Abstract
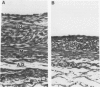
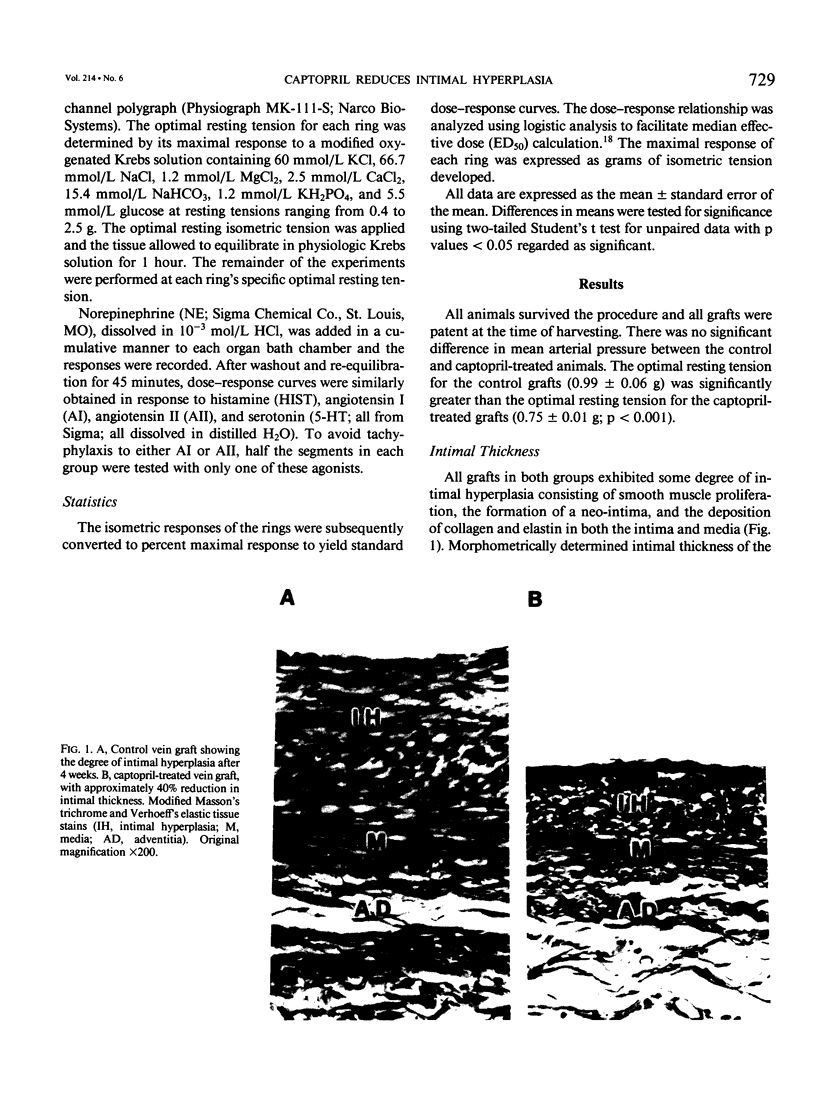
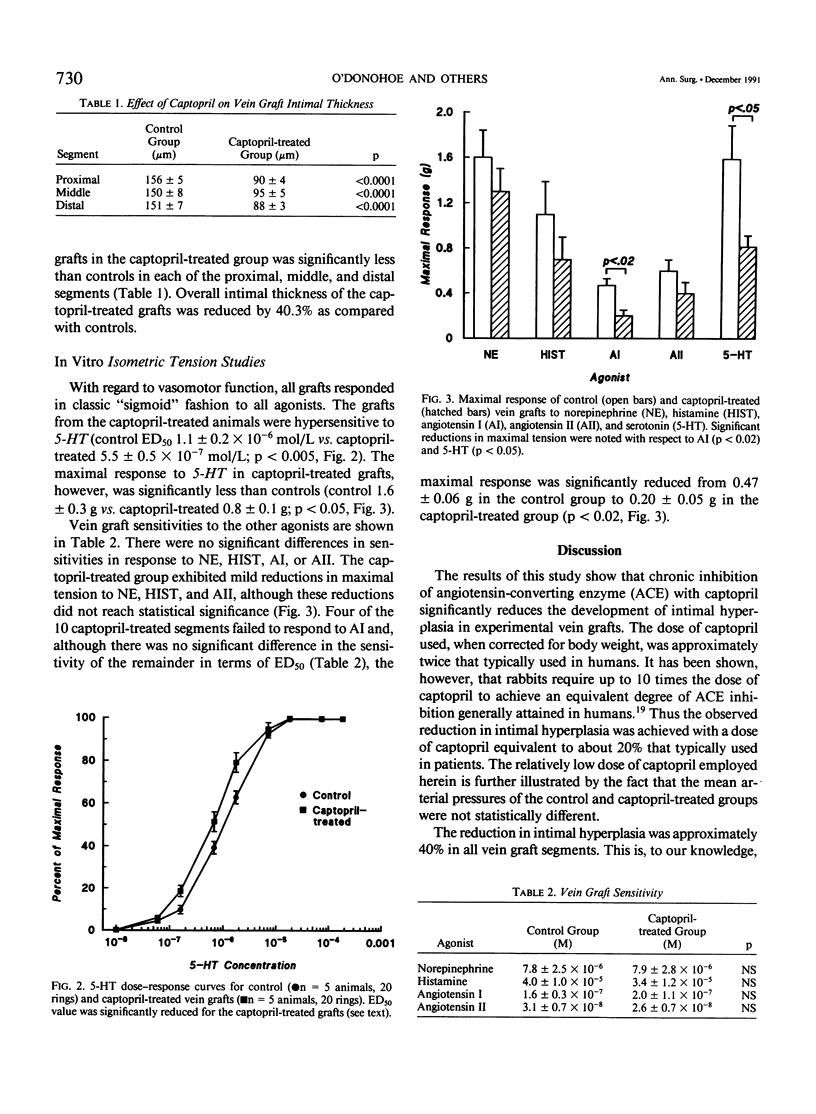
Intimal hyperplasia is an important factor in the pathophysiology of vein graft failure. Local renin-angiotensin systems recently have been shown to modulate the development of intimal hyperplasia in arteries after intimal injury. The effect of chronic angiotensin-converting enzyme (ACE) inhibition on the development of intimal hyperplasia in experimental vein grafts was examined in this study. Ten New Zealand White rabbits received 10 mg/kg of captopril daily in their drinking water. One week later the right carotid artery was divided and bypassed with the reversed right external jugular vein in these rabbits and in 10 matched controls. Captopril was continued for 28 days after operation, when all the grafts were harvested. Five grafts from each group were perfusion fixed, and the intimal thickness in the proximal, middle, and distal segments was determined. Rings from the remaining grafts (n = 20 in each group) were studied in vitro under isometric tension, and their responses to norepinephrine (NE), histamine (HIST), serotonin (5-HT), angiotensin I (AI), and angiotensin II (AII) was measured. The intimal thickness of the proximal, middle, and distal segments of the captopril-treated grafts were significantly less than controls, being reduced in all segments by approximately 40% (p less than 0.0001). With regard to vasoreactivity, the captopril-treated grafts were hypersensitive to 5-HT (control ED50 5.5 +/- 0.5 X 10(-7) mol/L vs. captopril-treated 1.1 +/- 0.2 X 10(-6) mol/L; p less than 0.005) although the maximal response was significantly reduced (control 1.6 +/- 0.3 g vs. captopril-treated 0.8 +/- 0.1 g; p less than 0.05). There were no differences in sensitivity between control and captopril-treated rings with respect to NE, HIST, AI, or AII. Four of the ten captopril-treated segments, however, failed to respond to AI, and the maximal active tension of the responders was significantly reduced (control 0.47 +/- 0.06 g vs. 0.20 +/- 0.05 g; p less than 0.02). These results suggest that ACE is involved in the modulation of vein graft intimal hyperplasia, and that ACE inhibitors may have therapeutic applications in patients undergoing vein bypass procedures.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aberg G., Ferrer P. Effects of captopril on atherosclerosis in cynomolgus monkeys. J Cardiovasc Pharmacol. 1990;15 (Suppl 5):S65–S72. [PubMed] [Google Scholar]
- Berkowitz H. D., Greenstein S., Barker C. F., Perloff L. J. Late failure of reversed vein bypass grafts. Ann Surg. 1989 Dec;210(6):782–786. doi: 10.1097/00000658-198912000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brogden R. N., Todd P. A., Sorkin E. M. Captopril. An update of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in hypertension and congestive heart failure. Drugs. 1988 Nov;36(5):540–600. doi: 10.2165/00003495-198836050-00003. [DOI] [PubMed] [Google Scholar]
- Chobanian A. V., Brecher P., Chan C. Effects of propranolol on atherogenesis in the cholesterol-fed rabbit. Circ Res. 1985 May;56(5):755–762. doi: 10.1161/01.res.56.5.755. [DOI] [PubMed] [Google Scholar]
- Chobanian A. V., Haudenschild C. C., Nickerson C., Drago R. Antiatherogenic effect of captopril in the Watanabe heritable hyperlipidemic rabbit. Hypertension. 1990 Mar;15(3):327–331. doi: 10.1161/01.hyp.15.3.327. [DOI] [PubMed] [Google Scholar]
- Chopra M., Scott N., McMurray J., McLay J., Bridges A., Smith W. E., Belch J. J. Captopril: a free radical scavenger. Br J Clin Pharmacol. 1989 Mar;27(3):396–399. doi: 10.1111/j.1365-2125.1989.tb05384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clozel J. P., Hefti F. Effects of chronic therapy with cilazapril, a new angiotensin-converting enzyme inhibitor, on regional blood flows in conscious spontaneously hypertensive rats. J Cardiovasc Pharmacol. 1987 Sep;10(3):350–355. doi: 10.1097/00005344-198709000-00015. [DOI] [PubMed] [Google Scholar]
- Cross K. S., el-Sanadiki M. N., Murray J. J., Mikat E. M., McCann R. L., Hagen P. O. Mast cell infiltration: a possible mechanism for vein graft vasospasm. Surgery. 1988 Aug;104(2):171–177. [PubMed] [Google Scholar]
- Diccianni M. B., Cardin A. D., Britt A. L., Jackson R. L., Schwartz A. Effect of a sustained release formulation of diltiazem on the development of atherosclerosis in cholesterol-fed rabbits. Atherosclerosis. 1987 Jun;65(3):199–205. doi: 10.1016/0021-9150(87)90035-9. [DOI] [PubMed] [Google Scholar]
- Dzau V. J. Implications of local angiotensin production in cardiovascular physiology and pharmacology. Am J Cardiol. 1987 Jan 23;59(2):59A–65A. doi: 10.1016/0002-9149(87)90178-0. [DOI] [PubMed] [Google Scholar]
- Dzau V. J. Vascular renin-angiotensin: a possible autocrine or paracrine system in control of vascular function. J Cardiovasc Pharmacol. 1984;6 (Suppl 2):S377–S382. [PubMed] [Google Scholar]
- Fuchs J. C., Mitchener J. S., 3rd, Hagen P. O. Postoperative changes in autologous vein grafts. Ann Surg. 1978 Jul;188(1):1–15. doi: 10.1097/00000658-197807000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisterfer A. A., Peach M. J., Owens G. K. Angiotensin II induces hypertrophy, not hyperplasia, of cultured rat aortic smooth muscle cells. Circ Res. 1988 Apr;62(4):749–756. doi: 10.1161/01.res.62.4.749. [DOI] [PubMed] [Google Scholar]
- Grünwald J., Chobanian A. V., Haudenschild C. C. Smooth muscle cell migration and proliferation: atherogenic mechanisms in hypertension. Atherosclerosis. 1987 Oct;67(2-3):215–221. doi: 10.1016/0021-9150(87)90281-4. [DOI] [PubMed] [Google Scholar]
- Jackson T. R., Blair L. A., Marshall J., Goedert M., Hanley M. R. The mas oncogene encodes an angiotensin receptor. Nature. 1988 Sep 29;335(6189):437–440. doi: 10.1038/335437a0. [DOI] [PubMed] [Google Scholar]
- Kent K. C., Whittemore A. D., Mannick J. A. Short-term and midterm results of an all-autogenous tissue policy for infrainguinal reconstruction. J Vasc Surg. 1989 Jan;9(1):107–114. doi: 10.1067/mva.1989.vs0090107. [DOI] [PubMed] [Google Scholar]
- Loop F. D., Lytle B. W., Cosgrove D. M., Stewart R. W., Goormastic M., Williams G. W., Golding L. A., Gill C. C., Taylor P. C., Sheldon W. C. Influence of the internal-mammary-artery graft on 10-year survival and other cardiac events. N Engl J Med. 1986 Jan 2;314(1):1–6. doi: 10.1056/NEJM198601023140101. [DOI] [PubMed] [Google Scholar]
- Makhoul R. G., Davis W. S., Mikat E. M., McCann R. L., Hagen P. O. Responsiveness of vein bypass grafts to stimulation with norepinephrine and 5-hydroxytryptamine. J Vasc Surg. 1987 Jul;6(1):32–38. doi: 10.1067/mva.1987.avs0060032. [DOI] [PubMed] [Google Scholar]
- Myhre H. O., Halvorsen T. Intimal hyperplasia and secondary changes in vein grafts. Acta Chir Scand Suppl. 1985;529:63–67. [PubMed] [Google Scholar]
- Naftilan A. J., Pratt R. E., Eldridge C. S., Lin H. L., Dzau V. J. Angiotensin II induces c-fos expression in smooth muscle via transcriptional control. Hypertension. 1989 Jun;13(6 Pt 2):706–711. doi: 10.1161/01.hyp.13.6.706. [DOI] [PubMed] [Google Scholar]
- Norman J., Badie-Dezfooly B., Nord E. P., Kurtz I., Schlosser J., Chaudhari A., Fine L. G. EGF-induced mitogenesis in proximal tubular cells: potentiation by angiotensin II. Am J Physiol. 1987 Aug;253(2 Pt 2):F299–F309. doi: 10.1152/ajprenal.1987.253.2.F299. [DOI] [PubMed] [Google Scholar]
- O'Malley M. K., McDermott E. W., Mehigan D., O'Higgins N. J. Role for prazosin in reducing the development of rabbit intimal hyperplasia after endothelial denudation. Br J Surg. 1989 Sep;76(9):936–938. doi: 10.1002/bjs.1800760921. [DOI] [PubMed] [Google Scholar]
- Powell J. S., Clozel J. P., Müller R. K., Kuhn H., Hefti F., Hosang M., Baumgartner H. R. Inhibitors of angiotensin-converting enzyme prevent myointimal proliferation after vascular injury. Science. 1989 Jul 14;245(4914):186–188. doi: 10.1126/science.2526370. [DOI] [PubMed] [Google Scholar]
- Radic Z. S., O'Donohoe M. K., Schwartz L. B., Stein A. D., Mikat E. M., McCann R. L., Hagen P. O. Alterations in serotonergic receptor expression in experimental vein grafts. J Vasc Surg. 1991 Jul;14(1):40–47. doi: 10.1067/mva.1991.27700. [DOI] [PubMed] [Google Scholar]
- Rosenthal J. H., Pfeifle B., Michailov M. L., Pschorr J., Jacob I. C., Dahlheim H. Investigations of components of the renin-angiotensin system in rat vascular tissue. Hypertension. 1984 May-Jun;6(3):383–390. doi: 10.1161/01.hyp.6.3.383. [DOI] [PubMed] [Google Scholar]
- Someya N., Morotomi Y., Kodama K., Kida O., Higa T., Kondo K., Tanaka K. Suppressive effect of captopril on platelet aggregation in essential hypertension. J Cardiovasc Pharmacol. 1984 Sep-Oct;6(5):840–843. doi: 10.1097/00005344-198409000-00016. [DOI] [PubMed] [Google Scholar]
- el-Sanadiki M. N., Cross K. S., Murray J. J., Schuman R. W., Mikat E., McCann R. L., Hagen P. O. Reduction of intimal hyperplasia and enhanced reactivity of experimental vein bypass grafts with verapamil treatment. Ann Surg. 1990 Jul;212(1):87–96. doi: 10.1097/00000658-199007000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]