Abstract
Cytomegalovirus (CMV) has highly evolved mechanisms for avoiding detection by the host immune system. Recently, in the genomes of human and primate CMV, a novel gene comprising segments of noncontiguous open reading frames was identified and found to have limited predicted homology to endogenous cellular interleukin-10 (IL-10). Here we investigate the biological activities of the CMV IL-10-like gene product and show it to possess potent immunosuppressive properties. Both purified bacterium-derived recombinant CMV IL-10 and CMV IL-10 expressed in supernatants of human cells were found to inhibit proliferation of mitogen-stimulated peripheral blood mononuclear cells (PBMCs), with specific activity comparable to that of recombinant human IL-10. In addition, CMV IL-10 expressed from human cells inhibited cytokine synthesis, as treatment of stimulated PBMCs and monocytes with CMV IL-10 led to a marked decrease in production of proinflammatory cytokines. Finally, CMV IL-10 was observed to decrease cell surface expression of both major histocompatibility complex (MHC) class I and class II molecules, while conversely increasing expression of the nonclassical MHC allele HLA-G. These results demonstrate for the first time that CMV has a biologically active IL-10 homolog that may contribute to immune evasion during virus infection.
Cytomegalovirus (CMV) is a widespread human pathogen with the ability to persist as a lifelong latent infection. Successful coexistence with its host is facilitated by numerous mechanisms that the virus has acquired for modulating the host immune system (11). The US2, US3, US6, and US11 gene products interfere with antigen processing and presentation, resulting in reduced major histocompatibility complex (MHC) class I presentation (1). In addition, the UL18 gene product is an MHC class I homolog that may facilitate evasion of natural killer cells (2). An α-chemokine homolog (encoded by UL146) that is chemotactic for neutrophils (22) and a β-chemokine receptor (US28 gene product) that binds human chemokines have also been identified (20). Most recently, a homolog of the potent immune modulator interleukin-10 (IL-10) was discovered in the genomes of human CMV (HCMV) and rhesus macaque CMV (RhCMV) (13, 15).
The HCMV and RhCMV IL-10 proteins are distinctive in that they have only 27 and 25% identity with their host cellular IL-10 proteins, respectively. They are encoded from noncontiguous stretches in the viral genome, including open reading frame (ORF) UL111A and adjacent intergenic areas. The CMV gene structures are distinct from those of the host IL-10 genes in that the HCMV IL-10 gene has two introns and the RhCMV IL-10 gene has three introns (compared to four in human and rhesus cellular IL-10 genes). Although the HCMV genome (strain AD169) was sequenced several years ago (3), the existence of CMV IL-10 has remained cryptic until recently, probably because of the unusual gene structure and low homology to cellular IL-10. In contrast, viral IL-10 homologs have been identified in the genomes of Epstein-Barr virus (EBV) (12), equine herpesvirus 2 (27), and the orf virus of the Poxviridae family (7), but these are encoded as contiguous ORFs generating proteins with 67 to 90% identity to human IL-10. EBV-encoded BCRF1, the first viral IL-10 homolog described, displays many of the activities of human IL-10, such as cytokine synthesis inhibition and macrophage deactivation (5, 12). Despite the extensive sequence homology with human IL-10, EBV IL-10 does not stimulate proliferation of B cells (as human IL-10 does), suggesting that viral IL-10 homologs may retain only a subset of human IL-10 activities that are advantageous for the virus.
IL-10 is a pleiotropic cytokine that is important in the regulation of the immune response. IL-10 was initially identified as a product of murine Th2 cells that inhibited the proliferation and effector activity of Th1 cells, although stimulatory effects on the proliferation of B cells and mast cells have been reported (19). In addition to inhibiting cytokine production, IL-10 has been shown to inhibit expression of MHC class II and adhesion molecules on monocytes, contributing to suppression of the Th1 type response (4, 5). The functional domains of human IL-10 have been mapped to defined regions; amino acids 8 to 16 were found to induce proliferation of mast cells, while a nine-amino-acid region at the carboxy terminus has cytokine synthesis inhibitory function (8). EBV IL-10 is partially conserved at the carboxy terminus (six of nine residues) and has been shown to inhibit cytokine synthesis (12). The HCMV IL-10 homolog has only one conserved residue in this region, and the RhCMV IL-10 sequence has no amino acid homology with either rhesus cellular IL-10 or human IL-10 in this domain, suggesting that the human and primate CMV IL-10 homologs may have different activities than human IL-10.
Despite the low homology of the CMV IL-10-like proteins with their cellular counterparts, recent evidence suggests that HCMV IL-10 may bind to the cellular human IL-10 receptor (13). However, no experimental evidence has previously demonstrated the biological activity or potency of this molecule. Therefore, we examined whether the CMV IL-10 proteins were biologically active gene products with properties that might be favorable to CMV immune evasion. To investigate this, we developed in vitro assays utilizing the IL-10 homologs from the HCMV Towne strain (ATCC VR977) and RhCMV strain 68.1 (ATCC VR677). To demonstrate interspecies conservation of activities we expressed both CMV IL-10 genes as recombinant proteins in human cells. In addition purified recombinant HCMV IL-10 was produced in bacteria to elucidate the potency of this molecule with respect to recombinant human IL-10. Here we describe the activities of these viral proteins. CMV IL-10 expressed in human cells exhibited profound inhibition of human and rhesus peripheral blood mononuclear cell (PBMC) proliferation and decreased the production of proinflammatory cytokines in PBMC and monocyte cultures. In addition, CMV IL-10 induced down regulation of cell surface MHC class I and class II proteins on monocytes, while the nonclassical MHC class I protein HLA-G was up regulated on the same cells. Additionally purified recombinant bacterium-expressed HCMV IL-10 demonstrated a specific activity comparable to that of recombinant human IL-10 in reduction of mitogen-stimulated proliferation of PBMCs, and this activity was inhibited by a monoclonal antibody to the human IL-10 receptor. These results demonstrate that CMV IL-10 possesses many immunosuppressive properties that may interfere with the host immune response.
MATERIALS AND METHODS
Cells.
PBMCs were obtained from the peripheral blood of healthy rhesus macaques by hypotonic lysis of red blood cells or from the buffy coats of cells from healthy human donors by Ficoll density gradient centrifugation. PBMCs were maintained in RPMI (HyClone) plus 10% fetal calf serum (FCS) (HyClone) and recombinant human IL-2 (10 ng/ml; R&D Systems). Monocytes were purified from PBMCs with anti-CD14 microbeads (Miltenyi) and cultured in RPMI plus 10% FCS.
Recombinant CMV IL-10 expression.
Human embryonic kidney (HEK) 293 (ATCC CRL1573) cells were grown in DMEM (HyClone) plus 5% FCS and transiently transfected with a myc/His-tagged expression vector containing either the cDNA sequence of RhCMV IL-10 (pcDNA3.1 m/H-RhCMV IL-10) described previously (15) or the cDNA sequence of HCMV (Towne) IL-10 (pcDNA3.1 m/H-HCMV IL-10). Cells were transfected with Superfect transfection reagent according to the manufacturer’s instructions (Qiagen). Supernatants were collected after 48 h and clarified by low-speed centrifugation, and protein expression was verified by Western blotting with an anti-poly-His monoclonal antibody (Invitrogen). Detection was via TMB membrane peroxidase substrate (Kirkegaard & Perry Laboratories). Mock-conditioned medium was obtained from cells transfected with the empty vector (pcDNA3.1-m/H; Invitrogen).
The concentration of CMV IL-10 present in culture supernatants was determined by comparison with a protein standard. Purified recombinant histidine-tagged fractalkine (R&D Systems) of known concentration was serially diluted and blotted alongside an equal volume of supernatant samples onto a nitrocellulose membrane using a standard dot blot apparatus. Once the samples dried, the membrane was washed in Tris-buffered saline (TBS) and then blocked with TBS-0.05% bovine serum albumin. The membrane was then incubated with INDIA-Hisprobe-HRP detection reagent (Pierce Chemical Company) and washed three times in TBS-0.5% Tween 20, and then horseradish peroxidase was detected via West-Pico HRP detection reagent as per manufacturer’s instructions (Pierce Chemical Company) and exposure to Kodak X-Omat film. As fractalkine has a molecular mass of around 90 kDa and that of CMV IL-10 is around 30 kDa, concentrations were adjusted by a factor of 3.0.
Recombinant HCMV IL-10.
Purified recombinant HCMV IL-10 (strain Towne) was obtained from R&D Systems.
Proliferation assays.
Human or rhesus macaque PBMCs were plated in 96-well culture dishes at a density of 105 cells per well and stimulated with phytohemagglutinin (PHA) (5 μg/ml) or concanavalin A (Sigma) in the presence of either 5 to 50% (vol/vol) supernatants from transfectants expressing CMV IL-10, the control vector (mock), recombinant HCMV IL-10, or recombinant human IL-10 (R&D Systems). Where indicated, CMV IL-10 was immunoprecipitated from the transfectant supernatants by incubation with anti-poly-His antibody (10 μg/ml) and protein A-Sepharose beads for 14 h at 4°C. In some assays monoclonal antibody to the human IL-10 receptor (R&D Systems) was included at 15 μg/ml. The plates were incubated at 37°C for 72 h, and DNA synthesis was determined by [3H]thymidine uptake (0.25 μCi/well; Amersham) during the last 18 h of culture.
Cytokine ELISA.
Human PBMCs or positively selected CD14+ monocytes were plated in 96-well culture dishes at a density of 105 cells per well and stimulated with PHA or lipopolysaccharide (LPS) (Sigma) in the presence of mock- or CMV IL-10-conditioned medium. After 48 h, supernatants were harvested and cytokine production was measured by sandwich enzyme-linked immunosorbent assay (ELISA). Briefly, 96-well microtiter plates (Nalge Nunc) were coated with anticytokine capture antibody (4 μg/ml; anti-alpha interferon [IFN-α], -tumor necrosis factor alpha [TNF-α], -granulocyte-macrophage colony-stimulating factor [GM-CSF], -IL-1α, or -IL-6 from R&D Systems) for 16 h at 4°C. After blocking with 1% bovine serum albumin (in phosphate-buffered saline), 100 μl of culture supernatant was added. After washing, biotinylated anticytokine antibodies (2 μg/ml; R&D Systems) were added, followed by streptavidin-horseradish peroxidase (Gibco BRL). Detection was via Genzyme color reagents (Genzyme Diagnostics); the reaction was terminated with 50 μl of 2 N HCl solution per well, and samples were read at 450 nm in a microplate reader (Molecular Devices). Cytokine levels were determined by linear regression analysis using a standard curve.
Flow cytometry.
Monocytes were stained with phycoerythrin-conjugated antibodies to CD54 (immunoglobulin G1 [IgG1]); HLA-DR (IgG2a); HLA-A, -B, or -C (IgG1); or the appropriate isotype control (Pharmingen) and analyzed using a FACScan device and CellQuest software (Becton Dickinson). Staining with antibody 87G directed against HLA-G (14) was followed by incubation with goat anti-mouse immunoglobulin-phycoerythrin secondary antibody.
RESULTS
CMV IL-10 inhibits PBMC proliferation.
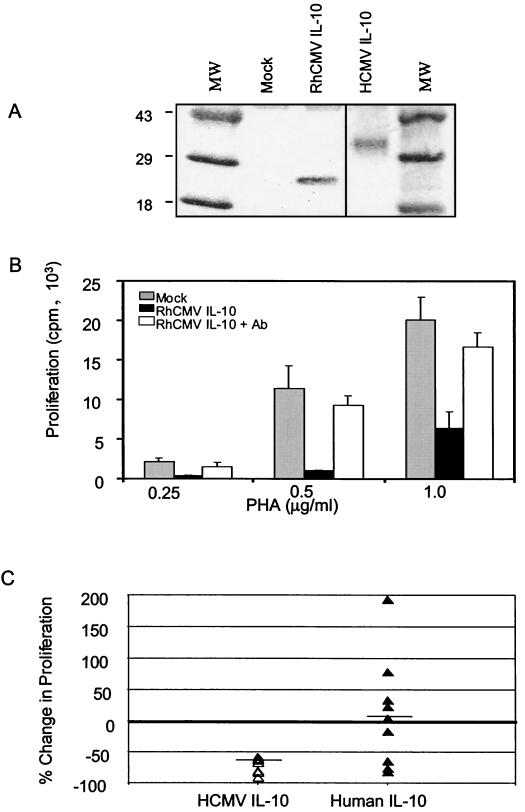
To elucidate the scope of biological activities of CMV IL-10 and to examine interspecies differences in these activities, recombinant CMV IL-10 was generated in HEK 293 cells transfected with plasmid DNA encoding a C-terminal myc/His epitope-tagged CMV IL-10 protein from either RhCMV strain 68.1 or HCMV strain Towne. Transfectant supernatants were harvested and examined by Western blotting with an antibody directed to the C-terminal poly-His tag. Supernatants from RhCMV IL-10-transfected cells were found to express a protein of 26 kDa, the predicted size of the epitope-tagged CMV IL-10 (15), for at least 48 h posttransfection (Fig. 1A). The HCMV IL-10 protein appeared as a 30- to 35-kDa protein, consistent with the predicted size of the protein with glycosylation (13). The diffuse protein band seen for HCMV IL-10 probably results from variability in the number of carbohydrate residues. In contrast, the RhCMV IL-10 protein, which lacks the corresponding N-linked glycosylation site, appeared as a distinct band (Fig. 1A), suggesting that the RhCMV protein is not glycosylated. The concentration of recombinant CMV IL-10 was determined by comparison to an identically histidine-tagged protein (fractalkine), assessed in a dot blot assay. All preparations of CMV IL-10 were reproducibly found to contain between 100 and 600 ng of IL-10/ml.
FIG. 1.
Supernatant CMV IL-10 inhibits PBMC proliferation. (A) Western blot analysis of supernatants from transfected HEK 293 cells with an anti-poly-His antibody. Cells were transiently transfected with pcDNA3.1-m/H-RhCMV IL-10, pcDNA3.1-m/H-HCMV IL-10, or an empty vector control (mock). MW, molecular weight marker (sizes at right, in thousands). (B) PBMCs from healthy human donors were stimulated with PHA for 72 h in the presence of RhCMV IL-10 or mock supernatants. Proliferation was measured by [3H]thymidine uptake during the final 18 h of culture. RhCMV IL-10 was immunoprecipitated from conditioned medium with an anti-poly-His antibody (CMV IL-10 + Ab). Error bars, standard deviations. (C) PBMCs from nine human donors were tested for effects on proliferation in the presence of HCMV IL-10-conditioned medium or recombinant human IL-10 (1 μg/ml). Results are expressed as percent increase in proliferation relative to control cultures from the same donor. The horizontal bar indicates mean change in proliferation for all donors.
The effects of CMV IL-10 on PBMC proliferation in the absence of other virally produced factors was assessed using a panel of mitogen-stimulated PBMCs from human and rhesus macaque donors. Every donor, regardless of species, showed marked inhibition of proliferation in the presence of RhCMV IL-10. Due to the greater availability of human PBMC, results for human PBMC are shown as representative throughout. Mitogen-stimulated PBMC exhibited robust proliferation, but this proliferation was inhibited in the presence of RhCMV IL-10 (Fig. 1B). While conditioned media constituted 50% of the total culture volume in these experiments, inhibition of proliferation was observed in cultures containing as little as 5% RhCMV IL-10-conditioned medium. Proliferation of cells cultured in the presence of the mock-conditioned medium was not found to differ from that of cells cultured in fresh RPMI. To confirm that the inhibitory activity was solely a province of CMV IL-10, culture supernatants were depleted of RhCMV IL-10 by adsorption with an antibody directed to the histidine tag. This resulted in a near total restoration of PBMC proliferation to levels observed with mock medium (Fig. 1B). In the representative experiment shown, RhCMV IL-10 inhibited the proliferation of human PBMC; however, equivalent inhibition of rhesus PBMC proliferation was also seen. Likewise, supernatant HCMV IL-10 was found to substantially reduce proliferation of human PBMC (data not shown), suggesting that both HCMV and RhCMV IL-10 homologs can suppress the replication of immune cells.
To compare this activity to that of the endogenous IL-10 a bank of PBMC samples was stimulated in the presence of HCMV IL-10 or recombinant human IL-10. While HCMV IL-10 inhibited PBMC proliferation for all donors tested (57 to 91% inhibition), variability was observed with human IL-10 treatment. PBMC proliferation for four donors was inhibited in the presence of recombinant human IL-10 at a concentration of 1 μg/ml (16 to 81% inhibition), while four other donors showed enhanced proliferation (increases of 24 to 194%) and one donor showed modest enhancement. The differing effects of recombinant human IL-10 on PBMC may reflect not only variability among human donors but also the mixed population of cells within the PBMC culture. It has been documented that human IL-10 can have inhibitory effects on monocytes and CD4+ T cells while having stimulatory effects on B cells and CD8+ T cells (19); thus, increased proliferation of some PBMC cultures could represent outgrowth of these cell types. In contrast, HCMV IL-10 suppressed proliferation over a wide range of concentrations on PBMC cultures from multiple donors. These data may suggest that HCMV IL-10 lacks some of the stimulatory activities of human IL-10.
CMV IL-10 inhibits cytokine synthesis.
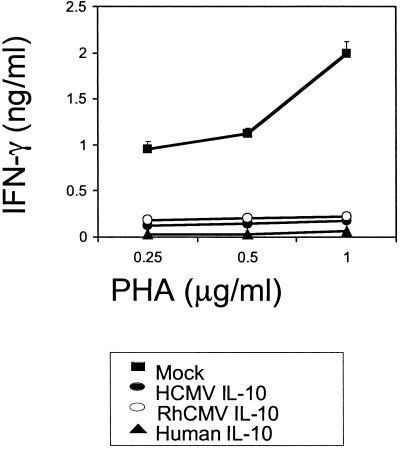
The hallmark activity of human IL-10 is inhibition of cytokine synthesis; therefore, we examined whether CMV IL-10 would alter the level of cytokine production in PBMC cultures. PHA-stimulated human PBMCs were cultured in the presence of mock-, HCMV IL-10-, or RhCMV IL-10-conditioned medium. After 48 h, the supernatants from these cultures were assayed for cytokine levels by ELISA. While control PBMC cultures produced IFN-γ in response to PHA stimulation, cytokine levels were decreased in cultures containing RhCMV IL-10 or HCMV IL-10 (Fig. 2). IFN-γ production was almost completely abolished in the presence of either viral cytokine. As an additional control, PBMC cultures were treated with recombinant human IL-10 (1 μg/ml), which also dramatically inhibited cytokine production. The results demonstrate that, like human IL-10, HCMV and RhCMV IL-10 homologs can exert profound inhibition of proinflammatory cytokine production by human leukocytes.
FIG. 2.
CMV IL-10 inhibits cytokine production by activated human PBMCs. PHA-stimulated PBMCs were incubated in the presence of mock-, RhCMV IL-10-, or HCMV IL-10-conditioned medium or recombinant human IL-10 (1 μg/ml). Supernatants were harvested after 48 h and assayed for IFN-γ production by sandwich ELISA.
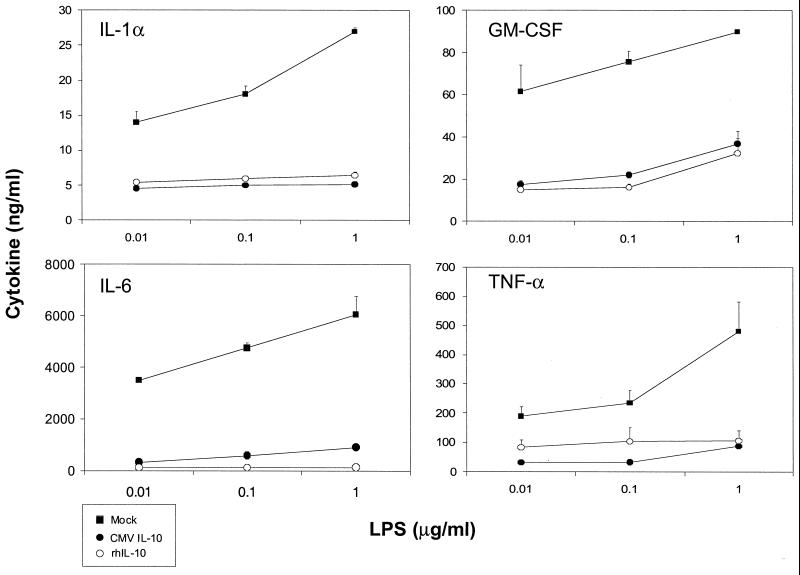
Because PBMC cultures represent a mixed population of cells, we next examined the effects of CMV IL-10 on a homogenous cell population. Primary human monocytes were purified from PBMCs by positive selection with CD14 microbeads. Flow cytometric analysis revealed the resulting cell population to be >99% CD14 positive (data not shown). Monocytes were stimulated with LPS in the presence of mock- or CMV IL-10-conditioned medium or recombinant human IL-10. Supernatants from these cultures were harvested after 48 h and assayed for proinflammatory cytokines by ELISA. In LPS-stimulated monocyte cultures, a robust dose-dependent production of IL-1α, IL-6, GM-CSF, and TNF-α was observed, and this production was greatly reduced in the presence of HCMV IL-10 or recombinant human IL-10. (Fig. 3). Similar results were observed upon treatment of human monocytes with RhCMV IL-10 (data not shown). Thus, CMV-encoded IL-10 has direct suppressive effects on monocytes, causing a profound decrease in the production of immunoregulatory cytokines.
FIG. 3.
HCMV IL-10 inhibits expression of proinflammatory cytokines by human monocytes. Monocytes were stimulated with LPS and incubated in the presence of mock- or HCMV IL-10-conditioned medium or recombinant human IL-10 (rhIL-10) (1 μg/ml). Supernatants were harvested after 48 h and assayed for IL-1α, IL-6, GM-CSF, and TNF-α production by sandwich ELISA. Error bars, standard deviations.
Cell surface MHC expression is altered by CMV IL-10.
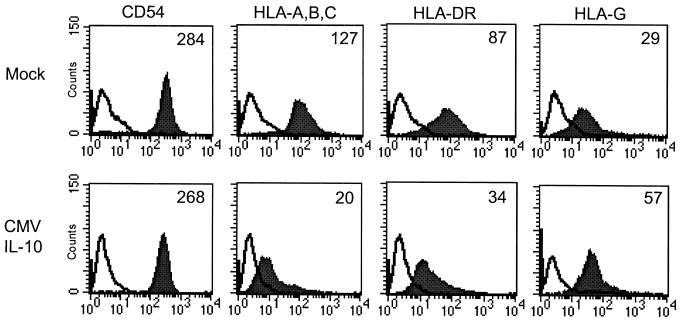
IL-10 has been reported to lead to decreased levels of MHC class II molecules and ICAM-1 on monocytes (5, 31), a putative mechanism by which immune responses are modulated and homeostasis is maintained. Decreased cellular surveillance may also potentiate viral infection. Human LPS-stimulated monocytes were first treated with RhCMV IL-10. Flow cytometric analysis showed that cell surface expression of ICAM-1 on LPS-stimulated monocytes was not significantly affected by RhCMV IL-10 treatment (Fig. 4, left panels). In contrast, cell surface levels of both MHC class I and class II antigens were reduced on the entire cell population by incubation with RhCMV IL-10 (Fig. 4, center panels). MHC class II levels were decreased by RhCMV IL-10 treatment in every donor tested, with the mean fluorescence intensity of MHC class II staining for RhCMV IL-10 treated cells being nearly threefold lower than that for cells treated with the mock-conditioned medium for all donors. The effect of RhCMV IL-10 on monocyte MHC class I protein expression was more variable; decreased cell surface expression was observed in four of six donors, while two of six donors were unaffected. This may reflect donor variability or differences in cellular activation states. Similar results were obtained following treatment with HCMV IL-10 (data not shown). Overall, CMV IL-10 treatment was observed to lead to decreased cell surface expression of both class I and class II MHC molecules on LPS stimulated monocytes, a scenario that may contribute to viral escape from immune surveillance.
FIG. 4.
MHC expression is altered by RhCMV IL-10. Flow cytometric analysis of LPS-stimulated monocytes cultured in the presence of mock- or RhCMV IL-10-conditioned medium. After 48 h the cells were stained for cell surface expression of CD54 (ICAM), MHC class I (HLA-A, -B, and -C), MHC class II (HLA-DR), and the nonclassical class I MHC molecule HLA-G. Filled histograms represent staining with the indicated antibody; open histograms indicate isotype controls. The mean fluorescence intensity is indicated in the upper right corner of each plot.
Finally, we examined the ability of CMV IL-10 to influence expression of nonclassical MHC antigens. HLA-G has been observed to confer protection from natural killer cell-mediated lysis (28), and human IL-10 has been linked to HLA-G up regulation on monocytes and trophoblasts (18). Since other CMV proteins have been implicated in the resistance of infected cells to NK cell lysis (11), we asked whether CMV IL-10 might play a role in evading detection by NK cells. Again cells were treated with supernatants containing RhCMV IL-10. As shown in Fig. 4, the intensity of HLA-G cell surface staining was increased following RhCMV IL-10 treatment (right panels). Similar results were seen with HCMV IL-10-containing supernatants. Up regulation of cell surface HLA-G expression was seen in all donors tested. Thus, in contrast to the decreased cell surface expression of classical MHC class I molecules observed in the presence of CMV IL-10, levels of HLA-G expression on monocytes were increased. The results demonstrate that CMV IL-10 modulates multiple aspects of the cell-mediated immune response by effecting major changes in class I and class II MHC expression.
Recombinant HCMV IL-10 functions through the IL-10 receptor.
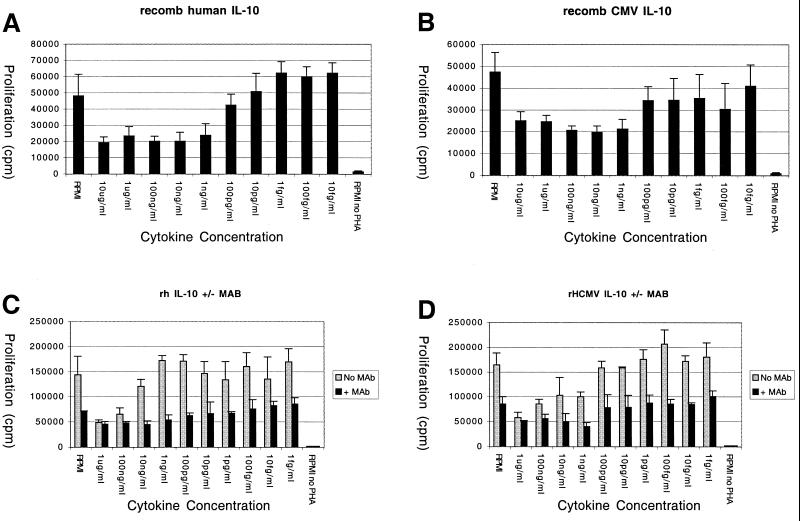
To more directly compare the activities of CMV IL-10 and human IL-10, purified recombinant HCMV IL-10 was obtained (R&D Systems). Recombinant human or CMV IL-10 was serially diluted, and their effects on PHA-stimulated proliferation of PBMC were measured by tritium incorporation. As with the CMV IL-10 supernatants, the recombinant HCMV IL-10 had inhibitory effects on proliferation. Both molecules significantly inhibited proliferation down to 10 to 100 pg/ml in replicate assays (Fig. 5A and B), suggesting that the molecules have a comparable specific activity in this assay.
FIG. 5.
Recombinant HCMV IL-10 inhibits PBMC proliferation. PBMCs from healthy human donors were stimulated with PHA for 72 h in the presence of serial dilutions of recombinant HCMV IL-10 (B and D [black bars]) or recombinant human IL-10 (A and C [black bars]). Proliferation was measured by [3H]thymidine uptake during the final 18 h of culture. Activity of recombinant proteins was abolished by the presence of monoclonal anti-human IL-10 receptor antibody at 15 μg/ml (C and D [gray bars]). Error bars, standard deviations.
To ascertain that the inhibitory activity was modulated specifically through the IL-10 receptor, the assay was repeated in the presence of neutralizing antibody to the human IL-10 receptor. Monoclonal anti-human IL-10 receptor antibody markedly increased proliferation of PBMCs at all assay points (Fig. 5C and D, gray bars) compared to parallel samples with no antibody (Fig. 5C and D, black bars), probably through neutralizing endogenous IL-10 produced by the cells during the incubation period. This effect of anti-IL-10 receptor antibodies on PBMC proliferation is a typical observation in assays of this nature (M. Howard, personal communication). However the antibody also neutralized the effects of the presumably greater quantity of exogenously provided recombinant human IL-10 (Fig. 5C) and recombinant HCMV IL-10 (Fig. 5D). Only high levels of recombinant protein were able to inhibit proliferation of PBMCs compared to the RPMI control. These results demonstrate that recombinant HCMV IL-10 has strong antiproliferative activity, comparable to that of human IL-10, and mediates its activity through the human IL-10 receptor.
DISCUSSION
Immunosuppression is a well-documented feature of acute CMV infection (16). Although these effects have in part been attributed to virus-induced dysfunction of the monocyte/macrophage population, it has been difficult to define factors that contribute to systemic immunosuppression due to the size and complexity of the CMV genome. In this report, we demonstrate that the HCMV- and RhCMV-encoded IL-10 homologs have a broad spectrum of immunosuppressive properties. The CMV IL-10 proteins, despite their low homology to endogenous cellular IL-10 proteins, can inhibit PBMC proliferation, suppress cytokine production, and down regulate the antigen-presenting and accessory cell functions of monocytes. Hence, CMV IL-10 may contribute to CMV-induced immunosuppression and be an important factor in viral pathogenesis.
IL-10 is an important regulator of inflammation; the essential role of this cytokine in limiting the immune response is evidenced by chronic inflammatory disease in mice lacking the IL-10 gene (24). Furthermore, the production of IL-10 by tumor cells for evasion of immune surveillance has been well documented (9). The observation that CMV IL-10 inhibits immune proliferation and cytokine production indicates that this protein likely plays a major role in the interaction of the virus with the host immune system. Previous work has shown that the CMV IL-10 gene is expressed in HCMV-infected cells (13) and in RhCMV-infected cells, where it was shown to be an early transcript expressed abundantly during infection (15). Polyclonal antiserum raised against the RhCMV IL-10 protein recognizes a 26-kDa band in infected cell lysates, demonstrating that the protein is expressed during infection. Furthermore, serum from chronically infected rhesus macaques was found to recognize the same 26-kDa protein from infected cell lysates as well as the recombinantly expressed epitope-tagged protein (15), suggesting that CMV IL-10 is a target of the host immune response. Given the previous findings, the antiproliferative activity of CMV IL-10 observed here in vitro could reflect a relevant in vivo modulation of the host immune system important for viral pathogenesis.
Interestingly, results from our studies suggest that CMV IL-10 has the capacity to impair the proliferation of a wide range of cell types at concentrations ranging from 25 up to 250 ng/ml. In contrast, human IL-10 was observed to have both inhibitory and stimulatory effects on proliferation of cells from various donors. Stimulation of PBMC proliferation was observed over a range of concentrations of recombinant human IL-10, from 1 ng to 1 μg/ml (data not shown). The stimulatory activities of human IL-10 on some cell types, including CD8+ T cells, have been documented (19, 29). It is interesting to speculate that this may represent a key difference in activities, as CD8+ T-cell-mediated immunity is crucial in control of CMV infection both in murine models and in humans following bone marrow transplantation (25, 26). Although the levels of CMV IL-10 produced in vivo during infection have yet to be determined, the extensive inhibitory effects of CMV IL-10 on PBMC suggest that the viral homolog may have evolved functions distinct from its cellular counterpart that are beneficial to virus replication, perhaps underscored by the sequence divergence within functionally important domains.
Despite differences in antiproliferative activity, levels of inhibition of cytokine production were comparable and profound for both CMV IL-10 and human IL-10. Treatment with CMV IL-10 virtually abolished production of cytokines by activated PBMCs and monocytes, as did treatment with recombinant human IL-10. In vivo studies with murine CMV have shown IFN-γ to be crucial for the antiviral response (10); thus, the reduction in cytokine production effected by CMV IL-10 may result in delayed activation and/or recruitment of immune cells to the sites of virus infection, favoring virus replication and dissemination. Also striking was the effect of CMV IL-10 on cell surface levels of MHC class II antigen in our system. MHC class II expression is induced by IFN-γ; therefore, down modulation of class II by CMV IL-10 could be via direct action of the viral cytokine or through indirect effects resulting from IFN-γ suppression. Interestingly, HCMV has been reported to interfere with transcription of class II genes through disruption of the Jak/Stat signaling pathway, although the factors mediating this event have not been defined (17). Our observations demonstrate that CMV IL-10 induces reduction in class II MHC expression on monocytes, and this correlates with the finding that CMV IL-10 binds to the cellular IL-10 receptor and induces signal transduction that leads to Jak/Stat signaling (13). Human IL-10 is known to activate Stat 1 and Stat 3, suppressing a number of IFN-γ inducible genes, including class II MHC genes (6). Murine CMV has been reported to cause down regulation of class II MHC antigen expression through induction of endogenous IL-10 at early times following infection (23); these same activities are likely carried out directly by the HCMV and RhCMV IL-10 homologs during infection. In addition, elevated levels of human IL-10 in plasma have been reported during CMV infection in some renal transplant recipients (21), suggesting further redundancy in efforts of the virus to attain replicative advantage by modulation of the host immune response.
While interference with class II MHC is an effective mechanism for suppression of cell-mediated immunity, we have also observed a role for CMV IL-10 in down modulation of MHC class I on monocytes. CMV encodes several proteins (US2, US3, US6, and US11) that interfere with the class I antigen processing and presentation pathway (11). We have shown that CMV IL-10 causes decreased surface expression of class I MHC, a function that has also been demonstrated for cellular and EBV IL-10 (31, 32). While the mechanism for class I down modulation has yet to be explored, it is possible that CMV IL-10 is acting through the regulation of transcription, a checkpoint upstream of the actions of other CMV encoded class I interference proteins.
The down regulation of classical MHC class I molecules observed in the presence of CMV IL-10 is in direct contrast to the effects on the nonclassical MHC protein HLA-G. HLA-G is important in feto-maternal tolerance by inhibiting lysis of fetal cells by maternal NK cells (28). Expression of HLA-G on trophoblasts and monocytes has been linked to IL-10 (18). Our results demonstrate that CMV IL-10 can increase expression of HLA-G, suggesting that CMV has evolved a novel mechanism for subversion of NK cell reactivity. Another CMV ORF, UL18, encodes a class I homolog that may confer protection from NK cell lysis (2) and recently gpUL40 has been implicated in the up regulation of HLA-E, another nonclassical MHC protein (30). Given the multiple strategies for subversion of the class I antigen presentation system, it would not be surprising if CMV had acquired several different mechanisms for avoiding NK cell lysis. Additional investigations are under way to examine in detail the regulation of HLA-G by CMV IL-10.
Evasion of immune detection is a central feature of a successful latent virus infection. We demonstrate that the HCMV- and RhCMV-encoded IL-10 homologs have a broad spectrum of immunosuppressive properties that may be utilized to escape immune detection. Characterization of biological functions conserved by CMV IL-10 contributes to our knowledge of virus-host interactions and also provides greater understanding of the regulation of the host immune system.
Acknowledgments
We thank D. E. Geraghty (Fred Hutchinson Cancer Research Center, Seattle, Wash.) for kindly providing antibody 87G and Jennifer Burns and Jennifa Gosling for helpful discussions and for critical reading of the manuscript.
This work was supported in part by NIH grant 1-R01-HL57883 (to P.A.B.).
REFERENCES
- 1.Ahn, K., A. Angula, P. Chazal, Y. Yang, and K. Fruh. 1996. Human cytomegalovirus inhibits antigen presentation by a sequential multistep process. Proc. Natl. Acad. Sci. USA 93:10990–10995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beck, S., and B. G. Barrel. 1988. Human cytomegalovirus encodes a glycoprotein homologous to MHC class-I antigens. Nature 331:269–272. [DOI] [PubMed] [Google Scholar]
- 3.Chee, M. S., A. T. Bankier, S. Beck, R. Bohni, C. M. Brown, R. Cerny, T. Horsnell, C. A. Hutchison, T. Kouzarides, J. A. Martignetti, et al. 1990. Analysis of the protein-coding content of the sequence of human cytomegalovirus strain AD169. Curr. Top. Microbiol. Immunol. 154:125–169. [DOI] [PubMed] [Google Scholar]
- 4.de Waal Malefyt, R., J. Abrams, B. Bennett, C. G. Figdor, and J. E. de Vries. 1991. Interleukin 10 (IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J. Exp. Med. 174:1209–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Waal Malefyt, R., J. Haanen, H. Spits, M. G. Roncarolo, A. te Velde, C. Figdor, K. Johnson, R. Kastelein, H. Yssel, and J. E. de Vries. 1991. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J. Exp. Med. 174:915–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donnelly, R. P., H. Dickensheets, and D. S. Finbloom. 1999. The interleukin-10 signal transduction pathway and regulation of gene expression in mononuclear phagocytes. J. Interferon Cytokine Res. 19:563–573. [DOI] [PubMed] [Google Scholar]
- 7.Fleming, S. B., C. A. McCaughan, A. E. Andrews, A. D. Nash, and A. A. Mercer. 1997. A homolog of interleukin-10 is encoded by the poxvirus orf virus. J. Virol. 71:4857–4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gesser, B., H. Leffers, T. Jinquan, C. Vestergaard, N. Kirstein, S. Sindet-Pedersen, S. L. Jensen, K. Thestrup-Pedersen, and C. G. Larsen. 1997. Identification of functional domains on human interleukin 10. Proc. Natl. Acad. Sci. USA 94:14620–14625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gotlieb, W. H., J. S. Abrams, J. M. Watson, T. J. Velu, J. S. Berek, and O. Martinez-Maza. 1992. Presence of interleukin 10 (IL-10) in the ascites of patients with ovarian and other intra-abdominal cancers. Cytokine 4:385–390. [DOI] [PubMed] [Google Scholar]
- 10.Heise, M. T., and H. W. Virgin. 1995. The T-cell-independent role of gamma interferon and tumor necrosis factor alpha in macrophage activation during murine cytomegalovirus and herpes simplex virus infections. J. Virol. 69:904–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hengel, H., W. Brune, and U. H. Koszinowski. 1998. Immune evasion by cytomegalovirus—survival strategies of a highly adapted opportunist. Trends Microbiol. 6:190–197. [DOI] [PubMed] [Google Scholar]
- 12.Hsu, D. H., R. de Waal Malefyt, D. F. Fiorentino, M. N. Dang, P. Vieira, J. de Vries, H. Spits, T. R. Mosmann, and K. W. Moore. 1990. Expression of interleukin-10 activity by Epstein-Barr virus protein BCRF1. Science 250:830–832. [DOI] [PubMed] [Google Scholar]
- 13.Kotenko, S. V., S. Saccani, L. S. Izotova, O. V. Mirochnitchenko, and S. Pestka. 2000. Human cytomegalovirus harbors its own unique IL-10 homolog (cmvIL-10). Proc. Natl. Acad. Sci. USA 97:1695–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee, N., A. R. Malacko, A. Ishitani, M. C. Chen, J. Bajorath, H. Marquardt, and D. E. Geraghty. 1995. The membrane-bound and soluble forms of HLA-G bind identical sets of endogenous peptides but differ with respect to TAP association. Immunity 3:591–600. [DOI] [PubMed] [Google Scholar]
- 15.Lockridge, K. M., S.-S. Zhou, R. H. Kravitz, J. L. Johnson, E. T. Sawal, E. L. Blewett, and P. A. Barry. 2000. Primate cytomegaloviruses encode and express an IL-10-like protein. Virology 268:272–280. [DOI] [PubMed] [Google Scholar]
- 16.McChesney, M. B., and M. B. Oldstone. 1987. Viruses perturb lymphocyte functions: selected principles characterizing virus-induced immunosuppression. Annu. Rev. Immunol. 5:279–304. [DOI] [PubMed] [Google Scholar]
- 17.Miller, D. M., B. M. Rahill, J. M. Boss, M. D. Lairmore, J. E. Durbin, W. J. Waldman, and D. D. Sedmak. 1998. Human cytomegalovirus inhibits major histocompatibility complex class II expression by disruption of the Jak/Stat pathway. J. Exp. Med. 187:675–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moreau, P., F. Adrian-Cabestro, C. Menier, V. Guiard, L. Gourand, J. Dausset, E. D. Carosella, and P. Paul. 1999. IL-10 selectively induces HLA-G expression in human trophoblasts and monocytes. Int. Immunol. 11:803–811. [DOI] [PubMed] [Google Scholar]
- 19.Mosmann, T. R. 1994. Properties and functions of interleukin-10. Adv. Immunol. 56:1–26. [PubMed] [Google Scholar]
- 20.Neote, K., D. DiGregorio, J. Y. Mak, R. Horuk, and T. J. Schall. 1993. Molecular cloning, functional expression, and signaling characteristics of a C-C chemokine receptor. Cell 72:415–425. [DOI] [PubMed] [Google Scholar]
- 21.Nordoy, I., F. Muller, K. P. Nordal, H. Rollag, E. Lien, P. Aukrust, and S. S. Froland. 2000. The role of the tumor necrosis factor system and interleukin-10 during cytomegalovirus infection in renal transplant recipients. J. Infect. Dis. 181:51–57. [DOI] [PubMed] [Google Scholar]
- 22.Penfold, M. E., D. J. Dairaghi, G. M. Duke, N. Saederup, E. S. Mocarski, G. W. Kemble, and T. J. Schall. 1999. Cytomegalovirus encodes a potent alpha chemokine. Proc. Natl. Acad. Sci. USA 96:9839–9844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Redpath, S., A. Angulo, N. R. J. Gascione, and P. Ghazal. 1999. Murine cytomegalovirus infection down-regulates MHC class II expression on macrophages by induction of IL-10. J. Immunol. 162:6701–6707. [PubMed] [Google Scholar]
- 24.Rennick, D., N. Davidson, and D. Berg. 1995. Interleukin-10 gene knock-out mice: a model of chronic inflammation. Clin. Immunol. Immunopathol. 76:S174–S178. [DOI] [PubMed] [Google Scholar]
- 25.Reusser, P., S. R. Riddell, J. D. Meyers, and P. D. Greenberg. 1991. Cytotoxic T-lymphocyte response to cytomegalovirus after human allogeneic bone marrow transplantation: pattern of recovery and correlation with cytomegalovirus infection and disease. Blood 78:1373–1380. [PubMed] [Google Scholar]
- 26.Riddell, S. R., and P. D. Greenberg. 1997. T cell therapy of human CMV and EBV infection in immunocompromised hosts. Rev. Med. Virol. 7:181–192. [DOI] [PubMed] [Google Scholar]
- 27.Rode, H. J., W. Janssen, A. Rosen-Wolff, J. J. Bugert, P. Thein, Y. Becker, and G. Darai. 1993. The genome of equine herpesvirus type 2 harbors an interleukin 10 (IL10)-like gene. Virus Genes 7:111–116. [DOI] [PubMed] [Google Scholar]
- 28.Rouas-Freiss, N., R. M. Goncalves, C. Menier, J. Dausset, and E. D. Carosella. 1997. Direct evidence to support the role of HLA-G in protecting the fetus from maternal uterine natural killer cytolysis. Proc. Natl. Acad. Sci. USA 94:11520–11525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rowbottom, A. W., M. A. Lepper, R. J. Garland, C. V. Cox, and E. G. Corley. 1999. Interleukin-10-induced CD8 cell proliferation. Immunology 98:80–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tomasec, P., V. M. Braud, C. Rickards, M. B. Powell, B. P. McSharry, S. Gadola, V. Cerundolo, L. K. Borysiewicz, A. J. McMichael, and G. W. G. Wilkinson. 2000. Surface expression of HLA-E, and inhibitor of natural killer cells, enhanced by human cytomegalovirus gpUL40. Science 287:1031–1033. [DOI] [PubMed] [Google Scholar]
- 31.Yue, F. Y., R. Dummer, R. Geertsen, G. Hofbauer, E. Laine, S. Manolio, and G. Burg. 1997. Interleukin-10 is a growth factor for human melanoma cells and down-regulates HLA class-I, HLA class-II, and ICAM-1 molecules. Int. J. Cancer 71:630–637. [DOI] [PubMed] [Google Scholar]
- 32.Zeidler, R., G. Elssner, P. Meissner, S. Uebel, R. Tampo, S. Lazis, and W. Hammerschmidt. 1997. Downregulation of TAP1 in B lymphocytes by cellular and Epstein-Barr virus encoded interleukin-10. Blood 90:2390–2397. [PubMed] [Google Scholar]