Abstract
In high-capacity adenovirus (HC-Ad) vectors the size and/or composition of the vector genome influences vector stability during production and the expression profile following gene transfer. Typically, an HC-Ad vector will contain both a gene or an expression cassette and stuffer DNA that is required to balance the final vector genome to a size of between 27 and 36 kb. To gain an improved understanding of factors that may influence gene expression from HC-Ad vectors, we have generated a series of vectors that carry different combinations of human alpha-1 antitrypsin (hAAT) expression constructs and stuffer DNAs. Expression in vitro did not predict in vivo performance: all vectors expressed hAAT at similar levels when tested in cell culture. Hepatic expression was evaluated following in vivo gene transfer in C57BL/6J mice. hAAT levels obtained from genomic DNA were significantly higher than levels achieved with small cDNA expression cassettes. Expression was independent of the orientation and only marginally influenced by the location of the expression cassette within the vector genome. The use of lambda stuffer DNA resulted in low-level but stable expression for at least 3 months when higher doses were applied. A potential matrix attachment region element was identified within the hAAT gene and caused a 10-fold increase in expression when introduced in an HC-Ad vector genome carrying a phosphoglycerate kinase (pgk) hAAT cDNA construct. We also illustrate the influence of the promoter on anti-hAAT antibody formation in C57BL/6J mice: a human cytomegalovirus but not a pgk promoter resulted in an anti-hAAT antibody response. Thus, the overall design of HC-Ad vectors may significantly influence amounts and duration of gene expression at different levels.
Somatic gene therapy for many inherited disorders will require, in addition to an efficient gene transfer into target cells, high-level, long-lasting, tissue-specific and/or regulated transgene expression. This is to be achieved in the absence of a toxic or inflammatory response to either viral functions or to the therapeutic protein.
High-capacity (also called helper-dependent or gutless) adenovirus (HC-Ad) vectors have two main advantages over earlier generation Ad vectors lacking E1. First, all viral coding sequences are deleted from the vector genome. Therefore, viral proteins cannot be expressed from the vector, reducing toxicity and the chances of unexpected adverse events. Second, concomitant with the absence of viral coding sequences, the capacity for the incorporation of heterologous DNA is increased to 36 kb, allowing the simultaneous expression of several genes, large cDNAs, and the flexible use of regulatory elements to control gene expression (reviewed in reference 26).
Several lines of evidence, direct and indirect, indicate that the size and/or nature of the vector genome in HC-Ad vectors may have significant functional consequences during production or following gene transfer. Earlier findings revealed that the smallest virus genome that was observed with different Ad type 5 (Ad5)-simian virus 40 hybrid viruses was about 25 kb (23), suggesting a lower size limit for the successful rescue of vector DNA. This was formally confirmed in studies using the Cre-LoxP system for production of HC-Ad vectors, indicating that only vector genomes with sizes of at least 27 kb allowed efficient and stable amplification during production (38). Also, vector rearrangements and amplifications have been a consistent finding with the use of small genomes as starting material for the rescue of HC-Ad vectors. Following rescue, the vector genomes structurally were either symmetric dimeric molecules (34) or were mixtures of head-to-head, head-to-tail, or tail-to-tail concatemers (19, 22, 27).
Using a helper virus-independent production system, vector genomes with sizes of less than 10 kb were packaged into Ad capsids (29). However, these vectors were found to be functionally incompetent. Following in vitro and in vivo gene transfer, vector DNA levels in the target cells were very low and transgene expression was detectable only for a few hours. While the mechanism for this inferior performance of small-vector-genome-containing particles is currently unclear, these studies together with the above summarized evidence point to a central role of size and potentially also nature of the vector genome for efficient vector production and for expression following gene transfer.
Together, these studies firmly established that additional stuffer DNA has to be included in an HC-Ad vector genome if the gene or the expression cassette that is incorporated is less than 27 kb in size. Although this additional DNA could be simply inert and without its own function, in principle it could also include sequences that could influence the duration and levels of expression in a positive or negative fashion. Thus, levels and duration of expression may be influenced by factors that are inherent to the expression construct (e.g., promoter cDNA versus genomic DNA introns) or to the stuffer DNA (e.g., enhancer, repressor, matrix attachment region [MAR]).
In order to gain an improved understanding of the factors that are important for gene expression in the context of HC-Ad vectors, we have varied different aspects of the expression construct and of the stuffer DNA, alone and in combination, and have analyzed the resulting effects on expression following in vivo gene transfer. We demonstrate advantages of using genomic DNA over small expression cassettes. We have analyzed potential influences of orientation and location of expression cassettes on gene expression, and we have identified a potential MAR that positively influences gene expression. We have also illustrated the influence of the choice of the promoter on antibody formation. The data presented suggest that the overall design of HC-Ad vectors may significantly influence the levels and duration of gene expression and that variables of both the expression construct and of the stuffer DNA need to be systematically considered.
MATERIALS AND METHODS
Cloning of a presumptive MAR from the hAAT locus.
A model for MAR structural motifs is summarized in reference 52. Based on these motifs, a computational model was established (http://www.futuresoft.org/MAR-Wiz). Using this software, we identified an 800-bp presumptive MAR element in the first intron of the human alpha-1 antitrypsin (hAAT) genomic locus. A 2-kb EcoRI-SpeI fragment containing this element was subcloned into pBluescript. This fragment was then introduced into several of the Ad constructs described below.
The human hypoxanthine-guanine phosphoribosyltransferase (HPRT) stuffer contains a DNA sequence located in the first intron that was shown to interact with the nuclear matrix in vitro (49). However, according to the computational model, this element does not bear significant sequence homology to typical MAR sequences. Except AdSTK135, all Ad constructs analyzed in this study contain this HPRT MAR element (Fig. 1).
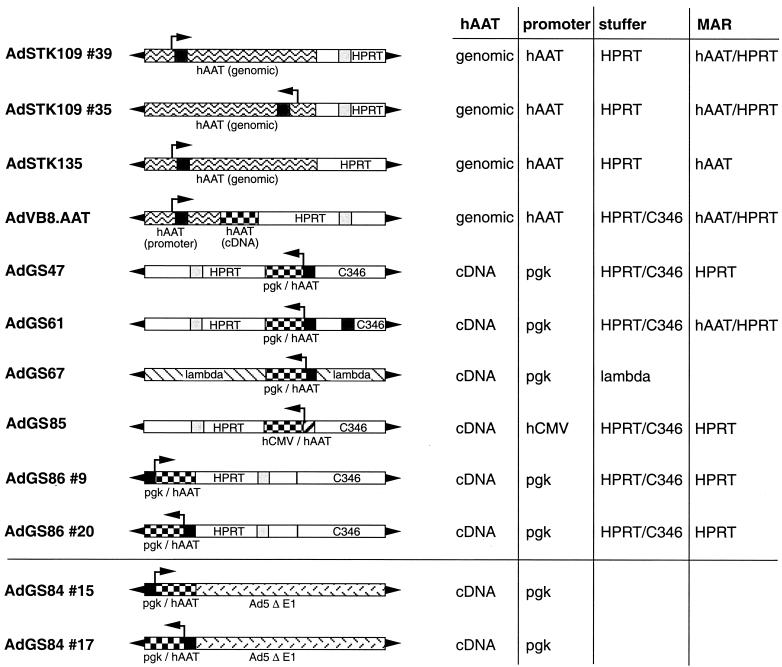
FIG. 1.
Schematic structure of HC-Ad vectors and Ad vectors lacking E1. The HC-Ad vectors have deletions of all viral genes and contain only the left and right termini of Ad5. In addition, AdSTK109 and AdSTK135 contain the genomic hAAT gene locus, including the liver- and macrophage-specific promoters. AdVB8.AAT contains the genomic hAAT promoter, the first hAAT exon and intron, the splice acceptor of the second intron, and the hAAT cDNA. AdGS47, AdGS61, AdGS67, and AdGS86 contain the murine pgk promoter and hAAT cDNA. In contrast to AdGS47, AdGS61, and AdGS86, which carry stuffer DNA derived from HPRT and C346, AdGS67 contains lambda DNA as the stuffer. AdGS85 contains the hCMV promoter and the hAAT cDNA plus HPRT and C346 stuffer. In addition, the HC-Ad vectors bear presumptive MARs located originally either in the HPRT stuffer or in the first intron of the hAAT gene locus. AdGS84 is an Ad vector lacking E1 which contains the murine pgk promoter and hAAT cDNA. The arrows indicated the transcriptional orientation of the hAAT expression cassette.
Ad constructs.
The following Ad vectors (e.g., AdGS47) were constructed as infectious plasmids (e.g., pGS47) based on high-capacity cloning plasmid pSTK120 or pSTK129. Most of these vectors contain noncoding stuffer sequences derived either from the human HPRT locus (18) or from the human cosmid C346 (3). The nucleotides of the stuffer sequences refer to the sequences listed in GenBank. In addition, all infectious plasmids contain the left (nucleotides [nt] 1 to 440) and the right (nt 35818 to 35935) termini of Ad5 DNA. Both left and right Ad termini are flanked by unique PmeI restriction sites. The plasmid backbone in all constructs is pBluescript II KS. In the list below, elements additional to these sequences are listed in left-to-right orientation on the vector genome. Schematic structures of the different Ad vectors are shown in Fig. 1. The details of the cloning procedure can be obtained upon request.
AdGS47.
AdGS47 contains the Ad5 left terminus, HPRT stuffer (nt 17853 to 1799), an expression cassette containing the murine phosphoglycerate kinase (pgk) promoter, hAAT cDNA, bovine growth hormone (bgh) poly(A), C346 stuffer (nt 21484 to 12421), and the Ad5 right terminus.
AdGS61.
AdGS61 contains the Ad5 left terminus, HPRT stuffer (nt 17853 to 1799), a pgk hAAT cDNA expression cassette, bgh poly(A), C346 stuffer (nt 21484 to 13143), sequences from the first intron of the hAAT gene locus (nt 2745 to 4800), C346 stuffer (nt 13143 to 12421), and the Ad5 right terminus.
AdGS67.
AdGS67 contains the Ad5 left terminus, stuffer sequences from phage lambda (nt 44141 to 48520 and nt 1 to 12188), a pgk hAAT cDNA expression cassette, bgh poly(A), phage lambda stuffer sequences (nt 12190 to 23130), and the Ad5 right terminus. In pGS67, the Ad inverted terminal repeats are flanked by additional unique SnaBI sites.
AdGS86.
AdGS86 contains the Ad5 left terminus, a pgk hAAT cDNA expression cassette, bgh poly(A), HPRT stuffer (nt 1799 to 21729), C346 stuffer (nt 10205 to 16750), and the Ad5 right terminus. Isolate #9 contains the expression cassette in left-to-right orientation, and isolate #20 contains it in right-to-left orientation.
AdGS85.
AdGS85 contains the Ad5 left terminus, HPRT stuffer (nt 1799 to 21729), an expression cassette containing the human cytomegalovirus (hCMV) promoter, hAAT cDNA, simian virus 40 poly(A), C346 stuffer (nt 10205 to 16750), and the Ad5 right terminus.
AdSTK109.
The construction of pSTK109 has been described previously (44). Briefly, pSTK109 contains the Ad5 left terminus, a 19-kb genomic fragment containing the hAAT locus, HPRT stuffer (nt 1777 to 10609), and the Ad5 right terminus. Isolate #39 contains the genomic hAAT locus in left-to-right orientation. Isolate #35 contains the same expression cassette in the reverse orientation.
AdSTK135.
pSTK135 was obtained by replacing a 3.8-kb fragment (HPRT nt 4636 to 8404) in the HPRT sequences of pSTK109 with lambda sequences (lambda nt 22652 to 18548).
AdVB8.ATT.
AdVB8.AAT contains the Ad5 left terminus, a 12-kb genomic fragment from the hAAT locus (embedding the liver- and macrophage-specific promoters, the first hAAT exon and intron, and the splice acceptor of the second exon), the hAAT cDNA, β-globin poly(A), HPRT stuffer (nt 17855 to 14588 and nt 8563 to 1799), and the Ad5 right terminus.
AdGS84.
AdGS84 is an first-generation Ad vector lacking E1 based on pGS66 (11). AdGS84 contains the Ad5 left terminus, the pgk hAAT expression cassette and the bgh poly(A) introduced into the unique PacI site, and the Ad5 sequences (nt 3523 to 35935). The Ad termini in pGS84 are flanked by unique SwaI sites. Isolate #15 contains the expression cassette in left-to-right orientation, and isolate #17 contains it in right-to-left orientation.
Rescue of HC-Ad vectors.
Plasmids pSTK109 #35, pSTK109 #39, pSTK135, pVB8.AAT, pGS47, pGS61, pGS85, pGS86 #9, and pGS86 #20 were digested with PmeI; plasmid pGS67 was digested with SnaBI. Following phenol-chloroform extraction, the plasmids were transfected into 293-based cre66 cells (Schiedner et al., unpublished data) which were coinfected with the loxP helper virus AdLC8cluc (37). Subsequent amplification steps and large-scale preparations were performed as described previously (44). All vector preparations were purified twice by CsCl equilibrium density centrifugation, and the particle titers were evaluated by optical density measurements. An average of 2 × 1012 to 6 × 1012 particles were produced from 3 × 108 cells. The infectious titers, as determined by slot blot analyses, ranged from a total of 0.5 × 1011 to 3 × 1011 infectious units. DNAs from CsCl-purified vectors were analyzed by restriction digest and did not show any rearrangements.
Rescue of Ad vectors lacking E1.
To produce AdGS84 #15 and 17, the corresponding plasmids were digested with SwaI. Following phenol-chloroform extraction, the plasmids were transfected into N52.E6 cells (45) and overlaid with agarose containing medium and serum. Plaques were isolated and subjected to a second plaque purification. The lysates were amplified in N52.E6 cells, and large-scale preparations were performed from 3 × 108 cells, resulting in approximately 6 × 1012 particles or 3 × 1011 PFU for each vector.
Experimental mice.
The mice used in this study were immunocompetent, 6- to 8-week-old C57BL/6J mice purchased from Charles River Wiga (Sulzfeld, Germany). Recombinant Ad vectors were diluted in phosphate-buffered saline and injected into the tail vein of mice in a total volume of 200 μl. Blood was obtained by weekly tail vein bleeding, and serum was frozen at −80°C.
Enzyme-linked immunosorbent assay (ELISA) for hAAT.
For analyses of in vitro expression of hAAT following gene transfer, 106 nonhepatic human lung carcinoma A549 cells (Cell Lines Service, Heidelberg, Germany) and 106 hepatic murine Hepa 1-6 cells (Deutsche Sammlung von Mikroorganismen und Zellkulturen, Braunschweig, Germany) were infected with the different HC-Ad vectors or Ad vectors lacking E1 at a multiplicity of infection of 100. At 24, 48, and 72 h after transduction, 1 ml of culture medium was collected.
For analyses of in vivo expression of hAAT following gene transfer, groups of 6 mice received injections into the tail vein of 109 infectious units of the different HC-Ad vectors or Ad vectors lacking E1. Mice were bled at indicated time points. An ELISA was used as described previously (25) to measure hAAT levels in cell culture medium or in the sera of mice.
Morphological analyses.
One, 6, and 12 weeks after injection, 2 animals from each group were sacrificed. Part of the liver was frozen in liquid nitrogen for DNA extraction. The remaining part of the liver was fixed in 10% formalin, and paraffin sections were stained with hematoxylin and eosin.
Antibody detection.
For detection of anti-hAAT antibodies in the serum of transduced mice, 96-well plates were coated with 100 ng of hAAT protein (ICN Biomedicals) for 1 h at 37°C. Serum samples were serially diluted 1:50, 1:500, 1:5,000, 1:50,000, and 1:500,000, loaded into the wells, and incubated for 2 h at 37°C. Subsequently, an anti-mouse antibody coupled to horseradish peroxidase (Jackson Immuno Research Laboratories, West Grove, Pa.) was added. After a 2-h incubation at 37°C, a substrate was added and the reaction was stopped after 15 min with 2 N H2SO4. The titer was determined as the reciprocal of the last dilution giving an optical density at 450 nm of >0.2.
Detection of viral DNA.
DNA was extracted from frozen liver sections by proteinase K digestion followed by phenol-chloroform extraction. DNA was digested with the appropriate enzymes, and Southern blot analyses were performed with a radiolabeled probe specific for the hAAT cDNA.
RESULTS
Construction of HC-Ad vectors expressing hAAT.
HC-Ad vectors are designed to retain only the viral elements required for replication and packaging of vector DNA and therefore allow incorporation of 35 kb of foreign DNA. This space can either be occupied by one or several cDNA expression cassette(s) plus noncoding stuffer DNA or by a large genomic locus encoding the transgene in any possible combination, location, or orientation. To analyze the potential influences of stuffer DNA, promoters, gene constructs, and MARs on the levels and durations of expression, we constructed the series of HC-Ad vectors shown in Fig. 1. These HC-Ad vectors contain either the hAAT cDNA expression cassette in different orientations or locations on the vector genome, different ubiquitous (pgk, hCMV) or tissue-specific (hAAT) promoters, different stuffer DNAs (HPRT, C346, lambda), different combinations of stuffers, additional MARs, or the genomic locus encoding hAAT. HC-Ad vectors were amplified using the Cre-LoxP helper-dependent system (37), and only vector preparations with ratios of vector particles to infectious units of 20 to 40 were used for in vitro and in vivo gene transfer experiments.
Expression of hAAT in vitro.
To analyze if any of the above-described elements had an influence on hAAT expression in tissue culture, a hepatic (Hepa 1-6) and a nonhepatic (A549) cell line were transduced with the different HC-Ad vectors in parallel in single-infection experiments. As expected, minor differences in the expression levels in the two cell lines were observed only with vectors carrying the liver-specific hAAT promoter (AdSTK109). All other vectors expressed comparable hAAT levels in Hepa 1-6 and A549 cells. In addition, comparable levels were obtained from cells transduced with different vectors (data not shown).
Expression of hAAT in vivo: genomic locus versus cDNA (AdSTK109 #39, AdGS47, AdVB8.AAT).
It has been shown previously that intravenous injection of AdSTK109 expressing hAAT from the genomic hAAT locus resulted in long-term and high hAAT levels in C57BL/6J mice (44).
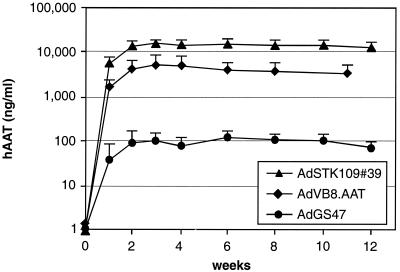
We wished to analyze the impact on levels of hAAT in serum when expressing hAAT from a cDNA (AdGS47) or the genomic locus (AdSTK109) in the context of an HC-Ad vector genome. Therefore, the hAAT cDNA and the murine pgk promoter were introduced into plasmid pSTK120, resulting in pGS47. C57BL/6J mice were transduced by tail vein injection with 109 infectious units of AdGS47 or AdSTK109 (isolate #39), and hAAT serum levels were determined by ELISA at different time points (Fig. 2). Both vectors resulted in stable transgene expression for 12 weeks (the duration of the experiment). However, in mice that received AdSTK109, hAAT levels were approximately 130-fold higher than in mice injected with AdGS47 (12 μg/ml versus 90 ng/ml).
FIG. 2.
Effects of using the genomic locus or cDNA on the expression of hAAT. Groups of 6 C57BL/6J mice were injected with 109 infectious units of either AdSTK109 #39, AdVB8.AAT, or AdGS47 in the tail vein. The amounts of hAAT in serum at the indicated time points were determined by ELISA. Error bars represent the standard deviations of each group.
To better understand the reasons for the differences in expression, AdVB8.AAT was constructed. In AdVB8.AAT the hAAT cDNA is transcribed from the original hAAT promoter. This vector was constructed to contain, in addition to the promoter, the first noncoding exon, the first intron, and the splice acceptor of the second exon. In C57BL/6J mice transduced with AdVB8.AAT, the levels of hAAT in serum were approximately 4 μg/ml and were stable for at least 12 weeks (Fig. 2). These levels are threefold lower than those obtained with the genomic hAAT locus in AdSTK109, suggesting only a minor positive influence on transgene expression by noncoding elements in the hAAT gene downstream from the splice acceptor site of exon 2.
Compared to mice transduced with AdGS47, a striking effect on transgene expression resulted from the use of the genomic hAAT promoter in AdVB8.AAT versus the pgk promoter (40-fold higher expression levels). As detailed in Materials and Methods, AdVB8.AAT contains the genomic hAAT locus accommodating the macrophage- and liver-specific promoters. Even when compared to expression levels in mice obtained with an HC-Ad vector containing an hCMV promoter-controlled hAAT cDNA expression cassette (AdGS85) the use of the genomic hAAT promoter resulted in higher hAAT levels.
It should be mentioned that we currently do not know whether the different polyadenylation signals used in AdSTK109, AdVB8.AAT, and AdGS47 [endogenous, β-globin, and bgh poly(A), respectively] might influence expression levels in the different vectors.
Impact of stuffer DNA on hAAT expression (AdGS47, AdGS67).
In principle, several specific requirements have to be demanded for stuffer DNA. Stuffer DNA should not interfere with but rather support stability and growth kinetics during vector amplification. Stuffer DNA preferably should not negatively influence transgene expression in vivo and itself should not be toxic or immunogenic. Stuffer sequences should be transcriptionally silent.
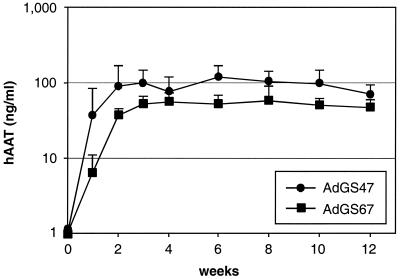
To test the influence of different stuffer DNAs on hAAT expression, an HC-Ad vector was constructed containing the pgk promoter hAAT cDNA expression cassette plus 27.5 kb of stuffer DNA from phage lambda (AdGS67). Injection of 109 infectious units into C57BL/6J mice resulted in nondetectable levels of hAAT in serum (data not shown). However, hAAT levels in the serum of mice transduced with 2 × 109 infectious units of AdGS67 reached approximately 50 ng/ml and were stable for 12 weeks. The kinetics of hAAT expression following injection of either AdGS67 (2 × 109 infectious units) and AdGS47 (109 infectious units) were comparable and reached a plateau 2 weeks after injection (Fig. 3). Injection of 2 × 109 infectious units of both AdGS47 or AdGS67, resulted in threefold-higher levels of hAAT in mice transduced with AdGS47 (data not shown). These results suggested that there were no differences in kinetics but significant, even though not dramatic, differences in the expression levels with HC-Ad vectors containing human or lambda stuffer sequences.
FIG. 3.
Influence of stuffer DNA from different origins (human and bacteriophage lambda) on transgene expression. Groups of 6 C57BL/6J mice were injected with either 109 infectious units of AdGS47 (HPRT, C346 stuffer) or 2 × 109 infectious units of AdGS67 (lambda stuffer) in the tail vein. The amounts of hAAT in the sera of transduced mice were analyzed by ELISA. Error bars represent the standard deviations of each group. Please note that the data for AdGS47 are the same as shown in Fig. 2.
Influence of MARs on hAAT expression levels (AdGS47, AdGS61, AdSTK109 #39, AdSTK135).
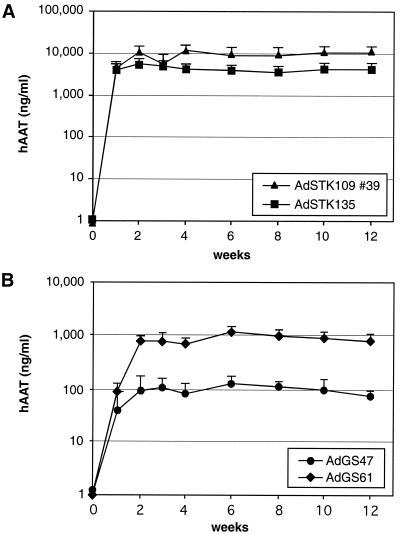
It has been shown previously that inclusion of a scaffold attachment region on a retroviral vector can influence transgene expression levels and the persistence of expression in vivo (2, 6, 16, 35). The presence of a MAR facilitated the replication, episomal maintenance, and stability of a circular yeast artificial chromosome vector in tissue culture (15). Therefore, it is conceivable that particular elements present on the HC-Ad vector genome potentially might help to stabilize DNA in the nucleus of the transduced cell. Noncoding stuffer DNA in AdSTK109 is derived from the human HPRT locus and contains a DNA element that has been shown to interact with the nuclear matrix in vitro (49). To test the influence on transgene expression of the MAR located in the HPRT, a 3.8-kb fragment in the HPRT stuffer of AdSTK109 containing the MAR element was replaced by DNA sequences from bacteriophage lambda, resulting in AdSTK135. Mice transduced with either AdSTK109 (#39) or AdSTK135 yielded high expression levels. The duration of expression did not differ between the two vectors and lasted for 12 weeks (Fig. 4A). In mice that received AdSTK135, the levels of hAAT were about 2.5-fold lower than those in mice transduced with AdSTK109 (5 versus 12 μg/ml). These experiments showed that deletion of the HPRT MAR in the stuffer DNA had no significant influence on transgene expression.
FIG. 4.
Influence of MARs on transgene expression. Groups of 6 C57BL/6J mice were injected with 109 infectious units of AdSTK109 #39 (containing the HPRT MAR) or AdSTK135 (HPRT MAR replaced by lambda sequences) (A) or AdGS47 (containing the HPRT MAR) or AdGS61 (containing the HPRT and hAAT MAR) (B) in the tail vein. The amounts of hAAT in the sera of mice at the indicated time points were determined by ELISA. Error bars represent the standard deviations of each group. Please note that the data for AdSTK109 #39 and AdGS47 are the same as shown in Fig. 2.
By computational analyses, we identified an additional presumptive MAR located in the first intron of the genomic hAAT locus. This 800-bp fragment fulfills in a computer model all criteria for a strong matrix binding sequence. To test if this element influenced transgene expression in vivo, a 2-kb fragment embedding this element was introduced in an HC-Ad vector that contained the pgk-driven hAAT cDNA plus stuffer DNA from HPRT and C346, resulting in AdGS61. C57BL/6J mice transduced with AdGS61 showed a 10-fold increase in hAAT expression compared to mice that were injected with AdGS47, a vector containing exactly the same elements except for the hAAT MAR (800 versus 80 ng/ml). Similar to the other HC-Ad vectors described so far, transgene expression in mice transduced with AdGS61 did not decline during the time of the experiment (Fig. 4B).
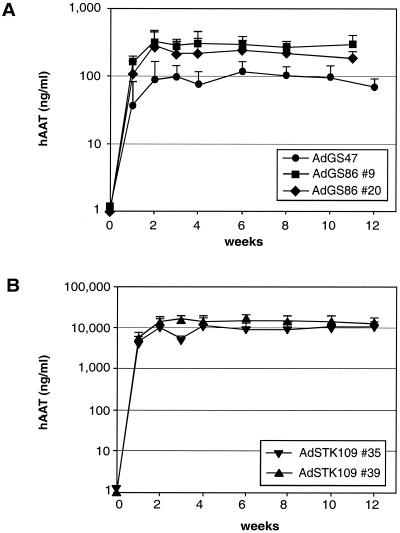
Effects of the location and the orientation of the expression cassette in the vector genome on hAAT expression (AdGS47, AdGS86 #9 and 20, AdSTK109 #35 and 39, AdGS84 #15 and 17).
Viral noncoding elements located within the left terminal Ad sequences have been shown to influence transgene expression in Ad vectors lacking E1 (7, 24, 41, 42). The same elements are also present in HC-Ad vectors. To test if these viral sequences could influence transgene expression from an HC-Ad vector, a pgk promoter-controlled hAAT expression cassette was introduced into an HC-Ad vector plasmid directly adjacent to the left Ad5 terminus. In the resulting vectors, AdGS86 #9 and 20, the expression cassettes were in opposite orientations (Fig. 1). Mice transduced with both vectors had comparable levels of hAAT in serum (AdGS86 #9, 300 ng/ml; AdGS86 #20, 230 ng/ml) which were stable over 12 weeks. These levels were slightly elevated in comparison to those of mice transduced with AdGS47, which contained an identical transgene cassette located between the HPRT and C346 stuffer (Fig. 5A). Also with a second set of vectors containing the genomic hAAT locus in different orientations (AdSTK109 #35 and 39) (Fig. 1), expression levels were comparable (AdSTK109 #35, 7.5 μg/ml; AdSTK109 #39, 12 μg/ml), indicating that the orientation of the expression cassette did not significantly influence expression in the two examples (Fig. 5B).
FIG. 5.
Effects of location and orientation of the transgene cassette on hAAT expression. Groups of 6 mice were injected with 109 infectious units of AdGS86 #9, AdGS86 #20 (expression cassette in different orientations located at the left terminus), or AdGS47 (expression cassette flanked by HPRT and C346 stuffer) (A) or AdSTK109 #35 or AdSTK109 #39 (expression cassette in different orientations) (B) in the tail vein. The amounts of secreted hAAT in the sera of transduced mice were determined by ELISA. Error bars represent the standard deviations of each group. Please note that the data for AdSTK109 #39 and AdGS47 are the same as shown in Fig. 2.
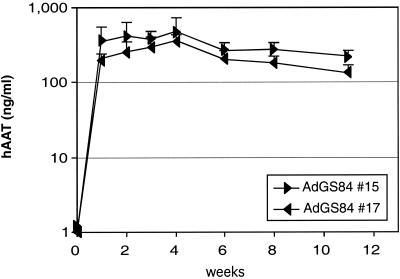
Two first-generation Ad vectors lacking E1, AdGS84 #15 and 17 (Fig. 1), were also constructed to contain the pgk promoter hAAT cDNA expression cassette in different orientations at the left terminus. Both vectors showed high and comparable transgene expression (AdGS84 #15, 350 ng/ml; AdGS84 #17, 250 ng/ml) with a 50% decrease in expression levels between 3 and 12 weeks after injection (Fig. 6). Morphological signs of toxicity were not detected at this low vector dose (data not shown). The expression levels achieved with the two first-generation Ad vectors were comparable to those that were obtained in mice transduced with AdGS86 #9 or 20.
FIG. 6.
Expression of hAAT in mice transduced with Ad vectors lacking E1. Groups of 6 mice were injected with 109 infectious units of either AdGS84 #15 or AdGS84 #17 (expression cassette in opposite orientation) in the tail vein, and the amounts of hAAT in the serum at different time points were determined by ELISA. Error bars represent the standard deviations of each group.
Influence of the promoter on duration of expression and antibody generation (AdGS85).
In mice injected with the described HC-Ad vectors which contain either the ubiquitously active pgk or the liver-specific hAAT promoter, the kinetics of transgene expression were different from those obtained with Ad vectors lacking E1, reaching a maximum level of expression 2 to 3 weeks after injection followed by stable expression levels for 12 weeks. In addition, none of the animals transduced with the HC-Ad vectors showed any sign of liver toxicity (data not shown). Southern blot experiments with genomic DNA from the livers of animals transduced with 109 infectious units revealed comparable transduction efficiencies with all HC-Ad vectors resulting in 0.1 to 0.2 copies per cell present at 1, 6, and 12 weeks after injection.
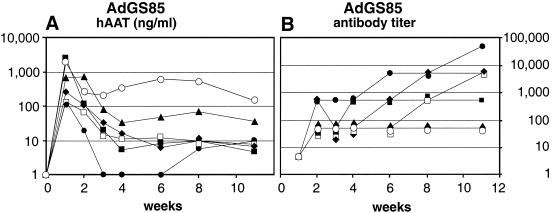
C57BL/6J mice are believed to not generate anti-hAAT antibodies following intravenous administration of Ad vectors expressing hAAT (8, 32, 46). To our surprise, different from all other HC-Ad vectors that we tested during this study, gene transfer of an HC-Ad vector expressing hAAT from the hCMV promoter resulted in very variable and frequently only transient hAAT expression. Expression levels were relatively high at 1 week after injection but were very variable, ranging from 150 to 2,500 ng/ml. In 4 out of 6 mice, hAAT expression levels dropped below the detection limit of slightly below 10 ng/ml within 3 to 4 weeks (Fig. 7A). These results suggested that antibodies to hAAT could have caused a loss of expression or could have limited the detection of hAAT in the sera of transduced animals. Indeed, in AdGS85-transduced animals, the appearance of high titers of antibodies to hAAT correlated with the disappearance of the protein from the serum (Fig. 7B). In mice transduced with the other HC-Ad vectors described above, only a few animals revealed very low levels of antibodies to hAAT and only at certain time points (data not shown). These low levels of antibodies did not result in a drop of the levels of hAAT in the serum. It should be mentioned that we did not observe any inflammatory changes in the livers of mice injected with AdGS85 (data not shown). The data obtained with AdGS85, AdGS47, AdVB8.AAT, or AdSTK109 suggested that rapid disappearance of hAAT from the blood of transduced animals as a result of the appearance of anti-hAAT antibodies was primarily determined by the choice of the promoter that was used to control transgene expression.
FIG. 7.
Influence of the promoter on antibody generation. Six C57BL/6J mice were injected with 109 infectious units of AdGS85, which carries the hAAT cDNA expressed from the hCMV promoter. Mice were bled at the indicated time points, and levels of hAAT in the serum (A) or titers of antibodies to hAAT (B) were determined by ELISA.
DISCUSSION
One of the attractive features that distinguish HC-Ad vectors from earlier generation Ad vectors is the potential to introduce very large DNA fragments onto the vector genome, allowing, for example, the parallel expression of several genes or the tight regulation of gene expression. Typically, HC-Ad vectors will contain stuffer DNA in addition to one or several genes or expression cassettes coding for therapeutic proteins or RNAs. Stuffer DNA is required to balance the final vector genome size to between 27 and 36 kb. The lower limit is determined by an instability of the vector genome during production that has been observed if the starting size of the vector genome was smaller than approximately 27 kb (38). The upper limit is determined by the overall packaging capacity of up to 38 kb (9).
Having the opportunity to introduce many different combinations of coding and noncoding DNA elements on an HC-Ad vector genome leads to the question of whether gene expression is influenced by more than simply promoter strength. In the present study we used in vivo hAAT expression from HC-Ad vectors as a model to analyze the influence on the expression of promoters, different stuffer DNAs, additional stabilizing elements (MARs), and the location and orientation of the expression cassette within an HC-Ad vector genome.
Expression of hAAT from the genomic hAAT locus or from the large hAAT promoter plus first intron resulted in improved expression compared to expression from the murine pgk or the hCMV promoter. The feasibility of introducing the genomic locus of the hAAT gene into an HC-Ad vector genome has recently been shown. This vector (AdSTK109) allowed tissue-specific, high-level, and long-term gene expression without detectable toxicity (33, 44). The use of the ubiquitous pgk (AdGS47) or hCMV (AdGS85) promoter to express hAAT from a cDNA did not provide comparably high hAAT levels. More than 100-fold lower expression was observed with both promoters compared to mice injected with AdSTK109. AdSTK109 includes both liver- and macrophage-specific promoters, all exons and introns, and the polyadenylation signal. Interestingly, AdVB8.AAT, an HC-Ad vector that had the upstream part of the hAAT gene incorporated (promoter region, first exon, intron, and splice acceptor of the second exon) to express hAAT from the cDNA resulted in expression levels that were only threefold lower than those in mice injected with AdSTK109. These results indicated that the noncoding 3′ prime part of the hAAT gene locus (i.e., introns 2 to 4, exons 2 to 5) did not significantly contribute to the increased expression of hAAT. Although we assume that the high levels of expression from AdSTK109 and AdVB8.AAT were largely determined by the strong hAAT promoter, it is conceivable that the first hAAT intron contributed to the enhanced expression since the insertion of an intron into a cDNA may boost expression. These results also exemplify the power of HC-Ad vectors in the performance of gene function and gene regulation studies in vivo.
In several previous studies using Ad vectors lacking E1, the duration and levels of expression have been analyzed with respect to the contribution of intronic or other untranslated DNAs to expression. In one study, expression was improved compared to the cDNA when the expression cassette included parts of the genomic locus (17). Many examples exist in which the inclusion of genomic elements such as introns or 5′ and 3′ untranslated regions into cDNAs increased expression in vitro (28) in transgenic animals (36) and in Ad constructs (14). It is likely that in many cases, particularly in highly expressed genes, use of the genomic locus will be the preferable method for expression if size permits.
Incorporation of a presumptive MAR element increased expression 10-fold. Through sequence analysis of AdSTK109 we located in the first intron of the hAAT a gene fragment with sequence features typical of MARs. Cellular DNA has been found to be tightly associated with the nuclear matrix through specific DNA elements termed MARs. MARs may be up to a few hundred nucleotides in size and many contain AT-rich stretches and clusters of topoisomerase II cleavage sites. Since gene transfer with AdVB8.AAT resulted in hAAT levels that were only slightly reduced compared to AdSTK109, we reasoned that this element might potentially contribute to the high expression levels observed. Therefore, we incorporated this element as an 800-bp fragment into an HC-Ad vector approximately 8 kb upstream from a pgk hAAT expression cassette. Injection of this vector (AdGS61) resulted in a 10-fold increase in hAAT levels in serum compared to those in mice injected with AdGS47, an otherwise identical vector. In eucaryotic and viral systems, MARs have been shown to contain regions of DNA involved in replication, partitioning, and transcription (reviewed in reference 10). Attachment of DNA to the nuclear matrix may lead to activation and inactivation of entire domains of DNA containing many genes. In addition, MARs can coincide with enhancer-like elements (20). Therefore, even though a clear distinction between MARs and enhancers might not be possible, we plan to analyze in future experiments whether the increased in vivo expression observed with AdGS61 is due to matrix binding or associated with an enhancer-like function.
AdSTK109 contains an additional element located in the HPRT stuffer which was found to bind to the nuclear matrix in vitro (49). However, replacing this HPRT MAR with sequences from phage lambda (AdSTK135) did not result in strikingly different hAAT levels when injected into C57BL/6J mice. These results suggested that the MAR located in the HPRT stuffer was not important for expression following gene transfer under the conditions used. These data are in agreement with studies in which the same HPRT MAR had been introduced into an HC-Ad vector expressing E. coli lacZ from the murine CMV promoter. No difference in expression was noted between vectors with and without the HPRT MAR (39).
Stuffer DNA from different origins (DNA of human origin or from phage lambda) did result in quantitative but not qualitative differences in the expression of hAAT. Independent from the choice of stuffer DNA (human or lambda), growth kinetics of the different HC-Ad vectors used in this study were comparable during production. Most HC-Ad vectors that have been generated in the past and that were used to express a variety of different genes in vivo have contained noncoding stuffer DNA from the human HPRT locus and/or the human cosmid C346. In the present study we have not observed that HPRT and/or C346 or lambda DNA-containing vectors are prone to rearrangements during cloning or production. This is in contrast to a study by Sandig et al. (43) in which an occasional instability during amplification of HPRT and/or C346 stuffer-containing vectors was noted. With all vectors of this study and with most published vectors, in vivo expression was long lasting if the protein expressed from the vector was not recognized as a neoantigen (12, 13, 30, 34, 39, 43, 44, 50). Here we demonstrate that the duration of expression of hAAT in the liver was stable for 3 months and did not differ between vectors carrying human or lambda DNA as the stuffer. However, the injection of higher doses of AdGS67 (a pgk hAAT vector with lambda stuffer) was needed to obtain expression levels that were comparable to those observed in mice transduced with AdGS47, a pgk hAAT vector with human DNA as the stuffer. Southern blot analyses with DNA from mice injected with 2 × 109 infectious units of AdGS67 revealed a higher copy number of genomes per hepatocyte (about 0.5 copies per cell) than in mice injected with 109 infectious units of AdGS47 (about 0.1 to 0.2 copies per cell), suggesting that AdGS67 was not impaired in transducing hepatocytes in vivo. Together, these data indicated that the presence of the lambda stuffer on the vector genome did not limit the duration of expression but rather negatively affected absolute expression levels. This is in contrast to a previous study in which E. coli β-galactosidase was expressed from the murine CMV promoter in an HC-Ad vector containing lambda stuffer. Transduction of this vector resulted in only transient expression of β-galactosidase when injected into healthy and lacZ transgenic mice, and lambda stuffer-directed cytotoxic T lymphocytes (CTL) were detected (39). The reasons for the differences between the two results are unclear, and further studies are required for clarification. In principle, several explanations alone or in combination might account for the differences. First, β-galactosidase is an intracellular protein, whereas hAAT is efficiently secreted from the cell. Second, β-galactosidase was expressed from the murine CMV promoter, whereas the murine pgk promoter was used to express hAAT. Third, the β-galactosidase-expressing HC-Ad vector contained a bacterial origin of replication and an ampicillin resistance gene in addition to the lambda stuffer. Fourth, the β-galactosidase-expressing HC-Ad vector was tested in FVB/n mice in contrast to the C57BL/6J mice used in the present study.
Location and orientation of the expression cassette on the vector genome did not influence expression levels of hAAT. In first- or second-generation Ad vectors, expression cassettes are usually introduced immediately adjacent to the left terminus. Several studies have indicated that the orientation of the expression cassette at this location may influence the magnitude of transgene expression in vitro (1, 48). Especially, tissue-specific or inducible transgene expression in vivo was impaired by viral regulatory elements located within the Ad left inverted terminal repeat (nt 1 to 340) but also within the pIX promoter (7, 47, 48). In the present study, potential orientation and/or location effects were analyzed by generating three vectors that carried identical pgk hAAT cassettes either embedded within stuffer DNA approximately in the middle of the vector genome or in both orientations at the left terminus. As a result of the present study we note that there were no significant differences in expression kinetics, duration, or absolute levels of hAAT among the different vectors, except that there was a tendency toward slightly lower hAAT levels when the expression cassette was located in the middle of the vector genome. In particular, injection of the two vectors AdGS86 #9 and 20, which carried the cassette in alternate orientations at the left terminus immediately adjacent to the E1 enhancer, resulted in identical hAAT levels. In addition, we also noted that the same expression cassette, when introduced in the two orientations into first-generation Ad vectors, also did not result in different expression levels. Comparable results were obtained by injection of two HC-Ad vectors carrying the genomic hAAT locus in different orientations (AdSTK109 #35 and 39) that resulted in equivalent expression levels.
Using the hCMV hAAT expression cassette in an HC-Ad vector resulted in the generation of anti-hAAT antibodies in transduced C57BL/6J mice. As shown in Fig. 2 to 5 , with most HC-Ad vectors the levels of hAAT in serum reached expression plateaus 2 to 3 weeks following injection and were followed by stable expression for 3 months (the duration of the experiment). Expression kinetics were independent of the combination of stuffer, gene construct, or promoter. None of the HC-Ad vectors resulted in detectable liver toxicity at the given doses. C57BL/6J mice were chosen because, in contrast to other mouse strains, in many studies they have been found to not generate anti-hAAT antibodies following intravenous administration of an Ad vector lacking E1 and expressing hAAT (8, 32, 46). In the present study we were surprised to note that injection of AdGS85, an HC-Ad vector expressing hAAT from the hCMV promoter, was followed by highly variable and rapidly declining hAAT levels in serum. In most mice, hAAT was undetectable in serum 3 to 4 weeks after injection. Several scenarios have been found responsible for transient gene expression from Ad vectors lacking E1: (i) CTL response to leaky expression of viral proteins and loss of transduced cells (53-58), (ii) immune responses against the transgene (31, 32, 51), and (iii) promoter shut-off (4, 5, 11, 21). HC-Ad vectors do not contain any viral coding sequences. Therefore, CTL responses to viral proteins in transduced animals can be excluded as a reason for the loss of transduced cells. In mice injected with AdGS85, hepatotoxicity was not observed (data not shown). However, rising anti-hAAT serum antibody titers were detected following vector injection. Interestingly, injection of AdGS47, an almost identical vector with the hCMV promoter replaced by the murine pgk promoter resulted in stable serum levels of hAAT (Fig. 2) in the absence of anti-hAAT serum antibodies (data not shown). These data indicated that the immune response against hAAT differed between two promoters that both fall into the category of constitutive promoters. In previous studies it has been observed that C3H/HeJ mice developed anti-hAAT antibodies when Ad vectors lacking E1 were injected that expressed hAAT from the ubiquitous pgk promoter but failed to develop antibodies when transduced with a vector expressing hAAT cDNA from the liver-specific albumin promoter (40). In view of data that indicate that antiviral immune responses may depend on the expression of viral proteins in professional antigen-presenting cells (APC) (58), this observation has been interpreted to mean that the generation of antibody responses against secreted foreign antigens following gene transfer might be induced by the expression of the antigen in APC due to the use of a promoter that is active in APC. In the studies presented here and in previously reported studies (40), even the pgk hAAT expression cassette in the context of an Ad vector lacking E1 did not result in anti-hAAT antibody formation in C57BL/6J mice (data not shown). Several conclusions can be drawn from these observations: (i) antibody response to the transgene expressed under the control of the pgk promoter is strain related and occurs in C3H/HeJ mice but not in C57BL/6J mice and (ii) expression of hAAT under the control of the hCMV promoter but not the pgk promoter results in anti-hAAT antibody formation in C57BL/6J mice. Future experiments should clarify a possible influence of the vector backbone (Ad vector lacking E1 versus HC-Ad vector) on the performance of different promoters in different mouse strains, and the experiments described in the present study in C57BL/6J mice should be extended to additional mouse strains.
In summary, we show that, except when using the hCMV promoter to express the transgene, the expression kinetics and duration of expression did not differ between the different vectors that were used in the present study. With all vectors, which were applied at a relatively low dose (109 infectious units), peak levels of hAAT in serum were observed 2 to 3 weeks following injection. However, absolute hAAT levels differed between the individual vectors. This was not surprising for vectors that contained different expression cassettes, e.g., different promoters or hAAT coding sequences. However, in vectors carrying identical expression cassettes, the nature of the stuffer DNA and the inclusion of a MAR significantly influenced expression. The differences in expression that were observed following gene transfer in vivo were not reflected in in vitro experiments. Therefore, the optimization of HC-Ad vectors should include the consideration of stuffer DNA, promoter, additional noncoding elements, and results obtained from in vivo testing. It is likely that, depending on the cell type to be transduced or the transgene to be expressed, each application will require a specific vector design. Thus, optimized vectors have the highest potential to achieve high transgene expression with low and nontoxic vector doses.
Acknowledgments
We thank Frank Graham for providing the helper virus Ad5LC8cluc.
This work was supported by grants FKZ 031178, FKZ 03-122235-6, and FKZ 01KS9502 from the Federal Ministry of Education and Research and the Center for Molecular Medicine Cologne (ZMMK) and by a grant from Coley Pharmaceuticals.
REFERENCES
- 1.Addison, C. L., M. Hitt, D. Kunsken, and F. L. Graham. 1997. Comparison of the human versus murine cytomegalovirus immediate early gene promoters for transgene expression by adenoviral vectors. J. Gen. Virol. 78:1653-1661. [DOI] [PubMed] [Google Scholar]
- 2.Agarwal, M., T. W. Austin, F. Morel, J. Chen, E. Bohnlein, and I. Plavec. 1998. Scaffold attachment region-mediated enhancement of retroviral vector expression in primary T cells. J. Virol. 72:3720-3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersson, B., F. Lu, D. M. Muzny, S. T. Warren, and R. A. Gibbs. 1995. Complete sequence of a 38.4-kb human cosmid insert containing the polymorphic marker DXS455 from Xq28. DNA Sequence 5:219-223. [DOI] [PubMed] [Google Scholar]
- 4.Armentano, D., J. Zabner, C. Sacks, C. C. Sookdeo, M. P. Smith, J. A. St. George, S. C. Wadsworth, A. E. Smith, and R. J. Gregory. 1997. Effect of the E4 region on the persistence of transgene expression from adenovirus vectors. J. Virol. 71:2408-2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armentano, D., M. P. Smith, C. C. Sookdeo, J. Zabner, M. A. Perricone, J. A. St. George, S. C. Wadsworth, and R. J. Gregory. 1999. E4ORF3 requirement for achieving long-term transgene expression from the cytomegalovirus promoter in adenovirus vectors. J. Virol. 73:7031-7034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Auten, J., M. Agarwal, J. Chen, R. Sutton, and I. Plavec. 1999. Effect of scaffold attachment region on transgene expression in retrovirus vector-transduced primary T cells and macrophages. Hum. Gene Ther. 20:1389-1399. [DOI] [PubMed] [Google Scholar]
- 7.Babiss, L. E., J. M. Friedman, and J. E. Darnell, Jr. 1986. Cellular promoters incorporated into the adenovirus genome: effects of viral regulatory elements on transcription rates and cell specificity of albumin and β-globin promoter. Mol. Cell. Biol. 6:3798-3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barr, D., J. Tubb, D. Ferguson, A. Scaria, A. Lieber, C. Wilson, J. Perkins, and M. A. Kay. 1995. Strain related variations in adenovirally mediated transgene expression from mouse hepatocytes in vivo: comparison between immunocompetent and immunodeficient inbred strains. Gene Ther. 2:151-155. [PubMed] [Google Scholar]
- 9.Bett, A. J., W. Haddara, L. Prevec, and F. L. Graham. 1994. An efficient and flexible system for construction of adenovirus vectors with insertions and deletions in early region 1 and 3. Proc. Natl. Acad. Sci. USA 91:8802-8806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boulikas, T. 1995. Chromatin domains and prediction of MAR sequences. Int. Rev. Cytol. 162A:279-388. [DOI] [PubMed]
- 11.Brough, D. E., C. Hsu, V. A. Kulesa, G. M. Lee, L. J. Cantolupo, A. Lizonova, and I. Kovesdi. 1997. Activation of transgene expression by early region 4 is responsible for a high level of persistent transgene expression from adenovirus vectors in vivo. J. Virol. 71:9206-9213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burcin, M. M., G. Schiedner, S. Kochanek, S. Y. Tsai, and B. W. O'Malley. 1999. Adenovirus-mediated regulable target gene expression in vivo. Proc. Natl. Acad. Sci. USA 96:355-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen, H.-H., L. M. Mack, R. Kelly, M. Ontell, S. Kochanek, and P. R. Clemens. 1997. Persistence in muscle of an adenoviral vector that lacks all viral genes. Proc. Natl. Acad. Sci. USA 94:1645-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Connelly, S., J. M. Gardner, A. McClelland, and M. Kaleko. 1996. High-level tissue-specific expression of functional human factor VIII in mice. Hum. Gene Ther. 7:183-195. [DOI] [PubMed] [Google Scholar]
- 15.Cossons, N., T. O. Nielsen, C. Dini, N. Tomilin, D. B. Young, K. T. Riabowol, J. B. Rattner, R. N. Johnston, M. Zannis-Hadjopoulos, and G. B. Price. 1997. Circular Yac vectors containing small mammalian origin sequences can associate with nuclear matrix. J. Cell. Biochem. 67:439-450. [DOI] [PubMed] [Google Scholar]
- 16.Dang, Q., J. Auten, and I. Plavec. 2000. Human beta interferon scaffold attachment region inhibits de novo methylation and confers long-term, copy number-dependent expression to retroviral vector. J. Virol. 74:2671-2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Geest, B., S. Van Linthout, M. Lox, D. Collen, and P. Holvoet. 2000. Sustained expression of human apolipoprotein A-I after adenoviral gene transfer in C57BL/6 mice: role of apolipoprotein A-I promoter, apolipoprotein A-I intron and human apolipoprotein E enhancer. Hum. Gene Ther. 11:101-112. [DOI] [PubMed] [Google Scholar]
- 18.Edwards, A., H. Voss, P. Rice, A. Civitello, J. Stegemann, C. Schwager, J. Zimmermann, H. Erfle, C. T. Caskey, and W. Ansorge. 1990. Automated DNA sequencing of the human HPRT locus. Genomics 6:593-608. [DOI] [PubMed] [Google Scholar]
- 19.Fisher, K. J., H. Choi, J. Burda, S.-J. Chen, and J. M. Wilson. 1996. Recombinant adenovirus deleted for all viral genes for gene therapy of cystic fibrosis. Virology 217:11-22. [DOI] [PubMed] [Google Scholar]
- 20.Gasser, S. M., and U. K. Laemmli. 1986. Cohabitation of scaffold binding regions with upstream/enhancer elements of three developmentally regulated genes of D. melanogaster. Cell 46:521-530. [DOI] [PubMed] [Google Scholar]
- 21.Grave, L., D. Dreyer, A. Dieterle, P. Leroy, A.-I. Michou, C. Doderer, A. Pavirani, M. Lusky, and M. Mehtali. 2000. Differential influence of E4 adenoviral genes on viral and cellular promoters. J. Gene Med. 2:433-443. [DOI] [PubMed] [Google Scholar]
- 22.Haecker, S. E., H. H. Stedman, R. J. Balice-Gordon, D. B. Smith, J. P. Greelish, M. A. Mitchell, A. Wells, H. L. Sweeney, and J. M. Wilson. 1996. In vivo expression of full-length human dystrophin from adenoviral vectors deleted for all viral genes. Hum. Gene Ther. 7:1907-1914. [DOI] [PubMed] [Google Scholar]
- 23.Hassell, J. A., E. Lukanidin, G. Fey, and J. Sambrook. 1978. The structure and expression of two defective adenovirus 2/simian virus 40 hybrids. J. Mol. Biol. 120:209-247. [DOI] [PubMed] [Google Scholar]
- 24.Imler, J. L., F. Dupuit, C. Chartier, N. Accart, A. Dieterle, H. Schultz, E. Puchelle, and A. Pavirani. 1996. Targeting cell-specific gene expression with an adenovirus vector containing the lacZ gene under the control of the CFTR promoter. Gene Ther. 3:49-58. [PubMed] [Google Scholar]
- 25.Kay, M. A., Q. Li, T. J. Liu, F. Leland, C. Toman, M. Finegold, and S. L. Woo. 1992. Hepatic gene therapy: persistent expression of human alpha 1-antitrypsin in mice after direct gene delivery in vivo. Hum. Gene Ther. 3:641-647. [DOI] [PubMed] [Google Scholar]
- 26.Kochanek, S. 1999. High-capacity adenoviral vectors for gene transfer and somatic gene therapy. Hum. Gene Ther. 10:2451-2459. [DOI] [PubMed] [Google Scholar]
- 27.Kumar-Singh, R., and D. B. Farber. 1998. Encapsidated adenovirus mini-chromosome-mediated delivery of genes to the retina: application to the rescue of photoreceptor degeneration. Hum. Mol. Genet. 7:1893-1900. [DOI] [PubMed] [Google Scholar]
- 28.Kurachi, S., Y. Hitomi, M. Furukawa, and K. Kurachi. 1995. Role of intron I in expression of human factor IX gene. J. Biol. Chem. 270:5276-5281. [DOI] [PubMed] [Google Scholar]
- 29.Lieber, A., C.-Y. He, I. Kirillova, and M. A. Kay. 1996. Recombinant adenovirus with large deletions generated by Cre-mediated excision exhibit different biological properties compared with first-generation vectors in vitro and in vivo. J. Virol. 70:8944-8960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maione, D., M. Wiznerowicz, P. Delmastro, R. Cortese, G. Ciliberto, N. La Monica, and R. Savi# 2000. Prolonged expression and effective readministration of erythropoietin delivered with a fully deleted adenoviral vector. Hum. Gene Ther. 11:859-868. [DOI] [PubMed] [Google Scholar]
- 31.Michou, A. I., L. Santoro, M. Christ, V. Julliard, A. Pavirani, and M. Mehtali. 1997. Adenovirus-mediated gene transfer: influence of transgene, mouse strain and type of immune response on persistence of transgene expression. Gene Ther. 4:473-482. [DOI] [PubMed] [Google Scholar]
- 32.Morral, N., W. O'Neal, H. Zhou, C. Langston, and A. L. Beaudet. 1997. Immune responses to reporter proteins and high viral dose limit duration of expression with adenoviral vectors: comparison of E2a wild type and E2a deleted vectors. Hum. Gene Ther. 8:1275-1286. [DOI] [PubMed] [Google Scholar]
- 33.Morral, N., R. J., Parks, H. Zhou, C. Langston, G. Schiedner, J. Quinones, F. L. Graham, S. Kochanek, and A. L. Beaudet. 1998. High doses of a helper-dependent adenoviral vector yield supraphysiological levels of α1-antitrypsin with negligible toxicity. Hum. Gene Ther. 9:2709-2716. [DOI] [PubMed] [Google Scholar]
- 34.Morsy, M. A., M. Gu, S. Motzel, J. Zhao, J. Lin, Q. Su, H. Allen, L. Franklin, R. J. Parks, F. L. Graham, S. Kochanek, A. J. Bett, and C. T. Caskey. 1998. An adenoviral vector deleted for all viral coding sequences results in enhanced safety and extended expression of a leptin transgene. Proc. Natl. Acad. Sci. USA 95:7866-7871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murray, L., M. Travis, K. Luens-Abitorabi, K. Olsson, I. Plavec, S. Forestell, E. G. Hanania, and B. Hill. 2000. Addition of human interferon beta scaffold attachment region to retroviral vector backbones increases the level of in vivo transgene expression among progeny of engrafted human hematopoietic stem cells. Hum. Gene Ther. 11:2039-2050. [DOI] [PubMed] [Google Scholar]
- 36.Palmiter, R. D., E. P. Sandgren, M. R. Avarbock, D. D. Allen, and R. L. Brinster. 1991. Heterologous introns can enhance expression of transgenes in mice. Proc. Natl. Acad. Sci. USA 88:478-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parks, R. J., L. Chen, M. Anton, U. Sankar, M. A. Rudnicki, and F. L. Graham. 1996. A helper-dependent adenovirus vector system: removal of helper virus by Cre-mediated excision of the viral packaging signal. Proc. Natl. Acad. Sci. USA 93:13565-13570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parks, R. J., and F. L. Graham. 1997. A helper-dependent system for adenovirus vector production helps define a lower limit for efficient DNA packaging. J. Virol. 71:3293-3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parks, R. J., J. L. Bramson, Y. Wan, C. L. Addison, and F. L. Graham. 1999. Effects of stuffer DNA on transgene expression from helper-dependent adenovirus vectors. J. Virol. 73:8027-8034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pastore, L., N. Morral, H. Zhou, R. Garcia, R. J. Parks, S. Kochanek, F. L. Graham, B. Lee, and A. L. Beaudet. 1999. Use of a liver-specific promoter reduced immune response to the transgene in adenoviral vectors. Hum. Gene Ther. 10:1773-1781. [DOI] [PubMed] [Google Scholar]
- 41.Quantin, B., L. D. Perricaudet, S. Tajbakhsh, and J. L. Mandel. 1992. Adenovirus as an expression vector in muscle cells in vivo. Proc. Natl. Acad. Sci. USA 89:2581-2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ring, C. J., J. D. Harris, H. C. Hurst, and N. R. Lemoine. 1996. Suicide gene expression induced in tumor cells transduced with recombinant adenoviral, retroviral and plasmid vectors containing the ERBB2 promoter. Gene Ther. 3:1094-1103. [PubMed] [Google Scholar]
- 43.Sandig, V., R. Youil, A. J. Bett, L. L. Franlin, M. Oshima, D. Maione, F. Wang, M. L. Metzker, R. Savino, and C. T. Caskey. 2000. Optimization of the helper-dependent adenovirus system for production and potency in vivo. Proc. Natl. Acad. Sci. USA. 97:1002-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schiedner, G., N. Morral, R. J. Parks, Y. Wu, S. C. Koopmans, C. Langston, F. L. Graham, A. L. Beaudet, and S. Kochanek. 1998. Genomic DNA transfer with a high-capacity adenovirus vector results in improved in vivo gene expression and decreased toxicity. Nat. Genet. 18:180-183. [DOI] [PubMed] [Google Scholar]
- 45.Schiedner, G., S. Hertel, and S. Kochanek. 2000. Efficient transformation of primary human amniocytes by E1 functions of Ad5: generation of new cell lines for adenoviral vector production. Hum. Gene Ther. 11:2105-2116. [DOI] [PubMed] [Google Scholar]
- 46.Schowalter, D. B., C. L. Himeda, B. L. Winther, C. B. Wilson, and M. A. Kay. 1999. Implication of interfering antibody formation and apoptosis as two different mechanisms leading to variable duration of adenovirus-mediated transgene expression in immune-competent mice. J. Virol. 73:4755-4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shi, Q., Y. Wang, and R. Worton. 1997. Modulation of the specificity and activity of a cellular promoter in an adenoviral vector. Hum. Gene Ther. 8:403-410. [DOI] [PubMed] [Google Scholar]
- 48.Steinwaerder, D. S., and A. Lieber. 2000. Insulation from viral transcriptional regulatory elements improves inducible transgene expression from adenovirus vectors in vivo and in vitro. Gene Ther. 7:556-567. [DOI] [PubMed] [Google Scholar]
- 49.Sykes, R. C., D. Lin, S. J. Hwang, P. E. Framson, and A. C. Chinault. 1988. Yeast ARS function and nuclear matrix association coincide in a short sequence from the human HPRT locus. Mol. Gen. Genet. 212:301-309. [DOI] [PubMed] [Google Scholar]
- 50.Thomas, C. E., G. Schiedner, S. Kochanek, M. G. Castro, and P. R. Loewenstein. 2000. Peripheral infection with adenovirus causes unexpected long-term brain inflammation in animals injected intracranially with first-generation, but not with high-capacity, adenovirus vectors: towards realistic long-term neurological gene therapy for chronic diseases. Proc. Natl. Acad. Sci. USA 97:7482-7487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tripathy, S. K., H. B. Black, E. Goldwasser, and J. M. Leiden. 1996. Immune responses to transgene-encoded proteins limit the stability of gene expression after injection of replication-defective adenovirus vectors. Nat. Med. 2:545-550. [DOI] [PubMed] [Google Scholar]
- 52.Walter, W. R., G. B. Singh, and S. A. Krawetz. 1998. MARs mission update. Biochem. Biophys. Res. Commun. 242:419-422. [DOI] [PubMed] [Google Scholar]
- 53.Yang, Y., F. A. Nunes, K. Berencsi, E. E. Furth, E. Gonczol, and J. M. Wilson. 1994. Cellular immunity to viral antigens limits E1-deleted adenoviruses for gene therapy. Proc. Natl. Acad. Sci. USA 91:4407-4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang, Y., H. C. Ertl, and J. M. Wilson. 1994. MHC class I-restricted cytotoxic T lymphocytes to viral antigens destroy hepatocytes in mice infected with E1-deleted recombinant adenoviruses. Immunity 1:433-442. [DOI] [PubMed] [Google Scholar]
- 55.Yang, Y., and J. M. Wilson. 1995. Clearance of adenovirus-infected hepatocytes by MHC class I-restricted CD4+ CTLs in vivo. J. Immunol. 155:2564-2570. [PubMed] [Google Scholar]
- 56.Yang, Y., Q. Li, H. C. Ertl, and J. M. Wilson. 1995. Cellular and humoral immune responses to viral antigens create barriers to lung-directed gene therapy with recombinant adenoviruses. J. Virol. 69:2004-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang, Y., Z. Xiang, H. C. Ertl, and J. M. Wilson. 1995. Up-regulation of class I major histocompatibility complex antigens by interferon gamma is necessary for T-cell mediated elimination of recombinant adenovirus-infected hepatocytes in vivo. Proc. Natl. Acad. Sci. USA 92:7257-7261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang, Y., K. U. Jooss, Q. Su, H. C. Ertl, and J. M. Wilson. 1996. Immune responses to viral antigens versus transgene product in the elimination of recombinant adenovirus-infected hepatocytes in vivo. Gene Ther. 3:137-144. [PubMed] [Google Scholar]