Abstract
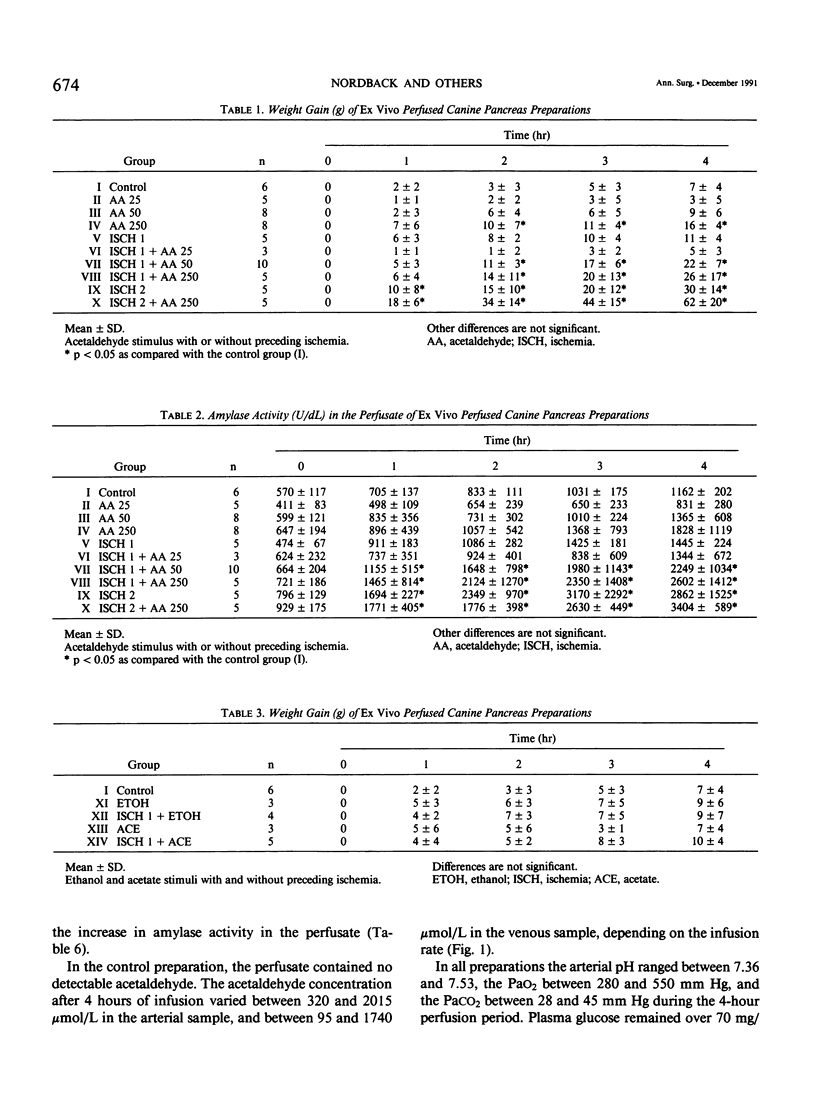
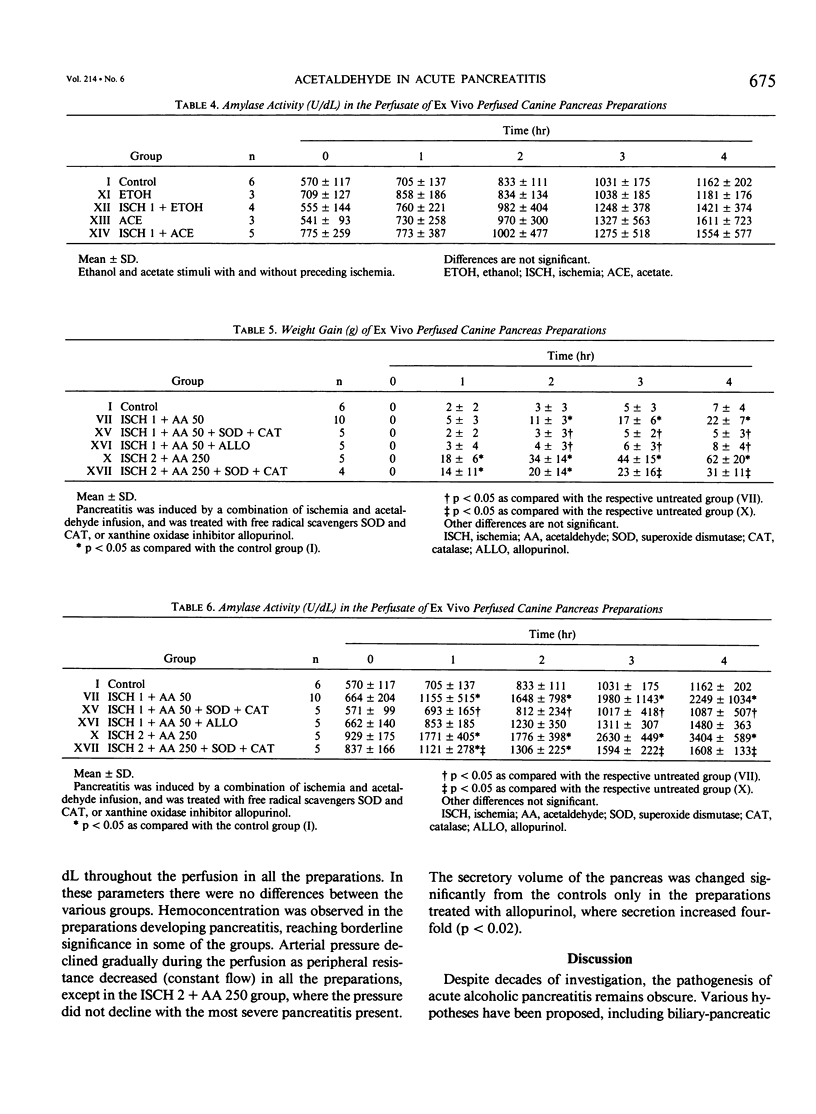
Acetaldehyde (AA), the first product of ethanol metabolism, has been suggested as an important mediator in alcoholic pancreatitis, but experimental evidence has not been convincing. Prior work using the isolated perfused canine pancreas preparation has suggested that toxic oxygen metabolites generated by xanthine oxidase (XO) may mediate the early injury in pancreatitis. Xanthine oxidase is capable of oxidizing AA, and during this oxidation free radicals are released. The hypothesis that acute alcoholic pancreatitis may be initiated by AA in the presence of active XO (converted from xanthine dehydrogenase [XD]) was tested in the authors' experimental preparation by converting XD to XO by a period of ischemia, and infusing AA. Control preparations remained normal throughout the 4-hour perfusion (weight gain, 7 +/- 4 g; amylase activity, 1162 +/- 202 U/dL). One hour of ischemia or infusion of AA at 25 mg/hr or at 50 mg/hr without ischemia did not induce changes in the preparation. Acetaldehyde at 250 mg/hr induced minimal edema and weight gain (16 +/- 4 g; p less than 0.05), but not significant hyperamylasemia. Changes also were not observed when 1-hour ischemia was followed by a bolus of ethanol (1.5 g) or sodium acetate (3.0 g), or by infusion of 25 mg/hr of AA. One hour of ischemia followed by infusion of AA at 50 mg/hr or at 250 mg/hr induced edema, hemorrhage, weight gain (22 +/- 7 g [p less than 0.05] and 26 +/- 17 g [p less than 0.05]) and hyperamylasemia (2249 +/- 1034 U/dL [p less than 0.05] and 2602 +/- 1412 U/dL [p less than 0.05]). Moreover infusion of AA at 250 mg/hr after 2 hours of ischemia potentiated the weight gain (62 +/- 20 g versus 30 +/- 14 g [p less than 0.05]), but not the hyperamylasemia (3404 +/- 589 U/dL versus 2862 +/- 1525 U/dL) as compared with 2 hours of ischemia alone. Pancreatitis induced by 1 hour of ischemia followed by AA at 50 mg/hr could be inhibited by pretreatment with the free radical scavengers superoxide dismutase and catalase and ameliorated with the XO inhibitor allopurinol. The authors conclude that AA, in the presence of active XO, can initiate acute pancreatitis in the isolated canine pancreas preparation and may be important in the initiation of acute alcoholic pancreatitis in man. Toxic oxygen metabolites appear to play an important intermediary role.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Battelli M. G., Lorenzoni E., Stripe F. Milk xanthine oxidase type D (dehydrogenase) and type O (oxidase). Purification, interconversion and some properties. Biochem J. 1973 Feb;131(2):191–198. doi: 10.1042/bj1310191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyeler C., Fisch H. U., Preisig R. The disulfiram-alcohol reaction: factors determining and potential tests predicting severity. Alcohol Clin Exp Res. 1985 Mar-Apr;9(2):118–124. doi: 10.1111/j.1530-0277.1985.tb05531.x. [DOI] [PubMed] [Google Scholar]
- Broe P. J., Cameron J. L. Experimental gallstone pancreatitis. Pathogenesis and response to different treatment modalities. Ann Surg. 1982 May;195(5):566–573. doi: 10.1097/00000658-198205000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broe P. J., Zuidema G. D., Cameron J. L. The role of ischemia in acute pancreatitis: studies with an isolated perfused canine pancreas. Surgery. 1982 Apr;91(4):377–382. [PubMed] [Google Scholar]
- Bühler R., Pestalozzi D., Hess M., Von Wartburg J. P. Immunohistochemical localization of alcohol dehydrogenase in human kidney, endocrine organs and brain. Pharmacol Biochem Behav. 1983;18 (Suppl 1):55–59. doi: 10.1016/0091-3057(83)90147-8. [DOI] [PubMed] [Google Scholar]
- Clare D. A., Blakistone B. A., Swaisgood H. E., Horton H. R. Sulfhydryl oxidase-catalyzed conversion of xanthine dehydrogenase to xanthine oxidase. Arch Biochem Biophys. 1981 Oct 1;211(1):44–47. doi: 10.1016/0003-9861(81)90427-6. [DOI] [PubMed] [Google Scholar]
- Darle N., Ekholm R., Edlund Y. Ultrastructure of the rat exocrine pancreas after long term intake of ethanol. Gastroenterology. 1970 Jan;58(1):62–72. [PubMed] [Google Scholar]
- Deitrich R. A. Tissue and subcellular distribution of mammalian aldehyde-oxydizing capacity. Biochem Pharmacol. 1966 Dec;15(12):1911–1922. doi: 10.1016/0006-2952(66)90220-6. [DOI] [PubMed] [Google Scholar]
- Dickson A. P., O'Neill J., Imrie C. W. Hyperlipidaemia, alcohol abuse and acute pancreatitis. Br J Surg. 1984 Sep;71(9):685–688. doi: 10.1002/bjs.1800710913. [DOI] [PubMed] [Google Scholar]
- Fridovich I. Oxygen radicals from acetaldehyde. Free Radic Biol Med. 1989;7(5):557–558. doi: 10.1016/0891-5849(89)90032-4. [DOI] [PubMed] [Google Scholar]
- Friedl H. P., Till G. O., Ryan U. S., Ward P. A. Mediator-induced activation of xanthine oxidase in endothelial cells. FASEB J. 1989 Nov;3(13):2512–2518. doi: 10.1096/fasebj.3.13.2806779. [DOI] [PubMed] [Google Scholar]
- Geokas M. C. Ethanol and the pancreas. Med Clin North Am. 1984 Jan;68(1):57–75. doi: 10.1016/s0025-7125(16)31241-x. [DOI] [PubMed] [Google Scholar]
- Granger D. N. Role of xanthine oxidase and granulocytes in ischemia-reperfusion injury. Am J Physiol. 1988 Dec;255(6 Pt 2):H1269–H1275. doi: 10.1152/ajpheart.1988.255.6.H1269. [DOI] [PubMed] [Google Scholar]
- Guynn R. W., Kuo Y. J., Shanbour L. L. Acute effect of ethanol on metabolite concentrations of dog pancreas in vivo. Alcohol Clin Exp Res. 1982 Fall;6(4):469–474. doi: 10.1111/j.1530-0277.1982.tb05009.x. [DOI] [PubMed] [Google Scholar]
- Hjortrup A., Andersen B. N., Kreutzfeldt M., Holm G., Schmidt A., Poulsen J. S. Effect of intra-arterial administration of acetaldehyde on pig pancreas. Dan Med Bull. 1983 Mar;30(2):125–127. [PubMed] [Google Scholar]
- Lieber C. S. Biochemical and molecular basis of alcohol-induced injury to liver and other tissues. N Engl J Med. 1988 Dec 22;319(25):1639–1650. doi: 10.1056/NEJM198812223192505. [DOI] [PubMed] [Google Scholar]
- Majumdar A. P., Vesenka G. D., Dubick M. A., Yu G. S., DeMorrow J. M., Geokas M. C. Morphological and biochemical changes of the pancreas in rats treated with acetaldehyde. Am J Physiol. 1986 May;250(5 Pt 1):G598–G606. doi: 10.1152/ajpgi.1986.250.5.G598. [DOI] [PubMed] [Google Scholar]
- Parks D. A., Granger D. N., Bulkley G. B., Shah A. K. Soybean trypsin inhibitor attenuates ischemic injury to the feline small intestine. Gastroenterology. 1985 Jul;89(1):6–12. doi: 10.1016/0016-5085(85)90738-3. [DOI] [PubMed] [Google Scholar]
- Parks D. A., Granger D. N. Xanthine oxidase: biochemistry, distribution and physiology. Acta Physiol Scand Suppl. 1986;548:87–99. [PubMed] [Google Scholar]
- Saharia P., Margolis S., Zuidema G. D., Cameron J. L. Acute pancreatitis with hyperlipemia: studies with an isolated perfused canine pancreas. Surgery. 1977 Jul;82(1):60–67. [PubMed] [Google Scholar]
- Sanfey H., Bulkley G. B., Cameron J. L. The pathogenesis of acute pancreatitis. The source and role of oxygen-derived free radicals in three different experimental models. Ann Surg. 1985 May;201(5):633–639. doi: 10.1097/00000658-198505000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanfey H., Bulkley G. B., Cameron J. L. The role of oxygen-derived free radicals in the pathogenesis of acute pancreatitis. Ann Surg. 1984 Oct;200(4):405–413. doi: 10.1097/00000658-198410000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanfey H., Cameron J. L. Increased capillary permeability: an early lesion in acute pancreatitis. Surgery. 1984 Sep;96(3):485–491. [PubMed] [Google Scholar]
- Singh M., LaSure M. M., Bockman D. E. Pancreatic acinar cell function and morphology in rats chronically fed an ethanol diet. Gastroenterology. 1982 Mar;82(3):425–434. [PubMed] [Google Scholar]
- Singh M., Simsek H. Ethanol and the pancreas. Current status. Gastroenterology. 1990 Apr;98(4):1051–1062. doi: 10.1016/0016-5085(90)90033-w. [DOI] [PubMed] [Google Scholar]
- Skibba J. L., Powers R. H., Stadnicka A., Kalbfleisch J. H. The effect of hyperthermia on conversion of rat hepatic xanthine dehydrogenase to xanthine oxidase. Biochem Pharmacol. 1988 Dec 1;37(23):4592–4595. doi: 10.1016/0006-2952(88)90681-8. [DOI] [PubMed] [Google Scholar]
- Stark K., Seubert P., Lynch G., Baudry M. Proteolytic conversion of xanthine dehydrogenase to xanthine oxidase: evidence against a role for calcium-activated protease (calpain). Biochem Biophys Res Commun. 1989 Dec 15;165(2):858–864. doi: 10.1016/s0006-291x(89)80045-2. [DOI] [PubMed] [Google Scholar]
- Stowell A., Hillbom M., Salaspuro M., Lindros K. O. Low acetaldehyde levels in blood, breath and cerebrospinal fluid of intoxicated humans as assayed by improved methods. Adv Exp Med Biol. 1980;132:635–645. doi: 10.1007/978-1-4757-1419-7_66. [DOI] [PubMed] [Google Scholar]
- Sultatos L. G. Effects of acute ethanol administration on the hepatic xanthine dehydrogenase/oxidase system in the rat. J Pharmacol Exp Ther. 1988 Sep;246(3):946–949. [PubMed] [Google Scholar]
- Suokas A., Kupari M., Pettersson J., Lindros K. The nitrefazole-ethanol interaction in man: cardiovascular responses and the accumulation of acetaldehyde and catecholamines. Alcohol Clin Exp Res. 1985 May-Jun;9(3):221–227. doi: 10.1111/j.1530-0277.1985.tb05739.x. [DOI] [PubMed] [Google Scholar]
- Watanabe A., Kobayashi M., Hobara N., Nakatsukasa H., Nagashima H., Fujimoto A. A report of unusually high blood ethanol and acetaldehyde levels in two surviving patients. Alcohol Clin Exp Res. 1985 Jan-Feb;9(1):14–16. doi: 10.1111/j.1530-0277.1985.tb05040.x. [DOI] [PubMed] [Google Scholar]
- Wilson J. S., Pirola R. C. Pathogenesis of alcoholic pancreatitis. Aust N Z J Med. 1983 Jun;13(3):307–312. doi: 10.1111/j.1445-5994.1983.tb04672.x. [DOI] [PubMed] [Google Scholar]


