Abstract
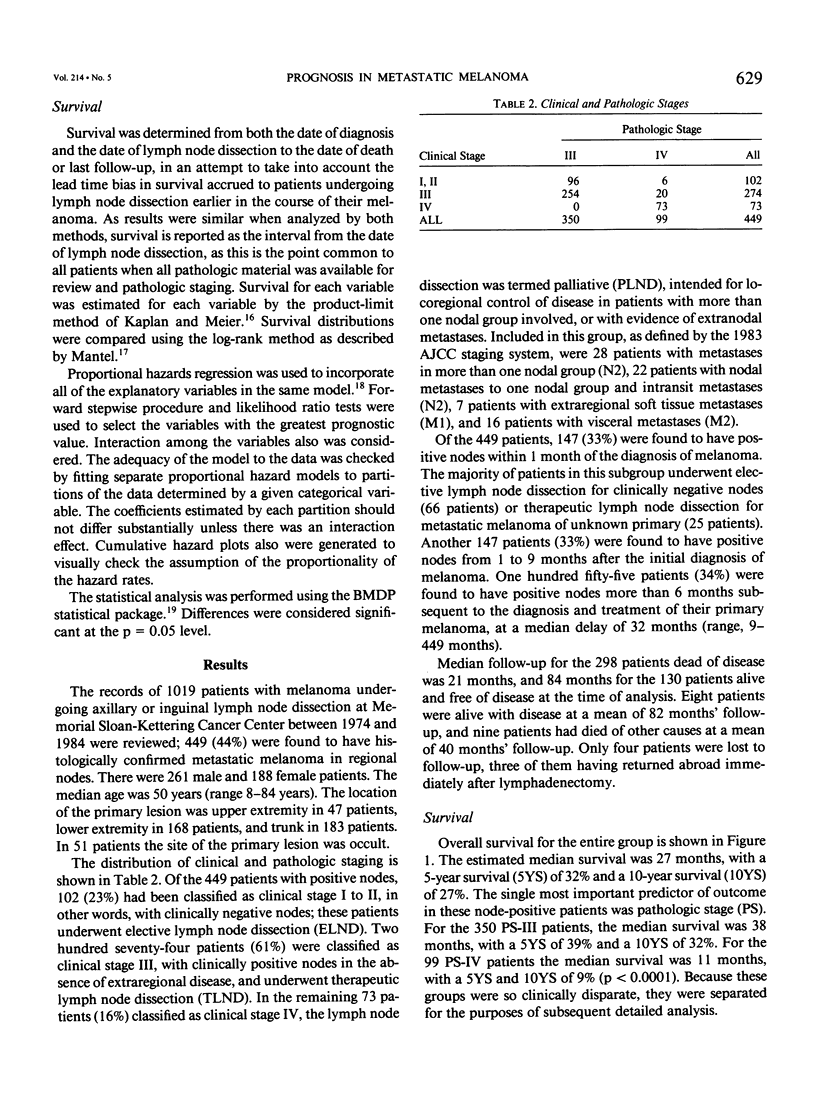
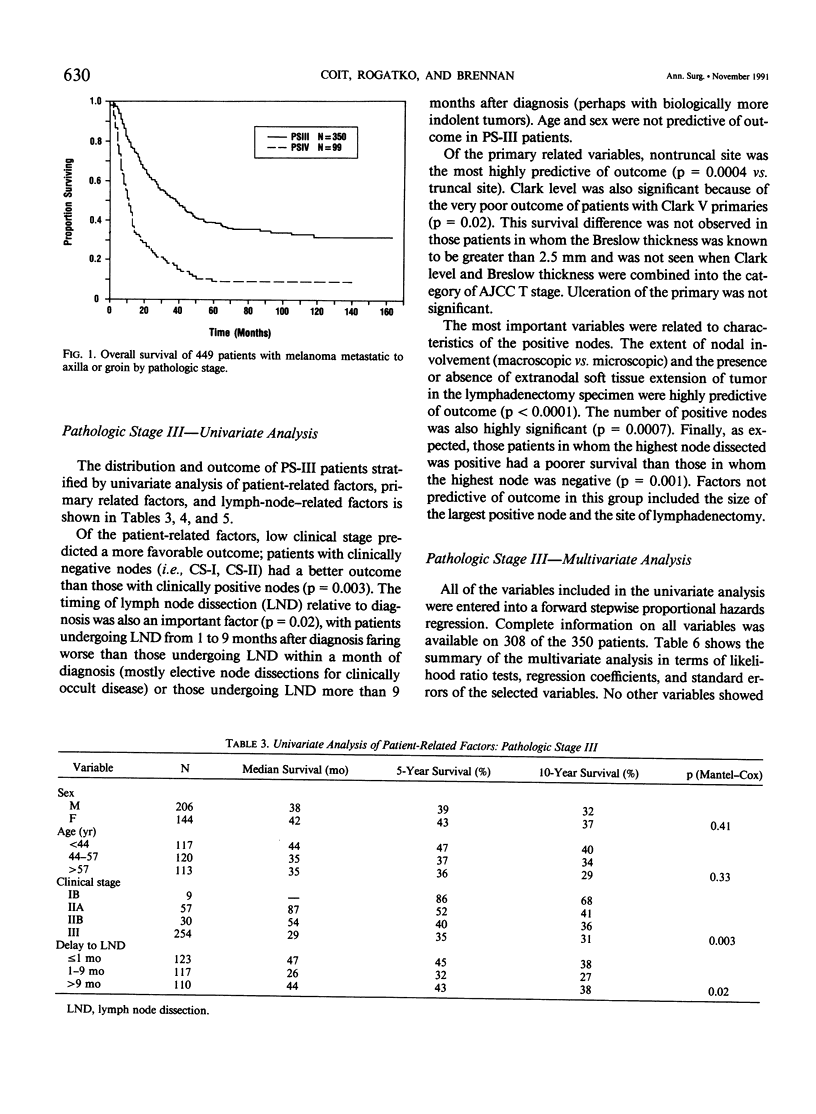
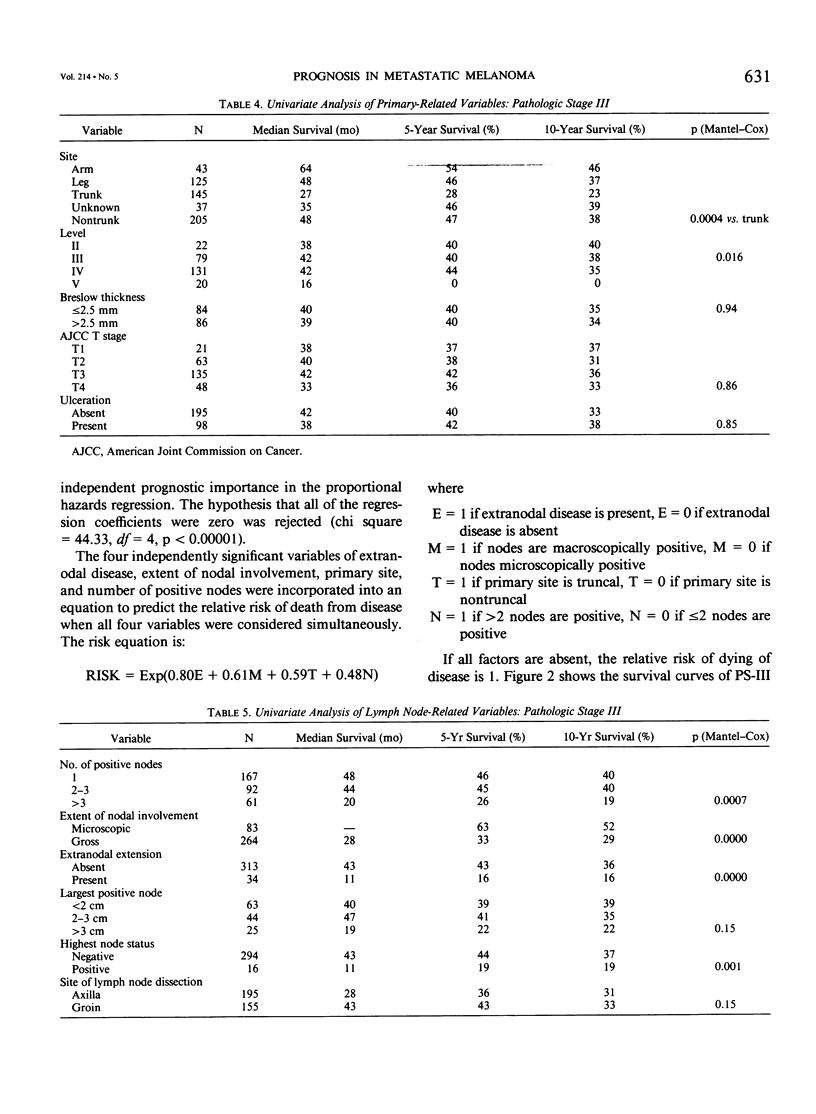
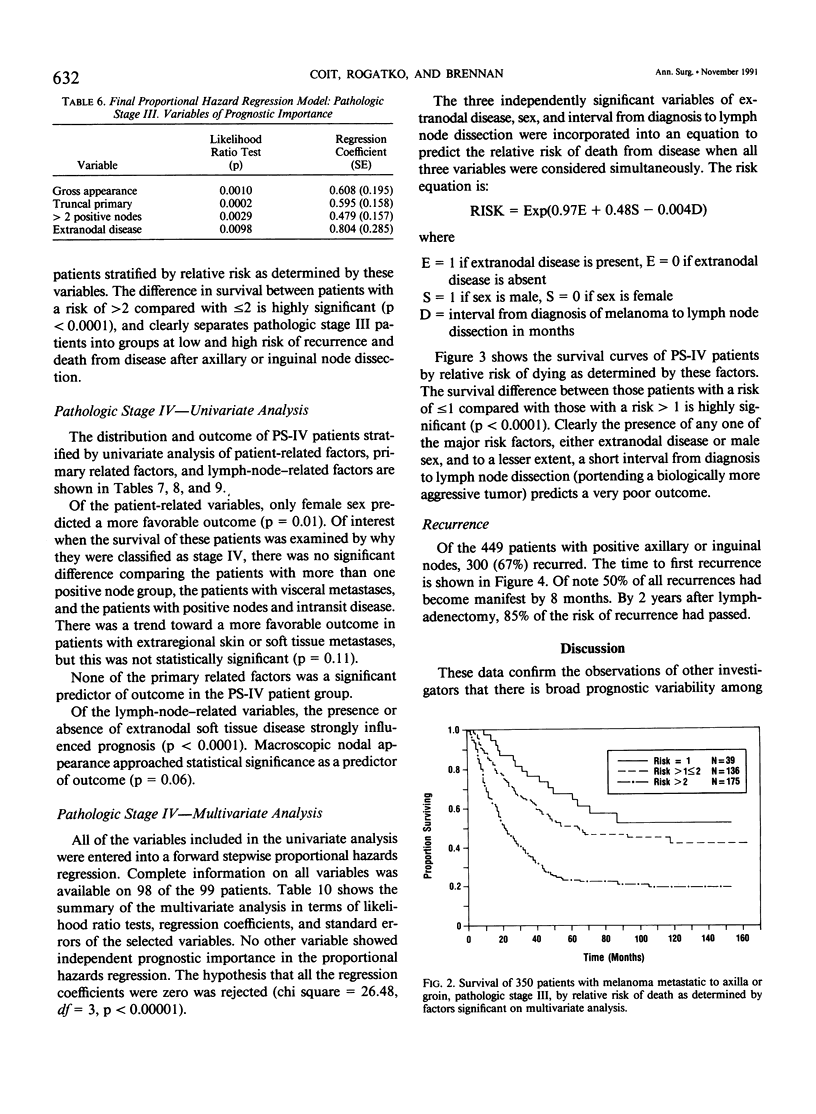
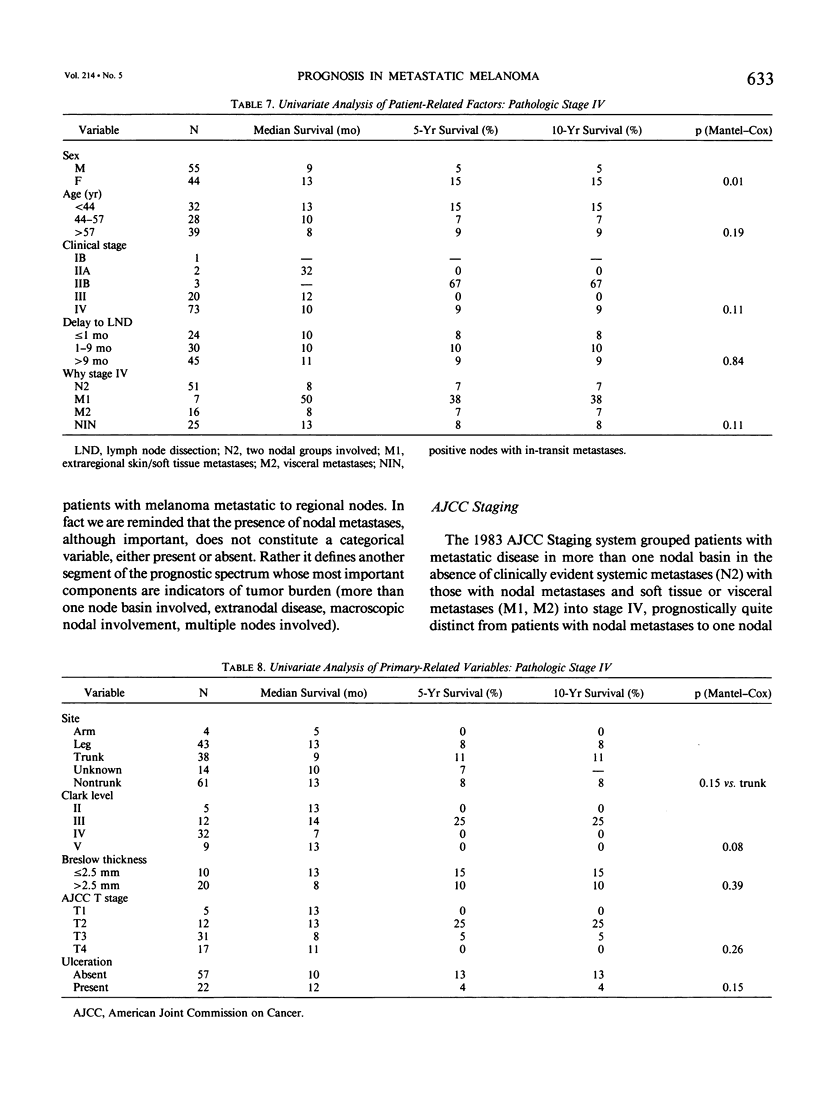
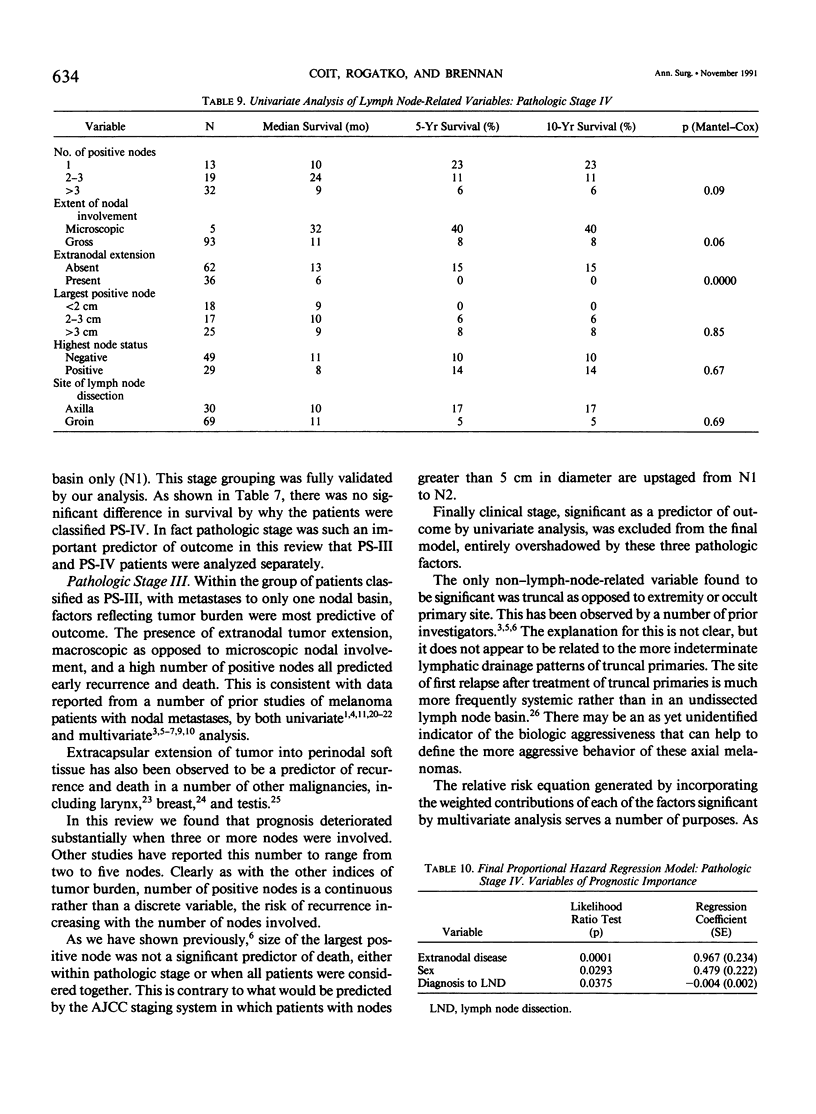
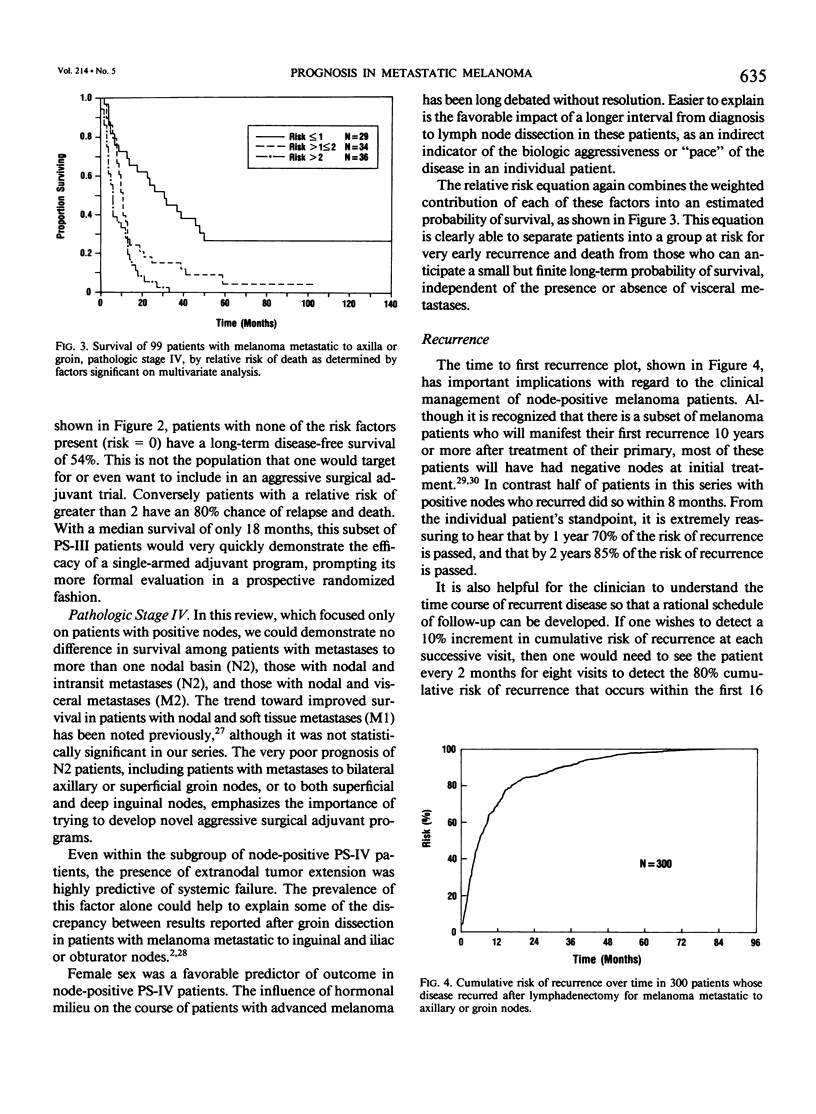
Although pathologic nodal status is a major determinant of outcome in melanoma, there is substantial prognostic heterogeneity among node-positive patients. This study was undertaken to further clarify significant variables predicting survival in patients with melanoma metastatic to axillary or groin nodes. From 1019 patients with melanoma undergoing axillary or groin dissection between 1974 and 1984, the authors identified 449 patients with histologically positive nodes. Both univariate and multivariate analyses were performed using the Kaplan-Meier product limit method and the Cox model of proportional hazard regression. The major determinant of survival was pathologic stage (PS) according to the 1983 AJCC staging system. Three hundred fifty patients (78%) were classified PS-III (one nodal group involved), with a survival of 39% at 5 years and 32% at 10 years. Factors independently predictive of a favorable outcome in these patients were nontruncal primary site (p = 0.0002), microscopic nodal involvement (p = 0.001), number of positive nodes less than three (p = 0.003), and absence of extranodal disease (p = 0.01). Ninety-nine patients (22%) were classified PS-IV, 51 with two nodal stations involved (N2), 25 with intransit disease and one nodal station involved (N2), 7 with extraregional soft tissue metastases (M1), and 16 with visceral metastases (M2). Survival for PS-IV patients was 9% at 5 and 10 years, respectively. Within PS-IV, factors independently predictive of a more favorable outcome were the absence of extranodal disease (p = 0.0001), female sex (p = 0.03), and a long interval from diagnosis to lymph node dissection (p = 0.04). These factors were incorporated into a model predicting relative risk of death from disease for both PS-III and PS-IV patients, separating patients into groups at high, intermediate, and low risk of recurrence after lymphadenectomy.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balch C. M., Soong S. J., Milton G. W., Shaw H. M., McGovern V. J., Murad T. M., McCarthy W. H., Maddox W. A. A comparison of prognostic factors and surgical results in 1,786 patients with localized (stage I) melanoma treated in Alabama, USA, and New South Wales, Australia. Ann Surg. 1982 Dec;196(6):677–684. doi: 10.1097/00000658-198212001-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balch C. M., Soong S. J., Murad T. M., Ingalls A. L., Maddox W. A. A multifactorial analysis of melanoma: III. Prognostic factors in melanoma patients with lymph node metastases (stage II). Ann Surg. 1981 Mar;193(3):377–388. doi: 10.1097/00000658-198103000-00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua R. G., Coit D. G., Rogatko A., Younes R. N., Brennan M. F. Axillary dissection in melanoma. Prognostic variables in node-positive patients. Ann Surg. 1990 Aug;212(2):125–131. doi: 10.1097/00000658-199008000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabro A., Singletary S. E., Balch C. M. Patterns of relapse in 1001 consecutive patients with melanoma nodal metastases. Arch Surg. 1989 Sep;124(9):1051–1055. doi: 10.1001/archsurg.1989.01410090061014. [DOI] [PubMed] [Google Scholar]
- Callery C., Cochran A. J., Roe D. J., Rees W., Nathanson S. D., Benedetti J. K., Elashoff R. M., Morton D. L. Factors prognostic for survival in patients with malignant melanoma spread to the regional lymph nodes. Ann Surg. 1982 Jul;196(1):69–75. doi: 10.1097/00000658-198207000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascinelli N., Vaglini M., Nava M., Santinami M., Marolda R., Rovini D., Clemente C., Bufalino R., Morabito A. Prognosis of skin melanoma with regional node metastases (stage II). J Surg Oncol. 1984 Apr;25(4):240–247. doi: 10.1002/jso.2930250404. [DOI] [PubMed] [Google Scholar]
- Cohen M. H., Ketcham A. S., Felix E. L., Li S. H., Tomaszewski M. M., Costa J., Rabson A. S., Simon R. M., Rosenberg S. A. Prognostic factors in patients undergoing lymphadenectomy for malignant melanoma. Ann Surg. 1977 Nov;186(5):635–642. doi: 10.1097/00000658-197711000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coit D. G., Brennan M. F. Extent of lymph node dissection in melanoma of the trunk or lower extremity. Arch Surg. 1989 Feb;124(2):162–166. doi: 10.1001/archsurg.1989.01410020032004. [DOI] [PubMed] [Google Scholar]
- Coit D., Sauven P., Brennan M. Prognosis of thick cutaneous melanoma of the trunk and extremity. Arch Surg. 1990 Mar;125(3):322–326. doi: 10.1001/archsurg.1990.01410150044009. [DOI] [PubMed] [Google Scholar]
- Crowley N. J., Seigler H. F. Late recurrence of malignant melanoma. Analysis of 168 patients. Ann Surg. 1990 Aug;212(2):173–177. doi: 10.1097/00000658-199008000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day C. L., Jr, Sober A. J., Lew R. A., Mihm M. C., Jr, Fitzpatrick T. B., Kopf A. W., Harris M. N., Gumport S. L., Raker J. W., Malt R. A. Malignant melanoma patients with positive nodes and relatively good prognoses: microstaging retains prognostic significance in clinical stage I melanoma patients with metastases to regional nodes. Cancer. 1981 Mar 1;47(5):955–962. doi: 10.1002/1097-0142(19810301)47:5<955::aid-cncr2820470523>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Fisher E. R., Gregorio R. M., Fisher B., Redmond C., Vellios F., Sommers S. C. The pathology of invasive breast cancer. A syllabus derived from findings of the National Surgical Adjuvant Breast Project (protocol no. 4). Cancer. 1975 Jul;36(1):1–85. doi: 10.1002/1097-0142(197507)36:1<1::aid-cncr2820360102>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Fortner J. G., Woodruff J., Schottenfeld D., Maclean B. Biostatistical basis of elective node dissection for malignant melanoma. Ann Surg. 1977 Jul;186(1):101–103. doi: 10.1097/00000658-197707000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes E. C., Clark W., Morton D. L., Eilber F. R., Bochow A. J. Regional lymph node metastases and the level of invasion of primary melanoma. Cancer. 1976 Jan;37(1):199–201. doi: 10.1002/1097-0142(197601)37:1<199::aid-cncr2820370128>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Jonk A., Kroon B. B., Rümke P., van der Esch E. P., Hart A. A. Results of radical dissection of the groin in patients with stage II melanoma and histologically proved metastases of the iliac or obturator lymph nodes, or both. Surg Gynecol Obstet. 1988 Jul;167(1):28–32. [PubMed] [Google Scholar]
- Karakousis C. P., Seddiq M. K., Moore R. Prognostic value of lymph node dissection in malignant melanoma. Arch Surg. 1980 Jun;115(6):719–722. doi: 10.1001/archsurg.1980.01380060021006. [DOI] [PubMed] [Google Scholar]
- Koh H. K., Sober A. J., Day C. L., Jr, Lew R. A., Kopf A. W., Lamar W., Ben Cosimi A., Wood W. C., Mihm M. C., Jr, Malt R. A. Prognosis of clinical stage I melanoma patients with positive elective regional node dissection. J Clin Oncol. 1986 Aug;4(8):1238–1244. doi: 10.1200/JCO.1986.4.8.1238. [DOI] [PubMed] [Google Scholar]
- Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966 Mar;50(3):163–170. [PubMed] [Google Scholar]
- McCarthy W. H., Shaw H. M., Thompson J. F., Milton G. W. Time and frequency of recurrence of cutaneous stage I malignant melanoma with guidelines for follow-up study. Surg Gynecol Obstet. 1988 Jun;166(6):497–502. [PubMed] [Google Scholar]
- Roses D. F., Provet J. A., Harris M. N., Gumport S. L., Dubin N. Prognosis of patients with pathologic stage II cutaneous malignant melanoma. Ann Surg. 1985 Jan;201(1):103–107. [PMC free article] [PubMed] [Google Scholar]
- Singletary S. E., Byers R. M., Shallenberger R., McBride C. M., Guinee V. F. Prognostic factors in patients with regional cervical nodal metastases from cutaneous malignant melanoma. Am J Surg. 1986 Oct;152(4):371–375. doi: 10.1016/0002-9610(86)90307-7. [DOI] [PubMed] [Google Scholar]
- Slingluff C. L., Jr, Vollmer R., Seigler H. F. Stage II malignant melanoma: presentation of a prognostic model and an assessment of specific active immunotherapy in 1,273 patients. J Surg Oncol. 1988 Nov;39(3):139–147. doi: 10.1002/jso.2930390302. [DOI] [PubMed] [Google Scholar]
- Snyderman N. L., Johnson J. T., Schramm V. L., Jr, Myers E. N., Bedetti C. D., Thearle P. Extracapsular spread of carcinoma in cervical lymph nodes. Impact upon survival in patients with carcinoma of the supraglottic larynx. Cancer. 1985 Oct 1;56(7):1597–1599. doi: 10.1002/1097-0142(19851001)56:7<1597::aid-cncr2820560722>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Vugrin D., Whitmore W. F., Cvitkovic E., Grabstald H., Sogani P., Barzell W., Golbey R. B. Adjuvant chemotherapy combination of vinblastine, actinomycin D, Bleomycin, and Chlorambucil following retroperitoneal lymph node dissection for stage II testis tumor. Cancer. 1981 Mar 1;47(5):840–844. doi: 10.1002/1097-0142(19810301)47:5<840::aid-cncr2820470503>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Wanebo H. J., Fortner J. G., Woodruff J., MacLean B., Binkowski E. Selection of the optimum surgical treatment of stage I melanoma by depth of microinvasion: Use of the combined microstage technique (Clark-Breslow). Ann Surg. 1975 Sep;182(3):302–315. doi: 10.1097/00000658-197509000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]


