholar]
- 58.Lewis, T. S., P. S. Shapiro, and N. G. Ahn. 1998. Signal transduction through MAP kinase cascade. Adv. Cancer Res. 74:49–139. [DOI] [PubMed] [Google Scholar]
- 59.Lilley, C. E., F. Grouts, Z. Han, J. A. Palmer, P. N. Anderson, D. S. Latchman, and R. S. Coffin. 2001. Multiple immediate-early gene-deficient herpes simplex vectors allowing efficient gene delivery to neurons in culture and widespread gene delivery to the central nervous system in vivo. J. Virol. 75:4343–4356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lin, H. J., V. Eviner, G. C. Prendergast, and E. White. 1995. Activated H-ras rescues E1A-induced apoptosis and cooperates with E1A to overcome p53-dependent growth arrest. Mol. Cell. Biol. 15:4536–4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Luo, J. H., C. C. Smith, M. Kulka, and L. Aurelian. 199. 1. A truncated protein kinase domain of the large subunit of herpes simplex virus type 2 ribonucleotide reductase (ICP10) expressed in Escherichia coli. J. Biol. Chem. 266:20976–20983. [PubMed] [Google Scholar]
- 62.Luo, J., and L. Aurelian. 1992. The transmembrane helical segment but not the invariant lysine is required for the kinase activity of the large subunit of herpes simplex virus type 2 ribonucleotide reductase (ICP10). J. Biol. Chem. 267:9645–9653. [PubMed] [Google Scholar]
- 63.Mah, S. P., L. T. Zhong, Y. Lin, A. Roghami, R. H. Edwards, and D. E. Bredesen. 1993. The proto-oncogene Bcl-2 inhibits apoptosis in PC12 cells. J. Neurochem. 60:1183–1186. [DOI] [PubMed] [Google Scholar]
- 64.Malek, A. M., G. G. Goss, L. Jiang, S. Izumo, and S. L. Alper. 1998. Mannitol at clinical concentrations activates multiple signaling pathways and induces apoptosis in endothelial cells. Stroke 29:2631–2640. [DOI] [PubMed] [Google Scholar]
- 65.Marsters, S. A., T. M. Ayres, M. Skubatch, C. L. Gray, M. Rothe, and A. Ashkenazi. 1997. Herpesvirus entry mediator, a member of the tumor necrosis factor receptor (TNFR) family, interacts with members of the TNFR-associated factor family and activates the transcription factors NF-κB and AP-1. J. Biol. Chem. 272:14029–14032. [DOI] [PubMed] [Google Scholar]
- 66.Marte, B. M., and D. J. Downward. 1997. PKB/Akt: connecting PI3-kinase to cell survival and beyond. Trends Biochem. Sci. 22:355–358. [DOI] [PubMed] [Google Scholar]
- 67.McLean, T. I., and S. L. Bachenheimer. 1999. Activation of c-Jun N-terminal kinase by herpes simplex virus type 1 enhances viral replication. J. Virol. 73:8415–8426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nahmias, A. J., R. J. Whitley, A. N. Visintine, Y. Takei, Y., and C. A. Alford, Jr., and the Collaborative Antiviral Study Group. 1982. Herpes simplex virus encephalitis: laboratory evaluations and their diagnostic significance. J. Infect. Dis. 145:829–836. [DOI] [PubMed] [Google Scholar]
- 69.Nelson, J. W., J. Zhu, C. C. Smith, M. Kulka, and L. Aurelian. 1996. ATP and SH3 binding sites in the protein kinase of the large subunit of herpes simplex virus type 2 of ribonucleotide reductase (ICP10). J. Biol. Chem. 271:17021–17027. [DOI] [PubMed] [Google Scholar]
- 70.Ouyang, Y.-B., Y. Tan, M. Comb, C.-L. Liu, M. E. Martone, B. K. Siesjo, and B.-R. Hu. 1999. Survival and death-promoting events after transient cerebral ischemia: phosphorylation of Akt, release of cytochrome c, and activation of caspase-like proteases. J. Cerebral Blood Flow Metab. 19:1126–1135. [DOI] [PubMed] [Google Scholar]
- 71.Peng, T., J. R. C. Hunter, and J. W. Nelson. 1996. The novel protein kinase of the RR1 subunit of herpes simplex virus has autophosphorylation and transphosphorylation activity that differs in its ATP requirements for HSV-1 and HSV-2. Virology 216:184–196. [DOI] [PubMed] [Google Scholar]
- 72.Perng, G.-C., C. Jones, J. Ciacci-Zanella, M. Stone, G. Henderson, A. Yukht, S. M. Slanina, F. M. Hofman, H. Ghiasi, A. B. Nesburn, and S. L. Wechsler. 2000. Virus-induced neuronal apoptosis blocked by the herpes simplex virus latency-associated transcript. Science 287:1500–1503. [DOI] [PubMed] [Google Scholar]
- 73.Portera-Cailliau, C., D. L. Price, and L. J. Martin. 1997. Excitotoxic neuronal death in the immature brain is an apoptosis-necrosis continuum. J. Comp. Neurol. 378:70–87. [PubMed] [Google Scholar]
- 74.Purifoy, D. J. M., and K. L. Powell. 1977. Interference between strains of type 1 and type2 herpes simplex virus. Virology 77:84–94. [DOI] [PubMed] [Google Scholar]
- 75.Riss, T. L., and R. A. Moravec. 1992. Comparison of MTT, XTT, and a novel tetrazolium compound for MTS for in vitro proliferation and chemosensitivity assays. Mol. Biol. Cell Suppl. 3:A184. [Google Scholar]
- 76.Sawa, A. 1999. Neuronal cell death in Down’s syndrome. J. Neural Transm. Suppl. 57:87–97. [DOI] [PubMed] [Google Scholar]
- 77.Shimohama, S. 2000. Apoptosis in Alzheimer’s disease—an update. Apoptosis 5:9–16. [DOI] [PubMed] [Google Scholar]
- 78.Smith, C. C., M. Kulka, J. P. Wymer, T. D. Chung, and L. Aurelian. 1992. Expression of the large subunit of herpes simplex virus type 2 ribonucleotide reductase (ICP10) is required for virus growth and neoplastic transformation. J. Gen. Virol. 73:1417–1428. [DOI] [PubMed] [Google Scholar]
- 79.Smith, C. C., J. H. Luo, J. C. Hunter, J. V. Ordonez, and L. Aurelian. 1994. The transmembrane domain of the large subunit of HSV-2 ribonucleotide reductase (ICP10) is required for protein kinase activity and transformation-related signaling pathways that result in ras activation. Virology 200:598–612. [DOI] [PubMed] [Google Scholar]
- 80.Smith, C. C., and L. Aurelian. 1997. The large subunit of herpes simplex virus type 2 ribonucleotide reductase (ICP10) is associated with the virion tegument and has PK activity. Virology 234:235–242. [DOI] [PubMed] [Google Scholar]
- 81.Smith, C. C., T. Peng, M. Kulka, and L. Aurelian. 1998. The PK domain of the large subunit of herpes simplex virus type 2 ribonucleotide reductase (ICP10) is required for immediate-early gene expression and virus growth. J. Virol. 72:9131–9141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Smith, C. C., J. Nelson, L. Aurelian, M. Gober, and B. B. Goswami. 2000. Ras-GAP binding/phosphorylation by HSV-2 RR1PK (ICP10) and activation of the Ras/MEK/MAPK mitogenic pathway are required for timely onset of virus growth. J. Virol. 74:10417–10429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stefanis, L., D. S. Park, C. Y. I. Yan, S. E. Farinelli, C. M. Troy, M. L. Shelansky, and L. A. Greene. 1996. Induction of CPP32-like activity in PC12 cells by withdrawal of trophic support. J. Biol. Chem. 271:30663–30671. [DOI] [PubMed] [Google Scholar]
- 84.Stone, D. R., and S. L. Gorbach. 2000. Head and neck infections, p.11–26. In Atlas of infectious diseases. W. B. Saunders Publishers, Philadelphia, Pa.
- 85.Sze, P., and R. C. Herman. 1992. The herpes simplex virus type 1 ICP6 gene is regulated by a “leaky” early promoter. Virus Res. 2:141–152. [DOI] [PubMed] [Google Scholar]
- 86.Tewari, M., L. T. Quan, K. O’Rourke, S. Desnoyers, Z. Zeng, D. R. Beidler, G. G. Poirier, Salvesen, and V. M. Dixit. 1995. Yama/CPP32β, a mammalian homolog of CED-3, is a CrmA-inhibitable protease that cleaves the death substrate poly(ADP-ribose) polymerase. Cell 81:801–809. [DOI] [PubMed] [Google Scholar]
- 87.Vlahos, C. J., W. F. Matter, K. Y. Hui, R. F. Brown. 1994. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002). J. Biol. Chem. 269:5241–5248. [PubMed] [Google Scholar]
- 88.Wagner, E. 1983. Transcription patterns in HSV infections, p.239–276. In G. Klein (ed.), Advances in viral oncology. Raven Press, New York, N.Y.
- 89.Wymer, J. P., T. D. Chung, Y. N. Chang, G. S. Hayward, and L. Aurelian. 1989. Identification of immediate-early type cis-response elements in the promoter for the ribonucleotide reductase large subunit from herpes simplex virus type 2. J. Virol. 63:2773–2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wymer, J. P., C. M. J. Aprhys, T. D. Chung, T. C.-P. Feng, M. Kulka, and L. Aurelian. 1992. Immediate early and functional AP-1 cis-response elements are involved in the transcriptional regulation of the large subunit of herpes simplex virus type 2 ribonucleotide reductase (ICP10). Virus Res. 23:253–270. [DOI] [PubMed] [Google Scholar]
- 91.Xia, Z., M. Dickens, J. Raingeaud, R. J. Davis, and M. E. Greenberg. 1995. Opposing effects of ERK and JNK-p38MAP kinases on apoptosis. Science 270:1326–1331. [DOI] [PubMed] [Google Scholar]
- 92.Yano, S., H. Tokumitsu, and T. R. Soderling. 1998. Calcium promotes cell survival through CaM-K kinase activation of the protein-kinase-B pathway. Nature 396:584–587. [DOI] [PubMed] [Google Scholar]
- 93.Zachos, G., B. Clements, and J. Conner. 1999. Herpes simplex virus type 1 infection stimulates p38/c-Jun N-terminal mitogen-activated protein kinase pathways and activates transcription factor AP-1. J. Biol. Chem. 274:5097–5103. [DOI] [PubMed] [Google Scholar]
- 94.Zhu, J., and L. Aurelian. 1997. AP-1 cis-response elements are involved in basal expression and Vmw110 transactivation of the large subunit of herpes simplex virus type 2 ribonucleotide reductase (ICP0). Virology 231:301–312. [DOI] [PubMed] [Google Scholar]