Abstract
The simian picornaviruses were isolated from various primate tissues during the development of general tissue culture methods in the 1950s to 1970s or from specimens derived from primates used in biomedical research. Twenty simian picornavirus serotypes are recognized, and all are presently classified within the Enterovirus genus. To determine the phylogenetic relationships among all of the simian picornaviruses and to evaluate their classification, we have determined complete VP1 sequences for 19 of the 20 serotypes. Phylogenetic analysis showed that A13, SV19, SV26, SV35, SV43, and SV46 are members of human enterovirus species A, a group that contains enterovirus 71 and 11 of the coxsackie A viruses. SA5 is a member of human enterovirus species B, which contains the echoviruses, coxsackie B viruses, coxsackievirus A9, and enterovirus 69. SV6, N125, and N203 are related to one another and, more distantly, to species A human enteroviruses, but could not be definitely assigned to a species. SV4 and SV28 are closely related to one another and to A-2 plaque virus, but distinct from other enteroviruses, suggesting that these simian viruses are members of a new enterovirus species. SV2, SV16, SV18, SV42, SV44, SV45, and SV49 are related to one another but distinct from viruses in all other picornavirus genera, suggesting that they may comprise a previously unknown genus in Picornaviridae. Several simian virus VP1 sequences (N125 and N203; SV4 and SV28; SV19, SV26, and SV35; SV18 and SV44; SV16, SV42, and SV45) are greater than 75% identical to one another (and/or greater than 85% amino acid identity), suggesting that the true number of distinct serotypes among the viruses surveyed is less than 20.
Picornaviruses have been isolated from many vertebrate species, including humans, nonhuman primates, horses, cattle, swine, rodents, and birds (19). The family Picornaviridae consists of six recognized genera (Aphthovirus, Cardiovirus, Enterovirus, Hepatovirus, Parechovirus, and Rhinovirus) and three proposed genera (Erbovirus, Kobuvirus, and Teschovirus) (19, 31). The genera are further subdivided into species. For example, the genus Enterovirus is composed of eight species: Bovine enterovirus (BEV; 2 serotypes), Human enterovirus A (HEV-A; 12 serotypes), Human enterovirus B (HEV-B; 36 serotypes), Human enterovirus C (HEV-C; 11 serotypes), Human enterovirus D (HEV-D; 2 serotypes), Poliovirus (PV; 3 serotypes), Porcine enterovirus A (PEV-A; 1 serotype), and Porcine enterovirus B (2 serotypes) (19). Traditionally, picornaviruses have been classified, identified, and differentiated on the basis of physical and antigenic properties, such as acid stability and virion density, and by neutralization with specific antisera. More recently, nucleotide sequencing has been applied to picornavirus classification and its use has aided in the establishment of echoviruses 22 and 23 (renamed human parechoviruses 1 and 2, respectively) (13), Aichi virus (37), equine rhinitis B virus (36), and several porcine enteroviruses (renamed porcine teschoviruses) (4, 15, 39) as members of four new or proposed new genera (19). Our laboratory and others have shown that enterovirus VP1 sequence correlates with serotype and that VP1 sequence can be used as a molecular surrogate for antigenic typing (2, 3, 24–28). Among viruses in the Enterovirus genus, VP1 sequences are monophyletic with respect to serotype, species, and genus. Genetic clusters based on VP1 sequence (27) contain the same members as those based on partial 3D or VP2 sequences (14, 30), but noncapsid sequences do not always correlate with serotype. The genetic clusters correspond to the currently recognized species (19), showing that VP1 is sufficient to assign isolates to one of the recognized enterovirus species.
The nonhuman primate picornaviruses were isolated in the 1950s to 1970s from primate cell cultures during the development of general tissue culture methods, from primary cell cultures used in vaccine production, or from specimens derived from captive or wild-caught primates used in biomedical research (5, 9, 16, 18, 32). All of the 20 recognized simian picornavirus serotypes are currently classified as tentative members of the Enterovirus genus, but they are not yet assigned to a species (19). Eleven of the simian picornaviruses were recently characterized by partial sequencing and phylogenetic analysis of their 5′-nontranslated regions (NTRs) and polymerase (3D) genes (29). That study suggested that at least one serotype (SV18) may belong to another genus, based on the 3D sequence. However, no 5′-NTR sequence was obtained for SV18, seven additional serotypes (SA5, SV2, SV16, SV42, SV44, SV45, and SV49) were not amplified in either region, and two serotypes (N125 and N203) were not tested. Because of the high frequency of genetic recombination in noncapsid regions of the genome (21, 22, 33), it is difficult to interpret phylogenetic relationships of closely related viruses by using 5′-NTR and 3D sequences. To better define the relationships among the simian picornaviruses and to assess their proper taxonomic classification, we have determined complete VP1 sequences for 19 of the 20 serotypes. Sequence alignments and phylogenetic analysis suggest that only 12 of the 19 are true enteroviruses, six belonging to HEV-A, one to HEV-B, and five in two proposed new species. The classification of the remaining seven viruses within the genus Enterovirus should be reconsidered.
MATERIALS AND METHODS
Viruses.
The prototype strains of 18 of the 20 simian picornavirus serotypes were obtained from American Type Culture Collection (ATCC) (Manassas, Va.) (Table 1). Two additional strains, N125 and N203, were kindly provided by S. Kalter and R. Heberling, Esoterix, Inc., San Antonio, Tex. (Table 1). Viral RNA was extracted from cell culture supernatant using the QiaAmp Viral RNA kit (Qiagen, Santa Clarita, Calif.) either directly from the original material or following one passage in LLC-MK2 cells (ATCC CCL 7).
TABLE 1.
Viruses analyzed
| Serotype | Strain | ATCC no. | Species of origina | Year | Sourceb | CPE groupc | Reference(s) |
|---|---|---|---|---|---|---|---|
| A13 | A13 | VR-274 | Papio anubis | c. 1962 | Feces | NKd | 5, 18 |
| SA4 | L79C3 | VR-951 | Cercopithecus aethiops | 1954–1957 | MKTC | 3 | 23 |
| SA5 | B165 | VR-952 | Cercopithecus aethiops | 1954–1957 | MKTC | 3 | 23 |
| SV2 | 2383 | VR-210 | Macaca mulatta | 1954 | MKTC | 2 | 12 |
| SV4 | 1715 UWB | VR-287 | Macaca mulatta | 1954 | MKTC | 3 | 12 |
| SV6 | 1631 | VR-944 | Macaca mulatta | 1955 | MKTC? | 4 | 12 |
| SV16 | 2450 SD | VR-211 | Macaca mulatta | 1955 | MKTC | 2 | 12 |
| SV18 | 2481 B2 | VR-212 | Macaca mulatta | c. 1958 | MKTC | 2 | 12 |
| SV19 | M19s (P2) | VR-213 | Macaca fascicularis | c. 1956 | RS | 2 | 9 |
| SV26 | 3163 | VR-290 | Macaca mulatta | c. 1957 | CNS | 4 | 11 |
| SV28 | 9128 | VR-291 | Macaca mulatta | c. 1957 | CNS | 3 | 11 |
| SV35 | A7987 | VR-292 | Macaca mulatta | c. 1957 | CNS | 4 | 11 |
| SV42 | M9 (P1) | VR-945 | Macaca fascicularis | c. 1956 | RS | 2 | 9 |
| SV43 | OM112t (P12) | VR-946 | Macaca fascicularis | c. 1956 | RS | 2 | 9 |
| SV44 | OM114s (P13) | VR-947 | Macaca mulatta | c. 1956 | RS | 2 | 9 |
| SV45 | M19h (P14) | VR-948 | Macaca fascicularis | c. 1964 | RS | NK | 8 |
| SV46 | OM22 (P15) | VR-949 | Macaca sp. | c. 1964 | NK | NK | 8 |
| SV49 | 2600 (P19) | VR-950 | Macaca mulatta | c. 1964 | RS | NK | 8 |
| N125 | N125 | NAe | Papio cynocephalus | c. 1972 | NK | NK | 32 |
| N203 | N203 | NA | Papio cynocephalus | c. 1972 | TS | NK | 32 |
Macaca mulatta, rhesus monkey; Macaca fascicularis, cynomolgus monkey; Cercopithecus aethiops, African green monkey (Vervet); Papio anubis (=doguera), baboon; Papio cynocephalus, baboon.
MKTC, monkey kidney tissue culture; RS, rectal swab; TS, throat swab.
According to the scheme of Hull et al. (12).
NK, not known.
NA, not available.
Molecular characterization.
Reverse transcription-PCR (RT-PCR), nucleotide sequencing, and sequence analysis were performed as described previously, using the primers listed in Table 2 (26). To determine whether the simian viruses are closely related to the human enteroviruses, all isolates were initially screened using diagnostic PCR primers that anneal at highly conserved sites in the 5′-NTR of all human enteroviruses and some rhinoviruses (38) (EV1 and EV2; Table 2). PCR amplification of a portion of VP1 was attempted for each strain by using primer pairs 012-011, 040-011, 187-222, 188-222, and 189-222 (Table 2) as described previously (25, 26). Together, primers 187, 188, 189, and 222 allow the amplification of all human enteroviruses and many nonhuman enteroviruses (25). Additional primers annealing to conserved sites in and surrounding VP1 were used for amplification and determination of complete VP1 sequences (Table 2). The sequences were compared to one another and to the VP1 sequences of other picornaviruses by using the program Gap (Wisconsin sequence analysis package, version 9.1, 1997; Genetics Computer Group, Madison, Wis.) as described previously (25, 26). The Ljungan virus VP1 sequence was generously provided prior to publication by Michael Lindberg, University of Kalmar, Kalmar, Sweden (personal communication). Other VP1 sequences were obtained from GenBank. Phylogenetic relationships were inferred using the programs DNADist and Neighbor (PHYLIP version 3.57 [PHYLIP: phylogeny inference package, version 3.5c, 1993; J. Felsenstein, University of Washington, Seattle]) and Puzzle (version 5.0 [35]). The maximum likelihood method of Kishino and Hasegawa (20), with a transition-transversion ratio of 8.0, was used to construct a distance matrix for neighbor-joining analysis. The statistical significance of phylogenies constructed using DNADist/Neighbor was estimated by bootstrap analysis with 1,000 pseudo-replicate data sets. Puzzle was executed using the distance method of Kishino and Hasegawa (20), with a transition-transversion ratio of 8.0, and the reliability of phylogenetic reconstructions was estimated by using 50,000 puzzling steps. Branch lengths of the neighbor-joining trees were calculated by the maximum likelihood method using Puzzle.
TABLE 2.
Primers used for PCR amplification or sequencing of simian picornaviruses
| Primera | Sequenceb | Gene | Positionc |
|---|---|---|---|
| EV2 | TCCGGCCCCTGAATGCGGCTAATCC | 5′ NTR | 446–470 |
| EV1 | ACACGGACACCCAAAGTAGTCGGTCC | 5′ NTR | 559–533 |
| 001 | NARITAYTAYRCICAITG | VP3 | 2077–2094 |
| 011 | GCICCIGAYTGITGICCRAA | 2A | 3408–3389 |
| 012 | ATGTAYGTICCICCIGGIGG | VP1 | 2951–2970 |
| 040 | ATGTAYRTICCIMCIGGIGC | VP1 | 2951–2970 |
| 050 | GTRCTYACIAIIAGRTCYCT | 2A | 3483–3464 |
| 055 | GGIACICAYRTIRTITGGGA | VP3 | 2186–2205 |
| 061 | GAITGYTGICCRAAYTTTCC | 2A | 3372–3356 |
| 110 | YTGYTCCATNGCYTCYTCRTC | 2A | 3802–3782 |
| 111 | YTGYTCCATNGCYTCYTCYTC | 2A | 3802–3782 |
| 112 | YTGYTCCATNGCRTCRTCYTC | 2A | 3802–3782 |
| 187 | ACIGCIGYIGARACIGGNCA | VP1 | 2612–2631 |
| 188 | ACIGCIGTIGARACIGGNG | VP1 | 2612–2630 |
| 189 | CARGCIGCIGARACIGGNGC | VP1 | 2612–2631 |
| 222 | CICCIGGIGGIAYRWACAT | VP1 | 2969–2951 |
| 224 | GCIATGYTIGGIACICAYRT | VP3 | 1977–1996 |
| 238 | CCIGGIWSIAAYCARTTIYTNAC | VP3 | 1787–1809 |
| 241 | ACIGCIGCIGARACIGGNGA | VP1 | 2612–2631 |
| 251 | GCCTAGCCTTTATCCTAG | 2A | (−) SV2 groupd |
| 254 | CTGTGCTTCAAAGTTGCTC | VP1 | (−) SV2 group |
| 281 | GGCAGCGGGAGAGAACAT | VP1 | 2987–2970 |
| 283 | TATCATCTTTCCACCACCA | 2A | (−) SV2 group |
| 302 | ACIATITGGTAYCARACNGC | VP3 | (+) SV2 group |
EV1 and EV2 are from reference 38; 011, 012, and 040 are from references 26 and 27; and 187, 188, 189, and 222 are from reference 25.
Sequences are shown 5′ to 3′, using standard IUB nucleotide ambiguity codes. I, deoxyinosine.
Nucleotide sequence coordinates are given relative to the sequence of the type strain of the Enterovirus genus, PV1-Mahoney (GenBank accession number J02281).
Specific primers derived from preliminary sequence of viruses in the SV2 group. The polarity of the primer is indicated as (+) or (−).
Nucleotide sequence accession numbers.
The sequences reported here were deposited in the GenBank sequence database, accession no. AF326750 to AF326766 and AF414372 to AF414373.
RESULTS
A previous molecular characterization of the simian picornaviruses suggested that some of the viruses may not belong in the Enterovirus genus, as PCR primers expected to amplify all enteroviruses did not amplify SA5, SV2, SV16, SV18, SV42, SV44, SV45, and SV49 (29). To confirm and extend their results, we tested all 20 simian picornavirus serotype prototype strains by using our own pan-enterovirus PCR primers, EV1 and EV2 (38). Primers EV1 and EV2 amplified the same viruses as those amplified by Pöyry et al. (29), except that EV1 and EV2 successfully amplified SA5 but failed to amplify SA4 (Table 3). All primer pairs we tested (Table 2), including the 5′-NTR primers used by Pöyry et al., failed to amplify SA4 (Table 3 and data not shown). As a result, we were unable to verify the presence of amplifiable RNA, even using two independently obtained vials of SA4, using both RNA extracted from passaged virus and RNA extracted directly from the material received from ATCC.
TABLE 3.
Summary of PCR, sequencing, and phylogenetic analyses
| Virus | PanEV PCR | VP1 primer setsa | VP1 length (nt) | Homologous strains | Genus-species |
|---|---|---|---|---|---|
| A13 | + | 224-222, 18X-11Xb | 852 | EV-A | |
| SA4 | − | ||||
| SA5 | + | 224-222, 187-254, 012-050 | 843 | EV-B | |
| SV2 | − | 224-222, 241-11X | 852 | Novel genus | |
| SV4 | + | 001-222, 18X-11X | 879 | SV28, A-2 plaque virus | EV-new species |
| SV6 | + | 224-222, 241-011 | 867 | EV-A? | |
| SV16 | − | 055-222, 241-283 | 879 | SV42, SV45 | Novel genus |
| SV18 | − | 224-222, 241-283 | 873 | SV44 | Novel genus |
| SV19 | + | 224-222, 18X-11X | 864 | SV26, SV35 | EV-A |
| SV26 | + | 224-222, 189-061 | 861 | SV19, SV35 | EV-A |
| SV28 | + | 224-222, 18X-11X | 879 | SV4, A-2 plaque virus | EV-new species |
| SV35 | + | 224-222, 189-061 | 858 | SV19, SV26 | EV-A |
| SV42 | − | 224-11X | 888 | SV16, SV45 | Novel genus |
| SV43 | + | 224-011 | 867 | EV-A | |
| SV44 | − | 055-222, 241-251 | 885 | SV18 | Novel genus |
| SV45 | − | 224-281, 241-283 | 879 | SV16, SV42 | Novel genus |
| SV46 | + | 238-240 | 876 | EV-A | |
| SV49 | − | 302-281, 241-283 | 876 | Novel genus | |
| N125 | + | 224-222, 18X-011 | 846 | EV-A? | |
| N203 | + | 224-222, 18X-011 | 846 | EV-A? |
Minimum primer set needed to amplify the complete VP1 gene. For most viruses, additional primer sets were also used for sequence determination.
18X, combination of 187, 188, and 189; 11X, combination of 110, 111, and 112.
Because enterovirus VP1 sequences have been shown to correlate with serotype and species identity (26, 27), VP1 was chosen for further genetic analyses. The amplification strategy used degenerate inosine-containing primers targeted to regions of VP3, VP1, and 2A that are highly conserved among the enteroviruses or among multiple picornavirus genera (Table 2 and Table 3). Table 3 lists the minimal primer sets needed to amplify the complete VP1 gene of each of the simian picornaviruses. For most viruses, additional primer sets were also used. Complete VP1 sequences were determined for the prototype strain of each of the recognized simian picornavirus serotypes except SA4, as noted above. The predicted VP1 proteins varied in length from 281 to 296 amino acids (843 to 888 nucleotides) based on comparisons with the VP1 cleavage sites of other picornaviruses.
The simian picornavirus VP1 nucleotide and deduced amino acid sequences were compared to one another as well as to other picornavirus VP1 sequences. Sequence comparisons used VP1 sequences of representatives of all picornavirus genera and included 68 enteroviruses, 12 aphthoviruses, 6 rhinoviruses, 4 cardioviruses, 2 parechoviruses, 2 hepatoviruses, 1 kobuvirus, 1 teschovirus, 1 erbovirus, and 2 unassigned picornaviruses (A-2 plaque virus and Ljungan virus). Enterovirus VP1 nucleotide and amino acid pairwise identity scores may be used to determine the species or serotype identity of a virus isolate (25–28). Clinical isolates of the same serotype are usually more than 75% identical to one another in VP1 nucleotide sequence (more than 85% amino acid sequence identity). Occasionally, viruses of the same serotype may be only 70 to 75% identical to one another, but they are always more closely related to viruses of the same serotype than to viruses of another serotype (26, 28). Viruses belonging to the same species (but different serotypes) are approximately 56 to 73% identical in nucleotide sequence (55 to 85% amino acid identity), whereas those belonging to different species (but the same genus) are about 44 to 58% identical in nucleotide sequence (34 to 55% amino acid identity). Viruses of different picornavirus genera are less than 45% identical to one another (less than 34% amino acid identity) (26, 27). The simian virus nucleotide sequences are less than 65% identical to those of any other picornavirus (less than 70% amino acid identity) in all comparisons, except to A-2 plaque virus (see below), confirming that they do not represent members of recognized serotypes (Table 4). The simian viruses cluster into two distinct groups on the basis of VP1 sequence identities (Table 4): one group related to members of the genus Enterovirus (SA5, SV4, SV28, SV6, A13, SV19, SV26, SV35, SV43, SV46, N125, and N203) and another group distinct from all other picornavirus genera (SV2, SV16, SV18, SV42, SV44, SV45, and SV49).
TABLE 4.
VP1 sequence relationships between simian picornaviruses and other picornaviruses
| Simian virusa | % Nucleotide identity between simian picornaviruses and other picornavirusese
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| HEV-A | HEV-B | HEV-C | HEV-D | BEVb | PEV9 | A-2c | HRVd | Other genera | |
| A13 | 59.2–52.8 | 46.4–52.5 | 45.7–52.3 | 52.4–54.0 | 52.1–54.6 | 52.3 | 48.2 | 48.9–51.0 | 35.0–42.7 |
| SA5 | 45.0–50.5 | 58.4–64.6 | 49.9–56.6 | 51.7–53.1 | 46.2–52.5 | 49.7 | 49.0 | 48.7–52.1 | 35.3–40.1 |
| SV2 | 41.0–46.4 | 40.1–46.3 | 40.5–48.0 | 43.9–45.0 | 43.6–45.8 | 45.6 | 42.7 | 40.7–46.4 | 33.8–42.2 |
| SV4 | 46.6–51.8 | 46.9–52.7 | 47.6–52.0 | 50.5–51.0 | 50.6–52.6 | 49.5 | 84.1 | 48.5–51.7 | 35.4–42.5 |
| SV6 | 52.0–55.9 | 45.8–52.8 | 45.6–52.5 | 47.5–49.6 | 51.0–53.5 | 51.6 | 47.8 | 48.1–50.5 | 34.4–41.1 |
| SV16 | 41.7–45.3 | 39.7–46.1 | 41.4–45.0 | 40.1–45.3 | 43.9–45.4 | 42.3 | 43.8 | 40.0–44.2 | 35.3–41.6 |
| SV18 | 39.7–46.2 | 40.3–45.3 | 39.4–44.4 | 43.5–46.1 | 42.9–44.9 | 44.2 | 44.1 | 41.7–45.7 | 33.8–41.7 |
| SV19 | 53.5–62.1 | 45.9–52.7 | 46.3–49.9 | 51.9–53.5 | 53.7–54.1 | 53.2 | 50.8 | 48.8–52.1 | 35.4–40.9 |
| SV26 | 57.8–62.3 | 46.5–53.8 | 46.9–50.9 | 52.5–54.8 | 53.8–55.0 | 51.6 | 52.0 | 48.1–52.4 | 35.3–43.3 |
| SV28 | 47.0–52.1 | 46.9–53.0 | 47.2–50.3 | 50.8–51.2 | 49.9–52.4 | 49.5 | 83.8 | 48.6–51.6 | 35.5–41.8 |
| SV35 | 55.8–62.2 | 45.7–52.7 | 46.4–51.3 | 52.8–56.2 | 53.2–54.9 | 51.7 | 52.1 | 48.9–52.9 | 34.8–44.7 |
| SV42 | 38.8–46.6 | 39.2–45.8 | 39.2–46.9 | 42.3–43.5 | 43.8–47.1 | 42.1 | 44.3 | 40.3–43.9 | 34.5–42.2 |
| SV43 | 58.9–63.1 | 46.0–51.0 | 46.9–51.5 | 50.4–52.6 | 53.5–54.0 | 51.6 | 50.6 | 46.0–51.0 | 35.0–41.3 |
| SV44 | 39.8–45.4 | 38.3–45.8 | 39.1–45.8 | 41.2–41.6 | 42.9–44.1 | 42.3 | 42.6 | 41.2–45.7 | 33.3–39.6 |
| SV45 | 39.4–46.3 | 38.6–46.2 | 39.4–44.8 | 44.6–44.8 | 42.1–44.7 | 44.0 | 44.1 | 40.8–42.8 | 35.1–42.9 |
| SV46 | 59.3–62.4 | 46.9–51.8 | 45.5–51.2 | 51.7–53.3 | 51.5–54.2 | 53.0 | 51.3 | 45.1–50.8 | 35.7–40.7 |
| SV49 | 39.0–46.2 | 39.8–47.0 | 38.2–44.1 | 44.5–45.2 | 40.5–43.8 | 41.8 | 44.7 | 42.8–46.2 | 36.1–41.7 |
| N125 | 50.7–56.9 | 44.8–49.1 | 45.3–51.7 | 49.9–51.8 | 48.8–53.2 | 48.4 | 48.6 | 47.2–50.9 | 33.3–40.3 |
| N203 | 51.7–55.0 | 43.7–49.9 | 47.2–51.6 | 52.4–54.2 | 50.4–50.9 | 49.1 | 51.6 | 46.4–52.2 | 33.8–42.0 |
VP1 sequence was not obtained for SA4.
BEV, bovine enteroviruses. Includes BEV1, BEV2a, and BEV2b.
A-2, A-2 plaque virus.
VP1 sequences are available only for HRV1B, HRV2, HRV3, HRV14, HRV16, and HRV89.
Comparisons of viruses of the same species are boldfaced, and comparisons of viruses of the same serotype are boldfaced and italicized.
For the simian viruses in the enterovirus genus, using the molecular classification scheme described above, A13, SV19, SV26, SV35, SV43, and SV46 should be assigned to HEV-A. All are 56 to 63% identical to members of species A and less than 57% identical to members of other species (Table 4). Only SV35 is not fully resolved from members of other species (55.8% identity to CA4 [HEV-A] and 56.2% identity to EV68 [HEV-D]), but its close relationship with SV19 and SV26 argues that it belongs with them in species A (Table 5). SA5 should be assigned to HEV-B (58 to 65% nucleotide [nt] identity with HEV-B viruses and less than 57% identity with members of other species) (Table 4). SV4 and SV28 are less than 53% identical to any other picornavirus other than A-2 plaque virus, indicating that SV4, SV28, and A-2 are the sole members of a new enterovirus species. SV6, N125, and N203 are 52.0 to 55.9% identical to HEV-A viruses and less than 53% identical to members of other species, suggesting that they probably represent a second new enterovirus species.
TABLE 5.
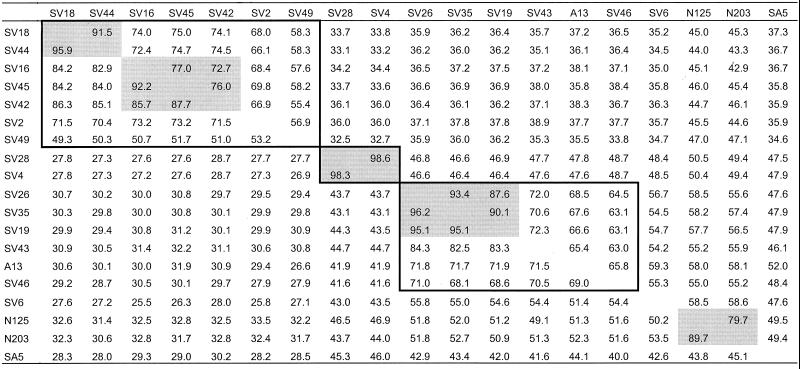
VP1 sequence relationships among simian picornavirusesa
Percent identity of the nucleotide sequences is above the diagonal, and percent identity of the amino acid sequence is below the diagonal. Comparisons of viruses of the same species are boxed, and viruses of the same serotype are shaded.
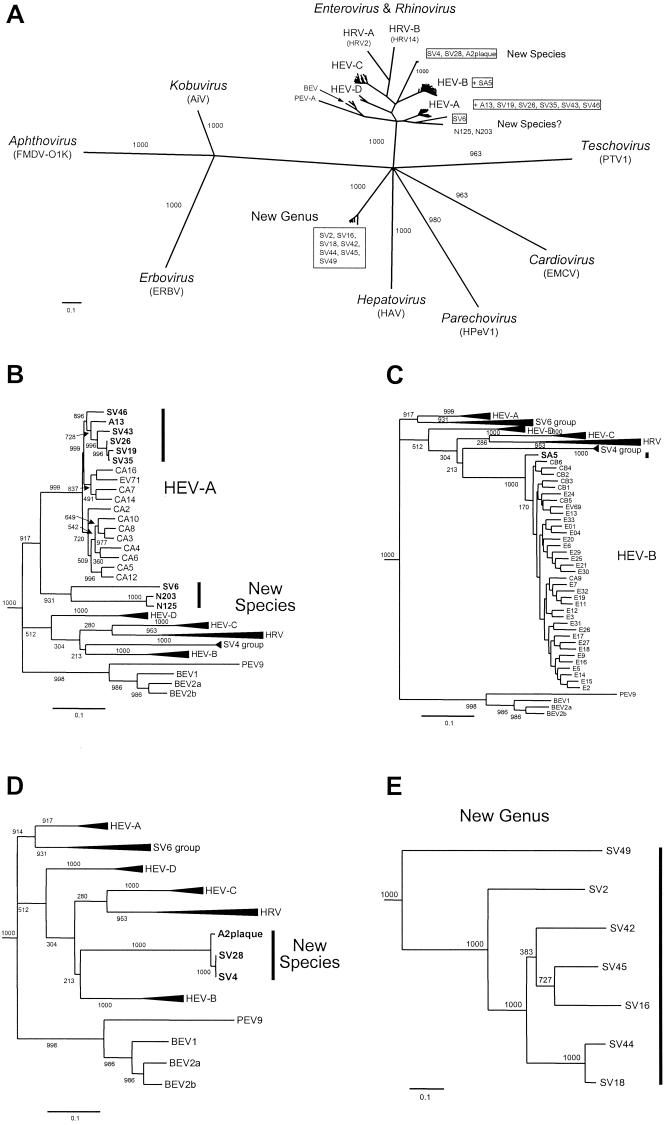
Phylogenetic relationships generally mirror those revealed by pairwise sequence comparisons, with high bootstrap support (Fig. 1). A13, SV19, SV26, SV35, SV43, and SV46 cluster together within HEV-A (Fig. 1A and B). SV6, N125, and N203 clustered together and are loosely related to HEV-A (Fig. 1A and B), but they are less than 56% identical to any HEV-A viruses (Table 4). SA5 clusters with the echoviruses and coxsackie B viruses in HEV-B (Fig. 1A and C). SV4, SV28, and A-2 plaque virus form a unique cluster in the Enterovirus genus (Fig. 1A and D), consistent with their pairwise identities with other enteroviruses.
FIG. 1.
Phylogram depicting the relationships of picornavirus VP1 sequences. The numbers indicate bootstrap values, out of 1,000, for the node to the right. (A) Radial neighbor-joining tree, with at least one virus from each of the six recognized and three proposed picornavirus genera (genus names in italics). For enteroviruses and rhinoviruses, the species names are also indicated. The names of the simian viruses are boxed. (B to E) Portions of the tree in panel A, redrawn as rectangular phylograms. Simian picornaviruses are indicated in bold-face type and by vertical bars to the right of the virus names. (B to D) Emphasis is on specific subbranches within the Enterovirus/Rhinovirus branch, which are drawn to the same scale. Other subbranches are collapsed for clarity (indicated by triangles at the branch tips). The length of the triangle is proportional to the genetic diversity within the collapsed subbranch.
The remaining simian viruses that are distinct from the enterovirus genus (SV2, SV16, SV18, SV42, SV44, SV45, and SV49) are monophyletic, forming a single cluster that is distinct from any other picornavirus genera, with 100% bootstrap support (Fig. 1A and E). Within this group, SV49 is distinct from the other viruses (100% bootstrap support), with the next-distal node branching to SV2 (100% bootstrap support). The remaining viruses are monophyletic (100% bootstrap support) and form three groups: (i) SV16 and SV45, (ii) SV42, and (iii) SV18 and SV44. SV18 and SV44 cluster tightly together (100% bootstrap support), consistent with their 91.5% sequence identity, but the topology of the SV16/SV45 and SV42 branches is not well supported (Fig. 1E).
Several simian virus VP1 sequences are greater than 75% identical to one another (greater than 85% amino acid identity), suggesting that the true number of distinct serotypes among the viruses surveyed is less than 20 (Tables 3, 4, and 5). The SV4 and SV28 VP1 sequences are 98.6% identical to one another (98.3% amino acid identity) (Table 5), supporting previous antigenic comparisons that identified these two viruses as variants of a single serotype (16). SV4 and SV28 are also 84.1 and 83.8% identical to A-2 plaque virus (95.9 and 95.6% amino acid identity), respectively, indicating that A-2 plaque virus is also a member of this same serotype (Table 4). N125 and N203 are 79.7% identical to one another (89.7% amino acid identity); SV19, SV26, and SV35 sequences are 87.6 to 93.4% identical to one another (95.1 to 96.2% amino acid identity); SV16, SV42, and SV45 sequences are 72.7 to 77.0% identical to one another (85.7 to 92.2% amino acid identity); and SV18 and SV44 sequences are 91.5% identical to one another (95.9% amino acid identity). The relationships among SV16, SV18, SV42, SV44, and SV45 could not be fully resolved, as they are all at least 72.4% identical to one another (84.0% amino acid identity) (Table 5).
DISCUSSION
During their initial characterization in the 1950s and 1960s, viruses of simian origin were categorized by physical properties, such as virion size, resistance to heat, plaque size, and morphology (8, 10, 12), and by the type of cytopathic effect (CPE) in tissue cultures (11). At the time, few human viruses had been well characterized, so this scheme was used to classify new agents as, for example, “adenovirus-like” or “enterovirus-like.” New viruses were also characterized antigenically by neutralization, complement fixation, hemagglutination, and hemagglutination-inhibition tests. SA4, SV4, and SV28 (CPE group 3) were shown by cross-neutralization to be antigenically related to one another (8, 11, 23). Similarly, an antigenic relationship was demonstrated among SV2, SV16, SV18, SV42, SV45, and SV49, all of which belonged to CPE group 2 (8, 11). Our VP1 sequence comparisons agree with the antigenic relationships among these viruses and confirm that SV4 and SV28 are strains of a single serotype. Although we were unable to analyze the SA4 prototype strain, a previously published analysis indicated that SA4 is closely related to SV4 and SV28 (29). The partial 5′-NTR sequences of SV4 and SV28 were virtually identical to one another (29), suggesting that the two strains diverged from one another very recently. A-2 plaque virus was isolated from the icteric phase serum of a hepatitis patient whose serum was also positive for hepatitis B antigen (34). The virus was originally isolated in human embryonic kidney culture and passaged in primary African green monkey kidney cells. Its close relationship to SV4 and SV28 raises the possibility that it was present in the original uninfected monkey cell cultures or that it was introduced during subsequent passages. Alternatively, it may represent the rare infection of a human by a simian enterovirus. Based on VP1 sequence analysis, SV19, SV26, and SV35 also appear to be strains of a single serotype, as do SV16, SV42, and SV45, as well as SV18 and SV44, thus reducing the number of distinct simian picornavirus serotypes from 20 to 13. SV16, SV18, SV42, SV44, and SV45 are all closely related to one another (Table 5), but additional studies are needed to determine whether they represent intratypic variants of a single serotype or a family of closely related serotypes.
Currently, picornavirus species are named according to the host with which they are most closely associated, e.g., Human enterovirus A, Bovine enterovirus, Porcine enterovirus A, etc. (19). The VP1 sequences of A13, SV19, SV26, SV35, SV43, and SV46 clearly places them within Human enterovirus A (Table 4 and Fig. 1), suggesting that the current naming convention may not be adequate to classify all enteroviruses.
The genetic relationships of the simian picornaviruses to the human enteroviruses were recently addressed by molecular methods (29). In those studies, 10 simian virus serotypes were amplified using primers specific for the 5′-NTR (A13, SA4, SV4, SV6, SV19, SV26, SV28, SV35, SV43, and SV46) and seven serotypes were amplified using 3D-specific primers (A13, SV6, SV18, SV26, SV35, SV43, and SV46). Seven serotypes (SA5, SV2, SV16, SV42, SV44, SV45, and SV49) were not amplified by either primer pair (29). With the exception of SA5, all of the serotypes that failed to amplify with both 5′-NTR and 3D primer pairs in that study are not closely related to the human enteroviruses on the basis of our VP1 sequence analysis. Our own enterovirus-specific 5′NTR primers that amplify all human enteroviruses (38) successfully amplified all of the simian picornaviruses that were enteroviruses by VP1 type (SA5, SV4, SV6, SV19, SV26, SV28, SV35, SV43, and SV46) but failed to amplify those viruses whose VP1 sequences distinguished them from the enteroviruses (Tables 3 and 4). SA4 is also likely to be a member of the Enterovirus genus, as described above. SV2, SV16, SV18, SV42, SV44, SV45, and SV49 appear to represent a new picornavirus genus, as phylogenetic comparisons showed that they were clearly distinct from members of the Enterovirus genus and from other existing picornavirus genera (Fig. 1). Although these data are consistent with previous reports, final resolution of their taxonomic status must await further detailed molecular characterization, and until then, they should be regarded as unclassified picornaviruses. These additional studies are in progress.
Comparison of our VP1 phylogeny (Fig. 1) with published simian picornavirus phylogenies based on partial 5′-NTR and partial 3D sequences (29) revealed that the three phylogenetic trees are noncongruent, suggesting that recombination has played a role in the evolution of some of the simian picornaviruses. For example, in the VP1 tree (Fig. 1), SV19, SV26, SV35, SV43, SV46, and A13 formed a single genetic cluster within HEV-A, whereas A13 was distinct from all other enteroviruses in the 3D tree (29). The A13 5′-NTR sequence was also distinct from, but related to, enterovirus 5′-NTR group I (corresponding to HEV-C and -D), while SV19, SV26, SV35, SV43, and SV46 clustered together and were related to enterovirus 5′-NTR group II (corresponding to HEV-A and B) (29). The SV18 partial 3D sequence was distinct from all enterovirus sequences, in agreement with our VP1 results (5′-NTR sequence was not obtained for SV18). Unfortunately, the simian picornavirus 5′-NTR and 3D sequences were not compared with those of genera other than Enterovirus (29). We were not able to make those comparisons ourselves because the sequences were not available in a public sequence database.
While it may be uncommon, picornaviruses appear to be capable of occasionally infecting a species other than the natural host(s). Antigenic and molecular comparisons have suggested that swine vesicular disease virus emerged in the past 50 years through infection of swine with the human pathogen coxsackievirus B5, followed by subsequent adaptation and evolution of the virus in the new host (1, 7, 40, 41). Serologic studies have suggested that the simian picornaviruses may infrequently infect humans, particularly those with natural or occupational exposure to wild primates (17). There is no evidence that simian picornaviruses are capable of causing disease in humans, but a number of other primate viruses, including herpesvirus B and monkeypox virus, are closely related to human pathogens and have the potential to directly cause serious disease in humans (6). The increasing encroachment of human activity on wild primate habitats may increase the risk of virus infection in species other than the natural host. Enteroviruses 70 and 71 (EV70 and EV71), agents of acute hemorrhagic conjunctivitis and hand, foot, and mouth disease, respectively, appear to have emerged as human pathogens relatively recently, but their origins remain unknown. EV70 emerged in a region of the world that is inhabited by wild primate populations, but none of the simian enteroviruses were members of HEV-D, the group that includes EV70. Likewise, none of the simian viruses were closely related to EV71, but many clearly belong to HEV-A, of which EV71 is also a member. Further studies are needed to study the natural enteroviral flora of wild primates, as very few species have been sampled to date, and to determine the potential for primate enteroviruses to infect and cause disease in humans. The availability of the relatively generic molecular reagents described here should greatly accelerate the identification and characterization of novel picornaviruses associated with human and animal disease.
Acknowledgments
We are grateful to Seymour Kalter and Richard Heberling for generously supplying simian picornaviruses N125 and N203 and to Nick Knowles for stimulating discussions.
REFERENCES
- 1.Brown, F., P. Talbot, and R. Burrows. 1973. Antigenic differences between isolates of swine vesicular disease virus and their relationship to Coxsackie B5 virus. Nature 245:315–316. [DOI] [PubMed] [Google Scholar]
- 2.Caro, V., S. Guillot, F. Delpeyroux, and R. Crainic. 2001. Molecular strategy for “serotyping” of human enteroviruses. J. Gen. Virol. 82:79–91. [DOI] [PubMed] [Google Scholar]
- 3.Casas, I., G. F. Palacios, G. Trallero, D. Cisterna, M. C. Freire, and A. Tenorio. 2001. Molecular characterization of human enteroviruses in clinical samples: comparison between VP2, VP1, and RNA polymerase regions using RT nested PCR assays and direct sequencing of products. J. Med. Virol. 65:138–148. [PubMed] [Google Scholar]
- 4.Doherty, M., D. Todd, N. McFerran, and E. M. Hoey. 1999. Sequence analysis of a porcine enterovirus serotype 1 isolate: relationships with other picornaviruses. J. Gen. Virol. 80:1929–1941. [DOI] [PubMed] [Google Scholar]
- 5.Fuentes-Marins, R., A. R. Rodriguez, S. S. Kalter, A. Hellman, and R. A. Crandell. 1963. Isolation of enteroviruses from the “normal” baboon (Papio doguera). J. Bacteriol. 85:1045–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gorbach, S. L., J. G. Bartlett, and N. R. Blacklow (ed.). 1992. Infectious diseases, 2nd ed. W. B. Saunders Company, Philadelphia, Pa.
- 7.Graves, J. H. 1973. Serological relationship of swine vesicular disease virus and Coxsackie B5 virus. Nature 245:314–315. [DOI] [PubMed] [Google Scholar]
- 8.Heberling, R. L., and F. S. Cheever. 1965. Some characteristics of the simian enteroviruses. Am. J. Epidemiol. 81:106–123. [DOI] [PubMed] [Google Scholar]
- 9.Hoffert, W. R., M. E. Bates, and F. S. Cheever. 1958. Study of enteric viruses of simian origin. Am. J. Hyg. 68:15–30. [DOI] [PubMed] [Google Scholar]
- 10.Hsiung, G. D., and J. L. Melnick. 1958. Orphan viruses of man and animals. Ann. N. Y. Acad. Sci. 70:342–361. [DOI] [PubMed] [Google Scholar]
- 11.Hull, R. M., J. R. Minner, and C. C. Mascoli. 1958. New viral agents recovered from tissue cultures of monkey kidney cells. III. Recovery of additional agents both from cultures of monkey tissues and directly from tissues and excreta. Am. J. Hyg. 68:31–44. [DOI] [PubMed] [Google Scholar]
- 12.Hull, R. N., J. R. Minner, and J. W. Smith. 1956. New viral agents recovered from tissue cultures of monkey kidney cells. I. Origin and properties of cytopathogenic agents S.V.1, S.V.2, S.V.4, S.V.5, S.V.6, S.V.11, S.V.12, and S.V.15. Am. J. Hyg. 63:204–215. [DOI] [PubMed] [Google Scholar]
- 13.Hyypiä, T., C. Horsnell, M. Maaronen, M. Khan, N. Kalkkinen, P. Auvinen, L. Kinnunen, and G. Stanway. 1992. A distinct picornavirus group identified by sequence analysis. Proc. Natl. Acad. Sci. USA 89:8847–8851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hyypiä, T., T. Hovi, N. J. Knowles, and G. Stanway. 1997. Classification of enteroviruses based on molecular and biological properties. J. Gen. Virol. 78:1–11. [DOI] [PubMed] [Google Scholar]
- 15.Kaku, Y., A. Sarai, and Y. Murakami. 2001. Genetic reclassification of porcine enteroviruses. J. Gen. Virol. 82:417–424. [DOI] [PubMed] [Google Scholar]
- 16.Kalter, S. S. 1982. Enteric viruses of nonhuman primates. Vet. Pathol. 19:33–43. [PubMed] [Google Scholar]
- 17.Kalter, S. S., and R. L. Heberling. 1971. Comparative virology of primates. Bacteriol. Rev. 35:310–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalter, S. S., C. S. Kim, and E. A. Sueltenfuss. 1967. Further characterization of agents isolated from normal baboon (Papio sp.). J. Infect. Dis. 117:301–306. [DOI] [PubMed] [Google Scholar]
- 19.King, A. M. Q., F. Brown, P. Christian, T. Hovi, T. Hyypiä, N. J. Knowles, S. M. Lemon, P. D. Minor, A. C. Palmenberg, T. Skern, and G. Stanway. 2000. Picornaviridae, p.657–678. In M. H. V. Van Regenmortel, C. M. Fauquet, D. H. L. Bishop, C. H. Calisher, E. B. Carsten, M. K. Estes, S. M. Lemon, J. Maniloff, M. A. Mayo, D. J. McGeoch, C. R. Pringle, and R. B. Wickner (ed.), Virus taxonomy. Seventh report of the International Committee on Taxonomy of Viruses. Academic Press, San Diego, Calif.
- 20.Kishino, H., and M. Hasegawa. 1989. Evaluation of the maximum likelihood estimate of the evolutionary tree topologies from DNA sequence data, and the branching order in Hominoidea. J. Mol. Evol. 29:170–179. [DOI] [PubMed] [Google Scholar]
- 21.Kopecka, H., B. Brown, and M. A. Pallansch. 1995. Genotypic variation in coxsackievirus B5 isolates from three different outbreaks in the United States. Virus Res. 38:125–136. [DOI] [PubMed] [Google Scholar]
- 22.Liu, H.-M., D.-P. Zheng, L.-B. Zhang, M. S. Oberste, M. A. Pallansch, and O. M. Kew. 2000. Molecular evolution of a type 1 wild-vaccine poliovirus recombinant during widespread circulation in China. J. Virol. 74:11153–11161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malherbe, H., and R. Harwin. 1963. The cytopathic effects of vervet monkey viruses. South Africa Med. J. 37:407–411. [PubMed] [Google Scholar]
- 24.Norder, H., L. Bjerregaard, and L. O. Magnius. 2001. Homotypic echoviruses share aminoterminal VP1 sequence homology applicable for typing. J. Med. Virol. 63:35–44. [PubMed] [Google Scholar]
- 25.Oberste, M. S., K. Maher, M. R. Flemister, G. Marchetti, D. R. Kilpatrick, and M. A. Pallansch. 2000. Comparison of classic and molecular approaches for the identification of “untypeable” enteroviruses. J. Clin. Microbiol. 38:1170–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oberste, M. S., K. Maher, D. R. Kilpatrick, M. R. Flemister, B. A. Brown, and M. A. Pallansch. 1999. Typing of human enteroviruses by partial sequencing of VP1. J. Clin. Microbiol. 37:1288–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oberste, M. S., K. Maher, D. R. Kilpatrick, and M. A. Pallansch. 1999. Molecular evolution of the human enteroviruses: correlation of serotype with VP1 sequence and application to picornavirus classification. J. Virol. 73:1941–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oberste, M. S., D. Schnurr, K. Maher, S. al-Busaidy, and M. A. Pallansch. 2001. Molecular identification of new picornaviruses and characterization of a proposed enterovirus 73 serotype. J. Gen. Virol. 82:409–416. [DOI] [PubMed] [Google Scholar]
- 29.Pöyry, T., L. Kinnunen, T. Hovi, and T. Hyypia. 1999. Relationships between simian and human enteroviruses. J. Gen. Virol. 80:635–638. [DOI] [PubMed] [Google Scholar]
- 30.Pöyry, T., L. Kinnunen, T. Hyypia, B. Brown, C. Horsnell, T. Hovi, and G. Stanway. 1996. Genetic and phylogenetic clustering of enteroviruses. J. Gen. Virol. 77:1699–1717. [DOI] [PubMed] [Google Scholar]
- 31.Pringle, C. R. 1999. Virus taxonomy at the XIth International Congress of Virology, Sydney, Australia, 1999. Arch. Virol. 144:2065–2070. [DOI] [PubMed] [Google Scholar]
- 32.Rodriguez, A. R., S. S. Kalter, R. L. Heberling, R. J. Helmke, and J. E. Guajardo. 1977. Viral infections of the captive Kenya baboon (Papio cynocephalus): a five-year epidemiologic study of an outdoor colony. Lab. Anim. Sci. 27:356–371. [PubMed] [Google Scholar]
- 33.Santti, J., T. Hyypiä, L. Kinnunen, and M. Salminen. 1999. Evidence of recombination among enteroviruses. J. Virol. 73:8741–8749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shaw, E. D., A. P. McKee, M. Rancourt, and L. Hollenbeck. 1973. Induction of hepatitis B antibody in experimental animals by immunization with A-2 plaque virus. J. Virol. 12:1598–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Strimmer, K., and A. von Haeseler. 1996. Quartet puzzling: a quartet maximum likelihood method for reconstructing tree topologies. Mol. Biol. Evol. 13:964–969. [Google Scholar]
- 36.Wutz, G., H. Auer, N. Nowotny, T. Skern, and, K. E. 1996. Equine Rhinovirus serotypes 1 and 2: relationship to each other and to aphthoviruses and cardioviruses. J. Gen. Virol. 77:1719–1730. [DOI] [PubMed] [Google Scholar]
- 37.Yamashita, T., K. Sakae, H. Tsuzuki, Y. Suzuki, N. Ishikawa, N. Takeda, T. Miyamura, and S. Yamazaki. 1998. Complete nucleotide sequence and genetic organization of Aichi virus, a distinct member of the Picornaviridae associated with acute gastroenteritis in humans. J. Virol. 72:8408–8412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang, C.-F., L. De, S.-J. Yang, J. Ruiz Gómez, J. Ramiro Cruz, B. P. Holloway, M. A. Pallansch, and O. M. Kew. 1992. Genotype-specific in vitro amplification of sequences of the wild type 3 polioviruses from Mexico and Guatemala. Virus Res. 24:277–296. [DOI] [PubMed] [Google Scholar]
- 39.Zell, R., M. Dauber, A. Krumbholz, A. Henke, E. Birch-Hirschfeld, A. Stelzner, D. Prager, and R. Wurm. 2001. Porcine teschoviruses comprise at least eleven distinct serotypes: molecular and evolutionary aspects. J. Virol. 75:1620–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang, G., D. T. Haydon, N. J. Knowles, and J. W. McCauley. 1999. Molecular evolution of swine vesicular disease virus. J. Gen. Virol. 80:639–651. [DOI] [PubMed] [Google Scholar]
- 41.Zhang, G., G. Wilsden, N. J. Knowles, and J. W. McCauley. 1993. Complete nucleotide sequence of a coxsackie B5 virus and its relationship to swine vesicular disease virus. J. Gen. Virol. 74:845–853. [DOI] [PubMed] [Google Scholar]