Abstract
The use of adenovirus vectors for human gene therapy is limited by potent inflammatory responses that result in significant morbidity. In kidney-derived epithelial cells (REC), activation of extracellular signal-regulated kinase 1/2 (ERK) and p38 kinase (p38) pathways occurred within 20 min of transduction with the serotype 5 adenovirus vector AdCMVβgal. Inhibition of ERK and p38 with U0126 and SB203580, respectively, reduced the expression of IP-10 mRNA following transduction with AdCMVβgal. To determine the role of the coxsackievirus-adenovirus receptor (CAR) or αv integrins in the activation of ERK and p38 and the expression of IP-10, REC cells were transduced with the fiber-modified and RGD-deleted adenovirus vectors AdL.F(RAEK-HA) and AdL.PB(HA), respectively. Compared with the wild-type capsid vector Ad5Luc, transduction with AdL.F(RAEK-HA) and AdL.PB(HA) resulted in reduced ERK-p38 activation and less IP-10 mRNA expression. The decreased IP-10 expression induced by the tropism-modified vectors was due to diminished transduction, since increasing multiplicity of infection resulted in increased IP-10 expression. Inhibition of adenovirus penetration with bafilomycin A1 or ammonium chloride attenuated the activation of ERK-p38 and IP-10 mRNA expression following infection, suggesting that endosomal escape was required to trigger these pathways. In vivo, direct inhibition of ERK and p38 signaling pathways inhibited adenovirus vector-induced IP-10 expression in mouse liver 1 h following transduction. These results demonstrate the importance of signaling via ERK and p38 in the early host response to adenovirus vectors and will permit the development of novel strategies to improve the safety and efficacy of these agents in human gene therapy.
The development of modified viruses as vectors for gene therapy has created a need to redefine the cellular and molecular biology of these infectious agents, especially in the context of their clinical use, which differs greatly from their wild-type counterparts. Adenovirus vectors are used experimentally and clinically, but potent host inflammatory and immune responses limit their use and in some cases have resulted in significant adverse effects (9, 23, 27). Within 1 week of administration, first-generation adenovirus vectors induce major histocompatibility complex class 1-restricted CD8+ cytotoxic T lymphocytes (CTL) directed against adenovirus proteins, a Th1 dominant immune response dependent on gamma interferon (47, 48). Ongoing expression of adenovirus genes is a significant factor in the development of the immune response against adenovirus vectors (38). For this reason, newer generations of adenovirus vectors are being developed that reduce the amount of viral DNA packaged within the virus capsid, eliminating genes encoding virus proteins (32). However, the adenovirus capsid itself can induce acute inflammation as well as antiviral cellular and humoral immune responses independent of viral transcription (20, 28, 30). Understanding the biology of the host immune response to replication-deficient adenoviruses will help bring these vectors closer to safe and effective clinical use.
We have previously demonstrated, in an animal model of adenovirus gene therapy, capsid-dependent induction of multiple chemokines occurring within 24 h of infection (30). Of the chemokines identified, the C-X-C chemokine IP-10 (interferon-inducible protein 10) was highly and rapidly induced in mice as early as 1 h following infection with first-generation and transcription-defective adenovirus vectors. IP-10 and its receptor, CXCR3, are increasingly being associated with Th1 immune responses (1, 5, 43). CXCR3 is expressed primarily on T lymphocytes of the Th1 phenotype, a finding consistent with the preferential chemotaxis of activated Th1 lymphocytes regulated by IP-10. Recently, IP-10 and CXCR3 have been shown to be important in the pathogenesis of Th1-dependent responses in models of antiviral immunity, allograft rejection, and immunity to Toxoplasma gondii (17, 21, 26). Given the association of IP-10 and CXCR3 with Th1 immune responses, it is likely that the induction of IP-10 by recombinant adenoviruses represents an important early step in the development of host immunity against these vectors.
The cellular entry of adenovirus is well studied. However, little is known regarding the effects of adenovirus cell entry on the activation of signaling pathways that lead to host recognition and subsequent antiviral immune responses. Serotype 2 adenovirus (Ad2) and Ad5 vectors first attach to the cell through an interaction between the carboxy terminus of the adenovirus fiber knob and a cell surface receptor, coxsackievirus-adenovirus receptor (CAR) (4, 34, 35). Following the high-affinity binding to CAR, a lower-affinity interaction between αv integrins (αvβ1, αvβ3, and αvβ5) and an arginine-glycine-aspartic acid (RGD) motif on the adenovirus penton base mediates virus internalization by receptor-mediated endocytosis through clathrin-coated vesicles (24, 40, 41, 46). Ten minutes following internalization, virus penetration into the cytoplasm occurs with subsequent delivery of viral DNA to the nucleus (14). Adenovirus penetration into the cytoplasm is believed to involve a pH-dependent conformational change in the adenovirus penton base and an interaction with αvβ5 integrins (14, 36, 42, 45). A virus-encoded 21-kDa cysteine protease is also needed for virus penetration (13). The early, capsid-dependent induction of IP-10 by adenovirus vectors suggests that events surrounding virus cell entry might trigger signaling that leads to the expression of this important immune mediator.
Understanding the biology of adenovirus vector induction of IP-10 as a precursor to host inflammatory and immune responses will have an impact on the development of future generations of adenovirus vectors and will identify new targets to reduce the immunogenicity of these vectors. In this paper, we further define the molecular mechanism of adenovirus vector-induced expression of the C-X-C chemokine IP-10. We demonstrate that early activation of extracellular signal-regulated kinase 1/2 (ERK) and p38 kinase (p38) signaling pathways during virus cell entry subsequently leads to the expression of this important immunoregulatory chemokine.
MATERIALS AND METHODS
Adenovirus vectors.
The type 5 E1-E3-deletion adenoviruses expressing Escherichia coli β-galactosidase, AdCMVβgal (2, 18), and luciferase, Ad5Luc (22), AdL.PB(HA) (19, 44), and AdL.F(RAEK-HA) (35), under the control of the cytomegalovirus promoter, were propagated in 293 cells and purified as previously described (3). Wild-type Ad3 (ATCC, Manassas, Va.) was propagated in HeLa cells. Ad3 was rendered transcription defective by UV-psoralen treatment as previously described (8). Virus vehicle (10 mM Tris [pH 8.0], 1 mM MgCl2, 10% glycerol) was used for controls.
Adenovirus vector particle number was determined by optical density measurement at 260 nm (29). For in vitro transductions, adenovirus vector titers are expressed as particle number per milliliter of cell culture medium. Multiplicity of infection (MOI) was expressed as particles per cell and calculated by dividing the total particle number by cell number used in transductions. Vectors were screened for replication-competent adenovirus by plaque assay on HeLa cells and remained consistently <1:1010 particles. All vectors contained <0.1 endotoxin unit of endotoxin/ml as per the Limulus amebocyte lysate assay.
Cell culture.
The immortalized, nontransformed, epithelium-derived DBA/2 kidney cell line (REC) (6) was maintained in Dulbecco modified Eagle medium (DMEM) containing 10% fetal calf serum (FCS). For all studies, cells were preincubated in DMEM containing 1% FCS 16 h prior to transduction with adenovirus vectors. Adenovirus transductions at specified titers were performed in 60-mm-diameter plates with cells 70 to 80% confluent (1.5 × 106 cells/plate). Cells were infected in 1 ml of DMEM containing 1% FCS for 90 min followed by incubation in fresh media for the remainder of the experimental period. Cells were preincubated with the MEK1-MEK2 inhibitor U0126 and the p38 kinase inhibitor SB203580 (Calbiochem, La Jolla, Calif.) 30 min prior to transduction with adenovirus vectors. Bafilomycin A1 (Sigma, St. Louis, Mo.) and ammonium chloride were added to the cell cultures 15 min prior to transduction with adenovirus vectors. Phorbol 12-myristate 13-acetate (PMA) and lysophosphatidic acid (Sigma) were added directly to cells at the onset of the experimenta period. Cell viability was tested with trypan blue following incubation with the above compounds.
Animal studies.
Animal studies were performed in accordance with the guidelines set forth by the Animal Care Committee at the University of Calgary. Male DBA/2 (H-2[supi]d) mice 10 to 12 weeks old (28 to 32 g) were obtained from Charles River (Wilmington, Mass.) and housed under standard conditions. Adenovirus vector injections were performed via the femoral vein using 1011 particles of AdCMVβgal in 100 μl of vehicle. Control animals were treated with 100 μl of vehicle alone (10 mM Tris [pH 8.0], 1 mM MgCl2, 10% glycerol). For the inhibitor studies, SB203580 and U0126 (Calbiochem) were dissolved in dimethyl sulfoxide (DMSO) at 100 and 160 mg/ml, respectively. Individual doses of SB203580 (100 mg/kg of body weight) or U0126 (160 mg/kg) were suspended in 40% polyethylene glycol (molecular weight, 3,000) to a final volume of 100 μl and injected intraperitoneally (i.p.) 30 min prior to and 6 h after the administration of AdCMVβgal. Control animals received an equivalent volume of DMSO in polyethylene glycol i.p.
RNase protection assays.
REC cells and whole-liver samples were processed for total RNA using RNeasy (Qiagen, Chatsworth, Calif.) per the manufacturer's protocol. RNase protection assays were performed with the RiboQuant Multi-Probe RNase Protection Assay System (Pharmingen, San Diego, Calif.) with the multiprobe template set mCK5 and a single probe set for IP-10 per the manufacturer's protocol. Autoradiographs were analyzed by phosphoimaging with a Personal FX phosphoimager and Quantity One software (Bio-Rad, Hercules, Calif.). Fold induction was determined as mRNA density ratio of IP-10 to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) within the same sample.
Enzymatic assays.
Luciferase assays were performed in six-well plates containing 2.5 × 105 REC cells/well. Ad5Luc, AdL.PB(HA), AdL.F(RAEK-HA), or vehicle was added directly to the cells and incubated at 37°C for 6 h. Cells were lysed in 300 μl of buffer (1% Triton X-100, 25 mM glycylglycine [pH 7.8], 15 mM MgSO4, 4 mM EGTA, and 1 mM dithiothreitol) and centrifuged. One hundred fifty microliters of supernatant was added to 300 μl of assay buffer (25 mM glycylglycine [pH 7.8], 15 mM K2PO4 [pH 7.8], 15 mM MgSO4, 4 mM EGTA, 1 mM dithiothreitol, 2 mM ATP), and luciferase activity was determined following addition of 100 μl of luciferin (0.3 mg/ml) (Promega, Madison, Wis.). Values are expressed as relative light units (RLU).
For β-galactosidase assays, AdCMVβgal-transduced REC cells or whole-liver tissue samples were lysed in 300 μl of lysis buffer (60 mM Na2HPO4, 40 mM NaH2PO4, 10 mM KCl, 1 mM MgSO4, 50 mM β-mercaptoethanol, 2.5 mM EDTA, 0.125% NP-40). Thirty microliters of cell extract was added to 270 μl of reaction buffer (1 mM MgCl2, 100 mM NaPO4, 1 mg of O-nitrophenyl-β-d-galactopyranoside/ml) and incubated for 30 min at 37°C. The reaction was terminated with 0.1 M NaCO3, and samples were read at 415 nm. Liver β-galactosidase activity was corrected for protein concentration and expressed as nanograms per milligram of protein.
Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) determinations were performed on mouse serum using the Sigma Diagnostics Transaminase kit per the manufacturer's protocol (Sigma). Results are expressed as Sigma-Frankel units per milliliter.
Immunoblotting.
REC cells were stimulated with adenovirus vectors for specified time points and lysed directly with hypotonic lysis buffer (50 mM NaF, 10 μg of leupeptin/ml, 200 μM Na3VO4). Lysate protein concentration was determined with the Bradford assay. Equivalent amounts of protein were separated on a sodium dodecyl sulfate (SDS)-10% polyacrylamide gel and then transferred to a polyvinylidene difluoride membrane. Membranes were blocked with skim milk and incubated overnight with a 1:750 dilution of antibodies against phosphorylated p38 kinase, p44/42 ERK, or p46/54 stress-activated protein kinase (SAPK) (New England Biolabs, Beverly, Mass.). Membranes were washed and then incubated for 1 h with a 1:7,500 dilution of peroxidase-conjugated anti-rabbit immunoglobulin. Proteins were visualized with ECL substrate reagent (Amersham Pharmacia, Piscataway, N.J.). To control for protein concentration, membranes were then stripped and reprobed for total p38 kinase, p44/42 ERK, and p46/54 SAPK as described above.
Southern analysis.
To obtain total DNA, adenovirus-transduced REC cells were trypsinized for 10 min at 37°C. Cells were centrifuged, and the pellets were lysed in 500 μl of lysis buffer (100 mM NaCl, 10 mM Tris [pH 8.0], 0.6% SDS, 10 mM EDTA, 100 μg of proteinase K/ml). The lysate was centrifuged, and the supernatant was extracted with phenol-chloroform-isoamyl alcohol (25:24:1). The aqueous layer was ethanol precipitated, and the DNA was resuspended in double-distilled water. For extraction of nuclear DNA, cells were first treated with buffer A (10 mM HEPES, 10 mM KCl, 0.1 M EDTA, 0.625% NP-40) to isolate the nuclei and then processed as above.
For Southern analysis, 2 to 5 μg of DNA was digested with HindIII and separated on an 0.8% agarose gel. The gel was washed in 0.25 M HCl solution and then in 0.4 M NaOH solution. DNA was transferred to a Hybond XL nylon membrane (Amersham Pharmacia) by capillary action in 0.4 M NaOH buffer. Twenty-five nanograms of the Ad5 fiber gene (nucleotide positions 827 to 1160) or the mouse cation exchanger gene, NCKX2 (housekeeping control), was labeled with [32P]dCTP using the Redprime random prime labeling system (Amersham Pharmacia). Hybridizations were performed using 2 ng of labeled probe/ml in 5 ml of ExpressHyb solution (Clontech, Palo Alto, Calif.) at 60°C for 1 h. The membrane was then washed in 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)-0.1% SDS for 30 min and twice in 0.2× SSC-0.1% SDS for 30 min at 60°C. The membrane was exposed to radiographic film, and band intensity was determined by densitometry using Quantity One software (Bio-Rad).
Statistical analysis.
All experiments were performed at least three times. Values are expressed as means ± standard deviations and were compared by Student's t test.
RESULTS
Adenovirus vectors activate ERK and p38 signaling pathways.
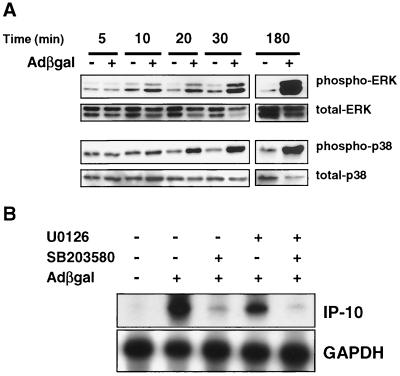
Adenovirus vectors, including transcription-defective adenovirus particles, induce the expression of the C-X-C chemokine IP-10 within 3 h of transduction in REC cells in vitro (6). We therefore hypothesized that events involving virus cell surface binding, viral entry, or nuclear transport might trigger intracellular signals that lead to the expression of IP-10. To determine whether any signaling pathways were triggered during adenovirus entry into the cell, we transduced 1.5 × 106 REC cells with 5 × 1010 particles of AdCMVβgal per ml (MOI, ∼3.3 × 104 particles/cell) or vehicle (10 mM Tris, 1 mM MgCl2, 10% glycerol) followed by immunoblotting for phosphorylated p38, ERK, and SAPK pathways at timed intervals. Compared to vehicle-treated cells, ERK and p38 activation was not evident at 5 min but was clearly detectable at 10 and 20 min, respectively, following transduction with AdCMVβgal. The activation of ERK and p38 was detectable for up to 3 h posttransduction (Fig. 1A). Interestingly, the amount of total p38 and ERK decreased over time in AdCMVβgal-treated but not vehicle-treated cells. The slightly delayed and prolonged activation of ERK and p38 was in contrast to the rapid and transient activation of ERK induced by 2 mM lysophosphatidic acid, which occurred within seconds, peaked at 10 min, and returned toward baseline at 30 min (data not shown). AdCMVβgal did not activate SAPK at any time point following transduction (data not shown).
FIG. 1.
ERK and p38 activation by adenovirus vectors. (A) Immunoblotting for phosphorylated and total p44/42 ERK and p38 kinase in REC cells transduced with AdCMVβgal. ERK and p38 pathways were activated within 10 min and activation persisted for up to 3 h following transduction with AdCMVβgal. (B) RNase protection assay for IP-10 mRNA. REC cells were transduced with AdCMVβgal in the presence of the specific p38 kinase inhibitor SB203580 (30 μM), the MEK1-MEK2 inhibitor U0126 (10 μM), or both. SB203580 and U0126 inhibited AdCMVβgal-induced expression of IP-10 mRNA at 6 h. GAPDH is used as a loading control.
ERK and p38 signaling pathways are involved in the regulation of numerous cytokines and chemokines. Given their temporal relationship to the expression of IP-10, we performed experiments to test whether p38 or ERK played a role in the adenovirus vector induction of this chemokine. REC cells were pretreated with 30 μM p38 kinase inhibitor SB203580 (10) or 10 μM MEK1-MEK2 inhibitor U0126 (11), 30 min prior to transduction with 5 × 1010 particles of AdCMVβgal per ml. Cell viability following treatment with SB203580 and U0126 as measured by trypan blue staining was >90% and similar to that of untreated cells. Six hours following transduction, total RNA was analyzed by RNase protection assay. U0126 partially and SB203580 almost completely attenuated the induction of IP-10 mRNA by AdCMVβgal (Fig. 1B). Pretreatment of cells with SB203580 (30 μM) and U0126 (10 μM) together reduced IP-10 mRNA expression more than did either agent alone, confirming that the activation of p38 and, to a lesser degree, ERK signaling pathways is essential for the adenovirus vector induction of IP-10.
Adenovirus vector-induced IP-10 expression is titer dependent in vitro.
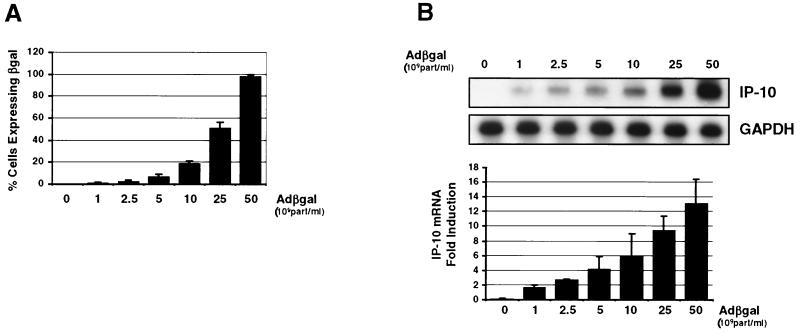
The adenovirus vector induction of IP-10 in vivo is dose dependent and increases with vector titer (30). To determine the IP-10 dose response to adenovirus vectors in vitro, 1.5 × 106 REC cells were transduced with 0.1 × 1010 (MOI, ∼0.7 × 103 particles/cell), 0.25 × 1010 (MOI, ∼1.6 × 103 particles/cell), 0.5 × 1010 (MOI, ∼3.3 × 103 particles/cell), 1 × 1010 (MOI, ∼6.6 × 103 particles/cell), 2.5 × 1010 (MOI, ∼1.6 × 104 particles/cell), and 5 × 1010 (MOI, ∼3.3 × 104 particles/cell) particles of AdCMVβgal per ml. Cells were analyzed for β-galactosidase expression or for IP-10 mRNA expression by RNase protection assay. At 6 h, the percentage of cells expressing β-galactosidase increased with increasing titer (Fig. 2A). Close to 98% of cells expressed the β-galactosidase transgene following transduction with a titer of 5 × 1010 particles/ml. IP-10 gene expression also increased with increasing vector titer. Transduction of REC cells with 0.1 × 1010 particles of AdCMVβgal per ml induced detectable IP-10 mRNA expression at 6 h. IP-10 mRNA expression increased proportionately with AdCMVβgal titer and was maximal at 5 × 1010 particles/ml (Fig. 2B). Increasing the titer of AdCMVβgal beyond 5 × 1010 particles/ml did not result in a further increase in IP-10 gene expression (data not shown). These results demonstrate that the adenovirus vector induction of IP-10 in REC cells correlates directly with vector titer and the MOI but reaches a maximum as transduction approaches 100%.
FIG. 2.
Dose response of adenovirus vector induction of IP-10. (A) β-Galactosidase expression in REC cells transduced with 1 × 109 particles (MOI, ∼0.7 × 103 particles/cell) to 50 × 109 particles (MOI, ∼3.3 × 104 particles/cell) of AdCMVβgal per ml. The percentage of cells expressing β-galactosidase at 6 h increases with increasing titer and approaches 100% for cells transduced at the titer of 50 × 109 particles/ml. (B) RNase protection assay for IP-10 mRNA in REC cells transduced with 1 × 109 particles to 50 × 109 particles of AdCMVβgal per ml. Adenovirus vector induction of IP-10 increases with increasing titer 6 h following transduction. GAPDH is used as a loading control. The bar graph is a graphic representation of IP-10 mRNA expression (fold induction ± standard deviation, n = 3).
Adenovirus vector-induced IP-10 expression is CAR and RGD independent.
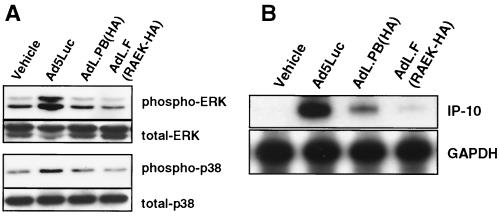
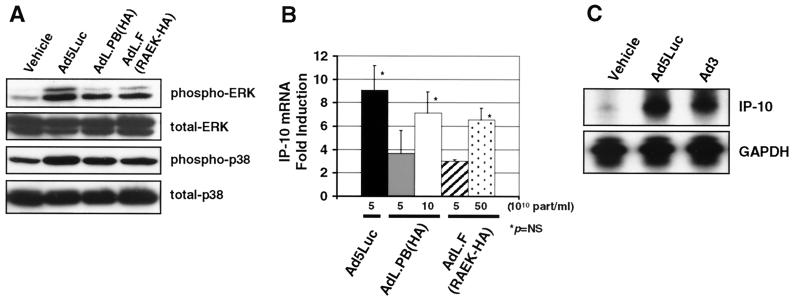
To test the role of the CAR and capsid RGD-αv integrin interactions in the activation of ERK-p38 and the induction of IP-10, we performed experiments with the tropism-modified adenovirus vectors AdL.F(RAEK-HA) and AdL.PB(HA). AdL.F(RAEK-HA) contains the mutations R412S, A415G, E416G, and K417G in the AB loop of the fiber protein (35), which inhibits its CAR binding ability, whereas AdL.PB(HA) has the RGD motif on the penton base deleted and therefore binds to CAR but does not interact with αv integrins (19, 44). REC cells (1.5 × 106) were transduced with 5 × 1010 particles of AdL.F(RAEK-HA), AdL.PB(HA), or the wild-type capsid vector Ad5Luc per ml (MOI, ∼3.3 × 104 particles/cell) and analyzed by immunoblotting for phosphorylated ERK and p38. Thirty minutes following transduction, activation of ERK and p38 by AdL.PB(HA) and AdL.F(RAEK-HA) was lower than activation induced by an equivalent titer of Ad5Luc (Fig. 3A). Consistent with this finding, 5 × 1010 particles of AdL.F(RAEK-HA) or AdL.PB(HA) per ml also resulted in significantly less IP-10 mRNA expression than did Ad5Luc 6 h following transduction as seen by RNase protection assay (Fig. 3B). Therefore, on a particle-to-particle basis, AdL.PB(HA) and AdL.F(RAEK-HA) activate ERK and p38 and induce IP-10 expression less than does the wild-type capsid vector Ad5Luc.
FIG. 3.
Effect of tropism-modified adenovirus vectors on ERK and p38 signaling and IP-10 expression. (A) Immunoblotting for phosphorylated and total p44/42 ERK and p38 kinase in REC cells transduced with 5 × 1010 particles of AdL.PB(HA), AdL.F(RAEK-HA), or the wild-type capsid vector Ad5Luc (MOI, ∼3.3 × 104 particles/cell) per ml. At 30 min, AdL.PB(HA) and AdL.F(RAEK-HA) activate ERK and p38 less than does Ad5Luc. (B) RNase protection assay for IP-10 mRNA in REC cells transduced with 5 × 1010 particles of AdL.PB(HA), AdL.F(RAEK-HA), or Ad5Luc per ml. At equivalent titers, AdL.PB(HA) and AdL.F(RAEK-HA) induced less IP-10 mRNA expression than did Ad5Luc 6 h following transduction. GAPDH is used as a loading control.
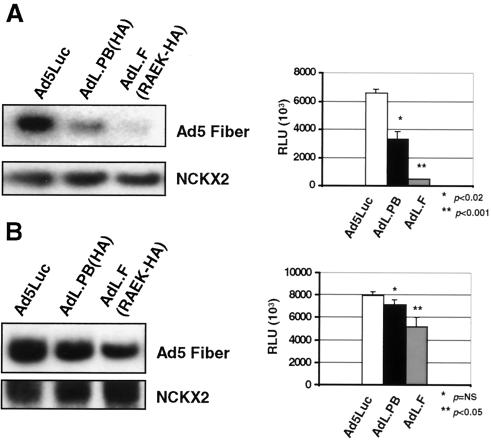
We expected that the tropism-modified vectors AdL.PB(HA) and AdL.F(RAEK-HA), due to modifications in capsid proteins and altered cell entry, would exhibit reduced transduction compared to that of Ad5Luc. Since the induction of IP-10 by adenovirus vectors is dose dependent, reduced cellular transduction exhibited by AdL.PB(HA) and AdL.F(RAEK-HA) may explain the differential effects on signaling and IP-10 gene expression. Therefore, we compared the transduction efficiencies of Ad5Luc, AdL.PB(HA), and AdL.F(RAEK-HA) by luciferase assay and direct assessment of cellular adenovirus DNA content using Southern analysis. REC cells were transduced with 5 × 1010 particles of Ad5Luc, AdL.PB(HA), or AdL.F(RAEK-HA) per ml and incubated for 6 h. Cells were trypsinized, and total DNA was extracted. A probe for the Ad5 fiber gene (nucleotide positions 827 to 1160) was used to detect adenovirus vector genomes in total DNA samples. At equivalent particle numbers, REC cells transduced with Ad5Luc contained substantially higher levels of adenovirus DNA than did cells transduced with AdL.PB(HA) or AdL.F(RAEK-HA) (Fig. 4A). At 6 h, luciferase activity was also reduced in the AdL.PB(HA)- and AdL.F(RAEK-HA)-transduced cells [Ad5Luc, (6.5 ± 0.3) × 106 RLU, versus AdL.PB(HA), (3.3 ± 0.5) × 106 RLU, P < 0.02, versus AdL.F(RAEK-HA), (0.5 ± 0.01) × 106 RLU, P < 0.001] (Fig. 4A), corresponding to the results obtained in the Southern analysis. These results confirm that the tropism-modified vectors transduce REC cells less than does their wild-type counterpart, Ad5Luc.
FIG. 4.
Transduction efficiency of tropism-modified adenovirus vectors. (A) Southern analysis probing for the Ad5 fiber gene (and the single-copy housekeeping gene NCKX2) in REC cells transduced with 5 × 1010 particles of Ad5Luc, AdL.PB(HA), or AdL.F(RAEK-HA) per ml (MOI, ∼3.3 × 104 particles/cell). REC cells transduced with Ad5Luc contained significantly higher levels of adenovirus DNA than did cells transduced with equivalent titers of AdL.PB(HA) and AdL.F(RAEK-HA). Luciferase activity in REC cells transduced with 5 × 1010 particles of AdL.PB(HA) (black bars) or AdL.F(RAEK-HA) (gray bars) per ml is significantly reduced compared to that for cells transduced with a similar titer of Ad5Luc (white bars) (RLU ± standard deviation, n = 3). (B) Southern analysis of total DNA and luciferase assay in REC cells transduced with 5 × 1010 particles of Ad5Luc per ml (MOI, ∼3.3 × 104 particles/cell) (white bars), 10 × 1010 particles of AdL.PB(HA) per ml (MOI, ∼6.6 × 104 particles/cell) (black bars), and 50 × 1010 particles of AdL.F(RAEK-HA) per ml (MOI, ∼3.3 × 105 particles/cell) (gray bars). Transduction with higher titers of AdL.PB(HA) and AdL.F(RAEK-HA) resulted in increased levels of cellular adenovirus DNA at 6 h. Luciferase activity in REC cells also increased following transduction with increased titers of the tropism-modified vectors (RLU ± standard deviation, n = 3).
Since AdL.PB(HA) and AdL.F(RAEK-HA) exhibited reduced transduction due to their modified capsids, the previous results did not confirm a role for CAR binding or capsid RGD-αv integrin interactions in the adenovirus vector induction of IP-10. For this reason, we assessed the expression of IP-10 mRNA using titers of AdL.PB(HA) or AdL.F(RAEK-HA) that achieved levels of transduction comparable to that of Ad5Luc. At 6 h, transduction of REC cells with 10 × 1010 particles of AdL.PB(HA) per ml (MOI, ∼6.6 × 104 particles/cell) and 50 × 1010 particles of AdL.F(RAEK-HA) per ml (MOI, ∼3.3 × 105 particles/cell) resulted in an increased cellular adenovirus DNA content approaching that achieved with 5 × 1010 particles of Ad5Luc per ml (MOI, ∼3.3 × 104 particles/cell) as determined by Southern analysis (Fig. 4B). Luciferase activity at 6 h in REC cells transduced with 10 × 1010 particles of AdL.PB(HA) per ml and 50 × 1010 particles of AdL.F(RAEK-HA) per ml also increased, although AdL.F(RAEK-HA)-transduced cells still expressed significantly less luciferase than did 5 × 1010 particles of Ad5Luc per ml [Ad5Luc, (8.0 ± 0.3) × 106 RLU, versus AdL.PB(HA), (7.2 ± 0.4) × 106 RLU, P = not significant (NS), versus AdL.F(RAEK-HA), (5.2 ± 0.9) × 106 RLU, P < 0.05] (Fig. 4B). Nevertheless, transduction of REC cells with increased titers of AdL.PB(HA) and AdL.F(RAEK-HA) resulted in ERK and p38 phosphorylation at 30 min (Fig. 5A). Furthermore, the RNase protection assay revealed increased IP-10 mRNA expression, indicating that escalating the MOI and hence transduction of AdL.PB(HA) or AdL.F(RAEK-HA) was sufficient to induce the expression of this chemokine (Fig. 5B).
FIG. 5.
Role of CAR and αv integrins in the induction of IP-10 by adenovirus vectors. (A) Immunoblotting for phosphorylated and total p44/42 ERK and p38 kinase in REC cells transduced with 5 × 1010 particles of Ad5Luc per ml (MOI, ∼3.3 × 104 particles/cell), 10 × 1010 particles of AdL.PB(HA) per ml (MOI, ∼6.6 × 104 particles/cell), and 50 × 1010 particles of AdL.F(RAEK-HA) per ml (MOI, ∼3.3 × 105 particles/cell). Increasing titers of AdL.PB(HA) and AdL.F(RAEK-HA) increased the phosphorylation of p42/44 ERK and p38 kinase at 30 min. (B) RNase protection assay for IP-10 mRNA in REC cells transduced with increased titers of AdL.PB(HA) and AdL.F(RAEK-HA). AdL.PB(HA) (10 × 1010 particles/ml) and AdL.F(RAEK-HA) (50 × 1010 particles/ml) increased the expression of IP-10 mRNA at 6 h (fold induction, mean ± standard deviation, n = 5). (C) RNase protection assay for IP-10 mRNA in REC cells transduced with 5 × 1010 particles of UV-psoralen-inactivated Ad3 or Ad5Luc per ml. Ad3 induced similar levels of IP-10 mRNA expression in REC cells as did Ad5Luc. GAPDH is used as a loading control.
To examine further the role of CAR in the induction of IP-10 by adenovirus vectors, we assessed the effect of infection with the group B Ad3, which does not utilize CAR for cellular attachment (34). Wild-type Ad3 produces a lytic infection in REC cells. To remove the confounding effect of virus replication, wild-type Ad3 was rendered transcription defective by UV-psoralen treatment (8). Infection of REC cells with 5 × 1010 particles of UV-psoralen-treated Ad3 per ml (MOI, ∼3.3 × 104 particles/cell) induced the expression of IP-10 mRNA comparably with an equivalent titer of Ad5Luc at 6 h, confirming that serotype or attachment to CAR per se was not essential for the induction of IP-10 by Ad5 vectors (Fig. 5C).
Taken together, these findings demonstrate that differences in signaling and IP-10 expression induced by AdL.PB(HA) and AdL.F(RAEK-HA) are due to differences in transduction. Furthermore, these results suggest that the adenovirus vector induction of IP-10 can occur independently of CAR binding and RGD-mediated interactions with αv integrins.
Adenovirus vector-induced signaling and IP-10 expression require virus penetration.
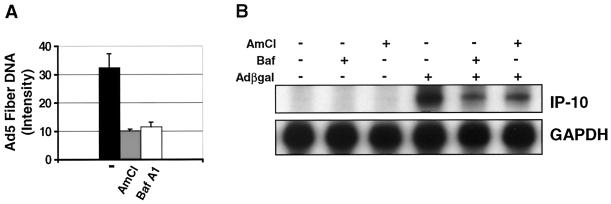
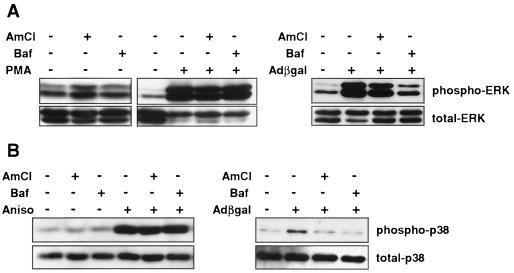
The activation of ERK and p38 coincided with the period of virus penetration into the cytoplasm, an event that begins 10 to 15 min following initial attachment to CAR and subsequent internalization through clathrin-coated vesicles (14). We performed experiments in the presence of either the selective vacuolar H+-ATPase inhibitor bafilomycin A1 or ammonium chloride to prevent endosomal acidification required for adenovirus penetration into the cytoplasm (14, 16, 49). The effects of bafilomycin A1 and ammonium chloride on adenovirus penetration were first determined by Southern analysis of nuclear adenovirus DNA content. Since bafilomycin A1 and ammonium chloride impair virus escape from the endosome but do not affect the initial phases of internalization, nuclear DNA was utilized to exclude virions trapped at various levels within the cytoplasm. Preincubation of REC cells with 200 nM bafilomycin A1 or 25 mM ammonium chloride 15 min prior to incubation with 5 × 1010 particles (MOI, ∼3.3 × 104 particles/cell) of AdCMVβgal per ml decreased nuclear adenovirus DNA content at 6 h, confirming that adenovirus penetration required a change in endosomal pH (Fig. 6A). Furthermore, preincubation of REC cells with 200 nM bafilomycin A1 or 25 mM ammonium chloride substantially reduced AdCMVβgal-induced IP-10 mRNA expression at 6 h as determined by RNase protection assay (Fig. 6B). Next, by immunoblotting, we tested the AdCMVβgal activation of ERK and p38 in the presence of these compounds. Bafilomycin A1 (200 nM) or ammonium chloride (25 mM) also attenuated the phosphorylation of p38 and ERK at 30 min following transduction with AdCMVβgal, suggesting that virus penetration out of the endosome was required to trigger these signaling pathways (Fig. 7). In contrast, activation of ERK by 100 nM PMA or of p38 by anisomycin was not affected by bafilomycin A1 or ammonium chloride, confirming that the effect of these compounds on adenovirus vector-induced signaling was specific (Fig. 7). The attenuation of ERK activation by ammonium chloride was less than that induced by bafilomycin A1 despite comparable effects on vector penetration. However, ammonium chloride alone activated ERK in the absence of adenovirus, making the decrease in activation difficult to monitor with precision (Fig. 7A). These data support the hypothesis that adenovirus vector penetration is required to activate ERK-p38 signaling and induce the expression of IP-10.
FIG. 6.
Effect of virus penetration on AdCMVβgal transduction and IP-10 gene expression. (A) Southern analysis of REC nuclear DNA probing for the Ad5 fiber gene. Pretreatment of cells with ammonium chloride (25 mM) or bafilomycin A1 (200 nM) diminished the transduction of AdCMVβgal as seen by reduced nuclear adenovirus DNA content at 6 h (band intensity, mean ± standard deviation, n = 3). (B) RNase protection assay for IP-10 mRNA. Ammonium chloride (25 mM) and bafilomycin A1 (200 nM) inhibited the induction of IP-10 mRNA by AdCMVβgal at 6 h. GAPDH is used as a loading control.
FIG. 7.
Effect of virus penetration on AdCMVβgal-induced signal transduction. Immunoblotting for phosphorylated and total p44/42 ERK (A) and p38 kinase (B) in AdCMVβgal-transduced REC cells pretreated with bafilomycin A1 (200 nM) or ammonium chloride (25 mM). Bafilomycin A1 and ammonium chloride attenuated the activation of ERK and p38 by AdCMVβgal transduction at 30 min. Bafilomycin A1 (200 nM) and ammonium chloride (25 mM) did not affect PMA (A)- or anisomycin (B)-induced activation of ERK or p38, respectively.
Adenovirus vector-induced IP-10 expression involves p38 and ERK in vivo.
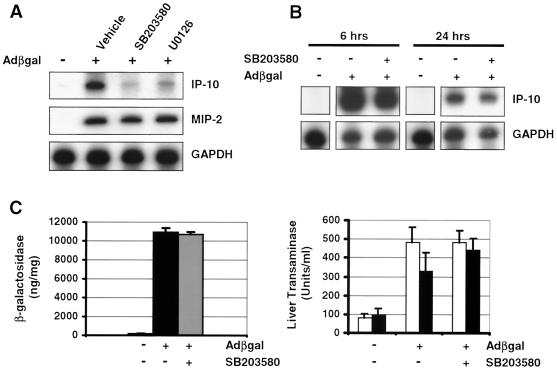
Since the inhibition of ERK and p38 signaling significantly attenuated the adenovirus vector induction of IP-10 in vitro, we sought to confirm our observations in vivo. DBA/2 mice were pretreated with U0126 (160 mg/kg) (n = 3), SB203580 (100 mg/kg) (n = 3), or DMSO-containing vehicle (n = 3) i.p. 30 min prior to the intravenous administration of 1011 particles of AdCMVβgal. Animals were sacrificed at predetermined time points, and the livers were harvested for analysis. At 1 h, AdCMVβgal-induced IP-10 mRNA expression in the liver was determined by RNase protection assay (Fig. 8A). Pretreatment with SB203580 or U0126, but not vehicle, significantly attenuated the AdCMVβgal induction of IP-10 mRNA in the liver at 1 h. SB203580 was slightly more effective than was U0126 in preventing the expression of IP-10, consistent with the findings in vitro. Interestingly, the induction of an unrelated chemokine, macrophage inflammatory protein 2 (MIP-2), was unaffected by pretreatment with U0126 or SB203580, demonstrating that the role of ERK and p38 in the adenovirus vector induction of IP-10 was specific (Fig. 8A).
FIG. 8.
Adenovirus vector-induced expression of IP-10 involves ERK and p38 in vivo. (A) RNase protection assay for IP-10 and MIP-2 mRNA in DBA/2 mouse liver 1 h following the intravenous administration of 1011 particles of AdCMVβgal. Mice were pretreated with SB203580 (n = 3), U0126 (n = 3), or vehicle (n = 3). SB203580 and U0126 blocked the induction of IP-10 mRNA by AdCMVβgal. In contrast, adenovirus-induced MIP-2 expression was unaffected by these compounds. (B) RNase protection assay for IP-10 mRNA 6 and 24 h following the intravenous administration of 1011 particles of AdCMVβgal. Pretreatment with SB203580 did not affect vector-induced gene expression at these time points. GAPDH is used as a loading control. (C) Liver β-galactosidase activity and serum transaminase levels in AdCMVβgal-transduced mice (2.5 × 1011 particles/mouse) treated with SB203580. β-Galactosidase activities were similar in SB203580- and vehicle-treated mice (n = 3). Serum AST (white bars) and ALT (black bars) levels in AdCMVβgal-transduced mice at 24 h were unaffected by treatment with SB203580.
Blocking p38, and to a lesser extent ERK, signaling effectively reduced the adenovirus vector induction of IP-10 at 1 h. To determine whether the attenuation of adenovirus vector-induced IP-10 expression persisted beyond 1 h, we conducted experiments to examine further the effects of SB203580 in vivo. DBA/2 mice were administered SB203580 (100 mg/kg) i.p. 30 min prior to and 12 h following the intravenous administration of 1011 particles of AdCMVβgal. At 6 and 24 h, AdCMVβgal-induced IP-10 mRNA expression was similar in SB203580-treated animals and in mice that received AdCMVβgal alone (n = 3) (Fig. 8B). We also tested the effects of SB203580 on transgene expression and adenovirus vector-induced inflammation in DBA/2 mice receiving a liver-toxic titer of AdCMVβgal. At 24 h, SB203580 (100 mg/kg i.p.) given 30 min prior to and 12 h following the intravenous administration of 2.5 × 1011 particles of AdCMVβgal did not affect liver β-galactosidase expression (10,909 ± 448 ng/mg versus 10,704 ± 255 ng/mg, P = NS) or adenovirus vector-induced liver toxicity as determined by serum AST and ALT levels (AST, 481 ± 82 versus 461 ± 64 U/ml; ALT, 327 ± 101 versus 443 ± 61 U/ml, P = NS) (Fig. 8C). These data demonstrate proof of the principle that the early induction of IP-10 by adenovirus vectors is dependent on p38 and ERK. However, the complexity of subsequent gene expression and the cascade of inflammation induced by adenovirus vectors in vivo could not be inhibited by SB203580 alone by this dosing regimen.
DISCUSSION
Despite numerous advantages such as the ability to transduce a wide variety of cells and the capability to deliver large quantities of DNA, adenovirus vectors have been significantly hampered by the inflammatory and immune responses that they induce in vivo. In this study, we shed further light on the biology of the host response to adenovirus vectors. We show that adenovirus vectors activate ERK and p38 signaling pathways during cell entry and that these pathways are directly involved in the early induction of the C-X-C chemokine IP-10 in vitro and in vivo. Adenovirus vector-induced signaling through p38 appears to play a larger role in the expression of IP-10 than does signaling via ERK, but our results suggest that these two pathways are synergistic. Our studies also demonstrate that the adenovirus vector induction of IP-10 can occur independently of CAR or capsid RGD-αv integrin interactions. The requirement of adenovirus endosomal penetration for optimal IP-10 upregulation supports the hypothesis that a postinternalization step in the entry process activates the early host response to adenovirus vectors.
The early activation of infected cells and the innate immune system is an essential component of host recognition and response to viral infection (15). In vivo, adenovirus vectors rapidly induce inflammation of transduced tissues in a dose-dependent manner (28, 30, 31). Chemokines play a pivotal role in the early inflammatory response triggered by adenovirus vectors (30, 31). Furthermore, chemokines likely contribute to the development of vector-specific adaptive immunity that generally occurs 5 to 7 days following transduction (15, 43). IP-10 and its receptor CXCR3 are increasingly being implicated in the development of Th1 immune responses (17, 33, 37). IP-10 and CXCR3 are important in the pathogenesis of acute allograft rejection, mouse hepatitis virus encephalomyelitis, and T. gondii infection (17, 21, 26). Since adenovirus vectors typically induce a Th1 dominant immune response (48), it is likely that the rapid and abundant expression of IP-10 following transduction with adenovirus vectors plays a significant role in the host response to these agents.
Our results confirm that the adenovirus vector induction of IP-10 is dose dependent and correlates with transduction. Modifications in vector capsid domains that mediate binding to CAR and αv integrins reduced the transduction efficiency of the tropism-modified adenovirus vectors, AdL.PB(HA) and AdL.F(RAEK-HA), and indirectly diminished IP-10 expression. Yet, AdL.PB(HA) and AdL.F(RAEK-HA) still induced IP-10 expression when administered in titers sufficient to increase cell transduction. This result suggests that CAR binding or RGD-mediated interaction with αv integrins is not essential for the adenovirus vector induction of IP-10. These findings may not completely rule out a role for these cell surface molecules in the adenovirus vector induction of IP-10. Since the exact entry mechanism of AdL.PB(HA) and AdL.F(RAEK-HA) is not known, it is possible that, at high titers, low-affinity interactions between CAR and/or the αv integrins and other capsid motifs are occurring. Furthermore, while our results suggest that CAR and capsid RGD motifs may not be directly involved in the induction of IP-10, their roles mediating adenovirus vector binding and internalization contribute indirectly by increasing transduction efficiency (19).
The early capsid-dependent induction of IP-10 by adenovirus vectors suggests that events leading to the expression of this immunoregulatory molecule are triggered during cell entry. The activation of ERK and p38 within minutes of adenovirus vector transduction is consistent with this hypothesis. Our results are also consistent with several studies examining adenovirus-induced signaling. Bruder and Kovesdi (7) demonstrated that, in HeLa cells, early activation of Raf-1 and p42 mitogen-activated protein kinase was required for the induction of the C-X-C chemokine interleukin-8 following transduction with Ad5 vectors. ERK activation during early Ad2 infection in epithelial cells has also been shown to play a role in cell migration (25). More recently, Suomalainen et al. (39) have demonstrated that early activation of p38 by Ad2 was required for efficient nuclear targeting in HeLa cells. Interestingly, although ERK was also activated following infection, signaling through this pathway had no effect on cellular virus trafficking. Our data confirm that the activation of p38 and ERK signaling, in addition to the roles of these kinases in mediating virus trafficking and cell migration, is important in regulating the host immune response to infection.
The exact point at which adenovirus vectors initiate ERK and p38 signaling is not fully known. Our data would suggest that it occurs postinternalization and requires virus penetration of the endosome. This is supported by the results of Suomalainen et al. (39), who showed a lack of signaling through p38 following infection with the temperature-sensitive, penetration-defective Ad2 mutant ts1. ERK signaling, on the other hand, is linked to integrins via the focal adhesion kinase (FAK) and the Src family tyrosine kinase Fyn (12). In SW480 human colon carcinoma cells, adenovirus infection phosphorylates FAK, an effect that is also induced by purified adenovirus penton base protein (25). However, phosphoinositide-3-OH kinase, not ERK, was found to be the downstream target of FAK in these cells. Nevertheless, the use of αv integrins for adenovirus internalization would implicate them in the activation of ERK by adenovirus vectors, a response that may differ between cell types. The activation of ERK by the RGD-deletion vector AdL.PB(HA) suggests that integrin-dependent and integrin-independent mechanisms for adenovirus vector ERK activation may exist.
Understanding the biology of adenovirus vectors is necessary to improve the effectiveness and safety of these agents for human gene therapy. Our results have significant implications and introduce several strategies to diminish the immunogenicity of adenovirus vectors. First, we have identified two signaling pathways that can be targeted to impair the induction of IP-10 following transduction with adenovirus vectors. Preventing the expression of IP-10 and other cytokines or chemokines by targeting ERK or p38 may reduce the inflammatory and immune properties of these vectors. In this study, we demonstrated proof of the principle that modulating adenovirus vector-induced signal transduction in vivo could attenuate IP-10 gene expression. However, our dosing regimen of SB203580 was ineffective in producing a lasting effect and did not alter transgene expression or acute liver inflammation. Although it is possible that testing various mitogen-activated protein kinase inhibitors, combinations, and dosing regimens may prove this approach to be feasible, at present these studies are impractical. We also demonstrate that altering vector tropism has a significant effect on the expression of IP-10. While it is intuitive that decreasing transduction results in reduction of vector-induced inflammation, avoiding the transduction of bystander tissues through selective targeting of adenovirus vectors in vivo will prevent inflammatory complications in nontargeted tissues.
Our results indicate that the onset of adenovirus vector-induced inflammation and immunity is linked to viral entry and occurs within minutes of transduction. The adenovirus capsid is critical in initiating the inflammatory process, creating significant implications for newer generations of targeted and helper-dependent adenovirus vectors. Increasing our understanding of adenovirus vector biology will permit the development of new strategies to develop safer and more effective vectors for clinical gene therapy.
Acknowledgments
This study was supported by the Canadian Institutes of Health Research and the Alberta Heritage Foundation for Medical Research.
We thank N. C. W. Wong for his advice regarding preparation of the manuscript.
REFERENCES
- 1.Annunziato, F., L. Cosmi, G. Galli, C. Beltrame, P. Romagnani, R. Manetti, S. Romagnani, and E. Maggi. 1999. Assessment of chemokine receptor expression by human Th1 and Th2 cells in vitro and in vivo. J. Leukoc. Biol. 65:691-699. [DOI] [PubMed] [Google Scholar]
- 2.Becker, T. C., H. Beltran del Rio, R. J. Noel, J. H. Johnson, and C. B. Newgard. 1994. Overexpression of hexokinase I in isolated islets of Langerhans via recombinant adenovirus. Enhancement of glucose metabolism and insulin secretion at basal but not stimulatory glucose levels. J. Biol. Chem. 269:21234-21238. [PubMed] [Google Scholar]
- 3.Becker, T. C., R. J. Noel, W. S. Coats, A. M. Gomez-Foix, T. Alam, R. D. Gerard, and C. B. Newgard. 1994. Use of recombinant adenovirus for metabolic engineering of mammalian cells. Methods Cell Biol. 43:161-189. [DOI] [PubMed] [Google Scholar]
- 4.Bergelson, J. M., J. A. Cunningham, G. Droguett, E. A. Kurt-Jones, A. Krithivas, J. S. Hong, M. S. Horwitz, R. L. Crowell, and R. W. Finberg. 1997. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science 275:1320-1323. [DOI] [PubMed] [Google Scholar]
- 5.Bonecchi, R., G. Bianchi, P. P. Bordignon, D. D'Ambrosio, R. Lang, A. Borsatti, S. Sozzani, P. Allavena, P. A. Gray, A. Mantovani, and F. Sinigaglia. 1998. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J. Exp. Med. 187:129-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borgland, S. L., G. P. Bowen, N. C. Wong, T. A. Libermann, and D. A. Muruve. 2000. Adenovirus vector-induced expression of the C-X-C chemokine IP-10 is mediated through capsid-dependent activation of NF-κB. J. Virol. 74:3941-3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bruder, J. T., and I. Kovesdi. 1997. Adenovirus infection stimulates the Raf/MAPK signaling pathway and induces interleukin-8 expression. J. Virol. 71:398-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cotten, M., M. Saltik, M. Kursa, E. Wagner, G. Maass, and M. L. Birnstiel. 1994. Psoralen treatment of adenovirus particles eliminates virus replication and transcription while maintaining the endosomolytic activity of the virus capsid. Virology 205:254-261. [DOI] [PubMed] [Google Scholar]
- 9.Crystal, R. G. 1995. Transfer of genes to humans: early lessons and obstacles to success. Science 270:404-410. [DOI] [PubMed] [Google Scholar]
- 10.Cuenda, A., J. Rouse, Y. N. Doza, R. Meier, P. Cohen, T. F. Gallagher, P. R. Young, and J. C. Lee. 1995. SB 203580 is a specific inhibitor of a MAP kinase homologue which is stimulated by cellular stresses and interleukin-1. FEBS Lett. 364:229-233. [DOI] [PubMed] [Google Scholar]
- 11.Favata, M. F., K. Y. Horiuchi, E. J. Manos, A. J. Daulerio, D. A. Stradley, W. S. Feeser, D. E. Van Dyk, W. J. Pitts, R. A. Earl, F. Hobbs, R. A. Copeland, R. L. Magolda, P. A. Scherle, and J. M. Trzaskos. 1998. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J. Biol. Chem. 273:18623-18632. [DOI] [PubMed] [Google Scholar]
- 12.Giancotti, F. G., and E. Ruoslahti. 1999. Integrin signaling. Science 285:1028-1032. [DOI] [PubMed] [Google Scholar]
- 13.Greber, U. F., P. Webster, J. Weber, and A. Helenius. 1996. The role of the adenovirus protease on virus entry into cells. EMBO J. 15:1766-1777. [PMC free article] [PubMed] [Google Scholar]
- 14.Greber, U. F., M. Willetts, P. Webster, and A. Helenius. 1993. Stepwise dismantling of adenovirus 2 during entry into cells. Cell 75:477-486. [DOI] [PubMed] [Google Scholar]
- 15.Guidotti, L. G., and F. V. Chisari. 2001. Noncytolytic control of viral infections by the innate and adaptive immune response. Annu. Rev. Immunol. 19:65-91. [DOI] [PubMed] [Google Scholar]
- 16.Guinea, R., and L. Carrasco. 1995. Requirement for vacuolar proton-ATPase activity during entry of influenza virus into cells. J. Virol. 69:2306-2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hancock, W. W., B. Lu, W. Gao, V. Csizmadia, K. Faia, J. A. King, S. T. Smiley, M. Ling, N. P. Gerard, and C. Gerard. 2000. Requirement of the chemokine receptor CXCR3 for acute allograft rejection. J. Exp. Med. 192:1515-1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herz, J., and R. D. Gerard. 1993. Adenovirus-mediated transfer of low density lipoprotein receptor gene acutely accelerates cholesterol clearance in normal mice. Proc. Natl. Acad. Sci. USA 90:2812-2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hidaka, C., E. Milano, P. L. Leopold, J. M. Bergelson, N. R. Hackett, R. W. Finberg, T. J. Wickham, I. Kovesdi, P. Roelvink, and R. G. Crystal. 1999. CAR-dependent and CAR-independent pathways of adenovirus vector-mediated gene transfer and expression in human fibroblasts. J. Clin. Investig. 103:579-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kafri, T., D. Morgan, T. Krahl, N. Sarvetnick, L. Sherman, and I. Verma. 1998. Cellular immune response to adenoviral vector infected cells does not require de novo viral gene expression: implications for gene therapy. Proc. Natl. Acad. Sci. USA 95:11377-11382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khan, I. A., J. A. MacLean, F. S. Lee, L. Casciotti, E. DeHaan, J. D. Schwartzman, and A. D. Luster. 2000. IP-10 is critical for effector T cell trafficking and host survival in Toxoplasma gondii infection. Immunity 12:483-494. [DOI] [PubMed] [Google Scholar]
- 22.Kirby, I., E. Davison, A. J. Beavil, C. P. Soh, T. J. Wickham, P. W. Roelvink, I. Kovesdi, B. J. Sutton, and G. Santis. 1999. Mutations in the DG loop of adenovirus type 5 fiber knob protein abolish high-affinity binding to its cellular receptor CAR. J. Virol. 73:9508-9514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knowles, M. R., K. W. Hohneker, Z. Zhou, J. C. Olsen, T. L. Noah, P. C. Hu, M. W. Leigh, J. F. Engelhardt, L. J. Edwards, K. R. Jones, et al. 1995. A controlled study of adenoviral-vector-mediated gene transfer in the nasal epithelium of patients with cystic fibrosis. N. Engl. J. Med. 333:823-831. [DOI] [PubMed] [Google Scholar]
- 24.Li, E., S. L. Brown, D. G. Stupack, X. S. Puente, D. A. Cheresh, and G. R. Nemerow. 2001. Integrin αvβ1 is an adenovirus coreceptor. J. Virol. 75:5405-5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li, E., D. Stupack, R. Klemke, D. A. Cheresh, and G. R. Nemerow. 1998. Adenovirus endocytosis via αv integrins requires phosphoinositide-3-OH kinase. J. Virol. 72:2055-2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu, M. T., B. P. Chen, P. Oertel, M. J. Buchmeier, D. Armstrong, T. A. Hamilton, and T. E. Lane. 2000. Cutting edge: the T cell chemoattractant IFN-inducible protein 10 is essential in host defense against viral-induced neurologic disease. J. Immunol. 165:2327-2330. [DOI] [PubMed] [Google Scholar]
- 27.Marshall, E. 2000. Gene therapy's web of corporate connections. Science 288:954-955. [DOI] [PubMed] [Google Scholar]
- 28.McCoy, R. D., B. L. Davidson, B. J. Roessler, G. B. Huffnagle, S. L. Janich, T. J. Laing, and R. H. Simon. 1995. Pulmonary inflammation induced by incomplete or inactivated adenoviral particles. Hum. Gene Ther. 6:1553-1560. [DOI] [PubMed] [Google Scholar]
- 29.Mittereder, N., K. L. March, and B. C. Trapnell. 1996. Evaluation of the concentration and bioactivity of adenovirus vectors for gene therapy. J. Virol. 70:7498-7509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muruve, D. A., M. J. Barnes, I. E. Stillman, and T. A. Libermann. 1999. Adenoviral gene therapy leads to rapid induction of multiple chemokines and acute neutrophil-dependent hepatic injury in vivo. Hum. Gene Ther. 10:965-976. [DOI] [PubMed] [Google Scholar]
- 31.Otake, K., D. L. Ennist, K. Harrod, and B. C. Trapnell. 1998. Nonspecific inflammation inhibits adenovirus-mediated pulmonary gene transfer and expression independent of specific acquired immune responses. Hum. Gene Ther. 9:2207-2222. [DOI] [PubMed] [Google Scholar]
- 32.Parks, R. J., L. Chen, M. Anton, U. Sankar, M. A. Rudnicki, and F. L. Graham. 1996. A helper-dependent adenovirus vector system: removal of helper virus by Cre-mediated excision of the viral packaging signal. Proc. Natl. Acad. Sci. USA 93:13565-13570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qin, S., J. B. Rottman, P. Myers, N. Kassam, M. Weinblatt, M. Loetscher, A. E. Koch, B. Moser, and C. R. Mackay. 1998. The chemokine receptors CXCR3 and CCR5 mark subsets of T cells associated with certain inflammatory reactions. J. Clin. Investig. 101:746-754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roelvink, P. W., A. Lizonova, J. G. Lee, Y. Li, J. M. Bergelson, R. W. Finberg, D. E. Brough, I. Kovesdi, and T. J. Wickham. 1998. The coxsackievirus-adenovirus receptor protein can function as a cellular attachment protein for adenovirus serotypes from subgroups A, C, D, E, and F. J. Virol. 72:7909-7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roelvink, P. W., G. Mi Lee, D. A. Einfeld, I. Kovesdi, and T. J. Wickham. 1999. Identification of a conserved receptor-binding site on the fiber proteins of CAR-recognizing adenoviridae. Science 286:1568-1571. [DOI] [PubMed] [Google Scholar]
- 36.Seth, P., D. Fitzgerald, H. Ginsberg, M. Willingham, and I. Pastan. 1984. Evidence that the penton base of adenovirus is involved in potentiation of toxicity of Pseudomonas exotoxin conjugated to epidermal growth factor. Mol. Cell. Biol. 4:1528-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sorensen, T. L., M. Tani, J. Jensen, V. Pierce, C. Lucchinetti, V. A. Folcik, S. Qin, J. Rottman, F. Sellebjerg, R. M. Strieter, J. L. Frederiksen, and R. M. Ransohoff. 1999. Expression of specific chemokines and chemokine receptors in the central nervous system of multiple sclerosis patients. J. Clin. Investig. 103:807-815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spergel, J. M., W. Hsu, S. Akira, B. Thimmappaya, T. Kishimoto, and S. Chen-Kiang. 1992. NF-IL6, a member of the C/EBP family, regulates E1A-responsive promoters in the absence of E1A. J. Virol. 66:1021-1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suomalainen, M., M. Y. Nakano, K. Boucke, S. Keller, and U. F. Greber. 2001. Adenovirus-activated PKA and p38/MAPK pathways boost microtubule-mediated nuclear targeting of virus. EMBO J. 20:1310-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Svensson, U., and R. Persson. 1984. Entry of adenovirus 2 into HeLa cells. J. Virol. 51:687-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Varga, M. J., C. Weibull, and E. Everitt. 1991. Infectious entry pathway of adenovirus type 2. J. Virol. 65:6061-6070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang, K., T. Guan, D. A. Cheresh, and G. R. Nemerow. 2000. Regulation of adenovirus membrane penetration by the cytoplasmic tail of integrin β5. J. Virol. 74:2731-2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ward, S. G., K. Bacon, and J. Westwick. 1998. Chemokines and T lymphocytes: more than an attraction. Immunity 9:1-11. [DOI] [PubMed] [Google Scholar]
- 44.Wickham, T. J., M. E. Carrion, and I. Kovesdi. 1995. Targeting of adenovirus penton base to new receptors through replacement of its RGD motif with other receptor-specific peptide motifs. Gene Ther. 2:750-756. [PubMed] [Google Scholar]
- 45.Wickham, T. J., E. J. Filardo, D. A. Cheresh, and G. R. Nemerow. 1994. Integrin alpha v beta 5 selectively promotes adenovirus mediated cell membrane permeabilization. J. Cell Biol. 127:257-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wickham, T. J., P. Mathias, D. A. Cheresh, and G. R. Nemerow. 1993. Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell 73:309-319. [DOI] [PubMed] [Google Scholar]
- 47.Yang, Y., H. C. Ertl, and J. M. Wilson. 1994. MHC class I-restricted cytotoxic T lymphocytes to viral antigens destroy hepatocytes in mice infected with E1-deleted recombinant adenoviruses. Immunity 1:433-442. [DOI] [PubMed] [Google Scholar]
- 48.Yang, Y., Z. Xiang, H. C. Ertl, and J. M. Wilson. 1995. Upregulation of class I major histocompatibility complex antigens by interferon gamma is necessary for T-cell-mediated elimination of recombinant adenovirus-infected hepatocytes in vivo. Proc. Natl. Acad. Sci. USA 92:7257-7261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yoshimori, T., A. Yamamoto, Y. Moriyama, M. Futai, and Y. Tashiro. 1991. Bafilomycin A1, a specific inhibitor of vacuolar-type H(+)-ATPase, inhibits acidification and protein degradation in lysosomes of cultured cells. J. Biol. Chem. 266:17707-17712. [PubMed] [Google Scholar]