Abstract
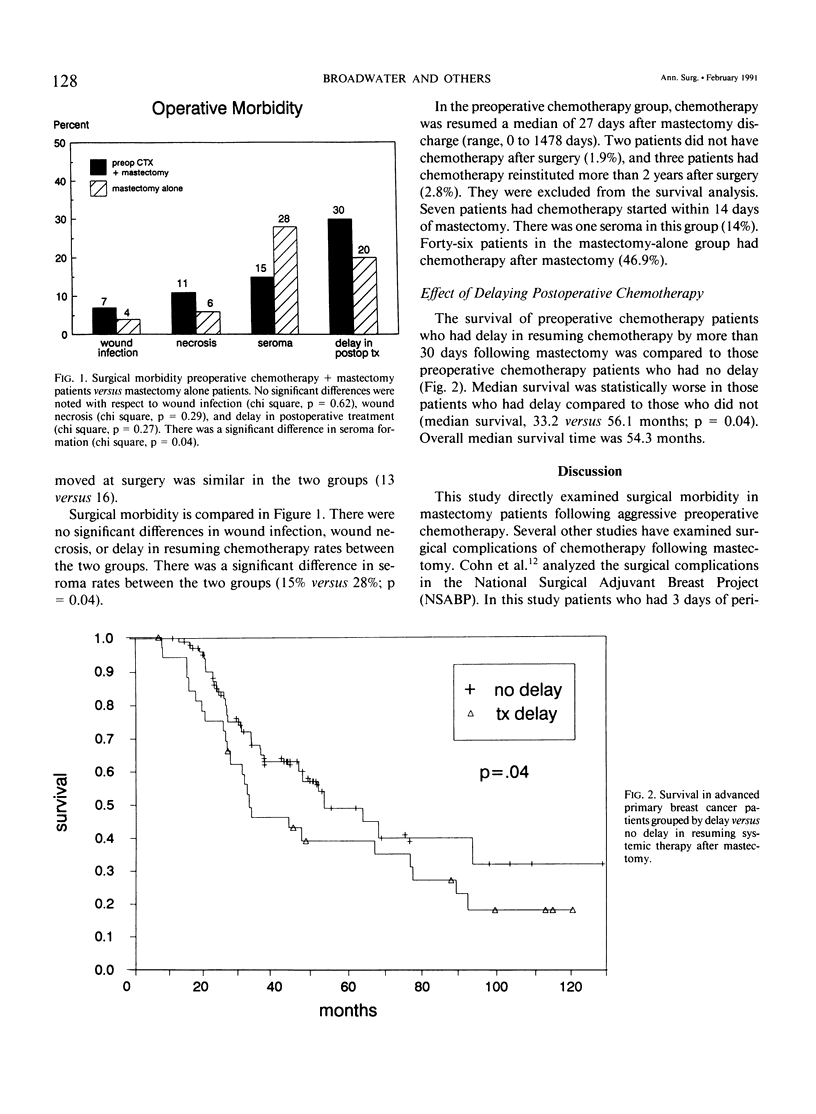
The surgical morbidity associated with aggressive preoperative chemotherapy in 106 patients with advanced primary breast cancer who had chemotherapy followed by mastectomy was examined. These patients were compared with a group of 91 consecutive patients who had mastectomy without preoperative chemotherapy. Strict operative criteria were used to determine the timing of mastectomy following chemotherapy. Wound infection rates were no different in the preoperative chemotherapy group compared to the mastectomy-alone groups (7% versus 4%; p = 0.62). The incidence of wound necrosis was similar (11% versus 6%; p = 0.29). Seroma formation was decreased significantly in the preoperative chemotherapy group compared to the mastectomy-alone group (15% versus 28%; p = 0.04). Intensive preoperative chemotherapy did not delay the reinstitution of postoperative treatment (30% versus 20%; p = 0.27). However, when delay in instituting postoperative chemotherapy was more than 30 days, there was a significant decrease in overall survival rate (p = 0.04). This study provides evidence that intensive preoperative chemotherapy and mastectomy can be performed without increased morbidity. Furthermore it is important to institute systemic chemotherapy within 30 days of mastectomy to achieve maximum survival.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balch C. M., Singletary S. E. Adjuvant therapy for node-negative breast cancer patients. Who benefits? Arch Surg. 1988 Oct;123(10):1189–1190. doi: 10.1001/archsurg.1988.01400340015001. [DOI] [PubMed] [Google Scholar]
- Bland K. I., Palin W. E., von Fraunhofer J. A., Morris R. R., Adcock R. A., Tobin G. R., 2nd Experimental and clinical observations of the effects of cytotoxic chemotherapeutic drugs on wound healing. Ann Surg. 1984 Jun;199(6):782–790. doi: 10.1097/00000658-198406000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn I., Jr Complications and toxic manifestations of surgical adjuvant chemotherapy for breast cancer. Surg Gynecol Obstet. 1968 Dec;127(6):1201–1209. [PubMed] [Google Scholar]
- Fisher B., Redmond C., Dimitrov N. V., Bowman D., Legault-Poisson S., Wickerham D. L., Wolmark N., Fisher E. R., Margolese R., Sutherland C. A randomized clinical trial evaluating sequential methotrexate and fluorouracil in the treatment of patients with node-negative breast cancer who have estrogen-receptor-negative tumors. N Engl J Med. 1989 Feb 23;320(8):473–478. doi: 10.1056/NEJM198902233200801. [DOI] [PubMed] [Google Scholar]
- GEHAN E. A. A GENERALIZED WILCOXON TEST FOR COMPARING ARBITRARILY SINGLY-CENSORED SAMPLES. Biometrika. 1965 Jun;52:203–223. [PubMed] [Google Scholar]
- Glick J. H. Meeting highlights: adjuvant therapy for breast cancer. J Natl Cancer Inst. 1988 Jun 1;80(7):471–475. doi: 10.1093/jnci/80.7.471. [DOI] [PubMed] [Google Scholar]
- Hobar P. C., Jones R. C., Schouten J., Leitch A. M., Hendler F. Multimodality treatment of locally advanced breast carcinoma. Arch Surg. 1988 Aug;123(8):951–955. doi: 10.1001/archsurg.1988.01400320037006. [DOI] [PubMed] [Google Scholar]
- Hortobagyi G. N., Ames F. C., Buzdar A. U., Kau S. W., McNeese M. D., Paulus D., Hug V., Holmes F. A., Romsdahl M. M., Fraschini G. Management of stage III primary breast cancer with primary chemotherapy, surgery, and radiation therapy. Cancer. 1988 Dec 15;62(12):2507–2516. doi: 10.1002/1097-0142(19881215)62:12<2507::aid-cncr2820621210>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Knight C. D., Jr, Martin J. K., Jr, Welch J. S., Ingle J. N., Gaffey T. A., Martinez A. Surgical considerations after chemotherapy and radiation therapy for inflammatory breast cancer. Surgery. 1986 Apr;99(4):385–391. [PubMed] [Google Scholar]
- Mansour E. G., Gray R., Shatila A. H., Osborne C. K., Tormey D. C., Gilchrist K. W., Cooper M. R., Falkson G. Efficacy of adjuvant chemotherapy in high-risk node-negative breast cancer. An intergroup study. N Engl J Med. 1989 Feb 23;320(8):485–490. doi: 10.1056/NEJM198902233200803. [DOI] [PubMed] [Google Scholar]
- McGonigal M. D., Martin D. M., Lucas C. E., Ledgerwood A. M., Brooks S. C., Grabow D. The effects of breast cancer chemotherapy on wound healing in the rat. J Surg Res. 1987 May;42(5):560–564. doi: 10.1016/0022-4804(87)90032-1. [DOI] [PubMed] [Google Scholar]
- Piccart M. J., de Valeriola D., Paridaens R., Balikdjian D., Mattheiem W. H., Loriaux C., Arrigo C., Cantraine F., Heuson J. C. Six-year results of a multimodality treatment strategy for locally advanced breast cancer. Cancer. 1988 Dec 15;62(12):2501–2506. doi: 10.1002/1097-0142(19881215)62:12<2501::aid-cncr2820621209>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Raaf J. H. Techniques for avoiding surgical complications in chemotherapy-treated cancer patients. Recent Results Cancer Res. 1985;98:46–52. doi: 10.1007/978-3-642-82432-6_6. [DOI] [PubMed] [Google Scholar]
- Shamberger R. Effect of chemotherapy and radiotherapy on wound healing: experimental studies. Recent Results Cancer Res. 1985;98:17–34. doi: 10.1007/978-3-642-82432-6_3. [DOI] [PubMed] [Google Scholar]


