Abstract
By comparing three expression vectors for the rabies virus (Rv) minigenome, we show that the characteristic of the Rv RNA is important for efficient rescue despite its not being crucial for replication. Moreover, we show that the coexpression of the viral proteins from helper Rv and Mokola virus could rescue the Rv minigenome while Rv-related European bat lyssavirus 1 could not, suggesting that the signals controlling transcription and replication are conserved in the distantly related Rv and Mokola virus.
Rabies virus (Rv) and related viruses from the genus Lyssavirus of the family Rhabdoviridae induce fatal encephalitis in mammals (5). The viral genome is a nonsegmented, single-stranded RNA (about 12 kb long) (8, 21) of negative polarity encapsidated by nucleoproteins. The genome codes for five successive genes. The 3" and 5" extremities have two short sequences, named the leader and trailer regions, that contain the essential signals promoting genome and antigenome transcription, replication, and encapsidation (20).
A rescue method has been developed for biologically active ribonucleocapsid assembly by coexpression in the cell cytoplasm of the N, P, and L proteins with a synthetic RNA minigenome (7, 15). This coexpression is under the control of a T7 promoter in which the T7 RNA polymerase (T7RNAP) is provided in trans by a recombinant vaccinia virus (9). By using this method, it was shown with vesicular stomatitis virus (VSV; another rhabdovirus) that the exact 3" end of the viral RNA genome is crucial for transcription and replication, whereas extra GGG nucleotides added at the 5" end for T7RNAP transcription were tolerated to some extent (14). In this study, we created different minigenome expression vectors to determine whether these extra nucleotides could affect rescue efficiency. Moreover, we developed a novel rescue protocol using a helper virus that allowed us to examine the possibilities of rescuing an Rv minigenome with heterologous lyssavirus.
Minigenome expression vectors.
All of the minigenome constructs used in this study are under the control of a T7RNAP promoter (Fig. 1A); it includes the trailer and leader sequences with a polylinker between the start and stop transcription signals, and it contains a hepatitis delta virus ribozyme (HdvRz) designed to cut the RNA at the leader-HdvRz junction, producing a minigenome with an exact 3" viral end (1, 18).
FIG. 1.
(A) Rv minigenome constructions. Leader and trailer sequences are represented reversed because all of the constructions encode a negative-strand Rv minigenome. The RNA produced after transcription and ribozyme cleavage is shown protruding. (B) Ribozyme sequences and secondary structures are shown flanking a negative-strand minigenome in which the luciferase gene has been cloned. The gray boxes indicate the nucleotides mutated in the HdvRz to insert an SmaI restriction site.
Construct pDI.GGG encoded three extra G nucleotides at the minigenome 5" end, since GGG is optimal for transcription initiation by the T7RNAP (10, 17). However, the additional GGG was thought to decrease the minigenome's replication efficiency. Thus, we designed the construct pDI.Ham, in which a hammerhead ribozyme (HamRz) (16) was inserted between the GGG and the minigenome; the ribozyme cleaves the RNA to produce an exact 5" viral end. Therefore, pDI.Ham combines efficient T7RNAP transcription (initiation on GGG) with optimal minigenome replication (authentic viral 5" end). pDI.Mut was similar to pDI.Ham, but both ribozymes were mutated to insert restriction sites. SmaI was selected because it is absent from the Rv genome and very rare in the lyssavirus genomes. The restriction sites were inserted into ribozymes so that they exactly flanked the viral sequences (Fig. 1B). The flexible structure of the HamRz allowed us to create the SmaI site simply by mutagenesis within helix III, which is not critical for RNA cleavage (16) as long as the helical structure is conserved. Mutation of the genomic HdvRz was more complex because much of the sequence is important for the structure and function of the ribozyme (1, 19). To our knowledge, this is the first time that a restriction site was created within a functionally important region of the HdvRz. Therefore, the activity of this mutated HdvRz was unknown.
Ribozyme activity in vitro.
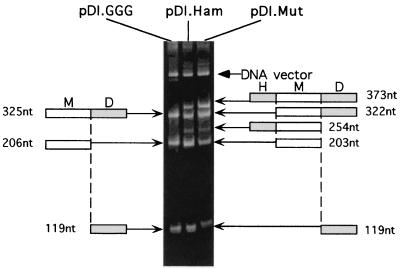
The three expression vectors were transcribed in vitro by the T7RNA transcription kit (Promega) for 90 min at 30°C. Under these conditions, ribozymes are able to cut RNA (Fig. 2). pDI.GGG generated the full-length RNA (325 nucleotides [nt], upper band) and two smaller RNA products (206 and 119 nt) resulting from HdvRz cleavage. Similarly, pDI.Ham and pDI.Mut produced all of the expected RNA cleavage intermediates (322 and 254 nt), demonstrating the functionality of both ribozymes. Interestingly, the amounts of minigenome product (203 and 206 nt) were roughly equivalent in all of the constructs, indicating that the HamRz does not significantly affect the amount of the final product, at least in vitro. Rather, the bands corresponding to RNA transcripts cut only by HamRz (322 nt) are more intense than those corresponding to RNA cut only by HdvRz (254 nt). This indicates that the HamRz cleaves the RNA more efficiently than does the HdvRz in vitro. The mutated HdvRz of the pDI.Mut construct slightly reduced in vitro activity compared to the wild-type HdvRz of pDI.Ham.
FIG. 2.
The minigenome-encoding vectors were transcribed in vitro. The products migrated on a 6% acrylamide gel colored with ethidium bromide. The little sketches represent the following cleavage products: H, HamRz; M, minigenome; D, HdvRz. Fragment sizes are indicated.
Rescue with a helper virus.
A luciferase reporter gene was inserted in a negative sense (relative to the T7 promoter) into the polylinker of the three expression vectors. Thus, the coding strand is under the control of the Rv transcription signals. Rescue of the minigenomes was assayed in BHK-21 cells that were first infected with Rv strain PV helper virus (9). These cells were superinfected 24 h postinfection (hpi) with the T7RNAP recombinant vaccinia virus and transfected 25 hpi with one of the constructs (Fig. 3A). Cells were harvested at 72 hpi, and a luciferase assay (Promega kit) was performed. The three vectors displayed a high nonspecific background in mock-infected cells, but they showed clearly higher specific activity (two- to fourfold) in cells infected with the helper virus.
FIG. 3.
Vectors pDI.GGG, pDI.Ham, and pDI.Mut were rescued by Rv. A multiplicity of infection (MOI) of 0 is actually a negative control. The results represent the average reporter gene expression in four experiments. Luciferase activity is expressed in flashes per second. (A) Luciferase activity in cell extract 24 h after transfection. (B) Passage; luciferase activity in cell extracts 24 h after fresh cells were infected with supernatant from preceding plates. (C) Rescue performed entirely with cloned cDNA. BHK-21 cells were infected with vaccinia virus expressing T7RNAP. One hour after infection, the minigenome-expressing vector was transfected along with plasmids encoding the N, P, M, G, and L proteins under control of the T7RNAP promoter. At 24 h after infection, the supernatant was used to infect fresh cells. Then, after 24 h of incubation, luciferase activity was measured.
To further examine the efficiency of rescue, a passage of the minigenome was tested. Cell supernatant collected at 72 hpi was used to reinfect fresh BHK-21 cells, which were assayed for luciferase activity 24 h later (Fig. 3B). This passage resulted in complete elimination of the nonspecific background in mock-infected cells. This allowed us to reliably compare the three different constructs. The pDI.Ham and pDI.Mut vectors showed, respectively, three and four times as much luciferase activity as did pDI.GGG. This indicates that the extra nucleotides 5" of the minigenome have an inhibitory effect on its transcription and/or replication. Also, the pDI.Mut vector appeared to be at least as efficient as the pDI.Ham vector, demonstrating that the mutations introduced into the HdvRz did not hamper minigenome vector rescue. This is indirect proof of the ribozyme's functionality in vivo. The specific signal decreased dramatically upon the second passage and disappeared after the third (data not shown).
Negative-strand RNA virus minigenomes rescued by helper virus have already been described, but they involved a natural defective interfering (DI) or direct RNA transfection (12, 13). The protocol presented here combines helper virus and recombinant vaccinia virus, so that only one plasmid needs to be transfected into the cell. This helper virus rescue protocol produced the same results as those using the proteins expressed from cloned genes. Thus, minigenomes created by helper rescue can be passaged through cells, although apparently no more than two or three times. We cannot explain the clearance of the minigenome by subsequent passages, although it could involve (i) mechanisms that reduce the defective interfering particle's replication or (ii) viral interference. Indeed, it makes sense for the virus to eliminate interfering particles.
Rescue entirely from cDNA.
pDI.Ham and pDI.Mut were rescued with viral proteins expressed from Rv genes cloned downstream of the T7 promoter: the N, P, M, and G proteins were from the Rv PV strain (pBluescript vector); the L protein expression vector (Rv SAD B19 strain) was kindly provided by Klaus Conzelmann (Fig. 3C) (7). Again, the minigenome with an authentic 5" end (pDI.Mut) produced twice as much reporter gene expression as did pDI.GGG, which definitively demonstrates that an exact viral 5" end is more efficient for viral rescue. Also, rescuing with the helper virus protocol described previously gave comparable results, indicating that the two methods are consistent. We conclude from these two experiments that extra 5" nucleotides somehow decrease rescue efficiency, most likely through polymerase-promoter interaction. Replication of these extra G nucleotides by the viral polymerase would give rise to an antigenome with three extra C nucleotides on the 3" end. This would presumably inhibit binding and/or initiation of replication by the viral polymerase. Surprisingly, it has been show in VSV that up to four extra nucleotides 5" of the trailer can be somehow corrected by viral replication (14). The same phenomenon probably occurs during pDI.GGG minigenome rescue.
Interestingly, the hammerhead construct increases rescue efficiency without reducing T7RNAP transcription or the amount of minigenome RNA processed. This is because the HamRz cleaves more efficiently than the HdvRz, at least in vitro, and does not appear to be the limiting step in the process. The HamRz could be used in any RNA-expressing vector in which a discrete 5" end is important, and it would be especially useful for RNA virus reverse genetics. The extra 3" nucleotides have been reported to dramatically inhibit RNA template activity for VSV, and we have now shown that extra 5" nucleotides also act as an inhibitor in Rv. It is important to note that this is true for rhabdoviruses, but it is probably not true for respiroviruses. Indeed Sendai virus DI tolerates up to 100 extra nucleotides on the 3" end without significantly decreasing its template efficiency (22). But the respiroviruses are subject to the “rule of six” (4), meaning that their promoters are active in a defined nucleocapsid context: the polymerase recognizes the RNA promoter precisely covered by nucleoproteins. There is no such “quality control” in rhabdoviruses, as they definitely are not subject to any kind of rule of six. This would explain why they need precisely defined 3" termini.
Rv minimgenome rescue by others lyssaviruses.
Rescue with a helper provides a useful tool with which to test cross-activity with heterologous rescue. The Rv minigenome produced by pDI.Mut was assayed in a helper rescue with two different Rv-related lyssaviruses: Mokola virus and European bat lyssavirus 1 (EBL1) (Fig. 4). Mokola virus, one of the more divergent lyssaviruses from Rv (2, 3, 11), rescued and passaged the strain PV Rv minigenome nearly as well as did Rv itself. This indicates that Mokola virus proteins are able to encapsidate and transcribe the Rv RNA minigenome and to promote viral miniparticle budding from the cell. In contrast, EBL1 failed to rescue the Rv minigenome. A small amount of reporter gene activity was detected above the background after transfection, but this totally disappeared after passage. It is not clear whether EBL1 failed to encapsidate/transcribe the Rv RNA minigenome or whether it would not allow viral budding. Interestingly, Mokola virus is genetically more divergent from Rv PV than is EBL1. These results are consistent with previous work showing that Mokola virus can complement an Rv defective for the L gene but EBL-1 cannot (6). This suggests that the failure of EBL-1 proteins to rescue the Rv minigenome probably involve the L polymerase-RNA template interaction. Thus, the encoded transcription and replication signals could be different from those of Rv and Mokola virus. This hypothesis will be tested once the EBL-1 leader and trailer have been sequenced.
FIG. 4.
Rv minigenome rescue by Rv PV, Mokola virus, and EBL-1. The cell were infected with each viruses at a multiplicity of infection of 1, except for the negative control, whose cells were not infected. The gray bars represent the average reporter gene expression of four experiments. Luciferase activity in cell extract is expressed in flashes per second. (A) Luciferase activity in cell extract collected 24 h after transfection. (B) Passage; luciferase activity in cell extract 24 h after infection by supernatant from experiment A.
Acknowledgments
We thank Klaus Conzelmann (Munich, Germany) for providing the plasmid encoding the SAD B-19 L polymerase and for helpful discussion and Yvette Forteville for technical assistance and moral support.
The Fondation pour la Recherche Medicale and a short-term FEBS fellowship supported this work.
REFERENCES
- 1.Been, M. D., and G. S. Wickham. 1997. Self-cleaving ribozymes of hepatitis delta virus RNA. Eur. J. Biochem. 247:741-753. [DOI] [PubMed] [Google Scholar]
- 2.Bourhy, H., B. Kissi, and N. Tordo. 1993. Molecular diversity of the Lyssavirus genus. Virology 194:70-81. [DOI] [PubMed] [Google Scholar]
- 3.Bourhy, H., N. Tordo, M. Lafon, and P. Sureau. 1989. Complete cloning and molecular organization of a rabies-related virus: Mokola virus. J. Gen. Virol. 70:2063-2074. [DOI] [PubMed] [Google Scholar]
- 4.Calain, P., and L. Roux. 1993. The rule of six, a basic feature for efficient replication of Sendai virus defective interfering RNA. J. Virol. 67:4822-4830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Charlton, K. 1994. The pathogenesis of rabies and other lyssaviral infections: recent studies. Curr. Top. Microbiol. Immunol. 187:95-119. [DOI] [PubMed] [Google Scholar]
- 6.Conzelmann, K.-K., J. Cox, and H.-J. Theil. 1991. An L (polymerase)-deficient rabies virus defective interfering particle RNA is replicated and transcribed by heterologous helper virus L proteins. Virology 184:655-663. [DOI] [PubMed] [Google Scholar]
- 7.Conzelmann, K.-K., and M. Schnell. 1994. Rescue of synthetic genomic RNA analogs of rabies virus by plasmid-encoded proteins. J. Virol. 68:713-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conzelmann, K. K., J. H. Cox, L. G. Schneider, and H. J. Thiel. 1990. Molecular cloning and complete nucleotide sequence of the attenuated rabies virus SAD B19. Virology 175:485-489. [DOI] [PubMed] [Google Scholar]
- 9.Fuerst, T., E. Niles, W. Studier, and B. Moss. 1986. Eukaryotic transient-expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proc. Natl. Acad. Sci. USA 83:8122-8126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ikeda, R., A. Lin, and J. Clarke. 1992. Initiation of transcription by T7 RNA polymerase at its natural promoters. J. Biol. Chem. 267:2640-2649. [PubMed] [Google Scholar]
- 11.Le Mercier, P., Y. Jacob, and N. Tordo. 1997. The complete Mokola virus genome sequence: structure of the RNA-dependent RNA polymerase. J. Gen. Virol. 78:1571-1576. [DOI] [PubMed] [Google Scholar]
- 12.Luytjes, W., M. Krystal, M. Enami, J. Parvin, and P. Palese. 1989. Amplification, expression and packaging of a foreign gene by influenza virus. Cell 59:1107-1113. [DOI] [PubMed] [Google Scholar]
- 13.Park, K., T. Huang, F. Correia, and M. Krystal. 1991. Rescue of a foreign gene by Sendai virus. Proc. Natl. Acad. Sci. USA 88:5537-5541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pattnaik, A., A. Ball, A. LeGrone, and G. Wertz. 1992. Infectious defective interfering particles of VSV from transcripts of a cDNA clone. Cell 69:1011-1020. [DOI] [PubMed] [Google Scholar]
- 15.Pattnaik, A., and G. Wertz. 1991. Cells that express all five proteins of vesicular stomatitis virus from cloned cDNAs support replication, assembly, and budding of defective interfering particles. Proc. Natl. Acad. Sci. USA 88:1379-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruffner, D., G. Stormo, and O. Uhlenbeck. 1990. Sequence requirements of the Hammerhead RNA self cleavage reaction. Biochemistry 29:10695-10702. [DOI] [PubMed] [Google Scholar]
- 17.Sen, R., and D. Dasgupta. 1993. Interaction of ribonucleotides with T7 RNA polymerase: probable role of GTP in transcription initiation. Biochem. Biophys. Res. Commun. 195:616-622. [DOI] [PubMed] [Google Scholar]
- 18.Tanner, N. K., S. Schaff, G. Thill, E. Petit-Koska, A. M. Crain-Denoyelle, and E. Westhof. 1994. A three-dimensional model of hepatitis delta virus ribozyme based on biochemical and mutational analyses. Curr. Biol. 4:448-498. [DOI] [PubMed] [Google Scholar]
- 19.Tanner, N. K. 1998. Biochemistry of the hepatitis delta virus catalytic RNAs, p. 23-38. In K. J. Scanlon and M. Kashani-Sabet (ed.), Ribozymes in gene therapy of cancer. R. G. Landes, Austin, Tex.
- 20.Tordo, N., O. Poch, A. Ermine, and G. Keith. 1986. Primary structure of leader RNA and nucleoprotein genes of the rabies genome: segmented homology with VSV. Nucleic Acids Res. 14:2671-2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tordo, N., O. Poch, A. Ermine, G. Keith, and F. Rougeon. 1988. Completion of the rabies virus genome sequence determination: highly conserved domains along the L (polymerase) proteins of unsegmented negative-strand RNA viruses. Virology 165:565-576. [DOI] [PubMed] [Google Scholar]
- 22.Vulliemoz, D., and L. Roux. 2001. Rule of six: how does Sendai virus RNA polymerase keep count? J. Virol. 75:4506-4518. [DOI] [PMC free article] [PubMed] [Google Scholar]