Abstract
The cyclin-dependent kinase (cdk) inhibitor Roscovitine (Rosco) reduces transcription of herpes simplex virus early genes significantly, even in the presence of wild-type levels of immediate-early (IE) viral proteins, suggesting that the transactivating functions of IE proteins may require the activities of one or more Rosco-sensitive cdk (L. M. Schang, A. Rosenberg, and P. A. Schaffer, J. Virol. 73:2161–2172, 1999). Based on this observation, we sought to determine whether Rosco alters the transactivating activity and posttranslational modification state of the IE protein, infected cell protein 0 (ICP0), in KOS6β-infected Vero cells. KOS6β is a KOS-derived recombinant virus containing an ICP0-inducible ICP6 promoter::lacZ cassette. To monitor ICP0’s transactivating activity, KOS6β-infected cells were released from a cycloheximide (CHX)-mediated protein synthesis block into medium with or without Rosco, and β-galactosidase activity was measured. Rosco inhibited the ability of ICP0 to transactivate the ICP6 promoter by 50-fold. This inhibition was shown not to be a consequence of inhibition of ICP6 basal promoter activity or aberrant nuclear localization of ICP0. Rosco also altered the electrophoretic mobility of a portion of ICP0 molecules derived from KOS-infected cells following reversal of a CHX block. Notably, however, Rosco had only a minimal effect on the phosphorylation state of ICP0. We conclude that ICP0’s transactivating activity requires Rosco-sensitive cdks and hypothesize that these cdks regulate the functions of cellular enzymes which modify ICP0, and are, consequently, required for its transactivating activity. Thus, we propose that Rosco regulates ICP0’s posttranslational state by mechanisms other than, or in addition to, phosphorylation.
Productive infection of actively dividing epithelial cells and fibroblasts by herpes simplex virus type 1 (HSV-1) is characterized by expression of all or nearly all viral genes, leading to the production of new infectious virus and cell death (reviewed in reference 62). The activities of HSV immediate-early (IE) regulatory proteins are required for the transcriptional activation of early and late genes and for the repression of IE gene transcription during productive infection (34). In nondividing neurons, however, the activities of IE regulatory proteins are repressed such that the productive phase of viral gene expression does not occur or occurs transiently, leading to latent infection. After stress, neurons again become permissive for viral gene expression leading to the production of new infectious virus (reactivation). The fact that nondividing neurons can support latent as well as productive infection (reactivation) implicates the differential expression and/or activation of cellular proteins in determining whether neuronal infection will be latent or productive. In support of this concept, Schang and coworkers have shown that inhibitors of cyclin-dependent kinases (cdks) block productive infection and reactivation from neuronal latency, implicating cdks as critical cellular determinants of whether infection will be productive or latent (67; L. M. Schang, A. Bantly, and P. A. Schaffer, unpublished data).
Among the five HSV IE regulatory proteins, infected cell protein 0 (ICP0) is a global transcriptional activator of viral and cellular gene expression (12, 17, 22, 26, 27, 54, 60, 81). As such, ICP0 plays a central role in facilitating efficient HSV replication in cell culture (11, 20, 64, 73) and in the efficient establishment and reactivation of HSV latency (9, 14, 29, 30, 31, 43). ICP0 is a 110-kDa nuclear phosphoprotein that activates viral gene expression at a pretranscriptional level (39, 59, 82). It appears to mediate its transcriptional activating activity, in part, through alterations in the levels and localization of distinct cellular proteins within centromeres (23, 45, 46) and nuclear structures (reviewed in reference 21) termed nuclear domain 10 (ND10) (49, 50) via the ubiquitin-proteasome pathway (25). Over a decade ago it was demonstrated that a mutant lacking ICP0 replicates inefficiently in Vero cells growth arrested in G0/G1, but in cells released from growth arrest, the mutant can be complemented by a cellular activity or activities expressed during the late G1/S phase of the cell cycle (10). More recently, ICP0 has been shown to arrest cells in the late G1/S and G2/M phases of the cell cycle (32, 45). While the latter observation indicates that ICP0 affects the stage of the cell cycle, the observation of Schang and coworkers demonstrates that cell cycle-associated cellular factors, specifically cdks, can alter the transactivating activities of IE proteins (69). Furthermore, two recent studies have demonstrated that the cdk inhibitor Roscovitine (Rosco) can affect the posttranslational modification of ICP0 (3, 69). Thus, ICP0 affects, and is affected by, cell cycle-associated cellular factors.
To determine whether cdks alter the functional activities and phosphorylation state of ICP0 in our system, we utilized the cdk inhibitor Rosco. In these studies, we show that Rosco inhibits ICP0’s ability to transactivate the ICP6 promoter and alters the electrophoretic mobility of a subset of ICP0 molecules but has no major effect on the phosphorylation state of ICP0 in Vero cells. These findings demonstrate that both the transactivating activity and posttranslational modification of ICP0 are regulated by cdks.
MATERIALS AND METHODS
Cells, viruses, and drugs.
Vero cells were propagated as previously described (39). L7 cells, a Vero cell line stably transformed with the ICP0 gene and able to complement the replication of ICP0 null mutants, were propagated as previously described (65). Low-passage (p9) HSV-1, strain KOS, was used as the wild-type virus and was propagated and assayed as described by Schaffer et al. (66). The KOS-derived ICP0 deletion mutant, dlx3.1, was grown and assayed as described by Sacks and Schaffer (64). The titers of dlx3.1 viral stocks were determined on ICP0 complementing L7 cells.
Drugs used in these studies were as follows: Rosco was prepared in dimethyl sulfoxide as a 50 mM stock and used in all experiments at a concentration of 100 μM (67). Cycloheximide (CHX) was prepared in serum-free Dulbecco modified Eagle medium (DMEM; 20 mg/ml) and used at concentrations of 50 or 75 μg/ml, as indicated. Actinomycin D (ActD) was prepared as a 1-mg/ml stock in 100% ethanol and used at a concentration of 1 μg/ml. Phosphonoacetic acid (PAA) was prepared in serum-free DMEM (10 mg/ml) and used at a concentration of 100 μg/ml.
Construction of plasmids and KOS6β.
A 4,138-bp PvuII-to-PstI fragment of HSV-1, strain KOS DNA, containing the UL49 and UL50 genes (Fig. 1A, diagram i) was cloned into plasmid pSP72 (Promega, Madison, Wis.) in which the BglII site in the multiple cloning site had been eliminated. The resulting plasmid, pUIC, contains a unique BglII site in the intergenic region between the UL49 and UL50 genes (Fig. 1A, diagram ii). This site was used to introduce a BamHI fragment containing the ICP6 promoter::lacZ cassette (from pD6p [28], gift of Sandra Weller, University of Connecticut Health Sciences Center, Farmington, Conn.) to create pUIClacZ (Fig. 1A, diagram iii). Vero cells were cotransfected with 1 μg of infectious KOS DNA and 2.5 μg of pUIClacZ as described by Cai and Schaffer (12). Progeny of the cotransfection were screened on Vero cell monolayers in medium containing 2% methylcellulose and the β-galactosidase (β-gal) chromogenic substrate, X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside). Recombinants containing the ICP6 promoter::lacZ cassette produced blue plaques. One blue plaque, KOS6β, was isolated and plaque purified three times.
FIG. 1.
Construction, Southern blot analysis, and one-step growth curve of KOS6β relative to wild-type strain KOS. (A) Construction of KOS6β. (i) Diagram of the HSV-1 genome (not drawn to scale) showing the unique long (UL) and unique short (US) regions of the genome, each flanked by inverted repeat sequences (ab and b′a′ [UL] and a′c′ and ca [US], respectively) shown as open boxes. (ii) Diagram of pUIC relative to the locations of the UL49 and UL50 genes in the cloned, subgenomic PvuII-PstI fragment. This plasmid contains a unique BglII site in the intergenic region between the UL49 and UL50 genes. Relevant restriction enzyme cleavage sites and map distances presented in base pairs are indicated. (iii) Diagram of pUIClacZ. The BamHI subfragment of plasmid pD6p containing the ICP6 promoter and E. coli lacZ protein coding sequence (open box) was cloned into the unique BglII site in pUIC. (B) Southern blot comparing KpnI digests of the UL49- and UL50-coding sequences of KOS and KOS6β DNA obtained from infected Vero cells by using 32P-labeled pUIClacZ as the probe (Fig. 1A, diagram iii). Expected sizes of fragments (in kilobases) are indicated to the left of the gel. (C) One-step growth curves of KOS6β and KOS in Vero cells at a multiplicity of 5 PFU per cell. Infectious virus was measured by standard plaque assay on Vero cell monolayers.
Southern blot analysis.
KOS and KOS6β viral DNAs were isolated from infected Vero cells (16) and digested with KpnI, and the resulting fragments were subjected to Southern blot analysis with 32P-labeled pUIClacZ probe as described by DeLuca et al. (16).
One-step growth curves.
Vero cells (1.2 × 105 cells) were plated in multiple 35-mm tissue culture dishes and infected 24 h later with 5 PFU of KOS or KOS6β per cell. After 1 h of absorption at 37°C, the inoculum was removed, cells were washed three times with phosphate-buffered saline (PBS), and Vero cell growth medium was added back to each dish. At the indicated times postinfection, cells were scraped into medium, and the resulting suspensions were frozen at −70°C. Samples were later thawed, sonicated, and clarified by low-speed centrifugation. Infectious virus in supernatant fluids was quantified by standard plaque assays on Vero cell monolayers (67).
Induction of the ICP6 promoter by ICP0.
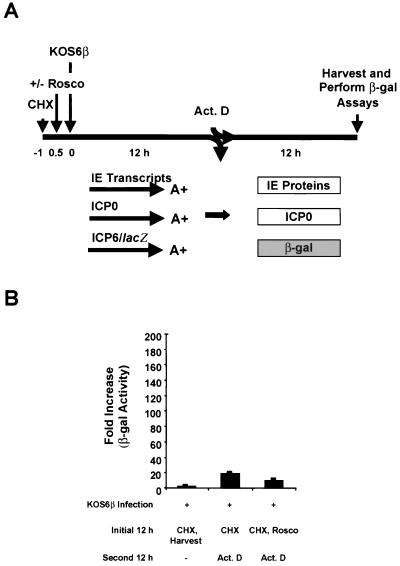
The experimental design of these tests is illustrated in Fig. 2A. Briefly, Vero cells were plated in 24-well plates (5 × 104 cells per well). After 23 h, the cells were pretreated with CHX (50 μg/ml) for 1 h and either mock infected or infected with 5 PFU of KOS6β per cell for 1 h at 37°C in the presence of CHX to inhibit viral protein synthesis. After virus absorption, the inoculum was removed, the monolayers were washed three times with PBS containing CHX to remove unabsorbed virus, 1 ml of Vero cell medium with CHX was added to each well, and the monolayers were incubated for an additional 4.5 h (t = 5.5 h postinfection [hpi]). At 5.5 hpi, cells were preincubated for 0.5 h in medium containing CHX in the absence or presence of each of the following: ActD, Rosco, or PAA. At 6 hpi, duplicate monolayers were either harvested directly or washed with, and released directly into, Vero cell medium in the presence or absence of CHX, ActD, Rosco, or PAA. At the end of the second 6-h treatment period (t = 12 hpi), cell extracts were prepared and β-gal assays were performed as described below.
FIG. 2.
Rosco inhibits ICP0-induced expression of β-gal from the ICP6 promoter. (A) Experimental design. Vero cells were pretreated with CHX (50 μg/ml) for 1 h (t = −1) and mock infected or infected with 5 PFU of KOS6β per cell at t = 0 in the presence of CHX for 6 h (t = 6 hpi). Cells were then either harvested or released into medium with or without CHX (50 μg/ml), ActD (1 μg/ml), Rosco (100 μM), or PAA (100 μg/ml) for an additional 6 h. At the end of the 6-h treatment period (t = 12 hpi), cell extracts were prepared and β-gal assays were performed. (B) Histogram showing results of a typical β-gal assay. All samples were compared to mock-infected cells as controls (far left sample); this control was given the arbitrary value of “1.” The data shown represent the means of duplicate samples; bars indicate the standard deviations of the means.
Basal ICP6 promoter activity.
The experimental design of these tests is shown in Fig. 3A. Vero cells were plated in duplicate 24-well plates (5 × 104 cells per well) and, 23 h later, monolayers were pretreated with medium containing CHX (75 μg/ml) for 1 h. One-half hour after CHX pretreatment had begun (t = −0.5), monolayers in one 24-well plate were pretreated with Rosco (100 μM) and monolayers in the second plate were left untreated. One-half hour later (t = 0), cells were either mock infected or infected with 5 PFU of KOS6β per cell for 1 h at 37°C in medium containing CHX with or without Rosco. After the 1-h absorption period, the inoculum was removed, cells were washed three times with PBS containing CHX with or without Rosco to remove unabsorbed virus, and 1 ml of medium containing CHX with or without Rosco was added per well. Monolayers were incubated for an additional 10.5 h (t = 11.5 hpi) at 37°C. At this time, the media on monolayers were changed to medium containing ActD for 0.5 h in the presence of CHX with or without Rosco. Infected cells were then washed and harvested (t = 12 hpi) directly or released into medium containing ActD for an additional 12 h (t = 24 hpi). The addition of ActD to the medium in the second 12-h incubation period permits the translation of IE transcripts that had accumulated during the 12-h CHX block and also inhibits ICP0-mediated transcriptional activation of the ICP6 promoter. At the end of the second 12-h treatment period (t = 24 hpi), cell extracts were prepared, and β-gal assays were performed as described below.
FIG. 3.
Rosco minimally affects basal expression of β-gal from the ICP6 Promoter. (A) Experimental design. Vero cells were pretreated with CHX (75 μg/ml) for 1 h (t = −1). At 0.5 h after CHX pretreatment was initiated (t = −0.5), one set of cultures was pretreated or not with Rosco (100 μM). Cells were then infected with 5 PFU of KOS6β per cell at t = 0 and incubated in the presence or absence of 100 μM Rosco for 12 h in medium containing CHX (t = 12 hpi). Infected cells were either harvested or released into medium containing the RNA synthesis inhibitor, ActD (1 μg/ml), for an additional 12 h (t = 24 hpi). At the end of the second 12-h treatment period, extracts were prepared and β-gal assays performed. (B) Histogram showing results of a typical β-gal assay. All samples are compared to KOS6β-infected cells harvested after the initial 12-h CHX block (far left sample), which was given the arbitrary value of “1.” The data shown represent the means of duplicate samples; bars indicate the standard deviations of the means. Cell viability was maintained in these cultures 24 hpi. Specifically, all KOS6β-infected cells were 93% viable compared to mock-infected, untreated samples in which 96% of the cells were viable, as determined by trypan blue exclusion.
β-gal and protein assays.
Cell extracts were prepared by lysing cells in 50 μl of cold Triton-Tris lysis buffer (1% Triton X-100, 20 mM Tris-HCl [pH 8.0], 150 mM NaCl, and fresh 1 mM dithiothreitol [85]). β-gal activity was assayed as described previously (63). Briefly, 10 μl of cell extract was added to 90 μl of a solution containing 11 mM KCl, 88 mM sodium phosphate buffer (pH 7.3), 1 mM MgCl2, 5 mM 2-mercaptoethanol, and freshly added 4.4 mM chlorophenol red-β-d-galactopyranoside (Calbiochem, San Diego, Calif.). Reaction mixtures in 96-well plates were incubated for 45 min at 37°C and overnight at room temperature. Optical densities were read at 595 nm on a plate reader (Molecular Dynamics, Sunnyvale, Calif.), and the amount of β-gal activity was determined by a use of a standard linear curve. β-gal assays were standardized relative to protein concentration (Bio-Rad Protein Assay; Bio-Rad, Hercules, Calif.) according to the manufacturer’s protocol.
Localization of ICP0 by immunofluorescence.
The localization of ICP0 was carried out essentially as described above for induction of the ICP6 promoter by ICP0, except that cells were mock infected or infected with wild-type HSV-1, strain KOS, or dlx3.1 and not KOS6β. At the end of the second 6-h treatment period (t = 12 hpi), the medium was removed, and the coverslips were washed twice with PBS, fixed in 3.7% buffered formalin phosphate (Fisher Scientific, Fair Lawn, N.J.) for 15 min on ice, washed twice with PBS, and then permeabilized with acetone for 2 min at −20°C. Cells were then rehydrated by two washes in sterile water and equilibrated by performing two washes in PBS containing 1% bovine serum albumin (BSA). Primary ICP0 antibody (murine monoclonal antibody H1112 [1]; Goodwin Institute for Cancer Research, Plantation City, Fla.) was diluted 1:800 in PBS containing 1% BSA, and 100 μl was added to each coverslip. Coverslips were incubated for 30 min at 37°C in a humidified chamber. Coverslips were washed three times with PBS containing 1% BSA, and 100 μl of secondary antibody (goat anti-mouse antibody [immunoglobulin G] conjugated with fluorescein isothiocyanate-conjugated antibody; Jackson ImmunoResearch, West Grove, Pa.) diluted 1:100 was added to each coverslip. Coverslips were incubated for 30 min at 37°C in a humidified chamber and then washed three times with PBS containing 1% BSA and three times with distilled water; 15 μl of Prolong Antifade Solution (Molecular Probes, Eugene, Oreg.) was then added to each coverslip. After being mounted on slides, cells were viewed by bright-field and fluorescence microscopy with a Nikon Eclipse TE300 fluorescence microscope at ×400 magnification, and the cells were photographed with an RT Slider digital camera (Diagnostic Instruments, Sterling Heights, Mich.) in Adobe Photoshop (Adobe Systems Inc., Mountain View, Calif.). Images were printed by using a Kodak ds 8650 PS printer (Kodak, Rochester, N.Y.).
Radiolabeling and sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) of 35S-labeled infected cell extracts.
Vero cells (5 × 105 cells per 60-mm dish) were plated 24 h prior to infection. At 23 h postplating, cells were pretreated with CHX (50 μg/ml) for 1 h and then mock infected or infected with KOS or dlx3.1 for 1 h at 37°C in the presence of CHX. After 1 h of absorption, the inoculum was removed, cells were washed three times with PBS containing CHX, 4 ml of Vero cell medium plus CHX was added per plate, and plates were incubated for 4.5 h (t = 5.5 hpi) at 37°C. At t = 5.5 hpi, cells were preincubated for 0.5 h in phosphate-free, methionine-free, or methionine-cystine-free DMEM containing CHX in the absence or presence of drugs (ActD and/or Rosco). At t = 6 hpi, medium was removed and infected cells were washed three times with phosphate-free, methionine-free, or methionine-cystine-free DMEM containing 1% fetal calf serum in the absence or presence of the indicated drugs. Cells were then labeled with 500 μCi of 32Pi, 50 μCi of [35S]methionine, or 50 μCi of [35S]methionine-cysteine (New England Nuclear, Boston, Mass.) in phosphate-free, methionine-free, or methionine-cystine-free DMEM containing 1% fetal calf serum, respectively. At the end of the second 6-h treatment period (t = 12 hpi), cells were washed twice with ice-cold PBS, scraped into 1 ml of ice-cold PBS, pelleted, and resuspended in 50 μl of standard radioimmunoprecipitation assay (RIPA) lysis buffer (150 mM NaCl, 50 mM Tris [pH 7.5], 0.1% sodium dodecyl sulfate, 1% Nonidet P-40, and 0.5% deoxycholic acid) containing the protease inhibitors phenylmethysulfonyl fluoride (PMSF; 1 mM), leupeptin (1 μg/ml), and aprotinin (1 μg/ml). Phosphatase inhibitors I and II (Sigma, St. Louis, Mo.) were included in the RIPA buffer in some experiments. Notably, the presence or absence of phosphatase inhibitors did not affect the phosphorylation pattern of ICP0, as determined by phosphotryptic peptide mapping. For [35S]methionine-labeled samples, 25 μl of extract was added to 25 μl of 2× Laemmli buffer (42) containing 2 mM PMSF. Samples were heated at 100°C for 5 min, placed on ice for 2 min, and analyzed on a 27-cm 6% acrylamide SDS-PAGE gel. Gels were visualized by PhosphorImager analysis (Molecular Dynamics).
Immunoprecipitation of ICP0.
To immunoprecipitate ICP0, 1 ml of RIPA buffer containing protease and phosphatase inhibitors was added to 25 μl of cell extract from the labeling experiment described above. Next, 1 μl of J17, an ICP0 polyclonal rabbit antibody (85), was added per sample, and the suspensions were agitated overnight at 4°C. The next day, 20 μl of protein A-agarose (Gibco-BRL/Life Technologies, Gaithersburg, Md.) was added to each sample, and the mixtures were agitated for 2 h at 4°C. Immune complexes were pelleted by centrifugation (2 min, 3,300 × g, 4°C). The supernatant fluid was removed, and each pellet was briefly washed and repelleted three times in RIPA buffer plus protease inhibitors. The resulting pellet was resuspended in 40 μl of 1× Laemmli buffer plus 1 mM PMSF (42). Samples were heated at 100°C for 5 min, placed on ice for 2 min, and centrifuged to pellet the protein A-agarose, and the supernatant was analyzed by SDS-9% PAGE. Proteins were visualized by PhosphorImager analysis.
Phosphotryptic peptide mapping. (i) Protein preparation and digestion with trypsin.
These procedures were carried out essentially as described by Boyle et al. (7). Briefly, 32P-labeled immunoprecipitated ICP0 was subjected to SDS-9% PAGE, and bands were excised from the gels. Gel slices were crushed with a Kontes pestle homogenizer, heated for 5 min at 100°C in a 1-ml total volume of 50 mM ammonium bicarbonate (pH 7.3) containing 0.1% SDS and 5% 2-mercaptoethanol, and rocked overnight at room temperature. Samples were centrifuged, the supernatant was removed and retained, and the pellet was heated once again at 100°C for 5 min in 50 mM ammonium bicarbonate containing 0.1% SDS and 5% 2-mercaptoethanol (total volume, 1.3 ml) and rocked overnight at room temperature. Suspensions were centrifuged, the supernatants from the two extractions were combined, and the eluted proteins were precipitated on ice for 2 h by the addition of 250 μl of 100% ice-cold trichloroacetic acid. Then, 20 μg of RNase A (Sigma) was added as the carrier protein. Pelleted protein was washed with 100% acetone (−20°C), resuspended in 70 μl of cold performic acid (9 parts 88% formic acid, 1 part 33% hydrogen peroxide), and incubated on ice for 1 h. Then, 100 μl of water was added to each sample, and the samples were frozen at −70°C and lyophilized. Each sample was resuspended in 50 μl of 50 mM ammonium bicarbonate (pH 8.0 to 8.3) and digested with 20 μg of trypsin containing TPCK (tosylsulfonyl phenylalanyl chloromethyl ketone; Worthington Biochemicals, Lakewood, N.J.) at 37°C overnight. In some cases, immunoprecipitates were used directly for analysis. Samples were heated in 50 mM ammonium bicarbonate for 5 min at 100°C, placed on ice for 2 min, and pelleted by centrifugation for 2 min (3,300 × g at 4°C), and protein in the supernatants was digested with trypsin. The results obtained from analysis of immunoprecipitates were comparable to those obtained with protein eluted from gel slices. Digested samples were washed with distilled water and lyophilized three times before a final wash and lyophilization in pH 1.9 buffer (88% formic acid-acetic acid-water [25:78:897, by volume]).
(ii) Two-dimensional separation of phosphopeptides.
Lyophilized samples were resuspended in 7 μl of pH 1.9 buffer. Approximately equal Cherenkov counts were used for analysis. At least 500 counts were loaded onto 20-by-20-cm cellular thin-layer chromatography (TLC) plates (EM Science, Gibbstown, N.J.). Peptides were spotted on TLC plates with 0.5 μl of tracking dye solution (5 mg of ɛ-DNP-lysine and 1 mg of xylene cyanol FF/ml, solubilized in water containing 50% [vol/vol] pH 4.72 buffer composed of n-butanol-pyridine-acetic acid-water [10:5:5:180, by volume]). Electrophoresis was carried out at 1,000 V for 34 min in pH 1.9 buffer in the Hunter Thin Layer Peptide Mapping Electrophoresis System (CBS Scientific, Del Mar, Calif.). Plates were dried, and ascending chromatography was performed in isobutyric acid buffer (isobutyric acid-pyridine-acetic acid-n-butanol-water [1,251:96:58:38:558, by volume]) overnight. Plates were dried, and peptides were visualized by PhosphorImager analysis.
RESULTS
Construction and characterization of KOS6β.
Of the five HSV-1 IE regulatory proteins, ICP0 is a potent and unique transactivator of the HSV early promoter, UL39, encoding ICP6, the large subunit of ribonucleotide reductase. ICP0 uniquely transactivates the ICP6 promoter both in the context of viral infection and in transient-transfection assays (15, 18, 28, 65, 71, 75, 77). Consequently, we utilized ICP6 promoter activity as a readout for the transactivating activity of ICP0 in the background of an HSV recombinant, KOS6β. KOS6β was constructed by cloning an ICP6 promoter::lacZ cassette (28) into the unique BglII site in the intergenic region between the UL49 and UL50 genes in the plasmid pUIC creating the plasmid pUIClacZ (Fig. 1A). The cassette and flanking sequences were excised from the plasmid and introduced into the viral genome by homologous recombination after cotransfection with infectious KOS DNA. The resulting recombinants were screened for their ability to produce blue plaques by staining for β-gal expression with the chromogenic substrate X-Gal.
One recombinant, KOS6β, was plaque purified. In parallel with KOS DNA, KOS6β DNA was isolated, digested with KpnI, and subjected to Southern blot analysis to ensure the correct size, location, and orientation of the cassette in the KOS6β genome (Fig. 1B). After KpnI digestion of KOS DNA, two fragments, 2.8 and 4.7 kb, were detected by using pUIClacZ probe (Fig. 1A, diagram ii). In contrast, three fragments (3.0, 4.0, and 4.7 kb) were detected after KpnI digestion of KOS6β DNA (Fig. 1A, diagrams ii and iii). The differences in the numbers and sizes of the KpnI fragments result from insertion of the ICP6 promoter::lacZ cassette (4.2 kb) into KOS DNA. The cassette in KOS6β contains an additional KpnI site in the ICP6 promoter producing a third fragment.
The replication efficiencies of KOS6β and KOS were then compared in one-step growth curves in Vero cells (Fig. 1C). The kinetics of replication and yields of the two viruses were essentially the same, indicating that the presence of the ICP6 promoter::lacZ cassette does not affect the replication of HSV detectably, making KOS6β a suitable tool for this study. In vivo, KOS6β replicated as efficiently as KOS in mouse eyes in a mouse ocular model of HSV-1 latency (74; D. J. Davido and D. A. Leib, unpublished data).
Rosco inhibits ICP0-induced expression of β-gal from the ICP6 promoter.
To determine the effects of the cdk inhibitor Rosco on the ability of ICP0 to induce β-gal expression from the ICP6 promoter, a protocol was designed in which KOS6β-infected Vero cells were released from a CHX block (Fig. 2A). The CHX block, initiated at or before the time of infection, results in the accumulation of IE transcripts, and the release from the CHX block allows these transcripts to be translated (34), allowing us to examine the effects of Rosco on ICP0’s posttranslational state and its transactivating activity. CHX, ActD (an RNA synthesis inhibitor), and PAA (a viral DNA synthesis inhibitor) were added individually at the time of release from the CHX block to confirm that the ICP6 promoter was indeed regulated as an early promoter in the context of KOS6β infection. This infection protocol was necessitated by the fact that Rosco inhibits transcription of IE genes (67).
As shown in Fig. 2B, relative to infected cells harvested at 6 hpi (i.e., immediately after release of the CHX block), an ∼110-fold induction of β-gal activity from the ICP6 promoter was apparent in KOS6β-infected cells released from the CHX block into medium containing no drug. As expected, the readdition of CHX or the addition of ActD at the time of release of the CHX block inhibited ICP0-mediated transactivation (70- and 30-fold, respectively), relative to the “no drug” control. PAA, which blocks viral DNA synthesis and leads to the accumulation of IE and early proteins (35), produced a further fourfold increase in transactivation of the ICP6 promoter compared to the “no drug” control. The results obtained with CHX, ActD, and PAA are consistent with the expression of ICP6 as an early gene in the context of KOS6β. Finally, Rosco inhibited ICP0’s ability to activate expression from the ICP6 promoter by 50-fold relative to KOS6β-infected cultures that contained no drug after removal of the CHX block. Thus, the ability of Rosco to inhibit ICP0-mediated transactivation of the ICP6 promoter was similar to the inhibitory effects of ActD or CHX.
Rosco has a minimal effect on the basal expression of β-gal from the ICP6 promoter.
To exclude the possibility that the inhibition of β-gal synthesis by Rosco was not at the level of basal ICP6 promoter activity, infections that included Rosco in one set of KOS6β-infected cultures during the CHX block were carried out. The design of this experiment is presented in Fig. 3A. Specifically, two 12-h incubation periods, the 12-h period of the CHX block and a 12-h period after release of the block in the presence of ActD, were required in this assay because the basal activity of the ICP6 promoter was barely detectable above the level of mock-infected cells after two 6-h incubation periods (data not shown). Release of the CHX block into medium containing ActD allows for the translation of all transcripts that accumulate during the block but inhibits ICP0-mediated transactivation of the ICP6 promoter. This experiment demonstrated a 14-fold increase and a 7-fold increase in ICP6 basal promoter activity in the absence and presence of Rosco, respectively, compared to KOS6β-infected cells harvested directly after the 12-h CHX block (Fig. 3B). Thus, Rosco had only a minimal effect (∼2-fold) on basal expression of β-gal from the ICP6 promoter.
Rosco does not affect the nuclear localization of ICP0.
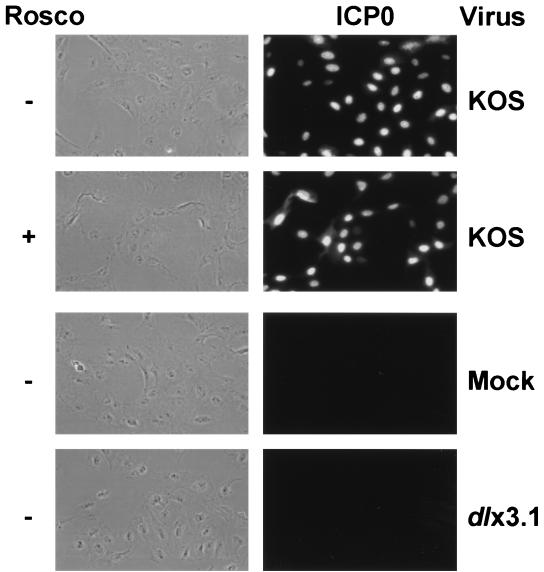
Because Rosco inhibited the transactivating activity of ICP0, we sought to determine whether it affects the localization of ICP0 to the cell nucleus as a possible explanation for the inhibition (1). In this experiment, we utilized the CHX block protocol illustrated in Fig. 2A, except that ICP0 was expressed from KOS and not KOS6β. Immunofluorescence tests with an ICP0-specific antibody were conducted after the second 6-h incubation period. As shown in Fig. 4, the fluorescent staining pattern of ICP0 was predominantly nuclear, both in the presence and in the absence of Rosco. Thus, Rosco did not affect the nuclear localization of ICP0. As anticipated, no ICP0 was detected in mock-infected or dlx3.1 (an ICP0 null mutant)-infected cells.
FIG. 4.
Rosco does not affect the nuclear localization of ICP0. Vero cells were pretreated with CHX (50 μg/ml) for 1 h, mock infected or infected with 5 PFU per cell of KOS or dlx3.1 (an ICP0 null mutant), and incubated for 6 h in the presence of CHX. Viruses used are indicated on the right of the figure. Cells were then released from the CHX block in the absence (−) or presence (+) of Rosco for 6 h, as indicated. At the end of the second 6-h treatment period, cells were washed, fixed, permeabilized, and incubated with a primary mouse monoclonal antibody against ICP0 (1). Primary antibody was detected with a fluorescein isothiocyanate-conjugated rabbit anti-mouse antibody. Monolayers were viewed by phase-contrast (left column) and fluorescence microscopy (right column) at ×400 magnification, and cells were photographed with a digital camera.
Rosco affects the electrophoretic mobility of ICP0.
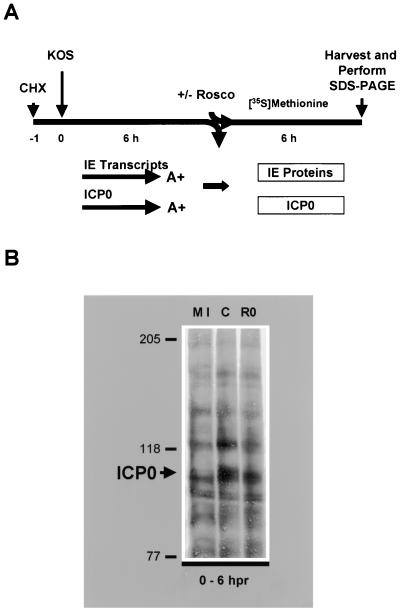
Since the subcellular localization of ICP0 was unaffected by Rosco treatment, we next sought to determine whether the level and/or electrophoretic mobility of ICP0 were altered in the presence of Rosco. For this purpose, KOS-infected cells were radiolabeled with [35S]methionine in the presence or absence of Rosco after release from the CHX block (Fig. 5A), and proteins in whole-cell extracts were resolved on a long SDS-PAGE gel. Although ICP0 protein levels were roughly equivalent in the presence and absence of Rosco in KOS-infected cells, a portion of ICP0 molecules exhibited reduced electrophoretic mobility in the absence of Rosco relative to the presence of the drug as previously reported (Fig. 5B) (69). Thus, Rosco-sensitive cdks affect the posttranslational state of a subset of ICP0 molecules. Notably, Rosco had no detectable effect on cellular protein synthesis.
FIG. 5.
Rosco affects the electrophoretic mobility of ICP0. (A) Experimental design. Vero cells were pretreated with CHX (50 μg/ml) for 1 h (t = −1) and mock infected or infected with 5 PFU of KOS per cell (t = 0 hpi) and incubated in the presence of CHX for 6 h (t = 6 hpi). Cells were released from the CHX block into methionine-free medium containing [35S]methionine in the absence or presence of Rosco (100 μM) for an additional 6 h (t = 12 hpi). At the end of the second 6 h (t = 12 hpi), extracts were prepared and proteins were resolved by SDS-PAGE and transferred to nitrocellulose. (B) SDS-PAGE analysis. Samples from mock-infected cells (MI), control cells infected with KOS in the absence of Rosco (C), and cells infected with KOS in the presence of Rosco (RO) were resolved by SDS-6% PAGE. Molecular mass markers (in kilodaltons) and the band specific for ICP0 (large arrow) are indicated on the left. Radiolabeled bands were visualized by PhosphorImager analysis.
Effect of Rosco on the phosphorylation state of ICP0.
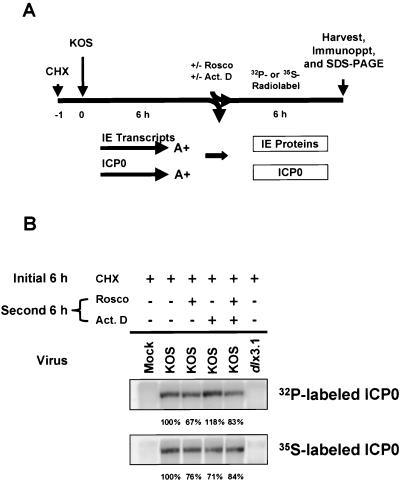
Because we observed an alteration in the electrophoretic mobility of ICP0 in the presence or absence of Rosco on two separate occasions (69; the present study), we hypothesized that ICP0 is directly phosphorylated by Rosco-sensitive cdks. To test this hypothesis, ICP0 was radiolabeled with [35S]methionine-cysteine or [32P]orthophosphate after reversal of the CHX block in the presence or absence of Rosco and/or ActD (Fig. 6A). ICP0 was subsequently immunoprecipitated from cell extracts and analyzed by SDS-PAGE. The results of this experiment indicate that differences in the levels of ICP0 synthesis in the four treatment groups were minimal as determined by 35S labeling (Fig. 6B). With regard to phosphorylation, any differences in the level of ICP0 phosphorylation were not apparent as determined by 32P labeling (Fig. 6B). Most of the 35S-labeled ICP0 synthesized was phosphorylated in the presence of Rosco, however, indicating that the majority of the phosphorylation of ICP0 is accomplished by Rosco-insensitive cdks or other kinases. In repeat experiments, the high level of ICP0 phosphorylation observed for ActD-treated samples was not reproducible. As expected, mock- and dlx3.1-infected cells did not express ICP0. It should be noted that the altered electrophoretic mobility of a portion of ICP0 molecules in the presence of Rosco observed in Fig. 5B was not evident in Fig. 6B since the percent acrylamide in the former gel was 6% and in the latter gel was 9%.
FIG. 6.
Rosco has no apparent effect on the level of phosphorylation of ICP0 (A) Experimental design. Vero cells were pretreated with CHX (50 μg/ml) for 1 h (t = −1), mock infected or infected with 5 PFU per cell of KOS or dlx3.1 (ICP0 null mutant) at t = 0, and incubated in the presence of CHX for 6 h (t = 6 hpi). Half of the cultures were then released from the CHX block into phosphate-free medium containing [32P]orthophosphate. The remaining cultures were released into methionine-cystine-free medium containing [35S]methionine-cysteine. Radiolabeling was carried out in the absence or presence of Rosco (100 μM) and/or ActD (1 μg/ml) for an additional 6 h (t = 12 hpi). After this second 6-h treatment period, extracts were prepared, samples were immunoprecipitated with an ICP0 rabbit polyclonal antibody. (B) SDS-PAGE analysis of radiolabeled, immunoprecipitated ICP0 on 9% gels. ICP0 32P- and 35S-specific bands are indicated on the left and were visualized by PhosphorImager analysis. Quantitation of the band intensities of ICP0 was determined by PhosphorImager analysis relative to the “no drug” control (expressed as 100%). In repeat experiments, the high level of ICP0 phosphorylation observed for ActD-treated sample was not reproducible.
Phosphotryptic peptide mapping.
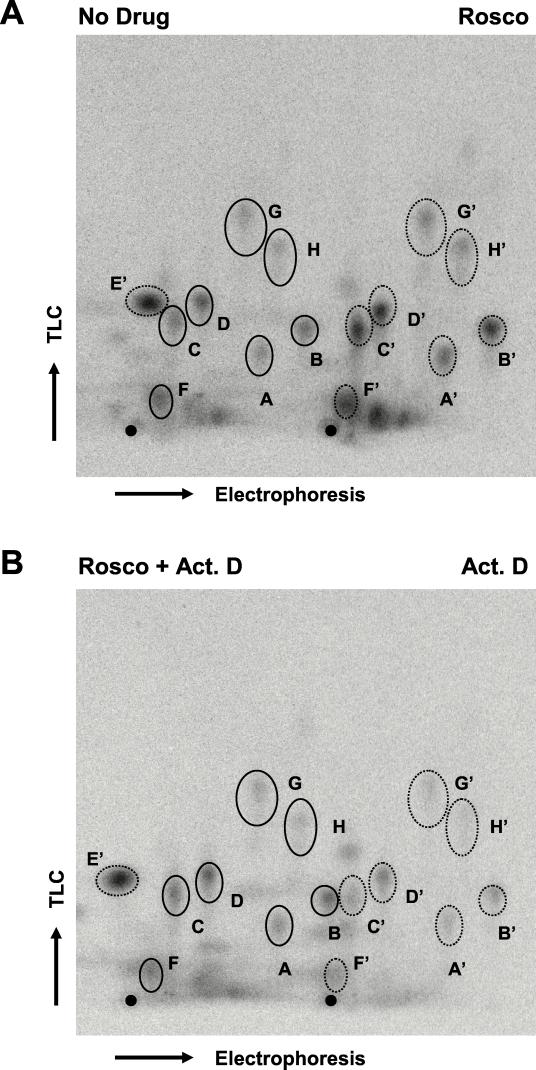
Since it is possible that only a subset of phosphorylation sites in ICP0 may be phosphorylated by cdks, phosphotryptic peptide analysis was performed to examine the phosphorylation of ICP0 in greater detail. 32P-labeled ICP0, either immunoprecipitated or extracted from gel slices, was digested with trypsin, generating smaller peptides which were resolved by two-dimensional tryptic-peptide analysis; electrophoresis was carried out in the first dimension, and ascending chromatography was carried out in the second (7). As shown in Fig. 7A, eight major ICP0 phosphopeptide signals from KOS-infected Vero cells synthesized in the absence of Rosco were observed (left side of Fig. 7A, labeled A through H). (Note that E [not visible] and E′ migrate to the left of the origin in the electrophoresis dimension; E and E′ appear to be free 32P [data not shown]). To examine the effects of Rosco on individual ICP0 phosphopeptides, 32P-labeled ICP0 synthesized in the absence of drug (Fig. 7A, No Drug) was compared side by side on the same plate with ICP0 synthesized in the presence of Rosco (Fig. 7A, Rosco). In these tests, the signals of ICP0 phosphopeptides from Rosco-treated samples relative to the “no drug” control were similar with regard to their relative intensities and mobilities. Similarly, the ICP0 phosphotryptic maps of peptides produced in the presence of both ActD and Rosco and in the presence of ActD alone (Fig. 7B) exhibited essentially the same migration patterns and phosphorylation signals as the “no drug” control shown in Fig. 7A. Notably, any differences observed in the intensities of phosphorylation signals between treatment groups (i.e., “no drug” versus ActD) can be attributed to variations in loading as the intensities of individual signals (A to H) were roughly maintained within each group. Our data suggest that the majority of posttranslational modifications affected by Rosco do not involve phosphorylation and that the phosphorylated peptides whose analysis is shown in Fig. 7A were not derived from the subset of molecules exhibiting reduced mobility as seen in Fig. 5B, since these peptides would be undetectable by this technique. Collectively, these findings indicate that although Rosco-sensitive cdks may well phosphorylate ICP0, they are not the principal kinases that phosphorylate the majority of this protein in Vero cells. On the other hand, Rosco-sensitive cdks appear to be required for other posttranslational modification(s) of ICP0 which, when inhibited, result in the altered electrophoretic mobility of a subset of ICP0 molecules as detected by one-dimensional SDS-PAGE.
FIG. 7.
Two-dimensional tryptic-peptide analysis of 32P-labeled ICP0. (A) ICP0 phosphopeptides derived from infected cells either left untreated (left side of plate, solid ovals) or Rosco treated (right side of plate, dotted ovals, prime marked) samples. Eight phosphopeptide signals (A through H) were detected in this analysis. (Note that the signal for E migrates in the opposite direction from the other phosphopeptide signals during the electrophoresis dimension.) (B) ICP0 phosphopeptides derived from samples treated with Rosco plus ActD (left side of plate, solid ovals) or ActD alone (right side of plate, dotted ovals, prime marked). The signal for E has migrated off the plate for the “no drug” samples and the samples treated with Rosco plus ActD. ICP0 was precipitated, digested with trypsin-TPCK, lyophilized, and analyzed on TLC plates. For each plate, electrophoresis was carried out for 34 min at 1,000 V in pH 1.9 buffer; plates were subsequently dried, and ascending chromatography performed according to the method of Boyle et al. (7). These results were reproduced in three independent experiments. The origin of loading is evident as a black dot at the bottom of each TLC plate.
DISCUSSION
Rosco-sensitive cdks are required for the transactivating activity of ICP0 and alter its posttranslational modification.
Previous studies have shown that the replication of ICP0 null mutants is significantly enhanced by a cellular activity expressed during the late G1/S phase of the cell cycle (10) and that ICP0 blocks the cell cycle at G1/S and G2/M in HSV-infected cells (32, 45). In the present study, we have shown that Rosco-sensitive cdks are required for the transactivating activity of ICP0 and that these cdks also affect the posttranslational modification of ICP0, demonstrating that Rosco-sensitive cdks, nearly all of which are active at specific stages of the cell cycle, are required for at least two properties of ICP0. Thus, an interesting dynamic is apparent in which ICP0 regulates aspects of the cell cycle, and cell cycle-associated activities regulate ICP0. These findings suggest that cdks, either directly or indirectly, mediate the posttranslational modification of ICP0 which, in turn, is required for its transactivating activity.
With regard to the mechanism of inhibition of ICP0’s transactivating activity by Rosco, our findings indicate that this inhibition is not primarily at the level of basal ICP6 promoter activity since Rosco had only a minimal effect on the basal activity of the ICP6 promoter (Fig. 4). To further support this observation, we performed Northern blot and S1 nuclease analyses of ICP6 transcripts synthesized in HSV-infected cells to examine basal transcription under CHX block conditions in the presence or absence of Rosco. Unfortunately, we were unable to detect ICP6 transcripts reproducibly in these experiments because of the very low levels of basal transcription (data not shown). Stingley et al. reported similar difficulties in detecting ICP6 transcripts in KOS-infected rabbit skin cells in the presence of CHX (72), and Samaniego et al. observed no measurable ICP6 protein synthesis in KOS-infected Vero cells after CHX reversal (65). Techniques which allow for greater sensitivity are currently being tested. Although this study was conducted with the ICP6 promoter, the available evidence (69) strongly suggests that Rosco-sensitive cdks are also required for the expression of all HSV early genes transactivated by ICP0 and for the synergistic transactivation of early and late genes by ICP0 and ICP4 (17, 22, 26, 60). Thus, Rosco significantly reduced the transcription of early genes in the presence of wild-type levels of IE proteins (including ICP0 and ICP4) but concomitantly altered the electrophoretic mobilities of these latter proteins (69).
Other DNA-containing viruses are also regulated by cell cycle-associated proteins. Thus, cdks affect the posttranslational modification of adenovirus E1A (48, 56), the E1 protein of human papillomavirus (47), and the large T antigens of polyomavirus (13, 61) and simian virus 40 (37, 52). Relevant to findings presented herein, cdk-mediated posttranslational modifications are required for critical regulatory functions of these proteins. Furthermore, cell cycle-associated activities, either directly or indirectly related to cdks, are a common feature of the life cycles of a number of herpesviruses. Specifically, the genomes of gammaherpesviruses, including Kaposi’s sarcoma herpesvirus (44), herpesvirus saimiri (55), and gammaherpesvirus 68 (33, 79), encode homologs of cellular cyclins, the partners and coactivators of cellular cdks. The alphaherpesviruses HSV-1 (67), HSV-2 (36), bovine herpesvirus type 1 (38), and varicella-zoster virus (83) and the betaherpesvirus human cytomegalovirus (8) either require or affect cdk activities during the course of infection. Our data are not only consistent with these observations but extend our understanding of the potential roles of cdks in HSV replication by demonstrating that two properties of the HSV-1 regulatory protein, ICP0, require cdks.
Rosco-sensitive cdks and their roles in the HSV-1 life cycle.
Rosco inhibits the kinase activities of cdk-1, cdk-2, cdk-5, cdk-7 and cdk-9 but not the activities of cdk-4, cdk-6, cdk-8, and 16 other kinases tested in vitro (53, 80; L. M. Schang, A. Bantley, M. Knockaert, F. Shaheen, L. Meijer, M. H. Malim, N. S. Gray, and P. A. Schaffer, unpublished data). Whether Rosco inhibits the activity of cdk-3 is not known. Rosco also inhibits erk-1 and erk-2 in vitro (53); however, these kinases are unlikely to be targets of Rosco in our experiments because their inhibition requires (i) a 10-fold-higher concentration of drug relative to the inhibition of Rosco-sensitive cdks (53) and (ii) a period of serum deprivation (at least 18 h) for inhibition in cell culture (78). Cells were not serum deprived in our experiments. Thus, the available evidence indicates that the phenotypes we observe with HSV-1 and ICP0 are due to inhibition of Rosco-sensitive cdk-1, cdk-2, cdk-5, cdk-7, and/or cdk-9.
As previously reported, Rosco-sensitive cdks are required for transcription (40, 67, 69) and replication (68) of the HSV-1 genome although the specific cdks required for these processes are unknown. Recent studies have shown that the activity of cdk-1 is activated (2) and that the activity of cdk-2 is inhibited (19) 12 and 8 hpi of highly transformed HeLa and U2OS cells, respectively. It has also been reported that a dominant-negative mutant form of cdk-1 inhibited expression of the HSV-1 US11 (γ2) late protein (but not ICP6 expression) 12 hpi of HEp-2 cells (4). Additionally, Rosco was recently reported to affect the posttranslational processing of ICP0 at 5 to 12 hpi of HEL cells (3). Importantly, all of these differences were detected after the ICP6 promoter is initially activated by ICP0 (i.e., at 4 hpi) (data not shown). Therefore, the changes in cdk-1 and cdk-2 activities and in the activities of Rosco-sensitive cdks in these reports (2, 3, 4, 19) may well represent later events in the HSV replication cycle and therefore be unrelated to the cdk-dependent ICP0-mediated transactivation of the ICP6 promoter described in this study. Notably, a direct correlation between the transactivating activity and posttranslational modification of ICP0 was not possible in the aforementioned reports involving cdk-1 and Rosco-sensitive cdks because of differences in the experimental design and reagents used in these studies (2–4).
Although the identities of the specific Rosco-sensitive cdks and the levels of these kinases required for ICP0 function during productive infection remain to be determined, even less is known about the roles of cdks in reactivation from neuronal latency. Although one might question the rationale underlying a requirement for Rosco-sensitive cdks in neurons given their postmitotic status, Rosco was recently shown to inhibit HSV reactivation from latency (L. M. Schang, A. Bantly, and P. A. Schaffer, unpublished). Given this requirement, one would predict that cdk gene expression and/or the activities of cdks are induced in reactivating neurons. Indeed, the presence of selected cdks in an increasing number of sensory neurons undergoing explant-induced stress has been demonstrated both in mock- and latently infected ganglia by Schang and coworkers (L. M. Schang, A. Bantly, and P. A. Schaffer, unpublished). Collectively, these observations, together with the requirement for ICP0 for efficient reactivation (9, 14, 29, 31, 43), and the findings presented in the present study suggest that Rosco-sensitive cdks regulate the functions of HSV-1 regulatory proteins, including ICP0, which are critical for reactivation.
Posttranslational modification of ICP0 by Rosco-sensitive cdks.
Because ICP0 is a highly phosphorylated protein (1, 82), a logical question is whether Rosco-sensitive cdks directly phosphorylate ICP0. Examination of the phosphorylation state of ICP0 by phosphotryptic-peptide analysis (Fig. 7A) led us to conclude that, although Rosco-sensitive cdks may phosphorylate ICP0 directly, they are not the principal kinases that phosphorylate this protein in Vero cells. Notably, however, transient or low levels of phosphorylation of a subset of ICP0 molecules by individual Rosco-sensitive cdks would be undetectable in our system. Indeed, in contrast to our findings, recent reports have indicated that one Rosco-sensitive cdk, cdk-1, is able to phosphorylate ICP0 in vitro (4) and that the phosphorylation of ICP0 in infected cells appears to be sensitive to Rosco (3) although, as noted above, these observations were made at later times after viral infection (6 to 10 hpi).
Aside from phosphorylation, our findings indicate that Rosco indirectly affects the synthesis or activities of other posttranslational modifying enzymes that directly alter the electrophoretic mobility of a subset of ICP0 molecules from 0 to 6 h postsynthesis (Fig. 5B). We hypothesize that these isoforms of ICP0 are required for its biological activities. This hypothesis is supported by the observations that the multiple modified isoforms of regulatory proteins of other DNA-containing viruses possess distinct functions. These include acetylation of the 12S E1A protein of adenovirus, which disrupts its binding to the cellular transcriptional corepressor, CtBP (84), and posttranslational modification of simian virus 40 large T antigen, which is important for its ATPase activity (adenylation) and subcellular localization (acylation, ribosylation, and glycosylation) (5, 41, 70, 76). An assessment of potential posttranslational modifications of ICP0 by amino acid sequence analysis (MacVector; Genetics Computer Group, Madison, Wis.) suggests that ICP0 is also modified by multiple posttranslational modifying enzymes which include phosphorylation, nucleotidylylation, acetylation, glycosylation, and myristoylation. Of these modifications, only phosphorylation (1, 82) and nucleotidylylation (6) have been demonstrated to date.
Indirect regulation of ICP0 function by Rosco-sensitive cdks.
In addition to direct or indirect changes in the posttranslational modification state of ICP0 by Rosco, other plausible mechanisms exist by which Rosco-sensitive cdks might indirectly affect ICP0 function. Thus, ICP0 has been shown to interact with several viral and cellular proteins (reviewed in reference 21), and these interactions contribute to the transactivating function of ICP0, since mutant forms of ICP0 which are unable to bind to these proteins are impaired for viral gene expression, viral growth, or pathogenesis. These ICP0-protein interactions may either be facilitated or disrupted by cdk-mediated phosphorylation of the interacting proteins. Rosco-sensitive cdks may also regulate ICP0 function via its effects on ND10, which are thought to serve as nuclear organization centers for HSV transcription and viral DNA replication (51) and appear to be critical for ICP0’s transactivating activity (49). Notably, cellular proteins that include ND10-associated antigens (24, 45, 58) are degraded in an ICP0-dependent manner via the ubiquitin-proteasome pathway, linking ICP0-mediated disruption of ND10 structures with its proteolytic induction of specific cellular proteins. Everett et al. have established that the ubiquitin-proteasome pathway is essential for ICP0-mediated transactivation (25). Therefore, it is possible that Rosco-sensitive cdks may control the transactivating activity of ICP0 via this pathway, in that Rosco-sensitive cdks have been reported to play a key role in controlling components of the ubiquitin-proteasome pathway (57). A more detailed understanding of these issues is fundamental to our understanding of ICP0 function.
Acknowledgments
This work was supported by Public Health Service grant RO1CA20260 from the National Cancer Institute. D.J.D. was supported by Public Health Service training grant T32AI07324-03 from the National Institute of Allergy and Infectious Diseases and an American Cancer Society fellowship (PF-00-021-01-MBC). D.A.L. was supported by Public Health Service grant RO1EY09083 and by a Robert E. McCormick Scholarship from Research to Prevent Blindness. Support from Research to Prevent Blindness and the NIH (P30-EY02687) to the Department of Ophthalmology is also gratefully acknowledged.
We thank members of the Schaffer lab for their helpful discussions and suggestions, Mark Yoa for providing excellent technical assistance with one-step growth curves, and Jill Meisenhelder from Tony Hunter’s lab for assistance with phosphotryptic peptide mapping.
REFERENCES
- 1.Ackermann, M., D. K. Braun, L. Pereira, and B. Roizman. 1984. Characterization of herpes simplex virus 1 alpha proteins 0, 4, and 27 with monoclonal antibodies. J. Virol. 52:108–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Advani, S. J., R. Brandimarti, R. R. Weichselbaum, and B. Roizman. 2000. The disappearance of cyclins A and B and the increase in activity of the G2/M-phase cellular kinase cdc2 in herpes simplex virus 1-infected cells require expression of the α22/US1.5 and UL13 viral genes. J. Virol. 74:8–15. [PMC free article] [PubMed] [Google Scholar]
- 3.Advani, S. J., R. Hagglund, R. R. Weichselbaum, and B. Roizman. 2001. Posttranslational processing of infected cell proteins 0 and 4 of herpes simplex virus 1 is sequential and reflects the subcellular compartment in which the proteins localize. J. Virol. 75:7904–7912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Advani, S. J., R. R. Weichselbaum, and B. Roizman. 2000. The role of cdc2 in the expression of herpes simplex virus genes. Proc. Natl. Acad. Sci. USA 97:10996–11001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baksi, K., H. Alkhatib, and M. E. Smulson. 1987. In vivo characterization of the poly(ADP-ribosylation) of SV40 chromatin and large T antigen by immunofractionation. Exp. Cell Res. 172:110–123. [DOI] [PubMed] [Google Scholar]
- 6.Blaho, J. A., C. Mitchell, and B. Roizman. 1993. Guanylylation and adenylylation of the alpha regulatory proteins of herpes simplex virus require a viral beta or gamma function. J. Virol. 67:3891–3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boyle, W. J., P. van der Geer, and T. Hunter. 1991. Phosphopeptide mapping and phosphoamino acid analysis by two-dimensional separation on thin-layer cellulose plates. Methods Enzymol. 201:110–149. [DOI] [PubMed] [Google Scholar]
- 8.Bresnahan, W. A., I. Boldogh, P. Chi, E. A. Thompson, and T. Albrecht. 1997. Inhibition of cellular Cdk2 activity blocks human cytomegalovirus replication. Virology 231:239–247. [DOI] [PubMed] [Google Scholar]
- 9.Cai, W., T. L. Astor, L. M. Liptak, C. Cho, D. M. Coen, and P. A. Schaffer. 1993. The herpes simplex virus type 1 regulatory protein ICP0 enhances virus replication during acute infection and reactivation from latency. J. Virol. 67:7501–7512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cai, W., and P. A. Schaffer. 1991. A cellular function can enhance gene expression and plating efficiency of a mutant defective in the gene for ICP0, a transactivating protein of herpes simplex virus type 1. J. Virol. 65:4078–4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cai, W., and P. A. Schaffer. 1992. Herpes simplex virus type 1 ICP0 regulates expression of immediate-early, early, and late genes in productively infected cells. J. Virol. 66:2904–2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cai, W. Z., and P. A. Schaffer. 1989. Herpes simplex virus type 1 ICP0 plays a critical role in the de novo synthesis of infectious virus following transfection of viral DNA. J. Virol. 63:4579–4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chatterjee, A., B. J. Bockus, O. V. Gjorup, and B. S. Schaffhausen. 1997. Phosphorylation sites in polyomavirus large T antigen that regulate its function in viral, but not cellular, DNA synthesis. J. Virol. 71:6472–6478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clements, G. B., and N. D. Stow. 1989. A herpes simplex virus type 1 mutant containing a deletion within immediate early gene 1 is latency-competent in mice. J. Gen. Virol. 70:2501–2506. [DOI] [PubMed] [Google Scholar]
- 15.Davido, D. J., and D. A. Leib. 1996. Role of cis-acting sequences of the ICP0 promoter of herpes simplex virus type 1 in viral pathogenesis, latency and reactivation. J. Gen. Virol. 77:1853–1863. [DOI] [PubMed] [Google Scholar]
- 16.DeLuca, N. A., M. A. Courtney, and P. A. Schaffer. 1984. Temperature-sensitive mutants in herpes simplex virus type 1 ICP4 permissive for early gene expression. J. Virol. 52:767–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeLuca, N. A., and P. A. Schaffer. 1985. Activation of immediate-early, early, and late promoters by temperature-sensitive and wild-type forms of herpes simplex virus type 1 protein ICP4. Mol. Cell. Biol. 5:1997–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Desai, P., R. Ramakrishnan, Z. W. Lin, B. Osak, J. C. Glorioso, and M. Levine. 1993. The RR1 gene of herpes simplex virus type 1 is uniquely trans activated by ICP0 during infection. J. Virol. 67:6125–6135. (Erratum, 68:1264, 1994.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ehmann, G. L., T. I. McLean, and S. L. Bachenheimer. 2000. Herpes simplex virus type 1 infection imposes a G1/S block in asynchronously growing cells and prevents G1 entry in quiescent cells. Virology 267:335–349. [DOI] [PubMed] [Google Scholar]
- 20.Everett, R. D. 1989. Construction and characterization of herpes simplex virus type 1 mutants with defined lesions in immediate early gene 1. J. Gen. Virol. 70:1185–1202. [DOI] [PubMed] [Google Scholar]
- 21.Everett, R. D. 2000. ICP0, a regulator of herpes simplex virus during lytic and latent infection. Bioessays 22:761–770. [DOI] [PubMed] [Google Scholar]
- 22.Everett, R. D. 1984. Transactivation of transcription by herpes virus products: requirement for two HSV-1 immediate-early polypeptides for maximum activity. EMBO J. 3:3135–3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Everett, R. D., W. C. Earnshaw, J. Findlay, and P. Lomonte. 1999. Specific destruction of kinetochore protein CENP-C and disruption of cell division by herpes simplex virus immediate-early protein Vmw110. EMBO J. 18:1526–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Everett, R. D., P. Freemont, H. Saitoh, M. Dasso, A. Orr, M. Kathoria, and J. Parkinson. 1998. The disruption of ND10 during herpes simplex virus infection correlates with the Vmw110- and proteasome-dependent loss of several PML isoforms. J. Virol. 72:6581–6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Everett, R. D., A. Orr, and C. M. Preston. 1998. A viral activator of gene expression functions via the ubiquitin-proteasome pathway. EMBO J. 17:7161–7169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gelman, I. H., and S. Silverstein. 1985. Identification of immediate early genes from herpes simplex virus that transactivate the virus thymidine kinase gene. Proc. Natl. Acad. Sci. USA 82:5265–5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gius, D., and L. A. Laimins. 1989. Activation of human papillomavirus type 18 gene expression by herpes simplex virus type 1 viral transactivators and a phorbol ester. J. Virol. 63:555–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goldstein, D. J., and S. K. Weller. 1988. Herpes simplex virus type 1-induced ribonucleotide reductase activity is dispensable for virus growth and DNA synthesis: isolation and characterization of an ICP6 lacZ insertion mutant. J. Virol. 62:196–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Halford, W. P., and P. A. Schaffer. 2001. ICP0 is required for efficient reactivation of herpes simplex virus type 1 from neuronal latency. J. Virol. 75:3240–3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Halford, W. P., and P. A. Schaffer. 2000. Optimized viral dose and transient immunosuppression enable herpes simplex virus ICP0-null mutants to establish wild-type levels of latency in vivo. J. Virol. 74:5957–5967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harris, R. A., R. D. Everett, X. X. Zhu, S. Silverstein, and C. M. Preston. 1989. Herpes simplex virus type 1 immediate-early protein Vmw110 reactivates latent herpes simplex virus type 2 in an in vitro latency system. J. Virol. 63:5313–5315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hobbs, W. E., II, and N. A. DeLuca. 1999. Perturbation of cell cycle progression and cellular gene expression as a function of herpes simplex virus ICP0. J. Virol. 73:8245–8255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoge, A. T., S. B. Hendrickson, and W. H. Burns. 2000. Murine gammaherpesvirus 68 cyclin D homologue is required for efficient reactivation from latency. J. Virol. 74:7016–7023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Honess, R. W., and B. Roizman. 1974. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J. Virol. 14:8–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Honess, R. W., and D. H. Watson. 1977. Herpes simplex virus resistance and sensitivity to phosphonoacetic acid. J. Virol. 21:584–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hossain, A., T. Holt, J. Ciacci-Zanella, and C. Jones. 1997. Analysis of cyclin-dependent kinase activity after herpes simplex virus type 2 infection. J. Gen. Virol. 78:3341–3348. [DOI] [PubMed] [Google Scholar]
- 37.Jans, D. A., M. J. Ackermann, J. R. Bischoff, D. H. Beach, and R. Peters. 1991. p34cdc2-mediated phosphorylation at T124 inhibits nuclear import of SV-40 T antigen proteins. J. Cell Biol. 115:1203–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jiang, Y., A. Hossain, M. T. Winkler, T. Holt, A. Doster, and C. Jones. 1998. A protein encoded by the latency-related gene of bovine herpesvirus 1 is expressed in trigeminal ganglionic neurons of latently infected cattle and interacts with cyclin-dependent kinase 2 during productive infection. J. Virol. 72:8133–8142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jordan, R., and P. A. Schaffer. 1997. Activation of gene expression by herpes simplex virus type 1 ICP0 occurs at the level of mRNA synthesis. J. Virol. 71:6850–6862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jordan, R., L. Schang, and P. A. Schaffer. 1999. Transactivation of herpes simplex virus type 1 immediate-early gene expression by virion-associated factors is blocked by an inhibitor of cyclin-dependent protein kinases. J. Virol. 73:8843–8847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Klockmann, U., and W. Deppert. 1983. Acylated simian virus 40 large T-antigen: a new subclass associated with a detergent-resistant lamina of the plasma membrane. EMBO J. 2:1151–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685. [DOI] [PubMed] [Google Scholar]
- 43.Leib, D. A., D. M. Coen, C. L. Bogard, K. A. Hicks, D. R. Yager, D. M. Knipe, K. L. Tyler, and P. A. Schaffer. 1989. Immediate-early regulatory gene mutants define different stages in the establishment and reactivation of herpes simplex virus latency. J. Virol. 63:759–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li, M., H. Lee, D. W. Yoon, J. C. Albrecht, B. Fleckenstein, F. Neipel, and J. U. Jung. 1997. Kaposi’s sarcoma-associated herpesvirus encodes a functional cyclin. J. Virol. 71:1984–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lomonte, P., and R. D. Everett. 1999. Herpes simplex virus type 1 immediate-early protein Vmw110 inhibits progression of cells through mitosis and from G1 into S phase of the cell cycle. J. Virol. 73:9456–9467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lomonte, P., K. F. Sullivan, and R. D. Everett. 2001. Degradation of nucleosome-associated centromeric histone H3-like protein CENP-A induced by herpes simplex virus type 1 protein ICP0. J. Biol. Chem. 276:5829–5835. [DOI] [PubMed] [Google Scholar]
- 47.Ma, T., N. Zou, B. Y. Lin, L. T. Chow, and J. W. Harper. 1999. Interaction between cyclin-dependent kinases and human papillomavirus replication-initiation protein E1 is required for efficient viral replication. Proc. Natl. Acad. Sci. USA 96:382–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mal, A., A. Piotrkowski, and M. L. Harter. 1996. Cyclin-dependent kinases phosphorylate the adenovirus E1A protein, enhancing its ability to bind pRb and disrupt pRb-E2F complexes. J. Virol. 70:2911–2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maul, G. G., and R. D. Everett. 1994. The nuclear location of PML, a cellular member of the C3HC4 zinc-binding domain protein family, is rearranged during herpes simplex virus infection by the C3HC4 viral protein ICP0. J. Gen. Virol. 75:1223–1233. [DOI] [PubMed] [Google Scholar]
- 50.Maul, G. G., H. H. Guldner, and J. G. Spivack. 1993. Modification of discrete nuclear domains induced by herpes simplex virus type 1 immediate early gene 1 product (ICP0). J. Gen. Virol. 74:2679–2690. [DOI] [PubMed] [Google Scholar]
- 51.Maul, G. G., A. M. Ishov, and R. D. Everett. 1996. Nuclear domain 10 as preexisting potential replication start sites of herpes simplex virus type-1. Virology 217:67–75. [DOI] [PubMed] [Google Scholar]
- 52.McVey, D., S. Ray, Y. Gluzman, L. Berger, A. G. Wildeman, D. R. Marshak, and P. Tegtmeyer. 1993. cdc2 phosphorylation of threonine 124 activates the origin-unwinding functions of simian virus 40 T antigen. J. Virol. 67:5206–5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Meijer, L., A. Borgne, O. Mulner, J. P. Chong, J. J. Blow, N. Inagaki, M. Inagaki, J. G. Delcros, and J. P. Moulinoux. 1997. Biochemical and cellular effects of roscovitine, a potent and selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and cdk5. Eur. J. Biochem. 243:527–536. [DOI] [PubMed] [Google Scholar]
- 54.Mosca, J. D., D. P. Bednarik, N. B. Raj, C. A. Rosen, J. G. Sodroski, W. A. Haseltine, G. S. Hayward, and P. M. Pitha. 1987. Activation of human immunodeficiency virus by herpesvirus infection: identification of a region within the long terminal repeat that responds to a trans-acting factor encoded by herpes simplex virus 1. Proc. Natl. Acad. Sci. USA 84:7408–7412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nicholas, J., K. R. Cameron, and R. W. Honess. 1992. Herpesvirus saimiri encodes homologues of G protein-coupled receptors and cyclins. Nature 355:362–365. [DOI] [PubMed] [Google Scholar]
- 56.Nomura, H., Y. Sawada, and S. Ohtaki. 1998. Interaction of p27 with E1A and its effect on CDK kinase activity. Biochem. Biophys. Res. Commun. 248:228–234. [DOI] [PubMed] [Google Scholar]
- 57.Pagano, M. 1997. Cell cycle regulation by the ubiquitin pathway. FASEB J. 11:1067–1075. [DOI] [PubMed] [Google Scholar]
- 58.Parkinson, J., S. P. Lees-Miller, and R. D. Everett. 1999. Herpes simplex virus type 1 immediate-early protein Vmw110 induces the proteasome-dependent degradation of the catalytic subunit of DNA-dependent protein kinase. J. Virol. 73:650–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Perry, L. J., F. J. Rixon, R. D. Everett, M. C. Frame, and D. J. McGeoch. 1986. Characterization of the IE110 gene of herpes simplex virus type 1. J. Gen. Virol. 67:2365–2380. [DOI] [PubMed] [Google Scholar]
- 60.Quinlan, M. P., and D. M. Knipe. 1985. Stimulation of expression of a herpes simplex virus DNA-binding protein by two viral functions. Mol. Cell. Biol. 5:957–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reynisdottir, I., S. Bhattacharyya, D. Zhang, and C. Prives. 1999. The retinoblastoma protein alters the phosphorylation state of polyomavirus large T antigen in murine cell extracts and inhibits polyomavirus origin DNA replication. J. Virol. 73:3004–3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Roizman, R., and A. Sears. 1996. Herpes simplex viruses and their replication, p.2231–2295. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 3rd ed., vol. 2. Raven Press, New York, N.Y.
- 63.Rouet, R., G. Raguenez, and J.-P. Salier. 1992. Optimized assays for quantifying transient expression of co-transfected β-galactosidase and CAT reporter genes. BioTechniques 13:700–702. [PubMed] [Google Scholar]
- 64.Sacks, W. R., and P. A. Schaffer. 1987. Deletion mutants in the gene encoding the herpes simplex virus type 1 immediate-early protein ICP0 exhibit impaired growth in cell culture. J. Virol. 61:829–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Samaniego, L. A., N. Wu, and N. A. DeLuca. 1997. The herpes simplex virus immediate-early protein ICP0 affects transcription from the viral genome and infected-cell survival in the absence of ICP4 and ICP27. J. Virol. 71:4614–4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schaffer, P. A., G. M. Aron, N. Biswal, and M. Benyesh-Melnick. 1973. Temperature-sensitive mutants of herpes simplex virus type 1: isolation, complementation and partial characterization. Virology 52:57–71. [DOI] [PubMed] [Google Scholar]
- 67.Schang, L. M., J. Phillips, and P. A. Schaffer. 1998. Requirement for cellular cyclin-dependent kinases in herpes simplex virus replication and transcription. J. Virol. 72:5626–5637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schang, L. M., A. Rosenberg, and P. A. Schaffer. 2000. Roscovitine, a specific inhibitor of cellular cyclin-dependent kinases, inhibits herpes simplex virus DNA synthesis in the presence of viral early protein. J. Virol. 74:2107–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schang, L. M., A. Rosenberg, and P. A. Schaffer. 1999. Transcription of herpes simplex virus immediate-early and early genes is inhibited by roscovitine, an inhibitor specific for cellular cyclin-dependent kinases. J. Virol. 73:2161–2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schmitt, M. K., and K. Mann. 1987. Glycosylation of simian virus 40 T antigen and localization of glycosylated T antigen in the nuclear matrix. Virology 156:268–281. [DOI] [PubMed] [Google Scholar]
- 71.Stabell, E. C., and P. D. Olivo. 1992. Isolation of a cell line for rapid and sensitive histochemical assay for the detection of herpes simplex virus. J. Virol. Methods 38:195–204. [DOI] [PubMed] [Google Scholar]
- 72.Stingley, S. W., J. J. Ramirez, S. A. Aguilar, K. Simmen, R. M. Sandri-Goldin, P. Ghazal, and E. K. Wagner. 2000. Global analysis of herpes simplex virus type 1 transcription using an oligonucleotide-based DNA microarray. J. Virol. 74:9916–9927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stow, N. D., and E. C. Stow. 1986. Isolation and characterization of a herpes simplex virus type 1 mutant containing a deletion within the gene encoding the immediate early polypeptide Vmw110. J. Gen. Virol. 67:2571–2585. [DOI] [PubMed] [Google Scholar]
- 74.Summers, B. M., T. P. Margolis, and D. A. Leib. 2001. Herpes simplex virus type 1 corneal infection results in periocular disease by zosteriform spread. J. Virol. 75:5069–5075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sze, P., and R. C. Herman. 1992. The herpes simplex virus type 1 ICP6 gene is regulated by a “leaky” early promoter. Virus Res. 26:141–152. [DOI] [PubMed] [Google Scholar]
- 76.Tack, L. C., J. H. Wright, and E. G. Gurney. 1988. Characterization of simian virus 40 large T antigen by using different monoclonal antibodies: T-p53 complexes are preferentially ATPase active and adenylylated. J. Virol. 62:1028–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tebas, P., E. C. Stabell, and P. D. Olivo. 1995. Antiviral susceptibility testing with a cell line which expresses beta-galactosidase after infection with herpes simplex virus. Antimicrob. Agents Chemother. 39:1287–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Troppmair, J., J. T. Bruder, H. Munoz, P. A. Lloyd, J. Kyriakis, P. Banerjee, J. Avruch, and U. R. Rapp. 1994. Mitogen-activated protein kinase/extracellular signal-regulated protein kinase activation by oncogenes, serum, and 12-O-tetradecanoylphorbol-13-acetate requires Raf and is necessary for transformation. J. Biol. Chem. 269:7030–7035. [PubMed] [Google Scholar]
- 79.van Dyk, L. F., H. W. T. Virgin, and S. H. Speck. 2000. The murine gammaherpesvirus 68 v-cyclin is a critical regulator of reactivation from latency. J. Virol. 74:7451–7461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang, D., C. de la Fuente, L. Deng, L. Wang, I. Zilberman, C. Eadie, M. Healey, D. Stein, T. Denny, L. E. Harrison, L. Meijer, and F. Kashanchi. 2001. Inhibition of human immunodeficiency virus type 1 transcription by chemical cyclin-dependent kinase inhibitors. J. Virol. 75:7266–7279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Weber, P. C., and B. Wigdahl. 1992. Identification of dominant-negative mutants of the herpes simplex virus type 1 immediate-early protein ICP0. J. Virol. 66:2261–2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wilcox, K. W., A. Kohn, E. Sklyanskaya, and B. Roizman. 1980. Herpes simplex virus phosphoproteins. I. Phosphate cycles on and off some viral polypeptides and can alter their affinity for DNA. J. Virol. 33:167–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ye, M., K. M. Duus, J. Peng, D. H. Price, and C. Grose. 1999. Varicella-zoster virus Fc receptor component gI is phosphorylated on its endodomain by a cyclin-dependent kinase. J. Virol. 73:1320–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang, Q., H. Yao, N. Vo, and R. H. Goodman. 2000. Acetylation of adenovirus E1A regulates binding of the transcriptional corepressor CtBP. Proc. Natl. Acad. Sci. USA 97:14323–14328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhu, Z., W. Cai, and P. A. Schaffer. 1994. Cooperativity among herpes simplex virus type 1 immediate-early regulatory proteins: ICP4 and ICP27 affect the intracellular localization of ICP0. J. Virol. 68:3027–3040. [DOI] [PMC free article] [PubMed] [Google Scholar]