Abstract
The G protein-coupled receptor encoded by Kaposi's sarcoma-associated herpesvirus, also referred to as ORF74, has been shown to stimulate oncogenic and angiogenic signaling pathways in a constitutively active manner. The biochemical routes linking ORF74 to these signaling pathways are poorly defined. In this study, we show that ORF74 constitutively activates p44/p42 mitogen-activated protein kinase (MAPK) and Akt via Gi- and phospholipase C (PLC)-mediated signaling pathways. Activation of Akt by ORF74 appears to be phosphatidylinositol 3-kinase (PI3-K) dependent but, interestingly, is also mediated by activation of protein kinase C (PKC) and p44/p42 MAPK. ORF74 may signal to Akt via p44/p42 MAPK, which can be activated by Gi, through activation of PI3-K or through PKC via the PLC pathway. Signaling of ORF74 to these proliferative and antiapoptotic signaling pathways can be further modulated positively by growth-related oncogene (GROα/CXCL1) and negatively by human gamma interferon-inducible protein 10 (IP-10/CXCL10), thus acting as an agonist and an inverse agonist, respectively. Despite the ability of the cytomegalovirus-encoded chemokine receptor US28 to constitutively activate PLC, this receptor does not increase phosphorylation of p44/p42 MAPK or Akt in COS-7 cells. Hence, ORF74 appears to signal through a larger diversity of G proteins than US28, allowing it to couple to proliferative and antiapoptotic signaling pathways. ORF74 can therefore be envisioned as an attractive target for novel treatment of Kaposi's sarcoma.
Kaposi's sarcoma (KS)-associated herpesvirus (KSHV), also termed human herpesvirus 8 (HHV-8), is a gammaherpesvirus that is implicated in the pathogenesis of KS and other proliferative disorders, such as primary effusion B-cell lymphoma and multicentric Castleman's disease (13, 15). KS is the most common malignancy in human immunodeficiency virus-infected individuals but has also been detected in non-human immunodeficiency virus-infected individuals (25). KS is a highly angiogenic, multicentric tumor associated with abundant vasculature and spindle cell proliferation. It is believed that spindle cells are the driving force of KS pathogenesis (25). However, it is unknown which factor(s) exactly initiates and sustains spindle cell growth.
The KSHV genome displays an unusual degree of genetic piracy compared to other herpesviruses (41). Many of its viral genes encode proteins whose cellular homologues are known to be involved in growth control, signal transduction, and other regulatory processes (25). Like several other herpesviruses, including cytomegalovirus (CMV) (16, 26), HHV-6 and -7 (29, 32, 47), and herpesvirus saimiri (48), KSHV contains a DNA sequence encoding a protein with homology to cellular G protein-coupled receptors (GPCRs) (14).
GPCRs play an essential role in cellular communication by transducing signals through coupling to heterotrimeric G proteins, generating a broad spectrum of physiological responses (8). It has become clear that various GPCRs induce proliferation and may induce oncogenic transformation and tumor formation in pathological conditions (31). Mechanisms involved in GPCR-induced proliferation include, e.g., activation of mitogen-activated protein kinase (MAPK) signaling pathways, including p44/p42, p38, and JNK kinases, and regulation of cell cycle machinery (30).
The function of virus-encoded GPCRs (vGPCRs) is not fully understood. vGPCRs show the greatest homology to the family of chemokine receptors (45, 46), which play a major role in immune response regulation. Since herpesviruses are associated with transformation of cells and, along with other factors, initiate oncogenesis, the GPCRs encoded by these viruses may be crucial determinants of viral action. The GPCR encoded by KSHV, also referred to as ORF74, shows the most resemblance to the chemokine receptor CXCR2 but, in contrast to most other cellular chemokine receptors, binds both the CC and CXC classes of chemokines (4, 46). Remarkably, ORF74 stimulates signaling pathways linked to cell proliferation in an agonist-independent manner (4). The occurrence of spontaneous activity of GPCRs, also referred to as constitutive activity, is an interesting new concept in GPCR-mediated drug action (36). Moreover, a variety of human diseases (including some proliferative disorders) have been ascribed to the constitutive activity of specific GPCRs due to naturally occurring mutations (56).
ORF74 constitutively initiates cellular transformation and tumorigenesis and induces a switch to an angiogenic phenotype in an agonist-independent manner (5). ORF74 induces the secretion of vascular endothelial growth factor (VEGF), an angiogenesis and KS spindle cell growth factor that is likely to be involved in sustained growth of spindle cells (5). The expression of VEGF was found to be associated with ORF74-induced activation of the p38 and p44/p42 MAPK pathways (55). Additionally, it has been shown that ORF74 induces expression of interleukin-1β (IL-1β), IL-6, and tumor necrosis factor alpha in THP-1 cells, bFGF, IL-8, and MCP-1 in human embryonic kidney (HEK) 293 cells, and IL-2 and IL-4 in Jurkat cells via activation of NF-κB (54). Since ORF74 in KS tumors is predominantly transcribed in a subpopulation of cells that express lytic-cycle genes and therefore are unlikely to proliferate, it has been proposed that these paracrine mechanisms may contribute to KS pathogenesis (33). Recently, transgenic expression of ORF74 in mice was found to induce angioproliferative lesions resembling KS, further supporting the notion that ORF74 acts as a viral oncogene, either directly or through paracrine mechanisms (58).
Although coupling of ORF74 to the MAPK cascade has been shown (55), little is known about the upstream mechanisms leading to activation of this pathway. In this study, we show which signaling components are utilized by ORF74 to activate p44/p42. Moreover, we observed that this pathway is linked to ORF74-induced signaling to Akt, a pathway implicated in antiapoptotic signaling (22). Activation of these pathways occurs in a constitutively active manner by activation of Gi and phospholipase C (PLC) signaling pathways. Signaling of ORF74 to p44/p42 MAPK and Akt signaling pathways does not seem to be universal for vGPCRs, as the CMV-encoded GPCR US28 does not couple to these pathways. Hence, ORF74 appears to signal through a larger diversity of G proteins than US28, allowing it to couple to proliferative and antiapoptotic signaling pathways.
MATERIALS AND METHODS
Materials.
Bovine serum albumin, chloroquine diphosphate, DEAE-dextran (chloride form), pertussis toxin (PTX), and phorbol 12-myristate 13-acetate (PMA) were obtained from Sigma (St. Louis, Mo.). U0126 was from Tocris Cookson Ltd. (Bristol, United Kingdom). Bisindolylmaleimide I hydrochloride (GF-109203X) and wortmannin were obtained from Calbiochem (La Jolla, Calif.). Cell culture media, l-glutamine, penicillin, and streptomycin were obtained from Life Technologies (Gaithersburg, Md.), and fetal calf serum was purchased from Integro B.V. (Dieren, The Netherlands). myo-[2-3H]inositol (17 Ci/mmol) was obtained from Perkin-Elmer Life Sciences. The human chemokines IL-8 (72-amino-acid form), GROα, and human gamma interferon-inducible protein 10 (IP-10) were obtained from PeproTech (Rocky Hill, N.J.).
DNA constructs.
The cDNA of HHV-8-encoded ORF74 was a gift from T. W. Schwartz. The cDNA of ORF74 (GenBank accession number U71368 with a silent G-to-T mutation at position 927) was inserted into pcDEF3 (a gift from J. A. Langen) after PCR amplification. The cDNA of US28 (encoded by the VHL/E human CMV strain; GenBank accession number L20501), inserted into pcDNA3, was a gift from R. Doms. The cDNA encoding Gαt was a gift from B. Defize.
Cell culture and transfection.
COS-7 cells were grown at 5% CO2 and 37°C in Dulbecco's modified Eagle's medium supplemented with 5% fetal calf serum, 2 mM l-glutamine, 50 IU of penicillin per ml, and 50 μg of streptomycin per ml. Transfection of COS-7 cells was performed with DEAE-dextran. The total amount of DNA in transfected cells was maintained constant by addition of the empty vector. Transfected cells were maintained in serum-free medium.
[3H]inositol phosphate production.
Transfected COS-7 cells were seeded in 24-well plates, and 24 h after transfection they were labeled overnight in inositol-free medium (modified Eagle's medium with Earle's salts) supplemented with 2 mM (each) l-glutamine, l-cysteine, l-leucine, l-methionine, l-arginine, and glucose and 1 μCi of myo-[2-3H]inositol per ml in the presence or absence of PTX (100 ng/ml). Subsequently, the labeling medium was aspirated, cells were washed for 10 min with Dulbecco's modified Eagle's medium containing 25 mM HEPES (pH 7.4) and 20 mM LiCl and incubated for 2 h in the same medium in the presence or absence of the indicated chemokines. The incubation was stopped by aspiration of the medium and addition of ice-cold 10 mM formic acid. After 90 min of incubation on ice, inositol phosphates were isolated by anion-exchange chromatography (Dowex AG1-X8 columns; Bio-Rad) and counted by liquid scintillation.
Binding experiments.
IL-8 was labeled with 125I using the Iodogen method (Pierce, Rockford, Ill.) in accordance with the manufacturer's protocol with some minor modifications. Shortly, an Eppendorf tube was coated with Iodogen by slow evaporation of 50 μl of Iodogen solution (1 mg/ml in dichloromethane). The tube was rinsed with label buffer (125 mM Tris [pH 6.0], 150 mM NaCl). Subsequently, 5 μg of IL-8, 35 μl of label buffer, and 0.5 mCi of carrier-free Na125I (Amersham Pharmacia Biotech Benelux, Roosendaal, The Netherlands) were added to the tube and incubated for 10 min at room temperature. The labeling was stopped by addition of 450 μl of 20 mM l-tyrosine (Merck KGaA, Darmstadt, Germany). 125I-IL-8 was separated from free iodine with a 25-cm, 10-ml Sephadex G25 (ICN Pharmaceuticals Inc., Costa Mesa, Calif.) gel filtration column.
Transiently transfected cells were seeded in 48-well plates. At 48 h after transfection, binding was performed on whole cells for 3 to 4 h at 4°C using 125I-IL-8 in binding buffer (50 mM HEPES [pH 7.4], 1 mM CaCl2, 5 mM MgCl2, 0.5% bovine serum albumin). Nonspecific binding was determined in the presence of 0.1 μM unlabeled GROα. After incubation, cells were washed four times with ice-cold binding buffer supplemented with 0.5 M NaCl. Cells were collected and counted in a Wallac Compugamma counter.
Western blot analysis.
Transiently transfected cells were lysed 48 h after transfection in radioimmunoprecipitation assay (RIPA) buffer (phosphate-buffered saline containing 1% Nonidet P-40, 0.1% sodium dodecyl sulfate, 0.5% sodium deoxycholate, 1 mM phenylmethylsulfonyl fluoride, 1 mM sodium orthovanadate, and 2 μg each of aprotinin and leupeptin per ml), sonicated, separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and blotted onto a polyvinylidene difluoride membrane. Antibodies recognizing p44/42 MAPK, Akt, and phospho-Akt (S473) (New England Biolabs, Inc., Beverly, Mass.) were used in combination with a goat anti-rabbit horseradish peroxidase-conjugated secondary antibody (Bio-Rad). The antibody recognizing phospho-p44/42 MAPK (T202/Y204) (New England Biolabs, Inc.) was used in combination with a goat anti-mouse horseradish peroxidase-conjugated secondary antibody (Bio-Rad). Protein bands were detected by an enhanced-chemiluminescence assay and directly quantified with an Image station (NEN Life Science Products, Inc., Boston, Mass.).
RESULTS
Agonist-independent activation of inositol phosphate production.
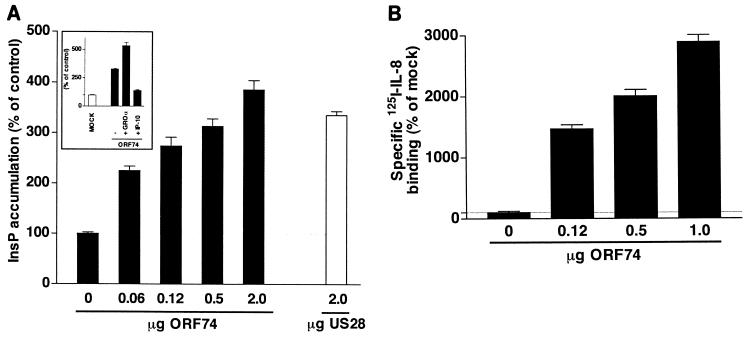
As previously reported (4, 52), increased expression of ORF74 in COS-7 cells, as shown by an increase in specific 125I-IL-8 binding, resulted in expression-dependent enhancement of [3H]inositol phosphate (InsP) production, measured in the absence of any ligand (Fig. 1A and B). This constitutive activity of ORF74 can be further increased by the agonist GROα (CXCL1, 100 nM) and can be inhibited by the inverse agonist IP-10 (CXCL10, 100 nM) (Fig. 1A, inset), as was shown by others (27, 28). Expression of the cellular homologue of ORF74, CXCR2, did not induce InsP production in either the absence or the presence of IL-8 (CXCL8, 100 nM) (data not shown). Expression of the constitutively active CMV-encoded GPCR US28 (12) resulted in an increase in InsP production comparable to that observed with ORF74 (Fig. 1A).
FIG. 1.
ORF74-mediated induction of inositol phosphate accumulation. (A) COS-7 cells (106 cells) were transiently transfected with increasing amounts of cDNA encoding ORF74 or a single amount of cDNA encoding US28 (2 μg/106 cells). At 48 h after transfection, inositol phosphate accumulation was measured. (Inset) Stimulation and inhibition of ORF74-induced inositol phosphate accumulation by the agonist GROα (100 nM, 2 h) and the inverse agonist IP-10 (100 nM, 2 h), respectively. (B) COS-7 cells (106 cells) were transiently transfected with increasing amounts of cDNA encoding ORF74, and 125I-IL-8 binding was performed as described in Materials and Methods. Data are presented as percentages of the values of control (mock-transfected) cells. Representative experiments performed in triplicate are shown. Each experiment was done at least three times.
Agonist-independent activation of p44/p42 MAPK activity.
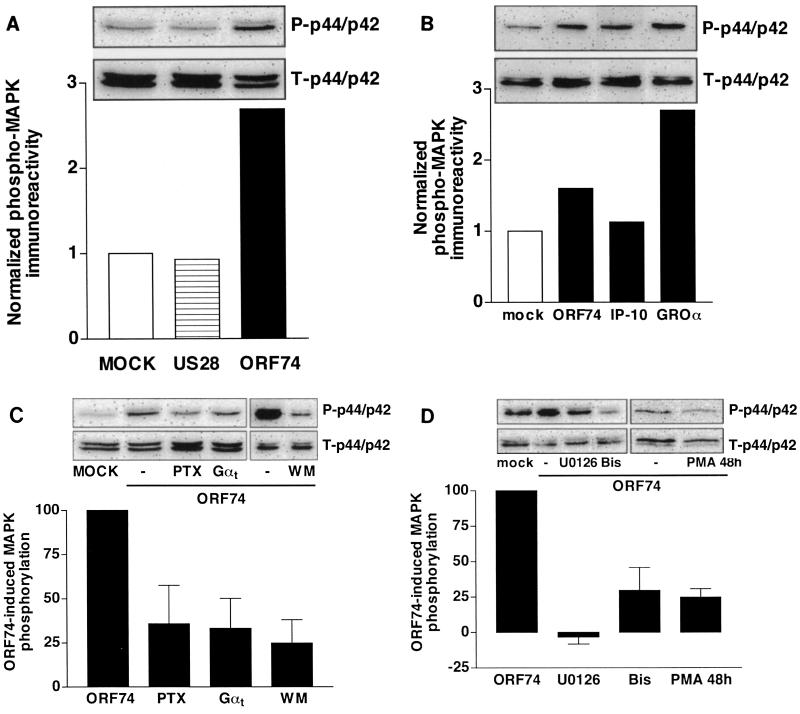
Since ORF74 is known to induce cellular transformation (5) and regulates expression of VEGF through activation of, e.g., p44/p42 MAPK (55), we examined the mechanisms involved in the activation of p44/p42 MAPK. Expression of ORF74 resulted in an increase in basal endogenous MAPK activity as detected by an increase in phosphorylation of p44/p42 MAPK, measured by using an antibody recognizing the phosphorylated form of p44/p42 MAPK (Fig. 2A). Interestingly, US28 was not able to induce an increase in MAPK phosphorylation (Fig. 2A). The ORF74-induced phosphorylation of MAPK can be further increased by incubation with GROα (CXCL1, 30 nM) and inhibited by exposure to IP-10 (CXCL10, 100 nM) (Fig. 2B). Incubation of COS-7 cells expressing ORF74 with the MEK inhibitor U0126 (10 μM) led to complete abolishment of MAPK phosphorylation, confirming the involvement of the Raf-MEK pathway (Fig. 2D).
FIG. 2.
ORF74-induced activation of p44/p42 MAPK. (A) Effect of ORF74 (0.5 μg/106 cells) and US28 (2.0 μg/106 cells) on the basal phosphorylation of p44/p42 MAPK in transiently transfected COS-7 cells as determined by Western blot analysis using specific anti-phosho-p44/p42 (P-p44/p42) antibodies. Phosphorylation was quantified by chemiluminescence and corrected for total MAPK (T-p44/p42) expression on stripped blots. Data are presented as fold control (mock-transfected) cell values. (B) Transiently transfected COS-7 cells were incubated with IP-10 (100 nM, 48 h) or with GROα (30 nM, 5 min) before the cells were lysed. (C) Involvement of the Gi signaling pathway in ORF74-induced p44/p42 MAPK phosphorylation. COS-7 cells transiently transfected with ORF74 alone (0.5 μg/106 cells) or cotransfected with Gαt (1 μg/106 cells) were grown in serum-free medium in the presence or absence of PTX (100 ng/ml, 48 h) or the PI3-K inhibitor wortmannin (WM) (100 nM, 2 h) before lysis and Western blot analysis. Data are presented as the percentage of ORF74-induced p44/p42 MAPK phosphorylation in at least three independent experiments, and representative blots are shown. (D) Role of the PLC signaling pathway in constitutive phosphorylation of p44/p42 MAPK by ORF74. COS-7 cells transiently transfected with ORF74 (0.5 μg/106 cells) were grown in serum-free medium in the presence or absence of the MEK1/2 inhibitor U0126 (10 μM, 2 h), the PKC inhibitor bisindolylmaleimide I (Bis; 1 μM, 2 h), or the phorbol ester PMA (100 nM, 48 h) before lysis and Western blot analysis. Data are presented as the percentage of ORF74-induced p44/p42 MAPK phosphorylation in at least two independent experiments, and representative blots are shown.
Since Gershengorn and colleagues have shown that NIH 3T3 cells expressing ORF74 induce tumor formation when injected into nude mice (5), we investigated the activation of p44/p42 MAPK by ORF74 in these cells also. ORF74 induced p44/p42 MAPK phosphorylation both constitutively and after incubation with GROα (138% ± 0.5% and 298% ± 106% over mock-transfected cells, respectively) in NIH 3T3 cells.
Both Gi- and Gq-coupled receptors are known to activate MAPK in COS-7 cells (18, 38, 39). MAPK activation by Gi-coupled receptors has been reported to be mediated by Gβγ subunits, leading to activation of PI3-K and Ras (34). Since ORF74 has been shown to couple to Gi proteins (10, 17, 40), the involvement of Gi in ORF74-induced MAPK activation was investigated. Pretreatment of cells with PTX (100 ng/ml, 48 h) resulted in a 64% reduction of an ORF74-induced increase in MAPK phosphorylation (Fig. 2C), indicating the involvement of Gi proteins. Coexpression of Gαt, which is known to scavenge Gβγ subunits, also led to a reduction (67%) in ORF74-induced phosphorylation of MAPK. Finally, the involvement of PI3-K was indicated by a 75% reduction of ORF74-induced phosphorylation of MAPK produced by pretreatment of cells for 2 h with wortmannin (100 nM) (Fig. 2C).
Receptors coupled to the PTX-insensitive Gq/11 family activate p44/p42 MAPK via activation of protein kinase C (PKC), which is known to stimulate Raf-1 independently of Ras (39). Since ORF74 stimulates PLC, thereby generating DAG, which is known to activate PKC, this kinase is likely to play a role in the ORF74-induced signaling to MAPK. As expected, downregulation of PKC by long-term treatment with the PKC activator PMA (100 nM, 48 h) or inhibition of PKC by preincubation of cells with the specific PKC inhibitor bisindolylmaleimide (1 μM) resulted in marked attenuation (70 and 75%, respectively) of the ORF74-induced increase in MAPK activity (Fig. 2D). Both treatments completely abolished MAPK activation by PMA (5 min, 1 μM) (data not shown).
Agonist-independent activation of Akt by ORF74.
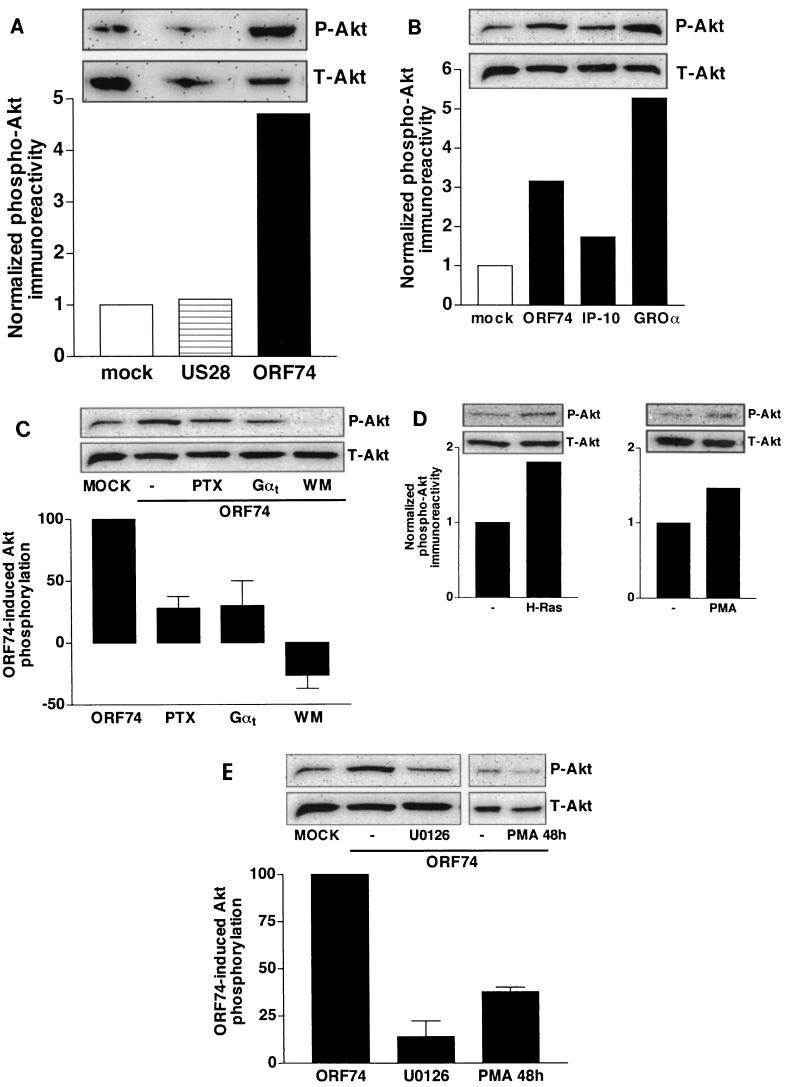
The serine/threonine kinase Akt has been shown to be implicated in signaling pathways leading to cell survival (22). ORF74 expression resulted in a marked increase in phosphorylation of a key regulatory serine residue in the carboxyl-terminal tail of endogenous Akt in COS-7 cells, as evidenced by immunoblotting with a phosphospecific antibody (Fig. 3A). US28 expression, on the other hand, did not influence phosphorylation of Akt (Fig. 3A). As anticipated, the constitutive increase in Akt phosphorylation caused by ORF74 can be further increased by the agonist GROα (CXCL1, 30 nM) and inhibited by the inverse agonist IP-10 (CXCL10, 100 nM) (Fig. 3B). Little is known about the mechanisms involved in signaling of GPCRs to Akt. Involvement of Gi signaling pathways in the ORF74-induced signaling to Akt was ascertained by treatment with PTX. As can be seen in Fig. 3C, PTX treatment resulted in inhibition (72%) of ORF74-induced phosphorylation of Akt. Inhibition of ORF74-induced Akt phosphorylation by coexpression of Gαt was similarly attenuated (70%), suggesting the involvement of Gβγ subunits in signaling of ORF74 to Akt (Fig. 3C). Since PI3-K is known to activate Akt (23), ORF74-expressing cells were treated with wortmannin. As expected, inhibition of PI3-K activity resulted in total abolishment of ORF74-induced phosphorylation of Akt (Fig. 3C). Basal Akt phosphorylation levels in mock-transfected cells were also affected by wortmannin treatment (data not shown). During the preparation of this report, Montaner et al. (40) showed the involvement of Gi, Gβγ subunits, and PI3-K in ORF74-induced activation of Akt by using transiently transfected, hemagglutinin-tagged Akt in COS-7 cells.
FIG.3.
ORF74-induced activation of Akt. (A) Effects of ORF74 (0.5 μg/106 cells) and US28 (2.0 μg/106 cells) on the basal phosphorylation of Akt in transiently transfected COS-7 cells as determined by Western blot analysis using specific anti-phosho-Akt (P-Akt) antibodies. Phosphorylation was quantified by chemiluminescence and corrected for total Akt (T-Akt) expression on stripped blots. (B) Regulation of the ORF74-induced constitutive phosphorylation of Akt by IP-10 (100 nM, 48 h) and GROα (30 nM, 5 min) in transiently transfected COS-7 cells. Data are presented as fold control (mock-transfected) cell values. Representative blots are shown. Each experiment was done at least three times. (C) Involvement of the Gi signaling pathway in ORF74-induced constitutive Akt phosphorylation. COS-7 cells transiently transfected with ORF74 alone (0.5 μg/106 cells) or cotransfected with Gαt (1 μg/106 cells) were grown in serum-free medium in the presence or absence of PTX (100 ng/ml, 48 h) or the PI3-K inhibitor wortmannin (WM; 100 nM, 2 h) before lysis and Western blot analysis. Data are presented as the percentage of ORF74-induced Akt phosphorylation of at least two independent experiments, and representative blots are shown. (D) Akt phosphorylation by mutationally activated H-Ras and via activation of PKC. COS-7 cells were transiently transfected with mutationally activated H-Ras (1 μg/106 cells) or empty vector and grown in serum-free medium for 48 h. PMA-treated cells were incubated with PMA (1 μM) 5 min before lysis. (E) Role of the PLC signaling pathway in constitutive phosphorylation of Akt by ORF74. COS-7 cells transiently transfected with ORF74 (0.5 μg/106 cells) were grown in serum-free medium in the presence or absence of the MEK1/2 inhibitor U0126 (10 μM, 2 h) or the phorbol ester PMA (100 nM, 48 h) before lysis and Western blot analysis. Data are presented as the percentage of ORF74-induced Akt phosphorylation in at least two independent experiments, and a representative blot is shown.
As previously shown by others, mutationally activated H-Ras (RasQL), which is known to activate endogenous PI3-K, also induced phosphorylation of Akt in COS-7 cells (Fig. 3D) (7, 40, 44). Interestingly, incubation of cells with the MEK inhibitor U0126 (10 μM) resulted in marked (86%) inhibition of ORF74-induced phosphorylation of Akt (Fig. 3E). Since ORF74 is able to activate p44/p42 MAPK through activation of PKC, we examined the involvement of PKC in the ORF74-induced phosphorylation of Akt. Treatment of mock-transfected cells with PMA (5 min, 1 μM) led to an increase in the phosphorylation of endogenous Akt, implying the involvement of PKC in the phosphorylation of Akt (Fig. 3D). Downregulation of PKC (treatment of cells for 48 h with PMA at 100 nM) indeed inhibited (63%) ORF74-induced phosphorylation of Akt, suggesting the involvement of PLC-mediated signaling of ORF74 to Akt (Fig. 3E).
DISCUSSION
There is compelling evidence that ORF74 activates oncogenic and angiogenic signaling pathways (5, 55, 58). ORF74 may therefore be a key determinant in the development of KS. Since ORF74 is expressed in only a small subpopulation of KS spindle cells (33), it has been suggested that ORF74 initiates tumorigenesis via indirect mechanisms, e.g., through production of growth factors and cytokines that act in a paracrine manner (54, 55). Activation of the transcription factor NF-κB through the PI3-K/Akt pathway was reported to be involved in the ORF74-induced secretion of IL-6, IL-8, and RANTES in KSIMM cells, an HHV-8-negative KS-derived cell line (49). However, the mechanisms underlying signaling of ORF74 to oncogenic and angiogenic pathways are poorly defined. Earlier studies of ORF74 linked this G protein-coupled receptor to activation of JNK and p38 MAPK signaling pathways when expressed in HEK 293 cells (5). Recent studies showed that besides activation of p38 MAPK, ORF74 also activates p44/p42 MAPK signaling pathways when expressed in COS-7 cells, leading to expression of VEGF via activation of the transcription factor hypoxia-inducible factor 1α (55). In this report, we describe the upstream mechanisms responsible for the ORF74-induced activation of p44/p42 MAPK. In addition, we show that ORF74 constitutively phosphorylates Akt, which is known to be involved in antiapoptotic signaling (22).
ORF74, unlike its cellular homologue CXCR2, initiates formation of InsP in a constitutively active manner when expressed in COS-7 cells, as was previously shown by others (4, 52). Activation of PLC by ORF74 is likely mediated via stimulation of Gq-mediated signaling pathways, although no direct evidence of this has been presented so far (4, 12, 52). p44/p42 MAPK is an important mediator of proliferative signaling, transducing the signal from the cell surface to the nucleus (31). Gi-coupled receptors are known to activate p44/p42 MAPK via Gβγ subunits involving PI3-K and Ras, while Gq-coupled receptors activate MAPK via Raf-1 through a PKC-dependent pathway independently of Gβγ and Ras (18, 38, 39). In this study, we show that ORF74 utilizes both pathways to activate p44/p42 MAPK, since inhibition of Gi, scavenging of Gβγ subunits, and inhibition of PKC activity markedly suppressed ORF74-induced activation of MAPK (Fig. 4). As expected, both MEK and PI3-K are key players in the ORF74-induced signaling to p44/p42 MAPK.
FIG. 4.
Hypothetical scheme of signal transduction pathways activated by ORF74. Based on data from this study, activation of p44/p42 MAPK by ORF74 seems to be Gi mediated via Gβγ subunits involving PI3-K and PLC dependent via activation of PKC, presumably at the level of Raf-1 (39). Activation of Akt by ORF74 appears to be PI3-K dependent, likely via Gβγ from Gi, and seems also to be mediated by activation of the PKC and Ras-Raf pathways.
Earlier studies in our laboratory showed that the CMV-encoded chemokine receptor US28, like ORF74, constitutively activates PLC (12). Interestingly, in COS-7 cells, the CMV-encoded GPCR US28, which is homologous to CC chemokine receptors, does not activate p44/p42 MAPK, even though US28 constitutively activates the production of InsP to an extent similar to that of ORF74. Yet, an earlier report described US28-mediated activation of p44/p42 MAPK by the CC chemokine RANTES (CCL5) in HEK 293 cells (6). In contrast, ORF74, preferentially binding CXC chemokines, does not activate p44/p42 MAPK signaling pathways when expressed in HEK 293 cells (5) while it does so when expressed in COS-7 and NIH 3T3 cells, as was shown in this study and by others (55). It therefore seems that the cell type rather than the chemokine family determines the ability of chemokine(-like) receptors to activate p44/p42 MAPK. The fact that US28 does not couple to the same broad range of signaling pathways as ORF74 when expressed in COS-7 cells (12) may account for its inability to activate p44/p42 MAPK.
Cellular transformation is often associated with the activation of antiapoptotic signaling pathways. Recently, cell type-specific activation of Akt by some GPCRs has been shown (42, 43, 53). Akt has been found to be an important intermediate in cell survival and in the control of apoptosis by regulation of several downstream effectors, including activation of Bad (20, 21), Forkhead (9), GSK3 (19), and transcription factors such as CREB (50) and NF-κB (51). Activation of CREB and NF-κB may lead to expression of factors implicated in the pathogenesis of KS. However, the signaling pathways linking GPCRs to Akt have been poorly characterized. PI3-K appears to be an important upstream activator of Akt since pharmacological inhibitors of PI3-K such as wortmannin abrogate activation of Akt (11, 24, 35). Moreover, constitutively active Ras (H-Ras), which is known to activate PI3-K, is able to activate Akt (40, 44). It is believed that the PI3-K phospholipid products PI(3,4,5)P3 and PI(3,4)P2 are responsible for the translocation of Akt, PDK-1, and yet to be identified PDK2 to the plasma membrane (3). As a consequence, these kinases phosphorylate Akt on the regulatory threonine 308 and serine 473 residues, respectively (2). However, activation of Akt via a PI3-K-independent pathway in response to cyclic AMP has also been reported but no mechanistic information is available (53).
Conflicting data exist about immediate signaling partners involved in GPCR-mediated activation of Akt. In one study, constitutively active Gαq was found to activate transiently transfected Akt in COS-7 cells (44) while another showed an inhibitory effect of Gαq on Gβγ (Gβ1γ2 or Gβ2γ2)- and H-Ras-mediated activation of Akt (7). The authors of the latter report therefore proposed that G proteins can regulate Akt by two distinct and opposing mechanisms.
In this study, we show that ORF74 constitutively phosphorylates endogenous Akt in COS-7 cells. As expected, PI3-K, which is likely to be activated via the release of Gβγ subunits upon interaction of ORF74 with Gi proteins, is fully implicated in the activation of Akt in ORF74-expressing cells. As described earlier by Gutkind and colleagues, the PI3-Kβ-isoform present in COS-7 cells is the likely candidate responsible for signaling of GPCRs to Akt (43). Interestingly, our paper reports, to our knowledge, for the first time that downstream signaling components of the Ras-Raf pathway also play a role in the signaling of a GPCR to Akt, as preincubation of cells with the MEK inhibitor U0126 inhibited ORF74-induced phosphorylation of Akt (Fig. 4). ORF74 may signal to Akt via p44/p42 MAPK, which can be activated by Gi through activation of PI3-K or through PKC via the PLC-dependent pathway (Fig. 4). Ectopic expression of PKC-α, which is present in COS-7 cells (1), has been reported to increase activation of Akt in myeloid progenitor and COS-1 cells (37, 57), suggesting the ability of endogenous PKC-α to activate Akt in COS-7 cells. Indeed, inhibition of PKC abrogates not only ORF74-induced activation of p44/p42 MAPK but also Akt. In COS-7 cells, the observed phosphorylation of Akt by direct stimulation of PKC is limited, suggesting that ORF74 utilizes additional signal transduction pathways to ensure optimal stimulation of Akt. Early studies by Gutkind and colleagues failed to show PKC-dependent activation of Akt directly or in signaling from the muscarinic m1 receptor and Gαq to Akt (44). This discrepancy may be explained by the fact that we studied ORF74-induced phosphorylation of endogenous Akt while others transiently coexpress Akt-1, an isoform with regulatory properties that are possibly different from those of the endogenous Akt isoform(s) present in COS-7 cells. Although we observed constitutive activation of p44/p42 MAPK by ORF74 in NIH 3T3 cells, we could not detect constitutive activation of Akt by ORF74 in these cells (unpublished observations). This may be explained by the presence of different Akt isoforms in these cells. Additional experiments need to be performed to further delineate the mechanisms by which downstream signaling components of the Ras-Raf pathway signal to Akt and whether PKC activates Akt via p44/p42 MAPK or by other means.
Despite the constitutive activation of PLC by US28, no increase in Akt phosphorylation is observed in US28-transfected cells. These observations are in line with our findings that US28 also does not activate p44/p42 MAPK, which plays an important role in the signaling of ORF74 to Akt. Alternatively, preferred coupling of US28 to the Gq pathway rather than the Gi pathway in COS-7 cells (12), along with the fact that Gq has been shown to inhibit Akt-induced signaling (7), may be another explanation for the inability of US28 to activate Akt.
Hence, by virtue of the ability of ORF74 to activate various distinct G proteins, ORF74 initiates an array of signaling pathways that interact to generate an integrated response. Further studies are required to delineate the mechanisms underlying signaling of ORF74 to proliferative and antiapoptotic signaling systems in more physiological cellular systems. However, our studies clearly indicate that ORF74 signals to these pathways in a constitutively active manner. Moreover, not only does ORF74 constitutively activate p44/p42 MAPK and Akt but its activity can also be modulated positively by GROα (CXCL1) and negatively by IP-10 (CXCL10), thus acting as an agonist and an inverse agonist, respectively. By virtue of its ability to respond either positively or negatively to these chemokines, ORF74 can influence proliferative and antiapoptotic signaling, depending on the presence of endogenously expressed chemokines.
During the preparation of this report, Gutkind and colleagues reported the ability of ORF74 to promote endothelial cell survival through activation of Akt-1 (40). Similar to one of the pathways linking ORF74 to Akt in this study, Gutkind and colleagues found ORF74-induced signaling to Akt-1 in COS-7 cells to be partially inhibited by PTX and dependent on PI3-K and Gβγ subunits. Based on the data presented in this report, the PLC-dependent PKC activation and p44/42 MAPK activation seem to play important roles as well.
Taken together, the data available indicate that ORF74 constitutively activates proliferative and antiapoptotic signaling pathways via Gi and PLC. The fact that ORF74 stimulates a broad range of signaling pathways accounts for the fact that this receptor, but not the CMV-encoded chemokine receptor US28, activates p44/p42 and Akt signaling pathways. Activation of p44/42 MAPK may play a significant role in ORF74-induced oncogenesis or induction of angiogenesis via expression of VEGF (55). Since transforming viruses require survival of infected cells to initiate oncogenesis, ORF74-mediated activation of antiapoptotic signaling pathways through activation of Akt may be of physiological relevance as well. ORF74 can be envisioned as an attractive target for the treatment of KS. Development of small ligands acting at this receptor is essential for further substantiation of the importance of this receptor in the viral action of KSHV.
Acknowledgments
M. J. Smit and D. Verzijl contributed equally to this report.
M. J. Smit and D. Verzijl were supported by the Royal Netherlands Academy of Arts and Sciences and The Netherlands Organization for Scientific Research (Chemische Wetenschappen), respectively. P. Casarosa was supported by Byk Nederland B.V. (Zwanenburg, The Netherlands).
REFERENCES
- 1.Adomeit, A., A. Graness, S. Gross, K. Seedorf, R. Wetzker, and C. Liebmann. 1999. Bradykinin B2 receptor-mediated mitogen-activated protein kinase activation in COS-7 cells requires dual signaling via both protein kinase C pathway and epidermal growth factor receptor transactivation. Mol. Cell. Biol. 19:5289-5297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alessi, D. R., M. Andjelkovic, B. Caudwell, P. Cron, N. Morrice, P. Cohen, and B. A. Hemmings. 1996. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 15:6541-6551. [PMC free article] [PubMed] [Google Scholar]
- 3.Andjelkovic, M., D. R. Alessi, R. Meier, A. Fernandez, N. J. Lamb, M. Frech, P. Cron, P. Cohen, J. M. Lucocq, and B. A. Hemmings. 1997. Role of translocation in the activation and function of protein kinase B. J. Biol. Chem. 272:31515-31524. [DOI] [PubMed] [Google Scholar]
- 4.Arvanitakis, L., E. Geras-Raaka, A. Varma, M. C. Gershengorn, and E. Cesarman. 1997. Human herpesvirus KSHV encodes a constitutively active G-protein-coupled receptor linked to cell proliferation. Nature 385:347-350. [DOI] [PubMed] [Google Scholar]
- 5.Bais, C., B. Santomasso, O. Coso, L. Aranitakis, E. Geras-Raaka, J. S. Gutkind, A. S. Asch, E. Cesarman, M. C. Gerhengorn, and E. A. Mesri. 1998. G-protein-coupled receptor of Kaposi's sarcoma-associated herpesvirus is a viral oncogene and angiogenesis activator. Nature 391:86-89. [DOI] [PubMed] [Google Scholar]
- 6.Billstrom, M. A., G. L. Johnson, N. J. Avdi, and G. S. Worthen. 1998. Intracellular signaling by the chemokine receptor US28 during human cytomegalovirus infection. J. Virol. 72:5535-5544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bommakanti, R. K., S. Vinayak, and W. F. Simonds. 2000. Dual regulation of Akt/protein kinase B by heterotrimeric G protein subunits. J. Biol. Chem. 275:38870-38876. [DOI] [PubMed] [Google Scholar]
- 8.Bourne, H. R. 1997. How receptors talk to trimeric G proteins. Curr. Opin. Cell Biol. 9:134-142. [DOI] [PubMed] [Google Scholar]
- 9.Brunet, A., A. Bonni, M. J. Zigmond, M. Z. Lin, P. Juo, L. S. Hu, M. J. Anderson, K. C. Arden, J. Blenis, and M. E. Greenberg. 1999. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96:857-868. [DOI] [PubMed] [Google Scholar]
- 10.Burger, M., J. A. Burger, R. C. Hoch, Z. Oades, H. Takamori, and I. U. Schraufstatter. 1999. Point mutation causing constitutive signaling of CXCR2 leads to transforming activity similar to Kaposi's sarcoma herpesvirus-G protein-coupled receptor. J. Immunol. 163:2017-2022. [PubMed] [Google Scholar]
- 11.Burgering, B. M., and P. J. Coffer. 1995. Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature 376:599-602. [DOI] [PubMed] [Google Scholar]
- 12.Casarosa, P., R. A. Bakker, D. Verzijl, M. Navis, H. Timmerman, R. Leurs, and M. J. Smit. 2001. Constitutive signaling of the human cytomegalovirus-encoded chemokine receptor US28. J. Biol. Chem. 276:1133-1137. [DOI] [PubMed] [Google Scholar]
- 13.Cesarman, E., Y. Chang, P. S. Moore, J. W. Said, and D. M. Knowles. 1995. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N. Engl. J. Med. 332:1186-1191. [DOI] [PubMed] [Google Scholar]
- 14.Cesarman, E., R. G. Nador, F. Bai, R. A. Bohenzky, J. J. Russo, P. S. Moore, Y. Chang, and D. M. Knowles. 1996. Kaposi's sarcoma-associated herpesvirus contains G protein-coupled receptor and cyclin D homologs which are expressed in Kaposi's sarcoma and malignant lymphoma. J. Virol. 70:8218-8223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang, Y., E. Cesarman, M. S. Pessin, F. Lee, J. Culpepper, D. M. Knowles, and P. S. Moore. 1994. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 266:1865-1869. [DOI] [PubMed] [Google Scholar]
- 16.Chee, M. S., S. C. Satchwell, E. Preddie, K. M. Weston, and B. G. Barrell. 1990. Human cytomegalovirus encodes three G protein-coupled receptor homologues. Nature 344:774-777. [DOI] [PubMed] [Google Scholar]
- 17.Couty, J. P., E. Geras-Raaka, B. B. Weksler, and M. C. Gershengorn. 2001. Kaposi's sarcoma-associated herpesvirus G protein-coupled receptor signals through multiple pathways in endothelial cells. J. Biol. Chem. 276:33805-33811. [DOI] [PubMed] [Google Scholar]
- 18.Crespo, P., N. Z. Xu, W. F. Simonds, and J. S. Gutkind. 1994. Ras-dependent activation of MAP kinase pathway mediated by G-protein βγ subunits. Nature 369:418-420. [DOI] [PubMed] [Google Scholar]
- 19.Cross, D. A., D. R. Alessi, P. Cohen, M. Andjelkovich, and B. A. Hemmings. 1995. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 378:785-789. [DOI] [PubMed] [Google Scholar]
- 20.Datta, S. R., H. Dudek, X. Tao, S. Masters, H. Fu, Y. Gotoh, and M. E. Greenberg. 1997. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 91:231-241. [DOI] [PubMed] [Google Scholar]
- 21.del Peso, L., M. Gonzalez-Garcia, C. Page, R. Herrera, and G. Nunez. 1997. Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science 278:687-689. [DOI] [PubMed] [Google Scholar]
- 22.Downward, J. 1998. Mechanisms and consequences of activation of protein kinase B/Akt. Curr. Opin. Cell Biol. 10:262-267. [DOI] [PubMed] [Google Scholar]
- 23.Franke, T. F., D. R. Kaplan, and L. C. Cantley. 1997. PI3K: downstream AKTion blocks apoptosis. Cell 88:435-437. [DOI] [PubMed] [Google Scholar]
- 24.Franke, T. F., S. I. Yang, T. O. Chan, K. Datta, A. Kazlauskas, D. K. Morrison, D. R. Kaplan, and P. N. Tsichlis. 1995. The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell 81:727-736. [DOI] [PubMed] [Google Scholar]
- 25.Ganem, D. 1997. KSHV and Kaposi's sarcoma: the end of the beginning? Cell 91:157-160. [DOI] [PubMed] [Google Scholar]
- 26.Gao, J. L., and P. M. Murphy. 1994. Human cytomegalovirus open reading frame US28 encodes a functional beta chemokine receptor. J. Biol. Chem. 269:28539-28542. [PubMed] [Google Scholar]
- 27.Geras-Raaka, E., A. Varma, H. Ho, I. Clark-Lewis, and M. C. Gershengorn. 1998. Human interferon-gamma-inducible protein 10 (IP-10) inhibits constitutive signaling of Kaposi's sarcoma-associated herpesvirus G protein-coupled receptor. J. Exp. Med. 188:405-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gershengorn, M. C., E. Geras-Raaka, A. Varma, and I. Clark-Lewis. 1998. Chemokines activate Kaposi's sarcoma-associated herpesvirus G protein-coupled receptor in mammalian cells in culture. J. Clin. Investig. 102:1469-1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gompels, U. A., J. Nicholas, G. Lawrence, M. Jones, B. J. Thomson, M. E. Martin, S. Efstathiou, M. Craxton, and H. A. Macaulay. 1995. The DNA sequence of human herpesvirus-6: structure, coding content, and genome evolution. Virology 209:29-51. [DOI] [PubMed] [Google Scholar]
- 30.Gudermann, T., R. Grosse, and G. Schultz. 2000. Contribution of receptor/G protein signaling to cell growth and transformation. Naunyn-Schmiedeberg's Arch. Pharmacol. 361:345-362. [DOI] [PubMed] [Google Scholar]
- 31.Gutkind, J. S. 1998. The pathways connecting G protein-coupled receptors to the nucleus through divergent mitogen-activated protein kinase cascades. J. Biol. Chem. 273:1839-1842. [DOI] [PubMed] [Google Scholar]
- 32.Isegawa, Y., Z. Ping, K. Nakano, N. Sugimoto, and K. Yamanishi. 1998. Human herpesvirus 6 open reading frame U12 encodes a functional β-chemokine receptor. J. Virol. 72:6104-6112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kirshner, J. R., K. Staskus, A. Haase, M. Lagunoff, and D. Ganem. 1999. Expression of the open reading frame 74 (G-protein-coupled receptor) gene of Kaposi's sarcoma (KS)-associated herpesvirus: implications for KS pathogenesis. J. Virol. 73:6006-6014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koch, W. J., B. E. Hawes, L. F. Allen, and R. J. Lefkowitz. 1994. Direct evidence that Gi-coupled receptor stimulation of mitogen-activated protein kinase is mediated by Gβγ activation of p21ras. Proc. Natl. Acad. Sci. USA 91:12706-12710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kohn, A. D., K. S. Kovacina, and R. A. Roth. 1995. Insulin stimulates the kinase activity of RAC-PK, a pleckstrin homology domain containing ser/thr kinase. EMBO J. 14:4288-4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leurs, R., M. J. Smit, A. E. Alewijnse, and H. Timmerman. 1998. Agonist-independent regulation of constitutively active G-protein-coupled receptors. Trends Biochem. Sci. 23:418-422. [DOI] [PubMed] [Google Scholar]
- 37.Li, W., J. Zhang, L. Flechner, T. Hyun, A. Yam, T. F. Franke, and J. H. Pierce. 1999. Protein kinase C-alpha overexpression stimulates Akt activity and suppresses apoptosis induced by interleukin 3 withdrawal. Oncogene 18:6564-6572. [DOI] [PubMed] [Google Scholar]
- 38.Luttrell, L. M., B. E. Hawes, T. van Biesen, D. K. Luttrell, T. J. Lansing, and R. J. Lefkowitz. 1996. Role of c-Src tyrosine kinase in G protein-coupled receptor- and Gβγ subunit-mediated activation of mitogen-activated protein kinases. J. Biol. Chem. 271:19443-19450. [DOI] [PubMed] [Google Scholar]
- 39.Marais, R., Y. Light, C. Mason, H. Paterson, M. F. Olson, and C. J. Marshall. 1998. Requirement of Ras-GTP-Raf complexes for activation of Raf-1 by protein kinase C. Science 280:109-112. [DOI] [PubMed] [Google Scholar]
- 40.Montaner, S., A. Sodhi, S. Pece, E. A. Mesri, and J. S. Gutkind. 2001. The Kaposi's sarcoma-associated herpesvirus G protein-coupled receptor promotes endothelial cell survival through the activation of Akt/protein kinase B. Cancer Res. 61:2641-2648. [PubMed] [Google Scholar]
- 41.Moore, P. S., C. Boshoff, R. A. Weiss, and Y. Chang. 1996. Molecular mimicry of human cytokine and cytokine response pathway genes by KSHV. Science 274:1739-1744. [DOI] [PubMed] [Google Scholar]
- 42.Moule, S. K., G. I. Welsh, N. J. Edgell, E. J. Foulstone, C. G. Proud, and R. M. Denton. 1997. Regulation of protein kinase B and glycogen synthase kinase-3 by insulin and β-adrenergic agonists in rat epididymal fat cells. Activation of protein kinase B by wortmannin-sensitive and -insensitive mechanisms. J. Biol. Chem. 272:7713-7719. [DOI] [PubMed] [Google Scholar]
- 43.Murga, C., S. Fukuhara, and J. S. Gutkind. 2000. A novel role for phosphatidylinositol 3-kinaseβ in signaling from G protein-coupled receptors to Akt. J. Biol. Chem. 275:12069-12073. [DOI] [PubMed] [Google Scholar]
- 44.Murga, C., L. Laguinge, R. Wetzker, A. Cuadrado, and J. S. Gutkind. 1998. Activation of Akt/protein kinase B by G protein-coupled receptors. A role for α and βγ subunits of heterotrimeric G proteins acting through phosphatidylinositol-3-OH kinaseγ. J. Biol. Chem. 273:19080-19085. [DOI] [PubMed] [Google Scholar]
- 45.Murphy, P. M. 2001. Viral exploitation and subversion of the immune system through chemokine mimicry. Nat. Immunol. 2:116-122. [DOI] [PubMed] [Google Scholar]
- 46.Murphy, P. M., M. Baggiolini, I. F. Charo, C. A. Hebert, R. Horuk, K. Matsushima, L. H. Miller, J. J. Oppenheim, and C. A. Power. 2000. International Union of Pharmacology. XXII. Nomenclature for chemokine receptors. Pharmacol. Rev. 52:145-176. [PubMed] [Google Scholar]
- 47.Nicholas, J. 1996. Determination and analysis of the complete nucleotide sequence of human herpesvirus 7. J. Virol. 70:5975-5989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nicholas, J., K. R. Cameron, and R. W. Honess. 1992. Herpesvirus saimiri encodes homologues of G protein-coupled receptors and cyclins. Nature 355:362-365. [DOI] [PubMed] [Google Scholar]
- 49.Pati, S., M. Cavrois, H. G. Guo, J. S. Foulke, Jr., J. Kim, R. A. Feldman, and M. Reitz. 2001. Activation of NF-κB by the human herpesvirus 8 chemokine receptor ORF74: evidence for a paracrine model of Kaposi's sarcoma pathogenesis. J. Virol. 75:8660-8673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pugazhenthi, S., A. Nesterova, C. Sable, K. A. Heidenreich, L. M. Boxer, L. E. Heasley, and J. E. Reusch. 2000. Akt/protein kinase B up-regulates Bcl-2 expression through cAMP-response element-binding protein. J. Biol. Chem. 275:10761-10766. [DOI] [PubMed] [Google Scholar]
- 51.Romashkova, J. A., and S. S. Makarov. 1999. NF-κB is a target of AKT in anti-apoptotic PDGF signalling. Nature 401:86-90. [DOI] [PubMed] [Google Scholar]
- 52.Rosenkilde, M. M., T. N. Kledal, H. Brauner-Osborne, and T. W. Schwartz. 1999. Agonists and inverse agonists for the herpesvirus 8-encoded constitutively active seven-transmembrane oncogene product, ORF-74. J. Biol. Chem. 274:956-961. [DOI] [PubMed] [Google Scholar]
- 53.Sable, C. L., N. Filippa, B. Hemmings, and E. Van Obberghen. 1997. cAMP stimulates protein kinase B in a wortmannin-insensitive manner. FEBS Lett. 409:253-257. [DOI] [PubMed] [Google Scholar]
- 54.Schwarz, M., and P. M. Murphy. 2001. Kaposi's sarcoma-associated herpesvirus G protein-coupled receptor constitutively activates NF-κB and induces proinflammatory cytokine and chemokine production via a C-terminal signaling determinant. J. Immunol. 167:505-513. [DOI] [PubMed] [Google Scholar]
- 55.Sodhi, A., S. Montaner, V. Patel, M. Zohar, C. Bais, E. A. Mesri, and J. S. Gutkind. 2000. The Kaposi's sarcoma-associated herpes virus G protein-coupled receptor up-regulates vascular endothelial growth factor expression and secretion through mitogen-activated protein kinase and p38 pathways acting on hypoxia-inducible factor 1α. Cancer Res. 60:4873-4880. [PubMed] [Google Scholar]
- 56.Spiegel, A. M. 1996. Defects in G protein-coupled signal transduction in human disease. Annu. Rev. Physiol. 58:143-170. [DOI] [PubMed] [Google Scholar]
- 57.Whelan, R. D., and P. J. Parker. 1998. Loss of protein kinase C function induces an apoptotic response. Oncogene 16:1939-1944. [DOI] [PubMed] [Google Scholar]
- 58.Yang, T. Y., S. C. Chen, M. W. Leach, D. Manfra, B. Homey, M. Wiekowski, L. Sullivan, C. H. Jenh, S. K. Narula, S. W. Chensue, and S. A. Lira. 2000. Transgenic expression of the chemokine receptor encoded by human herpesvirus 8 induces an angioproliferative disease resembling Kaposi's sarcoma. J. Exp. Med. 191:445-454. [DOI] [PMC free article] [PubMed] [Google Scholar]