Abstract
A member of the family of l-riboside benzimidazole compounds, 1263W94, was shown recently to inhibit replication of Epstein-Barr virus (EBV) (V. L. Zacny, E. Gershburg, M. G. Davis, K. K. Biron, and J. S. Pagano, J. Virol. 73:7271–7277, 1999). In the present report the effect of 1263W94 on the phosphorylation pattern of the EBV DNA polymerase processivity factor, EA-D, during viral reactivation in latently EBV-infected Akata cells is analyzed. This pattern specifically changes with progression of cytolytic infection. In the presence of 1263W94 the appearance of the hyperphosphorylated form of EA-D is mainly affected. Next, coexpression of the cloned EBV-encoded protein kinase (EBV PK), BGLF4, with EA-D demonstrated the ability of EBV PK to phosphorylate EA-D to its hyperphosphorylated form in transient assays. However, the phosphorylation of EA-D was not directly inhibited by 1263W94 in these coexpression assays. The results indicate that the EBV PK appears to be responsible for the hyperphosphorylation of EA-D, imply that the phosphorylation status of EA-D is important for viral replication, and suggest that 1263W94 acts at a level other than direct inhibition of EA-D phosphorylation by EBV PK.
Epstein-Barr virus (EBV) is a human gammaherpesvirus that causes infectious mononucleosis and is closely associated with several types of human malignancy, including B-cell lymphomas (19), some T-cell lymphomas (24), nasopharyngeal carcinoma, and Hodgkin’s disease (2, 11, 31). Despite the availability of antiviral drugs (reviewed in references 28 and 32) treatment for EBV infections remains undeveloped.
1263W94 [5,6-dichloro-2-(isopropylamino)-1-β-l-ribofuranosyl-1H-benzimidazole] (21), a member of the benzimidazole ribonucleoside family (10), was shown to possess both anti-human cytomegalovirus (HCMV) (21) and, later, anti-EBV activity (42). The compound does not inhibit replication of herpes simplex virus (HSV) and varicella-zoster virus (VZV) (V. L. Zacny, M. G. Davis, S. D. Chamberlain, L. B. Townsend, K. K. Biron, and J. S. Pagano, Abstr. 37th Intersci. Conf. Antimicrob. Agents Chemother., abstr. H69, 1997). It inhibits HCMV replication by a novel mechanism: although viral DNA synthesis is inhibited, the HCMV DNA polymerase is not a target (reviewed in reference 8). Recently, we have shown that the compound affects the phosphorylation pattern of the EBV DNA polymerase processivity factor (EA-D) (42). EA-D plays an important role in viral replication as an essential component of the EBV DNA polymerase complex (13, 14, 37, 38). It also interacts with the viral transactivator BZLF1 (43) and cellular transcription factors Sp1 and ZBP-89 at the origin of lytic replication (oriLyt) (3). It was shown years ago that EA-D is phosphorylated to different levels during viral reactivation (12, 33); however, the importance of the EA-D phosphorylation for viral replication remains unclear.
Resistance to 1263W94 in HCMV was mapped to the UL97 gene (C. L. Talarico, M. G. Davis, P. B. Sethna, W. H. Miller, M. R. Underwood, F. Baldanti, K. K. Biron, and R. J. Harvey, 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 140-H, 1998), the product of which is a phosphotransferase (16, 34, 36). Homologs of this protein are encoded by all known herpesviruses; they contain conserved cyclic AMP-dependent protein kinase key catalytic residues (23). Protein kinase activity has already been demonstrated for HSV-1 UL13 (30), VZV ORF47 (25), and HCMV UL97 (16) and recently for EBV BGLF4 (7, 18).
In this report we show that EA-D is a substrate for the EBV protein kinase (EBV PK), BGLF4. Despite the fact that the phosphorylation pattern of EA-D during viral reactivation is altered by 1263W94, the compound failed to inhibit phosphorylation of EA-D when the isolated gene products were overexpressed.
MATERIALS AND METHODS
Cell lines.
Akata is a Burkitt’s lymphoma cell line latently infected with EBV (35), DG75 is an EBV-negative Burkitt’s lymphoma cell line (4), and 293T is a transformed human embryonal kidney cell line. The cells were grown as described previously (42).
Treatment of cells with antiviral compounds and viral reactivation.
1263W94 and acyclovir (ACV) were supplied by GlaxoSmithKline. Exponentially growing cells were centrifuged at 800 × g and suspended in fresh medium containing different concentrations of either compound for an appropriate time as indicated. At the end of the treatment, cells were harvested and analyzed depending on the aim of experiment.
To reactivate lytic viral infection Akata cells were treated with goat anti-human immunoglobulin G (IgG) (0.1 mg/ml; Sigma). Treatment with antiviral compounds and viral reactivation were begun at the same time.
Plasmids and transfections.
The BGLF4 open reading frame (ORF) (genomic position 122327 to 123691) and the BMRF1 ORF (genomic position 79898 to 81112) from the BamHI-G fragment and pSG5-BMRF1, respectively, were PCR amplified using primer pair 5′-CCGGATCCCAGCGGGTGGAGG/5′-CCGGATCCCTCCACGTCGGCC for BGLF4 amplification and primer pair 5′-CCGGATCCCATGGAAACCACTCAGAC/5′-CCGGATCCAGGTGCTGCTTGCACGAG for BMRF1 amplification. Amplified BMRF1 ORF was cloned downstream of the cytomegalovirus immediate-early promoter in the pGEM2-based vector pHD101.3 (9) and the plasmid named pHD/BMRF1. The BGLF4 ORF was cloned downstream of the same promoter in pHD101.3, and the resulting plasmid was named pHD/BGLF4. The kinase-null mutant of BGLF4 was generated by site-directed mutagenesis that replaced lysine-128 with glutamine. The K128Q mutant was cloned into pHD101.3; the resulting plasmid was designated pHD/K128Q. The orientation of inserts was confirmed by restriction analysis and sequencing.
293T cells were transfected with 0.1 to 10 μg of plasmid DNA with the use of Effectine (QIAgen) following the manufacturer’s protocol. Transfected cells were cultured in Dulbecco’s modified Eagle medium supplemented by 10% fetal bovine serum and antibiotics for 24 to 48 h at 37°C and then harvested for analysis.
RNA extraction and RPA.
Total RNA was isolated from the cells with the use of the RNeasy total RNA isolation kit (QIAgen). RNase protection assays (RPA) were performed with 10 μg of total RNA with the use of the RNase Protection kit II (Ambion Inc.) following the manufacturer’s protocol. The hybridization temperature was 45°C. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) probe was supplied by U.S. Biochemical Inc. (probe size, 129 bp; protected region, 100 bp). BGLF4 probe was generated with NdeI-digested pHD/BGLF4 as a template and SP6 RNA polymerase (probe size, 185 bp; protected region, 157 bp). BMRF1 transcription served as a positive control, and the BMRF1 probe was generated with NotI-digested pHD/BMRF1 as a template and SP6 RNA polymerase (probe size, 339 bp; protected region, 311 bp).
Protein lysates and immunoblotting.
Cells were washed once with ice-cold phosphate-buffered saline solution and then resuspended in lysis buffer (50 mM Tris-HCl, 150 mM NaCl, 5 mM EDTA, 0.5% NP-40, 5 mM dithiothreitol, 0.2 mM Na3VaO4, 100 mM NaF, 1 mM phenylmethylsulfonyl fluoride, and a cocktail of protease inhibitors [Complete; Boehringer]). Protein concentration was determined by the Bradford protein assay (Bio-Rad).
For immunoblotting, equal amounts of protein lysates were separated by polyacrylamide gel electrophoresis on a sodium dodecyl sulfate polyacrylamide gel (10%). Proteins were transferred to NitroPlus (MSI) nitrocellulose and detected by appropriate antibody. For all Western analyses proteins were visualized with anti-mouse Ig-horseradish peroxidase (Amersham), SuperSignal (Pierce), and Kodak XAR-5 film.
Monoclonal antibodies to EA-D were purchased from Capricorn Products Inc. and used at a dilution of 1:100; monoclonal antibodies to β-actin and γ-tubulin (Sigma) were used at a dilution of 1:5,000.
RESULTS
1263W94 inhibits the phosphorylation of EA-D during viral reactivation.
To determine the phosphorylation status of EA-D during the viral cytolytic cycle, viral replication in latently EBV-infected Akata cells was induced with anti-IgG in the presence or absence of 10 μM 1263W94, a concentration 10 times greater than the 50% inhibitory concentration (42). Whole-cell lysates were prepared at different times after induction and analyzed by immunoblotting.
No EA-D was detected in uninduced cells (Fig. 1, lane 1); however, after induction two major phosphorylated forms of EA-D appeared. The forms are named “pp50” and “pp58,” where “pp” stands for “phosphoprotein” and the numbers refer to molecular masses in kilodaltons (33). The hyperphosphorylated form pp58 appeared 8 h after induction when it was the only form detected (lane 3). It reached its peak amount at 24 h and then declined 48 h after induction (lanes 5 and 6). The hypophosphorylated form pp50 appeared later (lane 4); however, its level remained high until the end of the experiment (lanes 5 and 6). From a comparison of these results with the 1263W94-treated samples it is clear that the compound reduced the overall amount of EA-D (Fig. 1, compare lanes 3 with 8, 4 with 9, 5 with 10, and 6 with 11, respectively). The level of pp58 was especially affected (compare lanes 5 with 10 and 4 with 11); it was strongly inhibited by the compound by 24 h and no longer detected at 48 h after viral reactivation.
FIG. 1.
Effect of 1263W94 on EBV EA-D in induced Akata cells. Akata cells were induced as described in Materials and Methods for 48 h. Cells were harvested at 4, 8, 12, 24, and 48 h, and equal amounts of the whole-cell lysates were analyzed by immunoblotting. Lanes 1 to 6, no 1263W94; lanes 7 to 11, samples treated with 10 μM 1263W94; lane 1, noninduced (n.i.) control.
These data show that the phosphorylation pattern of EA-D changes during the viral lytic cycle and imply that EA-D phosphorylation status might be important for its function. This hypothesis is enhanced by the fact that the waning of pp58 in the presence of 1263W94 accompanied the inhibition of viral replication (42).
RNA level of EBV PK during viral reactivation and effect of 1263W94.
We next began to characterize the BGLF4 gene product, which encodes the EBV PK (7, 18), by examining the time course of its mRNA. With the use of RPA we measured BGLF4 mRNA accumulation during viral reactivation in Akata cells in the presence of 1263W94 or ACV as a control. The cells were induced and treated with either compound as described above. Transcript levels of the EA-D-encoding gene, BMRF1, served as an early gene control. mRNAs of both genes appeared 4 h after induction (Fig. 2, lanes 2, 8 and 16); both levels peaked between 8 and 12 h after induction (lanes 3 and 4, 9 and 10, and 17 and 18) and declined to barely detectable levels 48 h after induction (lanes 6, 12, and 20). Treatment with either drug did not significantly affect BGLF4 or BMRF1 mRNA levels (compare lanes 7 to 12, 1 to 6, and 15 to 20), which were measured by normalizing of BGLF4 and BMRF1 mRNA levels to the GAPDH RNA level using a PhosphorImager. Neither BGLF4 nor BMRF1 mRNA was detected in the EBV-negative control cells (DG75) (lane 13) or in uninduced Akata cells (lanes 1, 7, and 15).
FIG. 2.
Kinetics of EBV PK (BGLF4) RNA during viral reactivation and effect of 1263W94. Induced Akata cells were harvested at 0, 4, 8, 12, 24, and 48 h after induction. Total RNA (10 μg) from each sample was analyzed by RPA. Lane 13 is a negative control (total RNA prepared from DG 75 cells); lanes 1, 7, and 15, noninduced Akata cells; lanes 8 to 12, Akata cells induced with IgG; lanes 2 to 6, Akata cells treated with 1263W94 and induced with IgG; lanes 16 to 20, Akata cells treated with ACV and induced with IgG. Yeast RNA (lane 14) was used as a negative control. Positions only of undigested probes are indicated by arrows.
These data demonstrate that BGLF4 is an early gene and its expression is temporally associated with the phosphorylation of EA-D (Fig. 1). 1263W94 and ACV did not affect BGLF4 mRNA levels, consistent with inhibition at translational or posttranslational levels.
EBV PK hyperphosphorylates EA-D protein.
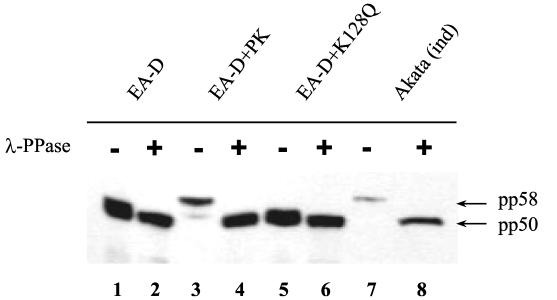
To determine if the viral protein kinase encoded by BGLF4 is indeed responsible for EA-D phosphorylation, we cloned both BMRF1 and BGLF4 ORFs under the control of a constitutive promoter and cotransfected the plasmids into an EBV-negative cell line, 293T. With the use of whole-cell lysates prepared from the transfected cells followed by immunoblotting, changes in EA-D phosphorylation status were detected by a shift in molecular mass upon sodium dodecyl sulfate-polyacrylamide gel electrophoresis. To confirm that this shift was due to phosphorylation the lambda phosphatase assay was employed. Expression of EA-D alone resulted in the appearance of a single band with a molecular mass of 50 kDa (pp50) that represents a hypophosphorylated form of EA-D. This result suggests that such a level of EA-D phosphorylation may be achieved by cellular kinases (Fig. 3, lane 1). Coexpression with EBV PK resulted in a complete shift to the hyperphosphorylated form (pp58) (lane 3). This shift up in molecular mass was greatly reduced when EA-D was coexpressed with the kinase-dead mutant of BGLF4, K128Q (lane 5). Finally, lambda phosphatase treatment resulted in a shift down in the molecular mass of the EA-D band to the nonphosphorylated level (46 to 48 kDa) (lanes 2, 4, and 6). The lysate from induced Akata cells was used as a molecular weight control (lane 7). No EA-D protein was detected in transfections with the vector (not shown).
FIG. 3.
Phosphorylation of EA-D by EBV PK in transient coexpression. 293T cells were either transfected with pHD/BMRF1 alone (lane 1) or cotransfected with pHD/BGLF4 (lane 3) or pHD/K128Q (lane 5). Lanes 2, 4, and 6 contain the same extracts treated with lambda phosphatase (λ-PPase). Akata cell protein extract (lanes 7 and 8) has been used as a size control. Cells were harvested 48 h after transfection, and equal amounts of the protein lysates were analyzed by immunoblotting. The differences in amount of EA-D in lanes 1 and 2 are due to unequal loading.
These results suggest that EA-D protein may be phosphorylated to some extent by cellular kinases, but it is hyperphosphorylated specifically by the viral protein kinase BGLF4.
1263W94 fails to inhibit hyperphosphorylation of EA-D by EBV PK.
The pp58 form of EA-D is affected by 1263W94 during viral reactivation (Fig. 1). Since many EBV proteins are expressed during viral reactivation, we coexpressed EA-D and BGLF4 alone in 293T cells in order to determine if the hyperphosphorylation of EA-D by BGLF4 is impaired by 1263W94. We transfected cells with decreasing amounts of BGLF4 and a constant amount of BMRF1 (0.5 μg). The results show that 1263W94 did not affect the appearance of pp58 (Fig. 4) and imply that the inhibition of BGLF4 kinase activity, as reflected by hyperphosphorylation of EA-D, is not directly affected by the compound.
FIG. 4.
1263W94 does not inhibit the phosphorylation of EA-D by EBV PK in coexpression assays. 293T cells were transfected as described in the legend to Fig. 3. Equal amounts (0.5 μg) of EA-D expression plasmid and serial dilutions of EBV PK expression plasmid (from 0.5 to 0.0625 μg) were used. Cells were harvested 48 h after transfection, and equal amounts of protein lysates were analyzed by immunoblotting. Lanes 7 to 11 contain cells treated with 1263W94.
DISCUSSION
1263W94, a member of the l-riboside benzimidazole family, possesses potent anti-EBV activity as shown previously (42). EA-D is a major phosphoprotein produced during the early phase of lytic infection (33) and an essential factor in an in vitro replication system that uses EBV oriLyt (13). In an extension of the earlier observations (42), we have shown that EA-D hyperphosphorylation is detected by 8 h and is temporally regulated; the hyperphosphorylated form (pp58) appears and wanes earlier than the hypophosphorylated form (pp50). Moreover, the predominant effect of 1263W94 appears to be on pp58, although the overall levels of both forms of EA-D are also affected (Fig. 1). The pp58 form of EA-D has previously been shown to contain both phosphoserine and phosphothreonine, but pp50 contains phosphoserine only (33), which supports the hypothesis that EA-D is hyperphosphorylated by a newly induced protein kinase. These observations imply that the phosphorylation status of EA-D is probably important for its functions. The results suggest that EA-D is probably phosphorylated by an induced protein kinase, presumably viral, and that 1263W94 inhibits EBV replication at least partially through its effect on hyperphosphorylated EA-D.
Beta- and gammaherpesviruses (including EBV) encode only one protein kinase (23) that is homologous to HSV UL13. UL13 has been shown to mediate the phosphorylation of viral proteins (such as ICP22 [30], gE and gI [26], and ICP0 [27]) and cellular factors (such as translation elongation factor 1 delta [20] and p60 [6]), thereby affecting viral replication and pathogenesis. EBV-encoded protein kinase BGLF4 has been recently shown to phosphorylate the translation elongation factor 1 delta (18) and appears to phosphorylate EA-D in vitro (7). We first examined a time course of BGLF4 mRNA expression. Since BGLF4 mRNA appeared 6 h after reactivation and was not affected by ACV treatment we concluded that BGLF4 is an early gene. Its RNA kinetics are temporally associated with the phosphorylation of EA-D (Fig. 1 and 2). Taken together, our results and the previous report show that BGLF4 is involved in EA-D hyperphosphorylation. 1263W94 did not affect significantly the mRNA level and presumably the protein level of BGLF4.
EA-D contains several potential sites for phosphorylation by protein kinase C and CKII cellular kinases. Indeed, our results demonstrate that transient expression of EA-D in EBV-negative cells results in hypophosphorylation of the protein, chiefly to the pp50 form (reference 42 and Fig. 3). However, coexpression of EA-D along with the BGLF4 gene product produces a complete shift from the pp50 to the pp58 form of EA-D, which confirms that BGLF4 indeed possesses protein kinase activity and that EA-D is one of its substrates.
These results and previous observations suggest a new mechanism of action for antiviral compounds active against herpesviruses. Essentially most drugs studied thus far act mainly on herpesvirus DNA polymerase and cause DNA chain termination. The β-d-riboside benzimidazole BDCRB, which inhibits HCMV but not EBV replication (42), is thought to inhibit the HCMV UL89 and UL56 (22, 39) gene products, as well as virus maturation (41).
Although in our system 1263W94 did not directly inhibit the hyperphosphorylation of EA-D by EBV PK (Fig. 4), it did affect the pp58 level during viral reactivation in Akata cells, suggesting that 1263W94 might act at other levels before or after the phosphorylation. Our preliminary results indicate that it might alter EA-D localization in the cell (not shown). Interestingly, although other herpesviruses have homologous DNA processivity factors (1, 5, 15, 40), HCMV and EBV are the only viruses that are reported to be sensitive to 1263W94 so far. It will be important to determine the phosphorylation status and the functionality or localization of the DNA processivity factors in herpesviruses both sensitive and insensitive to the compound. This information would be valuable for design of antiviral agents targeting these proteins and formation of the DNA polymerase complex.
Our results raise an important question. It has been reported that a 1263W94-resistant mutant of HCMV has a point mutation in the UL97 gene (Talarico et al., 38th ICAAC), which is homologous to EBV BGLF4. Moreover, we show the effect of the compound in altering the phosphorylation pattern of EA-D during viral reactivation in Akata cells (Fig. 1), which led us to the hypothesis that BGLF4 is involved in this process. Furthermore, a BGLF4 mutant bearing a mutation corresponding to that in UL97 (L397R) failed to hyperphosphorylate EA-D in a coexpression assay (not shown). However, 1263W94 did not directly affect EA-D hyperphosphorylation by BGLF4 when the two gene products were overexpressed in tandem. One possible explanation is that despite the homology, these kinases still have different targets; for example, the HSV-1 UL13 kinase induces directly or indirectly the phosphorylation of HSV-1 ICP22 and does not affect the phosphorylation of ICP4 (29), while VZV ORF47 (the homolog of UL13) phosphorylates VZV ORF62 (the homolog of HSV-1 ICP4) but not ORF63 (the homolog of HSV-1 ICP22) (17). Another possible explanation is that the compound affects another EBV protein(s) that in turn regulates BGLF4. Thus, to better characterize the role of BGLF4 in the EBV life cycle we are now attempting to identify its targets and regulators using purified protein and a number of antiviral compounds including 1263W94.
Acknowledgments
We thank S. Kenney for pSG5-BMRF1 and critical review of the manuscript and L. Zhang, M. Davis, and K. Biron for helpful discussions.
This work was supported by NCI grant CA-19014 and initially by a grant from Glaxo Wellcome Inc.
REFERENCES
- 1.Agulnick, A. D., J. R. Thompson, S. Iyengar, G. Pearson, D. Ablashi, and R. P. Ricciardi. 1993. Identification of a DNA-binding protein of human herpesvirus 6, a putative DNA polymerase stimulatory factor. J. Gen. Virol. 74:1003–1009. [DOI] [PubMed] [Google Scholar]
- 2.Ambinder, R. F., K. D. Robertson, S. M. Moore, and J. Yang. 1996. Epstein-Barr virus as a therapeutic target in Hodgkin’s disease and nasopharyngeal carcinoma. Semin. Cancer Biol. 7:217–226. [DOI] [PubMed] [Google Scholar]
- 3.Baumann, M., R. Feederle, E. Kremmer, and W. Hammerschmidt. 1999. Cellular transcription factors recruit viral replication proteins to activate the Epstein-Barr virus origin of lytic DNA replication, oriLyt. EMBO J. 18:6095–6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ben-Bassat, H., N. Goldblum, S. Mitrani, T. Goldblum, J. M. Yoffey, M. M. Cohen, Z. Bentwich, B. Ramot, E. Klein, and G. Klein. 1977. Establishment in continuous culture of a new type of lymphocyte from a ‘Burkitt like’ malignant lymphoma (line DG-75). Int. J. Cancer 19:27–33. [DOI] [PubMed] [Google Scholar]
- 5.Berthomme, H., S. J. Monahan, D. S. Parris, B. Jacquemont, and A. L. Epstein. 1995. Cloning, sequencing, and functional characterization of the two subunits of the pseudorabies virus DNA polymerase holoenzyme: evidence for specificity of interaction. J. Virol. 69:2811–2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruni, R., B. Fineschi, W. O. Ogle, and B. Roizman. 1999. A novel cellular protein, p60, interacting with both herpes simplex virus 1 regulatory proteins ICP22 and ICP0 is modified in a cell-type-specific manner and is recruited to the nucleus after infection. J. Virol. 73:3810–3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, M. R., S. J. Chang, H. Huang, and J. Y. Chen. 2000. A protein kinase activity associated with Epstein-Barr virus BGLF4 phosphorylates the viral early antigen EA-D in vitro. J. Virol. 74:3093–3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chulay, J., K. Biron, L. Wang, M. Underwood, S. Chamberlain, L. Frick, S. Good, M. Davis, R. Harvey, L. Townsend, J. Drach, and G. Koszalka. 1999. Development of novel benzimidazole riboside compounds for treatment of cytomegalovirus disease. Adv. Exp. Med. Biol. 458:129–134. [DOI] [PubMed] [Google Scholar]
- 9.Davis, M. G., S. C. Kenney, J. Kamine, J. S. Pagano, and E. S. Huang. 1987. Immediate-early gene region of human cytomegalovirus trans-activates the promoter of human immunodeficiency virus. Proc. Natl. Acad. Sci. USA 84:8642–8646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drach, J. C., L. B. Townsend, M. R. Nassiri, S. R. Turk, L. A. Coleman, R. V. Devivar, G. Genzlinger, E. D. Kreske, T. R. Renau, A. C. Westerman, C. Shipman, Jr., K. K. Biron, R. Dornsife, and E. R. Kern. 1992. Benzimidazole ribonucleosides: a new class of antivirals with potent and selective activity against human cytomegalovirus. Antivir. Res. 21:49. [Google Scholar]
- 11.Drouet, E., P. Brousset, F. Fares, J. Icart, C. Verniol, F. Meggetto, D. Schlaifer, H. Desmorat-Coat, F. Rigal-Huguet, A. Niveleau, and G. Delsol. 1999. High Epstein-Barr virus serum load and elevated titers of anti-ZEBRA antibodies in patients with EBV-harboring tumor cells of Hodgkin’s disease. J. Med. Virol. 57:383–389. [DOI] [PubMed] [Google Scholar]
- 12.Epstein, A. L. 1984. Immunobiochemical characterization with monoclonal antibodies of Epstein-Barr virus-associated early antigens in chemically induced cells. J. Virol. 50:372–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fixman, E. D., G. S. Hayward, and S. D. Hayward. 1992. trans-acting requirements for replication of Epstein-Barr virus ori-Lyt. J. Virol. 66:5030–5039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujii, K., N. Yokoyama, T. Kiyono, K. Kuzushima, M. Homma, Y. Nishiyama, M. Fujita, and T. Tsurumi. 2000. The Epstein-Barr virus pol catalytic subunit physically interacts with the BBLF4-BSLF1-BBLF2/3 complex. J. Virol. 74:2550–2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gottlieb, J., A. I. Marcy, D. M. Coen, and M. D. Challberg. 1990. The herpes simplex virus type 1 UL42 gene product: a subunit of DNA polymerase that functions to increase processivity. J. Virol. 64:5976–5987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He, Z., Y. S. He, Y. Kim, L. Chu, C. Ohmstede, K. K. Biron, and D. M. Coen. 1997. The human cytomegalovirus UL97 protein is a protein kinase that autophosphorylates on serines and threonines. J. Virol. 71:405–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heineman, T. C., and J. I. Cohen. 1995. The varicella-zoster virus (VZV) open reading frame 47 (ORF47) protein kinase is dispensable for viral replication and is not required for phosphorylation of ORF63 protein, the VZV homolog of herpes simplex virus ICP22. J. Virol. 69:7367–7370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kato, K., Y. Kawaguchi, M. Tanaka, M. Igarashi, A. Yokoyama, G. Matsuda, M. Kanamori, K. Nakajima, Y. Nishimura, M. Shimojima, H. T. Phung, E. Takahashi, and K. Hirai. 2001. Epstein-Barr virus-encoded protein kinase BGLF4 mediates hyperphosphorylation of cellular elongation factor 1delta (EF-1delta): EF-1delta is universally modified by conserved protein kinases of herpesviruses in mammalian cells. J. Gen. Virol. 82:1457–1463. [DOI] [PubMed] [Google Scholar]
- 19.Katz, B. Z., N. Raab-Traub, and G. Miller. 1989. Latent and replicating forms of Epstein-Barr virus DNA in lymphomas and lymphoproliferative diseases. J. Infect. Dis. 160:589–598. [DOI] [PubMed] [Google Scholar]
- 20.Kawaguchi, Y., C. Van Sant, and B. Roizman. 1998. Eukaryotic elongation factor 1delta is hyperphosphorylated by the protein kinase encoded by the UL13 gene of herpes simplex virus 1. J. Virol. 72:1731–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koszalka, G. W., S. D. Chamberlain, R. J. Harvey, L. W. Frick, S. S. Good, M. G. Davis, A. Smith, J. C. Drach, L. B. Townsend, and K. K. Biron. 1996. Benzimidazoles for the treatment of human cytomegalovirus infections. Antivir. Res. 30:A43. [Google Scholar]
- 22.Krosky, P. M., M. R. Underwood, S. R. Turk, K. W. Feng, R. K. Jain, R. G. Ptak, A. C. Westerman, K. K. Biron, L. B. Townsend, and J. C. Drach. 1998. Resistance of human cytomegalovirus to benzimidazole ribonucleosides maps to two open reading frames: UL89 and UL56. J. Virol. 72:4721–4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leader, D. P. 1993. Viral protein kinases and protein phosphatases. Pharmacol. Ther. 59:343–389. [DOI] [PubMed] [Google Scholar]
- 24.Meijer, C. J., N. M. Jiwa, D. F. Dukers, J. J. Oudejans, P. C. de Bruin, J. M. Walboomers, and A. J. van den Brule. 1996. Epstein-Barr virus and human T-cell lymphomas. Semin. Cancer Biol. 7:191–196. [DOI] [PubMed] [Google Scholar]
- 25.Ng, T. I., and C. Grose. 1992. Serine protein kinase associated with varicella-zoster virus ORF 47. Virology 191:9–18. [DOI] [PubMed] [Google Scholar]
- 26.Ng, T. I., W. O. Ogle, and B. Roizman. 1998. UL13 protein kinase of herpes simplex virus 1 complexes with glycoprotein E and mediates the phosphorylation of the viral Fc receptor: glycoproteins E and I. Virology 241:37–48. [DOI] [PubMed] [Google Scholar]
- 27.Ogle, W. O., T. I. Ng, K. L. Carter, and B. Roizman. 1997. The UL13 protein kinase and the infected cell type are determinants of posttranslational modification of ICP0. Virology 235:406–413. [DOI] [PubMed] [Google Scholar]
- 28.Pagano, J. S. 1995. Epstein-Barr virus: therapy of active and latent infection, p.155–195. In D. J. Jeffries and E. De Clercq (ed.), Antiviral chemotherapy. John Wiley & Sons Ltd., New York, N.Y.
- 29.Purves, F. C., W. O. Ogle, and B. Roizman. 1993. Processing of the herpes simplex virus regulatory protein alpha 22 mediated by the UL13 protein kinase determines the accumulation of a subset of alpha and gamma mRNAs and proteins in infected cells. Proc. Natl. Acad. Sci. USA 90:6701–6705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Purves, F. C., and B. Roizman. 1992. The UL13 gene of herpes simplex virus 1 encodes the functions for posttranslational processing associated with phosphorylation of the regulatory protein alpha 22. Proc. Natl. Acad. Sci. USA 89:7310–7314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raab-Traub, N. 1992. Epstein-Barr virus infection in nasopharyngeal carcinoma. Infect. Agents Dis. 1:173–184. [PubMed] [Google Scholar]
- 32.Reusser, P. 2000. Antiviral therapy: current options and challenges. Schweiz. Med. Wochenschr. 130:101–112. [PubMed] [Google Scholar]
- 33.Roeckel, D., and N. Mueller-Lantzsch. 1985. Biochemical characterization of two Epstein-Barr virus early antigen-associated phosphopolypeptides. Virology 147:253–263. [DOI] [PubMed] [Google Scholar]
- 34.Sullivan, V., C. L. Talarico, S. C. Stanat, M. Davis, D. M. Coen, and K. K. Biron. 1992. A protein kinase homologue controls phosphorylation of ganciclovir in human cytomegalovirus-infected cells. Nature 359:85. [DOI] [PubMed] [Google Scholar]
- 35.Takada, K. 1984. Cross-linking of cell surface immunoglobulins induces Epstein-Barr virus in Burkitt’s lymphoma lines. Int. J. Cancer 33:27–32. [DOI] [PubMed] [Google Scholar]
- 36.Talarico, C. L., T. C. Burnette, W. H. Miller, S. L. Smith, M. G. Davis, S. C. Stanat, T. I. Ng, Z. He, D. M. Coen, B. Roizman, and K. K. Biron. 1999. Acyclovir is phosphorylated by the human cytomegalovirus UL97 protein. Antimicrob. Agents Chemother. 43:1941–1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsurumi, T. 1993. Purification and characterization of the DNA-binding activity of the Epstein-Barr virus DNA polymerase accessory protein BMRF1 gene products, as expressed in insect cells by using the baculovirus system. J. Virol. 67:1681–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsurumi, T., T. Daikoku, R. Kurachi, and Y. Nishiyama. 1993. Functional interaction between Epstein-Barr virus DNA polymerase catalytic subunit and its accessory subunit in vitro. J. Virol. 67:7648–7653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Underwood, M. R., R. J. Harvey, S. C. Stanat, M. L. Hemphill, T. Miller, J. C. Drach, L. B. Townsend, and K. K. Biron. 1998. Inhibition of human cytomegalovirus DNA maturation by a benzimidazole ribonucleoside is mediated through the UL89 gene product. J. Virol. 72:717–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weiland, K. L., N. L. Oien, F. Homa, and M. W. Wathen. 1994. Functional analysis of human cytomegalovirus polymerase accessory protein. Virus Res. 34:191–206. [DOI] [PubMed] [Google Scholar]
- 41.Wolf, D. G., C. T. Courcelle, M. N. Prichard, and E. S. Mocarski. 2001. Distinct and separate roles for herpesvirus-conserved UL97 kinase in cytomegalovirus DNA synthesis and encapsidation. Proc. Natl. Acad. Sci. USA 98:1895–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zacny, V. L., E. Gershburg, M. G. Davis, K. K. Biron, and J. S. Pagano. 1999. Inhibition of Epstein-Barr virus replication by a benzimidazole l-riboside: novel antiviral mechanism of 5,6-dichloro-2-(isopropylamino)-1-β-l-ribofuranosyl-1H-benzimidazole. J. Virol. 73:7271–7277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang, Q., Y. Hong, D. Dorsky, E. Holley-Guthrie, S. Zalani, N. A. Elshiekh, A. Kiehl, and S. Kenney. 1996. Functional and physical interaction between the Epstein-Barr virus (EBV) proteins BZLF1 and BMRF1: effects on EBV transcription and lytic replication. J. Virol. 70:5131–5142. [DOI] [PMC free article] [PubMed] [Google Scholar]