Abstract
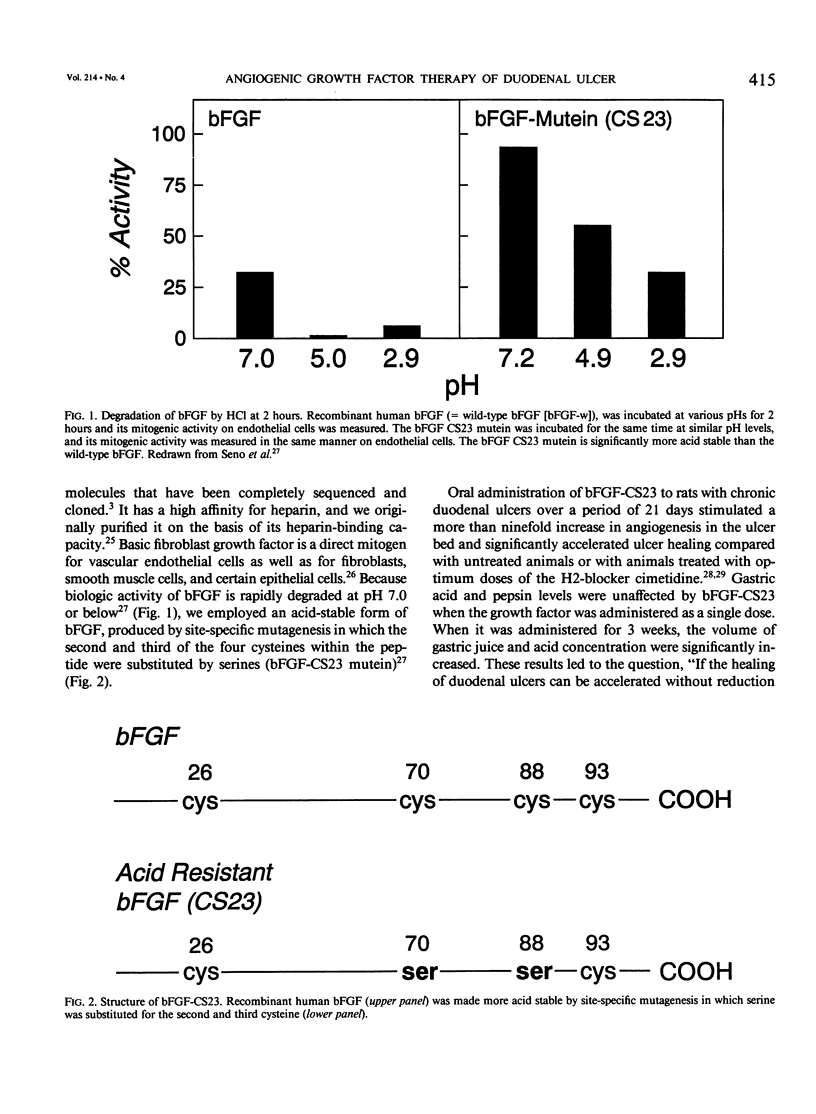
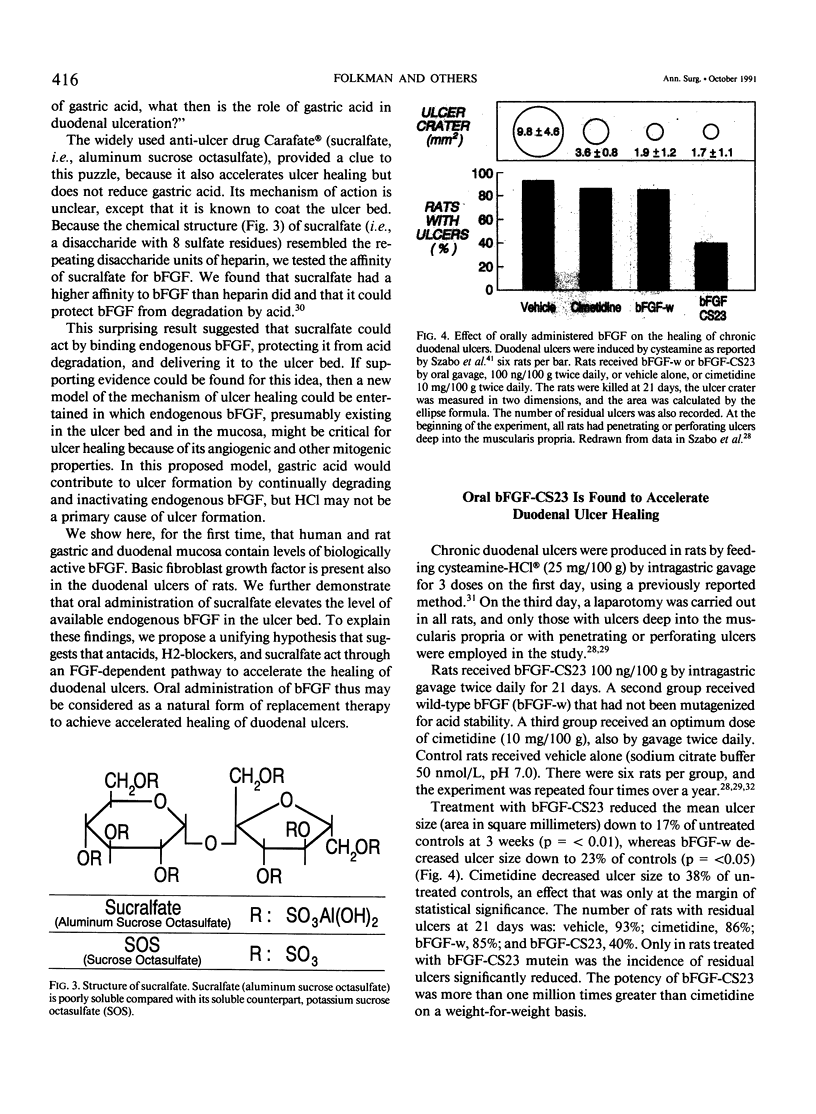
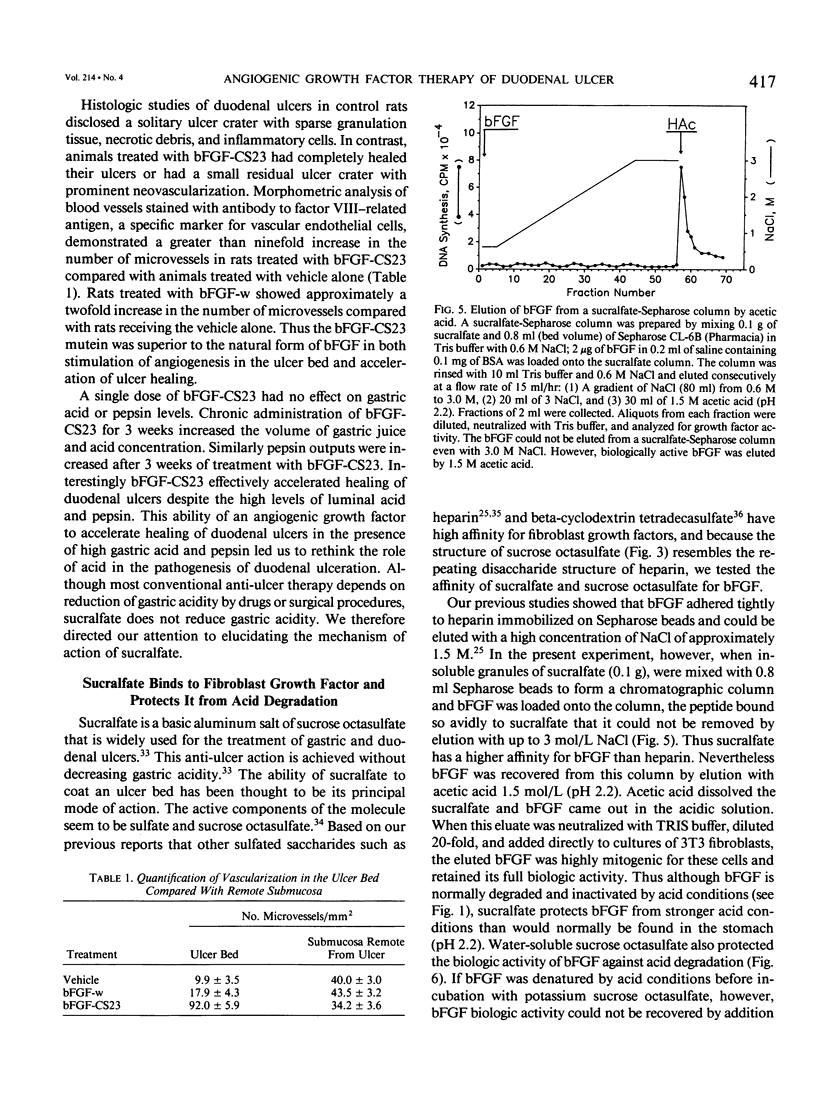
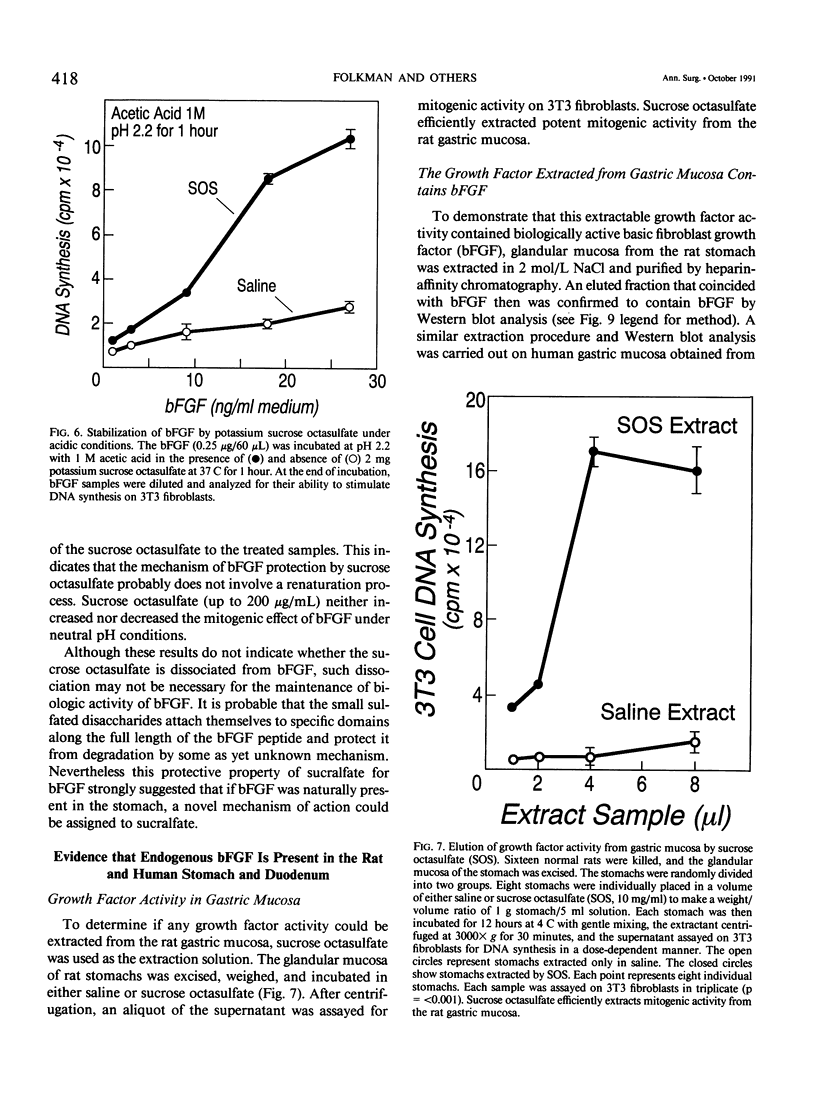
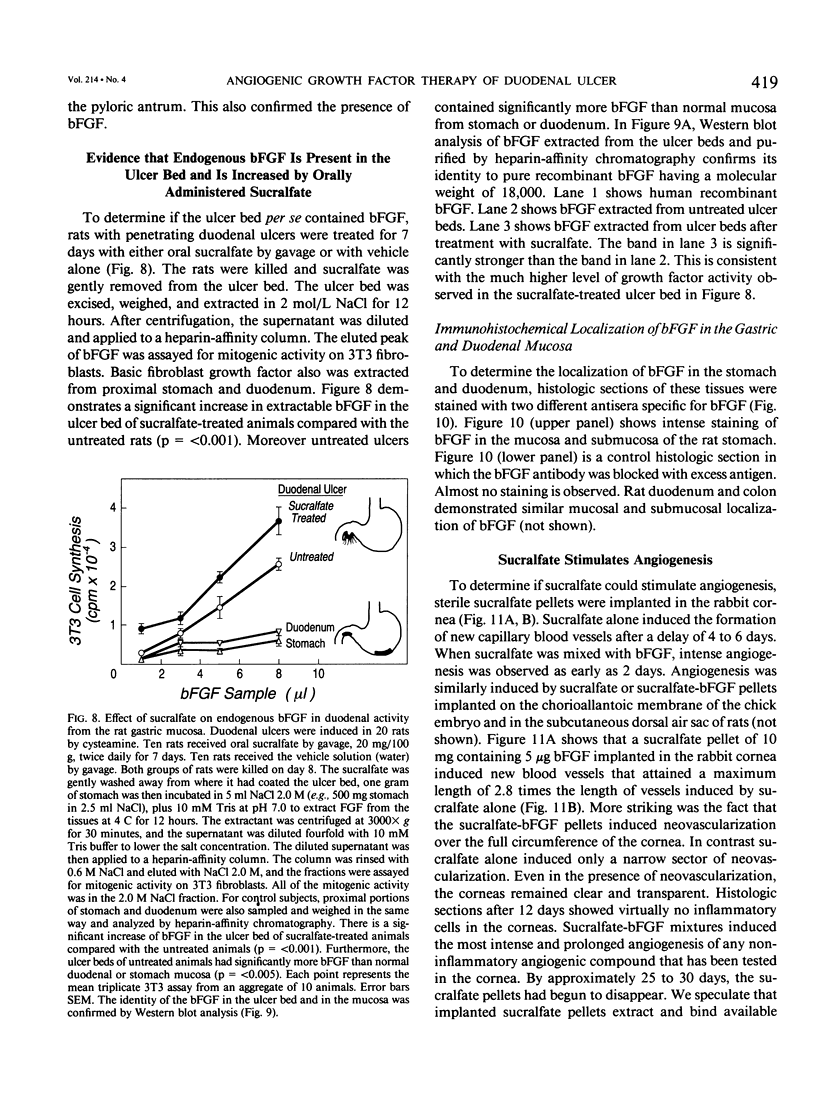
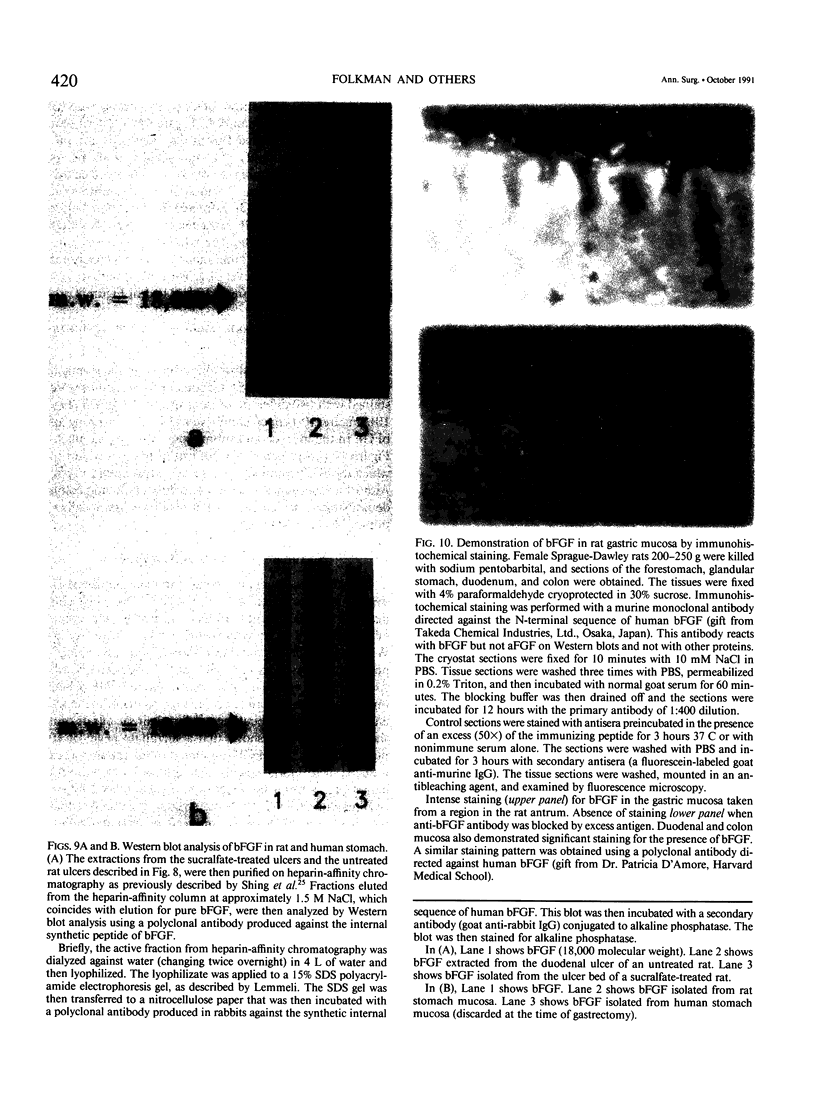
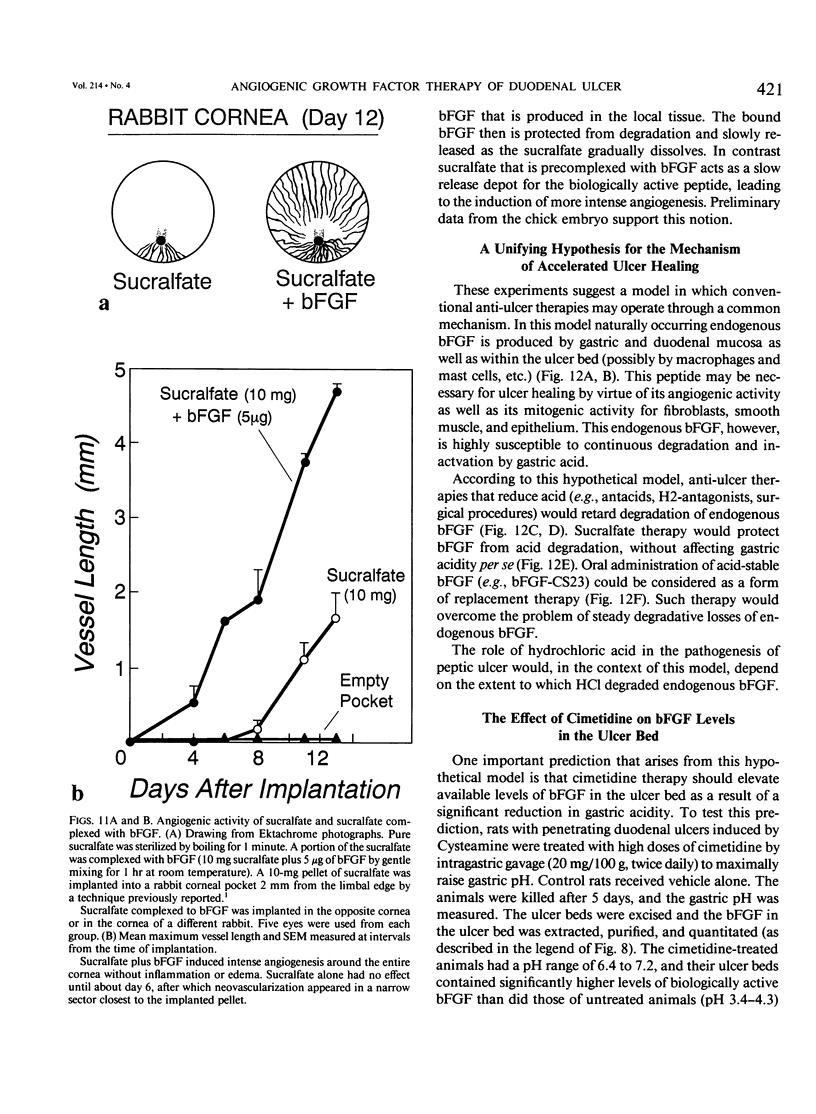
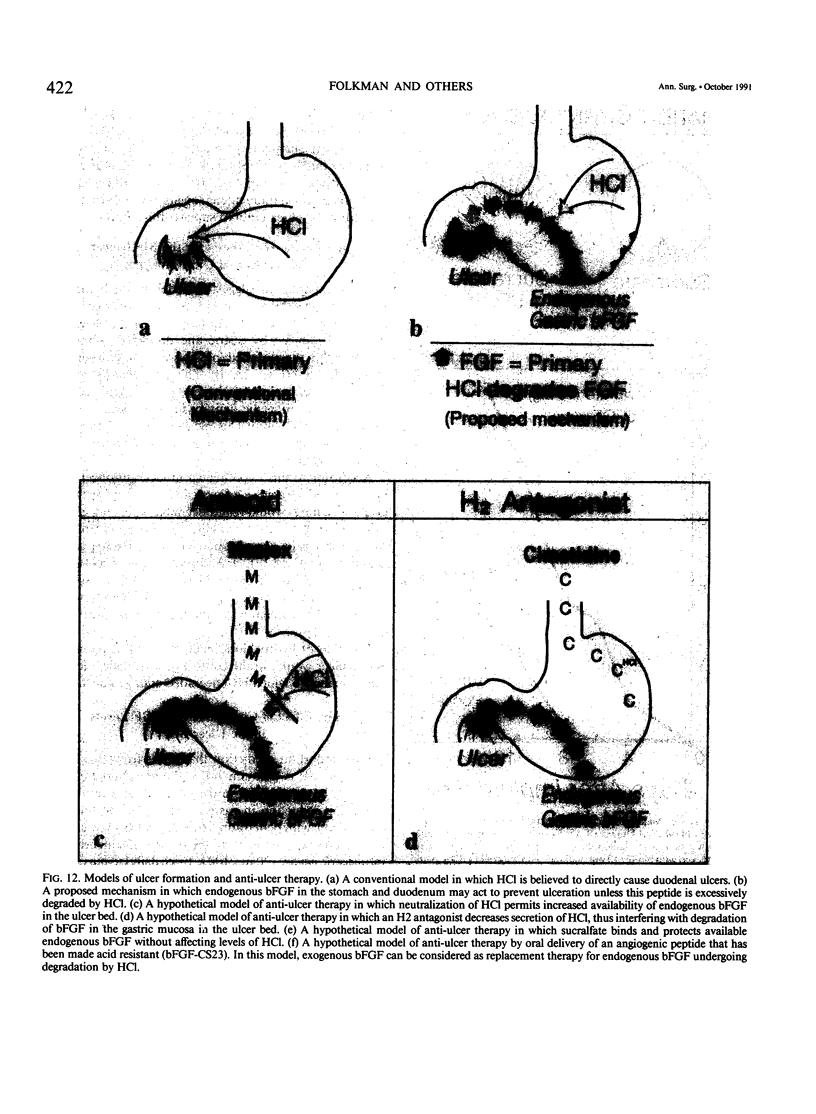
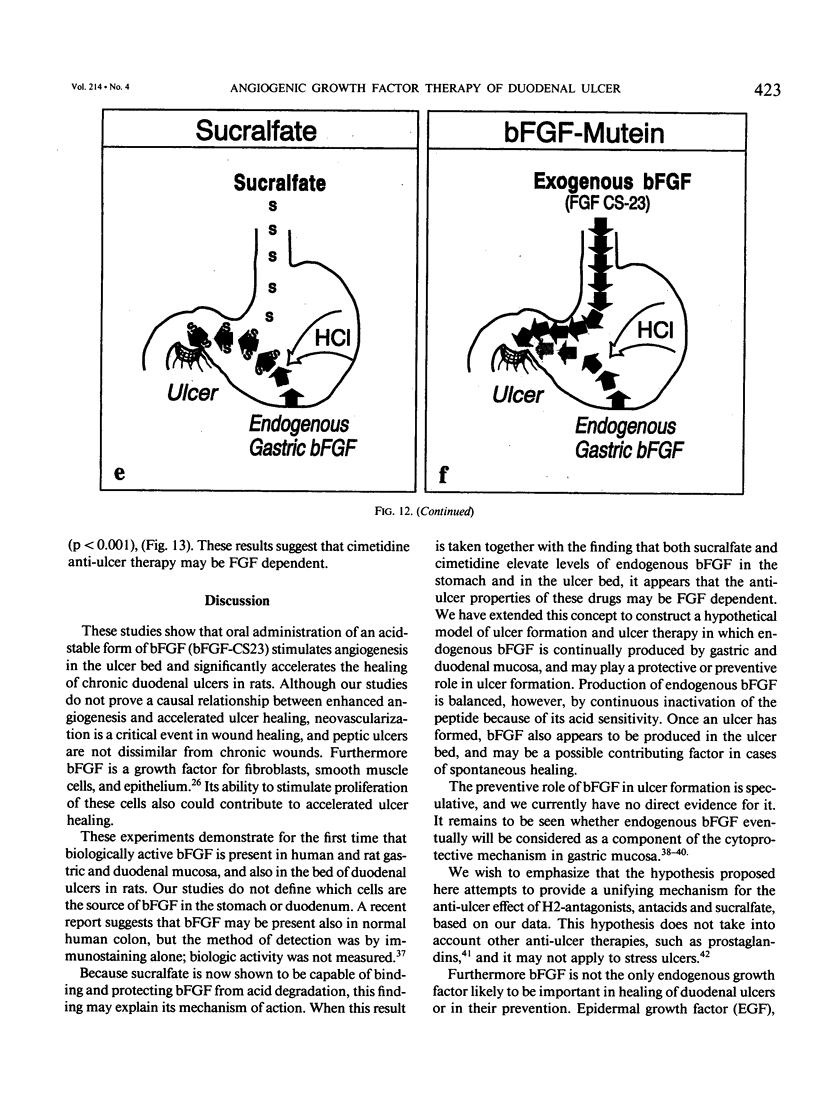
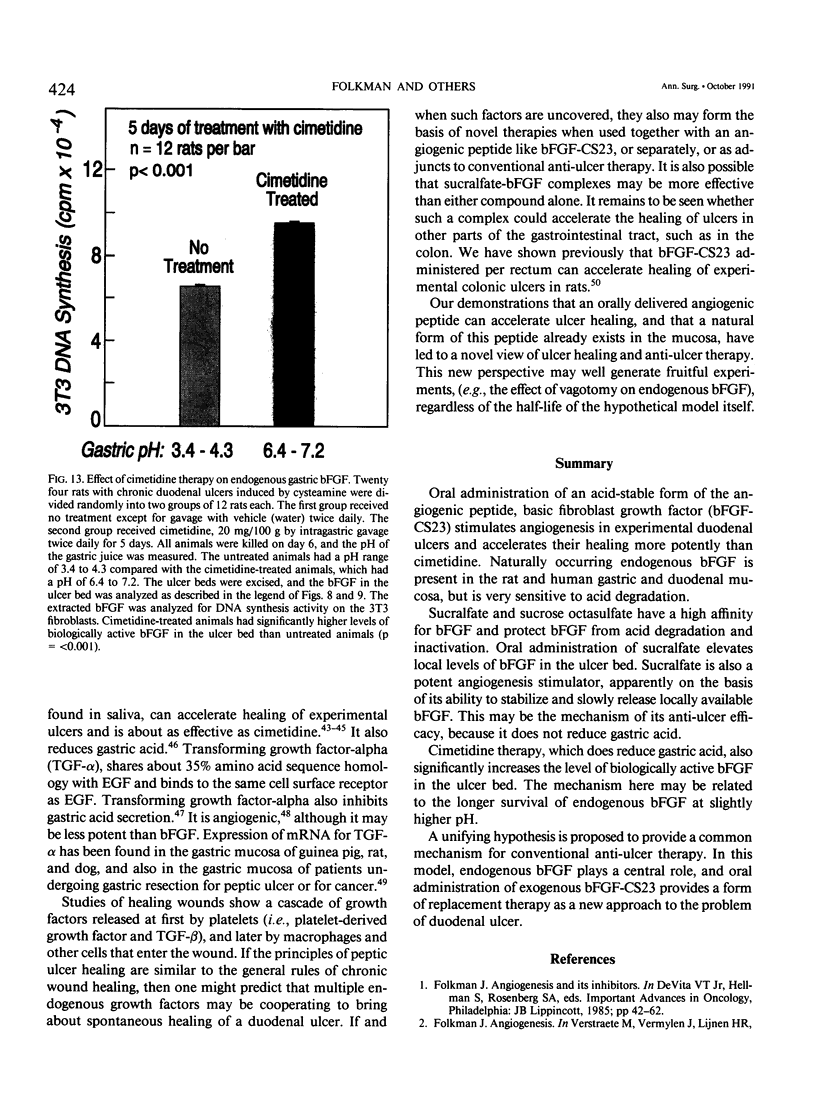
The complete purification of the first angiogenic molecule, basic fibroblast growth factor (bFGF), was carried out in the authors' laboratory in 1983. Application of this peptide to chronic wounds enhances angiogenesis and accelerates wound healing. The authors showed that an acid-stable form of bFGF (i.e., bFGF-CS23) could be administered orally to rats with duodenal ulcers. The peptide promoted a ninefold increase of angiogenesis in the ulcer bed and accelerated ulcer healing more potently than cimetidine. Basic fibroblast growth factor did not reduce gastric acid. The authors now show that bFGF exists as a naturally occurring peptide in rat and human gastric and duodenal mucosa. This endogenous bFGF is present also in the bed of chronic ulcers in rats. Sucralfate binds bFGF and protects it from acid degradation. The sucralfate is angiogenic, based on its affinity for bFGF. When sucralfate is administered orally to rats, it significantly elevates the level of bFGF in the ulcer bed. Cimetidine, by its capacity to reduce gastric acid, also elevates bFGF in the ulcer bed. A hypothetical model is proposed in which prevention of ulcer formation or accelerated healing of ulcers by conventional therapies may be FGF dependent. Acid-stable bFGF-CS23 may be considered as a form of replacement therapy in the treatment of duodenal ulcers.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beauchamp R. D., Barnard J. A., McCutchen C. M., Cherner J. A., Coffey R. J., Jr Localization of transforming growth factor alpha and its receptor in gastric mucosal cells. Implications for a regulatory role in acid secretion and mucosal renewal. J Clin Invest. 1989 Sep;84(3):1017–1023. doi: 10.1172/JCI114223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadley K. N., Aquino A. M., Woodward S. C., Buckley-Sturrock A., Sato Y., Rifkin D. B., Davidson J. M. Monospecific antibodies implicate basic fibroblast growth factor in normal wound repair. Lab Invest. 1989 Nov;61(5):571–575. [PubMed] [Google Scholar]
- Buntrock P., Jentzsch K. D., Heder G. Stimulation of wound healing, using brain extract with fibroblast growth factor (FGF) activity. I. Quantitative and biochemical studies into formation of granulation tissue. Exp Pathol. 1982;21(1):46–53. doi: 10.1016/s0232-1513(82)80051-0. [DOI] [PubMed] [Google Scholar]
- Buntrock P., Jentzsch K. D., Heder G. Stimulation of wound healing, using brain extract with fibroblast growth factor (FGF) activity. II. Histological and morphometric examination of cells and capillaries. Exp Pathol. 1982;21(1):62–67. doi: 10.1016/s0232-1513(82)80054-6. [DOI] [PubMed] [Google Scholar]
- Cordon-Cardo C., Vlodavsky I., Haimovitz-Friedman A., Hicklin D., Fuks Z. Expression of basic fibroblast growth factor in normal human tissues. Lab Invest. 1990 Dec;63(6):832–840. [PubMed] [Google Scholar]
- Davidson J. M., Klagsbrun M., Hill K. E., Buckley A., Sullivan R., Brewer P. S., Woodward S. C. Accelerated wound repair, cell proliferation, and collagen accumulation are produced by a cartilage-derived growth factor. J Cell Biol. 1985 Apr;100(4):1219–1227. doi: 10.1083/jcb.100.4.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finke U., Rutten M., Murphy R. A., Silen W. Effects of epidermal growth factor on acid secretion from guinea pig gastric mucosa: in vitro analysis. Gastroenterology. 1985 May;88(5 Pt 1):1175–1182. doi: 10.1016/s0016-5085(85)80077-9. [DOI] [PubMed] [Google Scholar]
- Folkman J., Klagsbrun M. Angiogenic factors. Science. 1987 Jan 23;235(4787):442–447. doi: 10.1126/science.2432664. [DOI] [PubMed] [Google Scholar]
- Folkman J. Successful treatment of an angiogenic disease. N Engl J Med. 1989 May 4;320(18):1211–1212. doi: 10.1056/NEJM198905043201811. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Ferrara N., Schweigerer L., Neufeld G. Structural characterization and biological functions of fibroblast growth factor. Endocr Rev. 1987 May;8(2):95–114. doi: 10.1210/edrv-8-2-95. [DOI] [PubMed] [Google Scholar]
- Greenhalgh D. G., Sprugel K. H., Murray M. J., Ross R. PDGF and FGF stimulate wound healing in the genetically diabetic mouse. Am J Pathol. 1990 Jun;136(6):1235–1246. [PMC free article] [PubMed] [Google Scholar]
- Hunt T. K., Knighton D. R., Thakral K. K., Goodson W. H., 3rd, Andrews W. S. Studies on inflammation and wound healing: angiogenesis and collagen synthesis stimulated in vivo by resident and activated wound macrophages. Surgery. 1984 Jul;96(1):48–54. [PubMed] [Google Scholar]
- Ingber D., Fujita T., Kishimoto S., Sudo K., Kanamaru T., Brem H., Folkman J. Synthetic analogues of fumagillin that inhibit angiogenesis and suppress tumour growth. Nature. 1990 Dec 6;348(6301):555–557. doi: 10.1038/348555a0. [DOI] [PubMed] [Google Scholar]
- Klagsbrun M., Shing Y. Heparin affinity of anionic and cationic capillary endothelial cell growth factors: analysis of hypothalamus-derived growth factors and fibroblast growth factors. Proc Natl Acad Sci U S A. 1985 Feb;82(3):805–809. doi: 10.1073/pnas.82.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knighton D. R., Hunt T. K., Thakral K. K., Goodson W. H., 3rd Role of platelets and fibrin in the healing sequence: an in vivo study of angiogenesis and collagen synthesis. Ann Surg. 1982 Oct;196(4):379–388. doi: 10.1097/00000658-198210000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konturek S. J. Role of growth factors in gastroduodenal protection and healing of peptic ulcers. Gastroenterol Clin North Am. 1990 Mar;19(1):41–65. [PubMed] [Google Scholar]
- Leibovich S. J., Ross R. The role of the macrophage in wound repair. A study with hydrocortisone and antimacrophage serum. Am J Pathol. 1975 Jan;78(1):71–100. [PMC free article] [PubMed] [Google Scholar]
- McGee G. S., Davidson J. M., Buckley A., Sommer A., Woodward S. C., Aquino A. M., Barbour R., Demetriou A. A. Recombinant basic fibroblast growth factor accelerates wound healing. J Surg Res. 1988 Jul;45(1):145–153. doi: 10.1016/0022-4804(88)90034-0. [DOI] [PubMed] [Google Scholar]
- Rhodes J. A., Tam J. P., Finke U., Saunders M., Bernanke J., Silen W., Murphy R. A. Transforming growth factor alpha inhibits secretion of gastric acid. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3844–3846. doi: 10.1073/pnas.83.11.3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber A. B., Winkler M. E., Derynck R. Transforming growth factor-alpha: a more potent angiogenic mediator than epidermal growth factor. Science. 1986 Jun 6;232(4755):1250–1253. doi: 10.1126/science.2422759. [DOI] [PubMed] [Google Scholar]
- Seno M., Sasada R., Iwane M., Sudo K., Kurokawa T., Ito K., Igarashi K. Stabilizing basic fibroblast growth factor using protein engineering. Biochem Biophys Res Commun. 1988 Mar 15;151(2):701–708. doi: 10.1016/s0006-291x(88)80337-1. [DOI] [PubMed] [Google Scholar]
- Shing Y., Folkman J., Sullivan R., Butterfield C., Murray J., Klagsbrun M. Heparin affinity: purification of a tumor-derived capillary endothelial cell growth factor. Science. 1984 Mar 23;223(4642):1296–1299. doi: 10.1126/science.6199844. [DOI] [PubMed] [Google Scholar]
- Silen W., Schiessel R., Kivilaakso E. The gastric mucosal barrier and ulceration. Brain Res Bull. 1980;5 (Suppl 1):3–6. doi: 10.1016/0361-9230(80)90296-8. [DOI] [PubMed] [Google Scholar]
- Silen W. The clinical problem of stress ulcers. Clin Invest Med. 1987 May;10(3):270–274. [PubMed] [Google Scholar]
- Silen W. What is cytoprotection of the gastric mucosa? Gastroenterology. 1988 Jan;94(1):232–235. doi: 10.1016/0016-5085(88)90635-x. [DOI] [PubMed] [Google Scholar]
- Skov Olsen P., Poulsen S. S., Therkelsen K., Nexø E. Oral administration of synthetic human urogastrone promotes healing of chronic duodenal ulcers in rats. Gastroenterology. 1986 Apr;90(4):911–917. doi: 10.1016/0016-5085(86)90867-x. [DOI] [PubMed] [Google Scholar]
- Sprugel K. H., McPherson J. M., Clowes A. W., Ross R. Effects of growth factors in vivo. I. Cell ingrowth into porous subcutaneous chambers. Am J Pathol. 1987 Dec;129(3):601–613. [PMC free article] [PubMed] [Google Scholar]
- Stenberg B. D., Phillips L. G., Hokanson J. A., Heggers J. P., Robson M. C. Effect of bFGF on the inhibition of contraction caused by bacteria. J Surg Res. 1991 Jan;50(1):47–50. doi: 10.1016/0022-4804(91)90008-a. [DOI] [PubMed] [Google Scholar]
- Szabo S., Brown A. Prevention of ethanol-induced vascular injury and gastric mucosal lesions by sucralfate and its components: possible role of endogenous sulfhydryls. Proc Soc Exp Biol Med. 1987 Sep;185(4):493–497. doi: 10.3181/00379727-185-4-rc1. [DOI] [PubMed] [Google Scholar]
- Szabo S. Duodenal ulcer disease. Animal model: cysteamine-induced acute and chronic duodenal ulcer in the rat. Am J Pathol. 1978 Oct;93(1):273–276. [PMC free article] [PubMed] [Google Scholar]
- Szabo S., Hollander D. Pathways of gastrointestinal protection and repair: mechanisms of action of sucralfate. Am J Med. 1989 Jun 9;86(6A):23–31. doi: 10.1016/0002-9343(89)90153-8. [DOI] [PubMed] [Google Scholar]
- Takeuchi K., Svanes K., Critchlow J., Magee D., Silen W. Prostaglandins stimulate and inhibit acid secretion in amphibian fundic musoca. Proc Soc Exp Biol Med. 1982 Sep;170(4):398–404. doi: 10.3181/00379727-170-41449. [DOI] [PubMed] [Google Scholar]
- Weidner N., Semple J. P., Welch W. R., Folkman J. Tumor angiogenesis and metastasis--correlation in invasive breast carcinoma. N Engl J Med. 1991 Jan 3;324(1):1–8. doi: 10.1056/NEJM199101033240101. [DOI] [PubMed] [Google Scholar]
- White C. W., Sondheimer H. M., Crouch E. C., Wilson H., Fan L. L. Treatment of pulmonary hemangiomatosis with recombinant interferon alfa-2a. N Engl J Med. 1989 May 4;320(18):1197–1200. doi: 10.1056/NEJM198905043201807. [DOI] [PubMed] [Google Scholar]
- Yuen S. N., Folkman J., Weisz P. B., Joullie M. M., Ewing W. R. Affinity of fibroblast growth factors for beta-cyclodextrin tetradecasulfate. Anal Biochem. 1990 Feb 15;185(1):108–111. doi: 10.1016/0003-2697(90)90263-9. [DOI] [PubMed] [Google Scholar]




