Abstract
In vitro proliferation of peripheral blood mononuclear cells was used to measure virus-specific cell-mediated immunity (vCMI) following neonatal woodchuck hepatitis virus (WHV) infection. Fifteen neonates were inoculated with the W8 strain of WHV. In 11, infection was resolved, and 4 became chronic carriers. Nineteen neonates were inoculated with the W7 strain and all became chronic carriers. Seven age-matched uninfected woodchucks served as controls. Virologic and vCMI profiles among the W8 and W7 infections were compared and related to the outcome of infection. Resolving woodchucks had robust, acute-phase vCMI to WHV antigens (core, surface, and x) and to several nonoverlapping core peptides. The acute-phase vCMI was associated temporally with the clearance of viral DNA and of surface antigen from serum at 14 to 22 weeks postinfection. In contrast, in approximately half of the W8 and W7 infections that progressed to chronicity, no significant acute-phase vCMI was detected. In the remaining carriers, acute-phase vCMI was observed, but it was less frequent and incomplete compared to that of resolved woodchucks. Serum viral load developed less rapidly in those carriers that had evidence of acute-phase vCMI, but it was still increased compared to that of resolving woodchucks. Thus, vigorous and multispecific acute-phase vCMI was associated with resolution of neonatal WHV infection. Absent or incomplete acute-phase vCMI was associated with the progression to chronic infection. By analogy, these results suggest that the onset of chronic hepatitis B virus (HBV) infection in humans may be associated with deficiencies in the primary T-cell response to acute HBV infection.
Adult humans infected with the hepatitis B virus (HBV) usually undergo an acute infection followed by recovery (i.e., resolution). Progression to chronic HBV infection is unusual in adults but frequent among unvaccinated children born to HBV-carrier mothers. Chronic HBV infection is characterized by severe hepatic disease sequelae, including chronic hepatitis, cirrhosis of the liver, and hepatocellular carcinoma (reviewed in reference 2). The early immunologic mechanisms that predispose to the development of chronic HBV infection are not completely understood (reviewed in references 2, 3, and 15).
Resolution of acute HBV infection is associated with the early induction of virus-specific, cell-mediated immunity (vCMI), which involves T helper (Th) cells and cytotoxic T lymphocytes. The successful cell-mediated immune response culminates in acute hepatitis, elimination of the virus from the liver, and subsequent recovery (reviewed in references 2, 3, and 32). Several epitopes of the HBV core (HBc) antigen (HBcAg) are important immunogenic stimuli for Th cells and cytotoxic T lymphocytes in patients that undergo acute, self-limited infection (11-13, 24, 29, 41). In the established chronic carrier, HBV persistence is usually associated with undetectable or weak T-cell responses to HBcAg and HBc peptides. Such responses become accentuated, however, during acute hepatic flare reactions and/or during seroconversion to antibody against HBV e antigen (11, 14, 21, 22, 25, 26, 30, 39, 45). Why such responses fail to result in more complete elimination of chronic HBV infection is not known. Although there have been numerous immunologic studies in established HBV carriers at various stages of disease progression (11-13, 22, 25, 29, 39), there have been no definitive studies of chronic infections as they evolve from the earliest acute stages.
Woodchuck hepatitis virus (WHV) and its natural host, the Eastern woodchuck (Marmota monax), have been used to study the immunopathogenesis of self-limited and chronic hepadnavirus infection (5, 6, 20, 33, 34, 35, 38; reviewed in references 7, 42, and 43). The vCMI associated with self-limited WHV infection of adult woodchucks appears analogous to that in human HBV infection (11-13, 24, 25, 29), with detectable in vitro activation of peripheral blood mononuclear cells (PBMC) following stimulation with WHV surface antigen (WHsAg), WHV core (WHc) antigen (WHcAg), and synthetic WHc peptides (8, 33, 34, 35). The cell-mediated immune response to WHcAg is associated with viral clearance in adult WHV infections, and this has been mapped to several WHcAg epitopes (33, 34). Immunization with the dominant WHcAg epitope sequence between amino acids 97 to 110 affords protection against WHV challenge (34). Thus, early vCMI appears to play a pivotal role in the resolution of acute WHV infection in adult woodchucks, and vCMI to WHcAg and to WHc peptides is instrumental in controlling viral infection.
In an initial comparative study, the acute hepatic inflammatory responses in WHV-infected neonatal woodchucks that developed chronic infection were shown to be less robust than those of neonatal woodchucks in which WHV infection was resolved (6). Follow-up studies of acute-phase liver tissues from this study indicated that the diminished acute hepatitis in carriers involved the reduced expression of intrahepatic gamma interferon and tumor necrosis factor alpha mRNAs, and this correlated with a markedly reduced sinusoidal inflammation by CD3+ T cells, as demonstrated by immunostaining using a universal anti-CD3 antibody reagent (38). Studies of such markers suggested that a deficiency in acute-phase T-cell effector mechanisms may contribute to the onset of chronicity. In the present study, in vitro PBMC proliferative responses were measured during the acute stages of representative neonatal WHV infections that became chronic or that resolved. The results indicate that progression to chronic WHV infection is associated temporally with increased viral load and diminished vCMI during the acute stages of WHV infection. This implies by analogy that the onset of chronic HBV infection in humans may be associated with deficiencies in the primary T-cell response during the acute stages of infection.
MATERIALS AND METHODS
Woodchucks.
Woodchucks were born to colony-bred, WHV-negative dams. In the first experiment, 15 woodchucks of both sexes from 4 litters that were born in the late winter of 1997 were infected with cWHV8P1 (W8) (5). In the second experiment, 19 woodchucks of both sexes from 7 litters born in the late summer of 1997 were infected with WHV7P1 (W7) (5). The latter group was born to dams and reared and bred under an austral light-dark cycle to reverse the circannual rhythm (40). Seven age-matched uninfected woodchucks of both sexes from litters born both in winter and summer served as controls. The use of woodchucks for this study was approved by the Cornell University Institutional Animal Care and Use Committee.
Study design.
Neonatal woodchucks were infected at 3 days of age by subcutaneous inoculation of 0.1 ml of standardized inocula (W8, 1 × 106 50% woodchuck infectious doses; W7, 5 × 106 50% woodchuck infectious doses). The viral inocula used (W8 and W7) were derived from two different woodchucks (no. 8 and 7) (5). The two lineages of WHV appear to represent two different strains of WHV based on the respective chronic carrier rates that occur after experimental infection of neonatal woodchucks (5). W8 and W7 are closely related genetically (4, 18), and the reasons they produce differing chronicity rates are not known (5). They have identical precore and core proteins, identical small and middle surface proteins, and there are only 2 amino acid substitutions each in the x protein and pre-S1 region of the envelope protein and 9 amino acid substitutions in the polymerase protein (4, 16, 18, 27). Inoculation of neonatal woodchucks with W8 in the present study resulted in 11 resolved infections and 4 chronic carriers out of 15 infected woodchucks (27% chronicity rate). Since the goal of this study was to further characterize acute-phase vCMI in WHV infections that progressed to chronic infection, neonates in the second experiment were infected with W7, which consistently produces higher rates of chronicity (5). In the present study, inoculation of 19 neonatal woodchucks with W7 produced 19 chronic carriers.
Blood samples were obtained from the W8-infected woodchucks at weeks 8, 14, 18, 22, and 26 postinfection (±1 week). W7-infected woodchucks were sampled for the first time at 4 weeks of age and then at 2-week intervals until 30 weeks postinfection. These sampling intervals enabled the monitoring of vCMI from the early to later acute stages of neonatal infection. Woodchucks whose acute infection resolved had no detectable WHV DNA in serum at 1 year and had developed antibodies against WHsAg (anti-WHs). Woodchucks whose acute infection progressed to chronicity had persistently high levels of WHV DNA and WHsAg in the serum at 1 year. The acute-phase vCMI of woodchucks that eventually resolved WHV infection (i.e., resolved woodchucks) were compared to those of woodchucks that eventually progressed to chronicity (i.e., chronic carriers).
Virological assays.
Serum was tested for antibodies to WHsAg (anti-WHs) and antibodies to WHcAg (anti-WHc) by using established enzyme-linked immunosorbent assays (9). WHsAg was quantified by a modification of the standard enzyme-linked immunosorbent assay (9). Briefly, the modification used rabbit polyclonal anti-WHs for solid-phase antigen capture. This modification enabled a 100- to 500-fold linear dynamic range of antigen detection above background when using a 1:50 dilution of test serum. Serum WHV DNA was quantified via a standard dot blot hybridization against the signals for known WHV DNA concentrations (sensitivity, ca. 107 genomic equivalents/ml).
Polyclonal activators, WHV antigens, and synthetic peptides.
Concanavalin A (ConA) and lipopolysaccharide (LPS) were purchased from Sigma (St. Louis, Mo.). Phytohemagglutinin (PHA) was obtained from Murex (Dartford, England). Human recombinant interleukin 2 (IL-2) was obtained from Cetus (Emeryville, Calif.). Bovine serum albumin (BSA) was used to assess nonspecific proliferation of PBMC (33, 34). Native 22-nm-diameter WHsAg was purified from the sera of chronic WHV carriers by ultracentrifugation (for an example, see reference 17) and used as described previously (8, 33, 34). Recombinant WHcAg (rWHcAg; 95% pure) was obtained and used as described previously (33, 34). Recombinant WHV x antigen (rWHxAg; 95% pure) was generated as described previously (23). Synthetic peptides of WHcAg were 12 to 20 amino acids in length and overlapped each other from 4 to 12 amino acids (Genosys, Cambridge, England). The panel consisted of 19 peptides covering the entire WHcAg protein, including WHc peptides C97-110 and C100-113, which contain a protective epitope (33, 34), and C129-140, which is not normally recognized by the PBMC of uninfected woodchucks or of woodchucks infected with WHV (33, 34).
Proliferative responses of PBMC.
PBMC proliferation assays were performed as described previously (33, 34). Briefly, whole blood was drawn into EDTA-containing Vacutainer tubes (Becton Dickinson, Franklin Lakes, N.J.), and PBMC were isolated by density gradient centrifugation using Ficoll-Paque (Pharmacia, Uppsala, Sweden). Washed PBMC were suspended in 0.9% NaCl (Baxter, Deerfield, Ill.) and counted with a hemocytometer (American Optical, Buffalo, N.Y.). PBMC were resuspended at 2.5 × 106 cells per ml in AIM-V medium (Gibco BRL, Grand Island, N.Y.) supplemented with 10% heat-inactivated fetal bovine serum (Sigma) and 5 × 10−5 mM 2-mercaptoethanol (Sigma) (complete medium). PBMC were cultured at 5 × 104 cells per 200 μl of complete medium per well in 96-well round bottom microtiter plates (Becton Dickinson). Cultured PBMC were incubated for 5 days at 37°C in a humidified atmosphere containing 5% CO2.
Unstimulated PBMC in medium alone served as the background control (8 samples). For stimulations (triplicate samples), polyclonal activators, antigens, and peptides were used at the following previously determined optimal concentrations (8, 33, 34): ConA, 8.0 μg/ml; PHA, 2.4 μg/ml; LPS, 0.5 μg/ml; human recombinant IL-2, 100 IU/ml; BSA, 1.0 μg/ml; WHsAg, 2.0 μg/ml; rWHcAg, 1.0 μg/ml; rWHxAg, 1.0 μg/ml; and WHc synthetic peptides, 10.0 μg/ml. Quality control measures were taken, and tests were performed prior to the present experiments to ensure that the viral antigen stimulators used were of high purity and were functionally free of contaminating materials that could produce false-positive stimulations.
[2-3H]adenine (37 kBq/well; specific activity = 703 GBq/mM) (Amersham Pharmacia Biotech, Inc., Arlington Heights, Ill.) was added to the cells on day 4, at least 16 to 20 h before harvesting on day 5, as described previously (34). Blastogenic transformation of lymphoid cell clusters was assessed by microscopy prior to the addition of [2-3H]adenine. Radioactively labeled PBMC were harvested onto glass-fiber mats (Wallac, Inc., Gaithersburg, Md.) by using an automatic 96-well cell harvester (Tomtek, Orange, Conn.), and incorporated radioactivity was measured by liquid scintillation (Betaplate Scint; Wallac, Inc.). Results for triplicate cultures were expressed as the stimulation index (SI), which was determined by dividing the total counts per minute obtained in the presence of stimulator by that obtained in the absence of stimulator.
The in vitro proliferation assay used in this study is comparable to those used for human PBMC and T cells (for examples, see references 12 and 24), except that [2-3H]adenine was substituted for [3H]thymidine (33). Woodchuck PBMC undergoing massive cell division in vitro in response to ConA or IL-2 incorporate high levels of [2-3H]adenine (e.g., maximal SI = 30 to 70) but only low levels of [3H]thymidine (8, 28, 31). The inefficient incorporation of [3H]thymidine apparently results because of inefficient transcription of the thymidine kinase 1 gene (31).
Parameters of cell-mediated immune responses.
SI values of ≥3.1 were considered positive for specific PBMC response. This positive cutoff is conservative in relationship to routine positive stimulations by antigens and peptides, i.e., the range of maximal antigen-specific SI in this study was 7.5 to 13.1. The 3.1 cutoff level, therefore, represents at least 25 to 45% of the observed response range for positive stimulations induced by antigens or peptides. At the 3.1 SI cutoff, the positive sample counts per minute for antigens and peptides was always greater than two standard deviations (SD) above the mean counts per minute of unstimulated control cultures (i.e., culture medium) from the same woodchuck and was characteristically more than two SD above the average counts per minute for antigen- and peptide-stimulated PBMC from uninfected control woodchucks.
The percentage of woodchucks responding to stimulation was determined as the number of woodchucks with one or more positive PBMC responses (≥3.1) divided by the total number of woodchucks in each group (×100). The cumulative frequency of positive PBMC responses for samples tested within a group and time interval was calculated as the number of positive PBMC responses (≥3.1) divided by the total number of observations in the experimental group (×100).
Statistics.
Mean WHV DNA concentrations in serum and levels of WHsAg in serum were compared by using the Mann-Whitney U test. Mean SI ratios were compared by using Student's t test. The percentage of woodchucks responding to stimulators and the cumulative frequency of positive PBMC responses were compared by Fisher's exact test (one tailed). For these tests, P values of <0.05 were considered statistically significant. Parameters for acute-phase vCMI were compared by infection outcome (i.e., resolved versus chronic) within each strain of WHV where possible and also by vCMI responder category, i.e., W8 and W7 chronic outcome groups segregated into carriers with and without significant acute-phase vCMI (i.e., vCMI positive [vCMI+] versus vCMI negative [vCMI−]). The results for vCMI in W8 and W7 carrier subgroups were compared and then pooled based on rational considerations for general comparison with those for the W8 resolved woodchucks.
RESULTS
The extent of acute-phase vCMI following neonatal WHV infection affects the outcome.
WHV infections were resolved in 11 of 15 woodchucks (73%) inoculated with W8 as neonates. In these 11 woodchucks there was significant vCMI to rWHcAg and to the protective WHc peptide C97-110 (34) that correlated temporally with the self-limited virologic response (Fig. 1A). In most, vCMI began at 14 to 18 weeks postinfection and continued for 4 to 12 weeks thereafter. vCMI to WHsAg and rWHxAg, and to several other WHc peptides, was also observed frequently during this time period. The acute-phase vCMI was relatively robust, was consistently detectable in most woodchucks once elicited (Fig. 1A), and coincided with the appearance and subsequent clearance of WHV DNA in serum (weeks 14 to 22) (Fig. 1A) and with seroconversion to anti-WHc (weeks 14 to 18) and to anti-WHs (weeks 14 to 22) (data not shown).
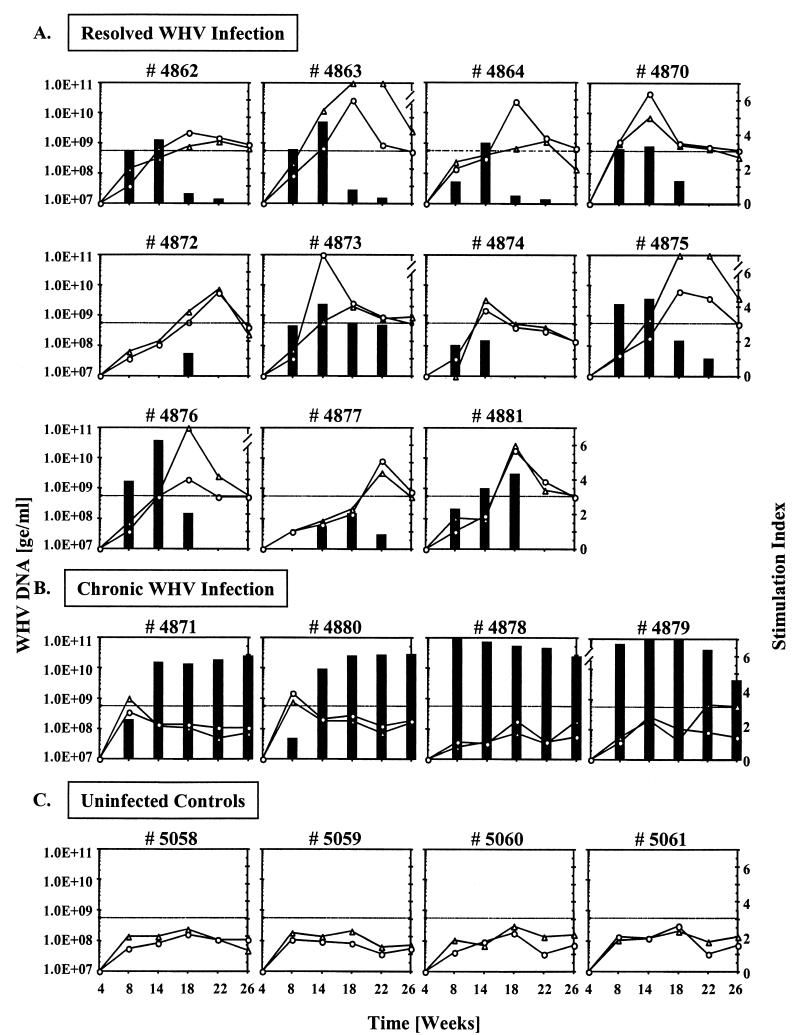
FIG. 1.
WHV DNA in serum and vCMI to rWHcAg and WHc peptide C97-110 in woodchucks with different outcomes following experimental neonatal infection with cWHV8P1 (W8). (A) Woodchucks in which W8 infection was resolved. (B) Woodchucks in which W8 infection became chronic. (C) Uninfected control woodchucks. SI values of ≥3.1 (cutoff line) were considered positive for specific vCMI. See Materials and Methods for other details. Black bars, WHV DNA in serum; ▵, vCMI (SI) to rWHcAg; ○, vCMI (SI) to WHc peptide C97-110. The mean counts per minute ± SD of the unstimulated PBMC from resolved woodchucks, from chronic carriers, and from uninfected control woodchucks were 3,304 ± 2,117, 3,209 ± 1,884, and 3,016 ± 1,822, respectively. Levels of WHV DNA are expressed in genomic equivalents (ge) per milliliter.
Four of 15 W8-infected woodchucks (27%) became chronic carriers. vCMI to rWHcAg and C97-110 was detected at week 8 postinfection in 2 of the 4 W8 carriers (no. 4871 and 4880) (Fig. 1B). vCMI to other WHV antigens and WHc peptides was also detected in these 2 woodchucks, mainly between weeks 8 to 14 postinfection. In the other 2 W8-infected woodchucks that became carriers, no comparable early vCMI was observed. One (no. 4879) developed vCMI at later times to rWHcAg (at weeks 22 and 26) (Fig. 1B) and to 2 of the WHc peptides (at week 18) that were least frequently recognized in woodchucks that resolved infection (see below). In the other chronic carrier (no. 4878), no vCMI was detected. No vCMI was detected in the 4 age-matched uninfected control woodchucks (Fig. 1C).
In the 4 W8-infected woodchucks that progressed to chronicity, the viral load was significantly higher than in woodchucks in which the WHV infection resolved (P value of <0.05 at weeks 14 to 26 for WHV DNA) (Fig. 1A and B). Similarly, the levels of WHsAg attained by the chronic carriers were higher than in those that resolved WHV infection (data not shown). The titers of WHV DNA (and of WHsAg) appeared to increase faster and reached maximal levels earlier in the 2 W8 carriers that did not exhibit significant vCMI during the early acute phase of infection (Fig. 1B). The nominal vCMI to rWHcAg observed at weeks 22 and 26 in one of the W8 carriers (no. 4879) was associated with a slight reduction in WHV DNA in serum, but it may have been ineffectual in bringing about resolution of the infection because it was too late and/or too weak. Such responses, therefore, were not considered significant in the temporal context, and such carriers were classified as vCMI−. The early vCMI detected in the other 2 W8 carriers (no. 4871 and 4880, week 8) (Fig. 1B) was minimal, but it was considered significant (vCMI+) because (i) it was usually coincident with one or more responses to other WHV antigens or WHc peptides and (ii) it was temporally associated with a reduced initial viral load.
The 19 W7-infected woodchucks that became chronic carriers consisted of 8 with minimal but significant acute-phase vCMI (vCMI+) and 11 without significant acute-phase vCMI (vCMI−) (Fig. 2A and B). Six of the 8 vCMI+ W7 carriers had early acute-phase vCMI to rWHcAg between weeks 4 and 14 postinfection (Fig. 2A) and 2 of the 6 had cotemporal vCMI to WHc peptide C97-110 (Fig. 2B). Two of the 8 vCMI+ W7 carriers that had no detectable vCMI to rWHcAg were classified as vCMI+ because one responded early to C97-110 (Fig. 2B) and the other had early cotemporal responses to both WHsAg and rWHxAg (data not shown). vCMI to other WHV antigens and to other WHc peptides was detected early and cotemporally in most of the W7 carriers that had significant vCMI to rWHcAg.
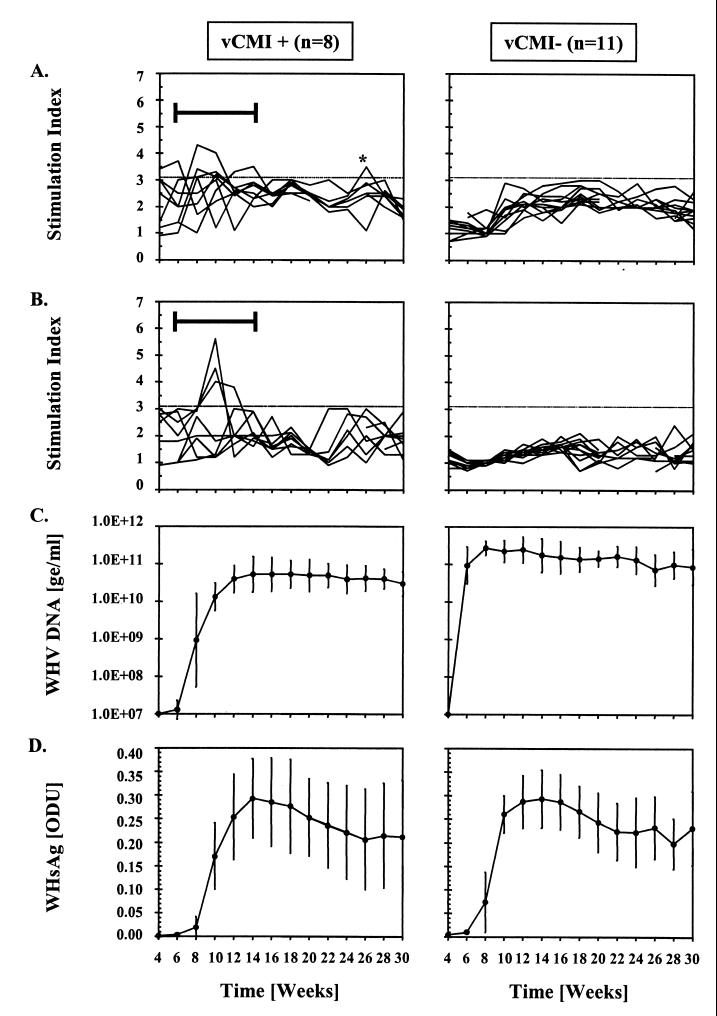
FIG. 2.
Comparison of vCMI to rWHcAg and WHc peptide C97-110, and of WHV DNA and WHsAg in serum in chronic carriers with significant acute-phase vCMI (SI ≥ 3.1, vCMI+) and without significant acute-phase vCMI (vCMI−) following experimental neonatal infection with WHV7P1 (W7). (A) vCMI to rWHcAg (SI). (B) vCMI to WHc peptide C97-110 (SI). (C) Titers of WHV DNA in serum (geometric mean log viral genomic equivalents [ge] ≥ per milliliter ± SD). (D) Relative levels of WHsAg in serum (mean optical density units [ODU] ± SD). SI values of ≥3.1 (cutoff line in panels A and B) were considered positive for specific vCMI. The interval during which significant acute-phase vCMI was observed is shown by a bar and brackets. The asterisk identifies a late and isolated result above the positive cutoff line. The mean counts per minute ± SD of the unstimulated PBMC was 3,688 ± 2,239 for the vCMI+ carriers and 3,593 ± 2,511 for the vCMI− carriers.
Eleven of the 19 W7 carriers were classified as vCMI− because they had no early vCMI to rWHcAg or to other WHV antigens (Fig. 2A and B). A few of these woodchucks had occasional and inconsistent responses to one or two WHc peptides late in the observation period. No significant acute-phase vCMI to WHsAg, rWHxAg, or WHc peptides was detected in the 3 age-matched, uninfected control woodchucks monitored with the W7 infections. Two of the 3 uninfected control woodchucks, however, had a single, weak response to rWHcAg at a time point fairly late in the observation period (e.g., weeks 26 and 28, respectively). This result was unexplained but was not considered relevant to the comparisons made during the earlier intervals of the acute phase of infection (Fig. 2A).
In the W7 vCMI+ carrier group, mean WHV DNA titers and WHsAg levels became detectable starting at weeks 6 to 8, reached maximal levels at week 14, and remained at the high levels thereafter (Fig. 2C and D). In the W7 vCMI− carrier group, mean WHV DNA titers and WHsAg levels increased faster and reached maximal levels earlier than in the vCMI+ W7 group (P < 0.05 at weeks 6 to 24 and 28 to 30 for WHV DNA and at weeks 6 to 10 for WHsAg). The differences in viral load between vCMI+ and vCMI− W7 carriers were analogous to those observed in W8 carriers.
The lack of detectable vCMI in certain woodchucks and PBMC samples was not a result of a general failure of the PBMC to proliferate since all woodchucks and essentially all PBMC samples responded to one or more polyclonal activators (e.g., ConA, PHA, IL-2, LPS). The highest frequencies of activation were observed for ConA, and these were similar regardless of viral strain, outcome of infection, and vCMI status (Table 1). There were no significant differences between the groups in the magnitudes or kinetics of the CMI responses with age (data not shown). Where samples were nonresponsive to one polyclonal activator, they frequently were activated by another, and such observations were similar among the groups (Table 1). PBMC responses to PHA, IL-2, and LPS were somewhat less frequent than for ConA, but there were no significant differences between the groups. WHV-infected woodchucks were no more sensitive than uninfected woodchucks in their response to these activators, and woodchucks in which infection was resolved were no more sensitive than those that became carriers (Table 1).
TABLE 1.
Percentage of woodchucks responding to and frequency of PBMC samples positive to polyclonal activators during the acute phase (4 to 30 weeks postinoculation) in outcome groups and controls after infection with W8 and W7 virus inocula
| Inoculum | Outcome of infectiona | n | % of woodchucks responding to:
|
No. of PBMC samples | % of PBMC samples positive to:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ConA | PHA | LPS | IL-2 | BSA | ConA | PHA | LPS | IL-2 | BSA | ||||
| W8 | Resolved | 11 | 100 | 100 | 73 | 100 | 0 | 42-55 | 91 | 86 | 52 | 84 | 0 |
| Chronic (vCMI+) | 2 | 100 | 100 | 100 | 100 | 0 | 8-10 | 100 | 75 | 63 | 88 | 0 | |
| Chronic (vCMI−) | 2 | 100 | 100 | 100 | 100 | 0 | 8-10 | 90 | 88 | 63 | 88 | 0 | |
| uninfected control | 4 | 100 | 100 | 100 | 100 | 0 | 16-20 | 95 | 71 | 44 | 88 | 0 | |
| W7 | Chronic (vCMI+) | 8 | 100 | 100 | 100 | 100 | 0 | 85-95 | 94 | 81 | 60 | 90 | 0 |
| Chronic (vCMI−) | 11 | 100 | 100 | 91 | 100 | 0 | 120-145 | 93 | 81 | 61 | 92 | 0 | |
| Uninfected control | 3 | 100 | 100 | 100 | 100 | 0 | 35-39 | 97 | 69 | 46 | 85 | 0 | |
vCMI+, chronic carriers with significant acute-phase WHV-specific CMI; vCMI−, chronic carriers without significant acute-phase WHV-specific CMI (see text for details).
PBMC samples from 3 of the 11 woodchucks with resolved W8 infections did not respond to LPS (Table 1), yet several of the samples that did not respond to LPS from these woodchucks did respond significantly to WHV antigens and to WHc peptides. The data obtained using the large panel of WHc peptides provide further evidence of specificity for the proliferation associated with the rWHcAg preparation (Fig. 1 and 2) (see Table 3).
TABLE 3.
Percentage of woodchucks responding to and frequency of PBMC samples positive to WHc peptides during the acute phase (4 to 30 weeks postinoculation) in outcome groups and controls after infection with W8 and W7 virus inoculaa
| WHc Peptide | % of woodchucks responding in group:
|
% of PBMC samples positive in groupb:
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| W8
|
W7
|
W8
|
W7
|
||||||||||||
| Resolved (n = 11) | Chronic (vCMI+) (n = 2) | Chronic (vCMI−) (n = 2) | Control (n = 4) | Chronic (vCMI+) (n = 8) | Chronic (vCMI−) (n = 11) | Control (n = 3) | Resolved (43-55) | Chronic (vCMI+) (9-10) | Chronic (vCMI−) (9-10) | Control (18-20) | Chronic (vCMI+) (97-103) | Chronic (vCMI−) (130-136) | Control (33-39) | ||
| C1-20 | 73cd | 50 | 0 | 0 | 38 | 0 | 0 | 27def | 10 | 0 | 0 | 5g | 0 | 0 | |
| C15-34 | 9 | 0 | 50h | 0 | 25 | 9 | 0 | 4 | 0 | 11h | 0 | 4i | 2 | 0 | |
| C28-47 | 46j | 50 | 0 | 0 | 25 | 0 | 0 | 17dk | 11 | 0 | 0 | 6 | 0 | 0 | |
| C38-57 | 18 | 50 | 0 | 0 | 25 | 18 | 0 | 4 | 11 | 0 | 0 | 4 | 3 | 0 | |
| C50-69 | 46j | 50 | 0 | 0 | 50l | 0 | 0 | 17cd | 20 | 0 | 0 | 13gm | 0 | 0 | |
| C61-80 | 9 | 0 | 0 | 0 | 38 | 9 | 0 | 8 | 0 | 0 | 0 | 12gm | 2 | 0 | |
| C70-89 | 27 | 50 | 0 | 0 | 38 | 9 | 0 | 11j | 10 | 0 | 0 | 10lm | 2 | 0 | |
| C82-101 | 36 | 50 | 0 | 0 | 50 | 9 | 0 | 19cd | 10 | 0 | 0 | 10gm | 2 | 0 | |
| C90-109 | 36 | 50 | 0 | 0 | 38 | 9 | 0 | 12n | 11 | 0 | 0 | 6 | 2 | 0 | |
| C97-110 | 100edop | 50 | 0 | 0 | 38 | 0 | 0 | 51defqr | 10 | 0 | 0 | 4g | 0 | 0 | |
| C100-113 | 63n | 50 | 0 | 0 | 25 | 0 | 0 | 25cdf | 11 | 0 | 0 | 4l | 0 | 0 | |
| C100-119 | 100edop | 50 | 0 | 0 | 38 | 0 | 0 | 44defqr | 10 | 0 | 0 | 4l | 0 | 0 | |
| C112-131 | 82dk | 50 | 0 | 0 | 25 | 0 | 0 | 28def | 11 | 0 | 0 | 2 | 0 | 0 | |
| C120-139 | 64n | 50 | 0 | 0 | 50l | 0 | 0 | 20cdp | 11 | 0 | 0 | 5l | 0 | 0 | |
| C136-155 | 0 | 50 | 0 | 0 | 38 | 0 | 0 | 0 | 11 | 0 | 0 | 10gm | 0 | 0 | |
| C146-165 | 9 | 50 | 0 | 0 | 25 | 0 | 0 | 2 | 11 | 0 | 0 | 12gm | 0 | 0 | |
| C156-175 | 0 | 0 | 0 | 0 | 25 | 0 | 0 | 0 | 0 | 0 | 0 | 9gm | 0 | 0 | |
| C169-188 | 9 | 0 | 50h | 0 | 13 | 0 | 0 | 4 | 0 | 11h | 0 | 3 | 0 | 0 | |
| C129-140 | 0 | 0 | 0 | 0 | 13s | 0 | 0 | 0 | 0 | 0 | 0 | 2s | 0 | 0 | |
vCMI+, chronic carriers with significant acute-phase WHV-specific vCMI; vCMI−, chronic carriers without significant acute-phase WHV-specific vCMI (see text for details).
Numbers in parentheses represent the number of samples tested.
P < 0.05 for resolved infection versus control or uninfected control within a viral strain.
P < 0.001 for resolved W8 infection versus chronic vCMI− W7 infection.
P < 0.01 for resolved infection versus control or uninfected control within a viral strain.
P < 0.001 for resolved W8 infection versus chronic vCMI+ W7 infection.
P < 0.01 for chronic vCMI+ infection versus chronic vCMI− infection within a viral strain.
Very late positive results of one vCMI− W8 carrier (see text for details).
P < 0.01 for chronic vCMI+ infection versus control or uninfected control within a viral strain.
P < 0.05 for resolved W8 infection versus chronic vCMI− W7 infection.
P < 0.05 for resolved W8 infection versus chronic vCMI+ W7 infection.
P < 0.05 for chronic vCMI+ infection versus chronic vCMI− infection within a viral strain.
P < 0.05 for chronic vCMI+ infection versus control or uninfected control within a viral strain.
P < 0.01 for resolved W8 infection versus chronic vCMI− W7 infection.
P < 0.05 for resolved infection versus chronic vCMI− infection within a viral strain.
P < 0.01 for resolved W8 infection versus chronic vCMI+ W7 infection.
P < 0.05 for resolved infection versus chronic vCMI+ infection within a viral strain.
P < 0.01 for resolved infection versus chronic vCMI− infection within a viral strain.
Late and isolated positive result (see text for details).
Chronicity as an outcome of neonatal WHV infection is associated with deficiencies in the extent of acute-phase vCMI to WHV antigens and WHc peptides.
W8 woodchucks in which WHV infection was resolved had significant acute-phase vCMI based on the percentage of woodchucks responding and on the cumulative frequency of PBMC samples positive for vCMI (Tables 2 and 3). There was negligible vCMI in the W7 and W8 vCMI− carrier categories and in the respective uninfected control woodchucks. The few late responses to WHc peptides in the W8 vCMI− carriers were to 2 peptides that were not often recognized by the W8 resolving group or by the W8 vCMI+ carrier group (Table 3). As mentioned above, there was the unusual detection of two very late positive responses to intact rWHcAg in 2 of the 3 uninfected control woodchucks that were monitored with the W7 carrier groups (Table 2). Also, 2 late PBMC samples from one W7 vCMI+ woodchuck were weakly positive for WHc peptide C129-140, which is not usually recognized by either WHV-infected or uninfected woodchucks (Table 3) (33, 34). Otherwise, the mass of results in both the W8 and W7 vCMI− carrier groups indicated that the chronic outcome was associated in some cases with an extensive deficiency in acute-phase vCMI (Tables 2 and 3). Results in both the vCMI+ W8 and W7 carrier groups, however, showed that chronicity could develop in the presence of vCMI that was less frequent and incomplete compared to that in woodchucks in which WHV infection was resolved.
TABLE 2.
Percentage of woodchucks responding to and frequency of PBMC samples positive to WHV antigens during the acute phase (4 to 30 weeks postinoculation) in outcome groups and controls after infection with W8 and W7 virus inocula
| Inoculum | Outcome of infectiona | n | % of woodchucks responding to:
|
No. of PBMC samples | % of PBMC samples positive to:
|
||||
|---|---|---|---|---|---|---|---|---|---|
| rWHcAg | WHsAg | rWHxAg | rWHcAg | WHsAg | rWHxAg | ||||
| W8 | Resolved | 11 | 100bc | 82cd | 91bce | 50-55 | 59bcefg | 34bceg | 34bceg |
| Chronic (vCMI+) | 2 | 100 | 50 | 50 | 9-10 | 20 | 11 | 11 | |
| Chronic (vCMI−) | 2 | 50h | 0 | 0 | 9-10 | 20h | 0 | 0 | |
| Uninfected control | 4 | 0 | 0 | 0 | 19-20 | 0 | 0 | 0 | |
| W7 | Chronic (vCMI+) | 8 | 75i | 50jk | 63i | 86-112 | 12i | 6i | 11ik |
| Chronic (vCMI−) | 11 | 0 | 0 | 0 | 136-149 | 0 | 0 | 0 | |
| Uninfected control | 3 | 67l | 0 | 0 | 36-37 | 5l | 0 | 0 | |
vCMI+, chronic carriers with significant acute-phase WHV-specific CMI; vCMI−, chronic carriers without significant acute-phase WHV-specific CMI (see text for details).
P < 0.01 for resolved infection versus control or uninfected control within a viral strain.
P < 0.001 for resolved W8 infection versus chronic vCMI− W7 infection.
P < 0.05 for resolved infection versus control or uninfected control within a viral strain.
P < 0.05 for resolved infection versus chronic vCMI− infection within a viral strain.
P < 0.05 for resolved infection versus chronic vCMI+ infection within a viral strain.
P < 0.001 for resolved W8 infection versus chronic vCMI+ W7 infection.
Very late positive results of one vCMI− W8 carrier (see text for details).
P < 0.01 for chronic vCMI+ infection versus chronic vCMI− infection within a viral strain.
P < 0.05 for chronic vCMI+ infection versus chronic vCMI− infection within a viral strain.
P < 0.05 for chronic vCMI+ infection versus control or uninfected control within a viral strain.
Late and isolated positive result (see text for details).
Chronicity as an outcome of neonatal WHV infection is associated with differential vCMI to distinct regions of WHcAg.
There was broad recognition of several WHc peptides representing apparently distinct regions of the WHcAg protein. As indicated above, all 11 woodchucks with resolved W8 infections had acute-phase vCMI to the important protective WHc peptide, C97-110 (Fig. 1; Table 3). The frequency of positive PBMC responses to C97-110 in this group (51%) was the highest among all groups and among all peptides tested (Table 3). In contrast, only 1 of the 2 vCMI+ W8 carriers and 3 of the 8 vCMI+ W7 carriers had responses to this peptide, and such responses were remarkably infrequent (Table 3). WHc peptides C1-20, C28-47, C50-69, C82-101, C100-113, C100-119, C112-131, and C120-139 were recognized in relatively high proportion and with relatively high frequency by woodchucks with resolved W8 infection (Table 3). Two of the 8 peptides (C100-113 and C100-119) overlap considerably with C97-110, while the other 6 do not. C120-139 overlaps with the nonreactive peptide, C129-140, suggesting that a second nonoverlapping site exists between residues 120 and 128. By comparison, the frequencies of response to C97-110 and C100-119 were significantly lower in the two W8 vCMI+ carriers and in the two W8 vCMI− carriers.
Additional statistical comparisons of core-specific responses were made between woodchucks with resolved W8 infections and W7 carriers. Twenty-five to 50% of the W7 vCMI+ carriers responded to WHc peptides C1-20, C97-110, C100-113, C100-119, C112-131, and C120-139, but the corresponding frequencies of positive PBMC samples were low in general (Table 3). In most cases these WHc peptide responses were significantly more frequent than those in uninfected control woodchucks and those in vCMI− W7 carriers (Table 3). But several of the PBMC response frequencies in vCMI+ W7 carriers were significantly less than those in the woodchucks with resolved W8 infections (e.g., C1-20, C28-47, C97-110, C100-113, C100-119, C112-131, and C120-139) (Table 3). Interestingly, the PBMC response frequencies to C136-155, C146-165, and C156-175 in vCMI+ W7 carriers were significantly higher than those in the woodchucks with resolved W8 infections, and the results were the same as the combined results for vCMI+ W8 and W7 carriers (data not shown).
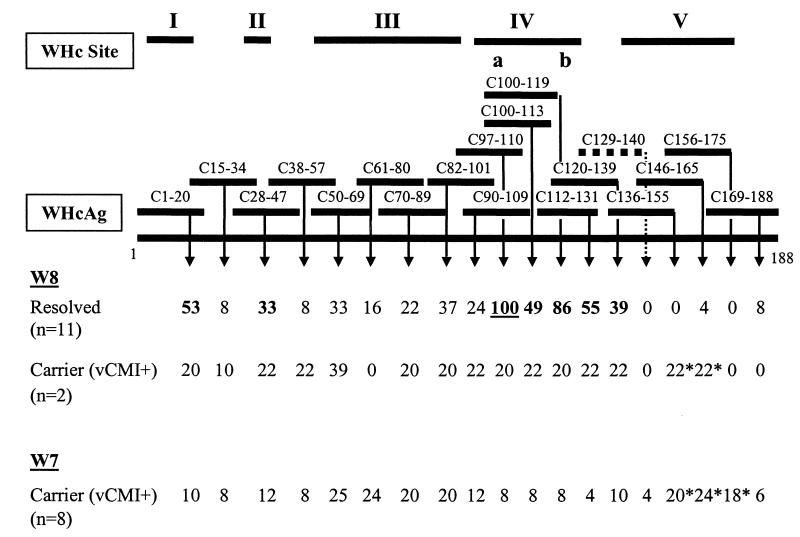
For comparison of the above results, values for the frequency of positive PBMC samples in the responding groups were expressed relative to the maximal frequency observed for C97-100 in the resolved W8 infection group (i.e., 51% frequency in Table 3 was set at 100% frequency in Fig. 3). The results show a broad acute-phase response by woodchucks to at least 5 distinct regions of WHcAg. Most apparent were the diminished frequencies of acute-phase vCMI of the vCMI+ W8 and vCMI+ W7 carriers to sites I, II, and IV. Responses to site III (residues 50 to 95) were equally evident in resolved- infection woodchucks and in vCMI+ W8 and vCMI+ W7 carriers. As mentioned above, only the acute-phase responses in vCMI+ W8 and vCMI+ W7 carriers extended to site V.
FIG. 3.
Summary of vCMI to WHc peptides in woodchucks with resolved WHV infection and in carriers with positive acute-phase vCMI. Values for the W8 and W7 outcome categories were expressed relative to the most frequently recognized WHc peptide, C97-110, in the resolved W8 infection group (i.e., 51% in Table 3 was set at 100%, underlined, here). Other values were calculated accordingly. Sites I to V were demarcated based on differential and similar response frequencies for the present groups of neonatally infected woodchucks and also on other published information (33, 34). In site IV, a and b represent at least 2 nonoverlapping CMI-related epitopes, one of which (C97-110) has been shown previously to be protective in experimental challenge studies (34). Sites I, II, and IV appeared to be preferred recognition sites for woodchucks with resolved infections (bold numbers). Site III appeared to be recognized similarly in both outcome groups. Site V may be recognized preferentially by neonatal carriers (numbers are marked with an asterisk). See the text for details and Table 3 for relevant statistical comparisons.
Deficiencies in the proportion, frequency, and magnitude of acute-phase vCMI are associated with the onset of chronic WHV infection in neonatal woodchucks.
The response data from all carrier groups were combined for a summary comparison with W8 resolving woodchucks. The necessary assumption was that acute-phase vCMIs leading to successful recovery responses are probably similar for woodchucks infected with W8 and W7. When compared to woodchucks with resolved W8 infections (82 to 100% of vCMI responders), acute-phase vCMI to WHV antigens and WHc peptide C97-110 was detected in significantly fewer W7 and W8 carriers (17 to 39% of vCMI responders) and was significantly less frequent overall (Table 4). The 39% of carriers that had acute-phase vCMI to rWHcAg were usually the same ones that responded to WHsAg, rWHxAg, and WHc peptide C97-110. With all antigens and significant vCMI responses considered, the overall percentage of responders among carriers was 44%. The remaining 13 of 23 carriers (56%) had no significant acute-phase vCMI.
TABLE 4.
Percentage of woodchucks responding to and frequency of PBMC samples positive to WHV antigens and WHc peptide C97-110 in resolved woodchucks and carriers during the acute phase (4 to 30 weeks postinoculation) in pooled outcome groups and controls after infection with W8 and W7 virus inoculaa
| Outcome of infection | % of woodchucks responding to:
|
% of PBMC samples positive to:
|
||||||
|---|---|---|---|---|---|---|---|---|
| rWHcAg | WHsAg | rWHxAg | C97-110 | rWHcAg | WHsAg | rWHxAg | C97-110 | |
| Resolved | 100 (11/11)b | 82 (9/11)b | 91 (10/11)b | 100 (11/11)b | 59 (32/54)b | 34 (18/53)b | 34 (17/50)b | 51 (28/55)b |
| Chronic | 39 (9/23) | 22 (5/23) | 26 (6/23) | 17 (4/23) | 6 (16/262) | 3 (6/242) | 4 (10/242) | 2 (5/265) |
| Uninfected control | 29 (2/7)c | 0 (0/7) | 0 (0/7) | 0 (0/7) | 5 (3/56)c | 0 (0/59) | 0 (0/59) | 0 (0/57) |
Numbers in parentheses represent number of positive observations/total number of observations.
P < 0.01 for woodchucks with resolved infections versus chronic carriers, and P < 0.01 for woodchucks with resolved infections versus uninfected control woodchucks.
Late and isolated positive result (see text for details).
In the resolved W8 infection group, the maximal positive SI values for acute-phase vCMI to rWHcAg among individual woodchucks ranged from 3.6 to 13.1 (Fig. 1). The higher peak SI values observed in 3 of the 11 resolved W8 woodchucks (SI > 7) correlated in part with higher initial viremia observed before the first evidence of vCMI (Fig. 1). This appeared to be characteristic for the resolved outcome only; the opposite was observed in W8 and W7 carriers. The maximal SI values for rWHcAg responses in the two W8 vCMI+ carriers were 3.3 and 3.5. The maximal positive SI values for rWHcAg responses among the W7 vCMI+ carriers ranged from 3.1 to 4.3. SI values for WHc peptide C97-110 in 11 of 11 resolved W8 woodchucks were between 3.1 and 7.5 (Fig 1), and they were between 3.8 and 5.3 in a total of 4 of 13 vCMI+ W8 and W7 carriers. The mean peak SI values for rWHcAg and for C97-110 among all W8 and W7 carriers (vCMI+ and vCMI−) were reduced significantly compared to resolved W8 woodchucks (P < 0.05 to 0.01). The mean peak SI values for WHsAg and rWHxAg were not significantly different between the two outcome groups (range for maximum, 3.1 to 5.0). Thus, in carriers, the reduced extent of acute-phase vCMI to intact WHcAg and C97-110 involved a reduced proportion, frequency, and magnitude, whereas the vCMI to other antigens was reduced only in proportion and frequency of response.
DISCUSSION
The outcome of experimental neonatal WHV infection characteristically is either self limited or chronic (5, 7, 43). This feature enables comparisons of viral and host responses at early time points postinfection, before the outcome can be established by standard serologic criteria (5, 7, 38). Studies of early immunologic events following HBV infection in adult humans usually are limited to the time interval after patients present with symptoms, although some exceptions exist (47). Nearly all such infections are self limited, however, leaving few opportunities to examine the acute-phase responses associated with evolution to chronic infection. This is also true for experimental studies of HBV infection in adult chimpanzees (10, 19). Our working hypothesis is that chronicity as an outcome of hepadnavirus infection results from a failed or suboptimal immune response to acute infection (6, 38). This cannot be fully addressed by studies in already established chronic carriers. Our goal is to better understand the early events associated with the onset of chronic hepadnavirus infection in woodchucks, and to apply the knowledge to the development of rational immunotherapy for chronic HBV infection in humans.
Monoclonal anti-woodchuck T-cell antibodies (e.g., CD3, CD4, and CD8) were not available at the time these studies were conducted. There is reasonable evidence that T cells were stimulated, although the subset could not be defined. A universal anti-CD3 antibody was used which stains histologically defined foci of cells in the healthy woodchuck spleen (P. J. Cote and W. C. Hall, unpublished data) that in other species are known to consist of T cells. Accordingly, this antibody has been applied to stain infiltrating T cells in the livers from WHV-infected adult (20) and neonatal (38) woodchucks. In vitro PBMC proliferative responses to most antigens, and especially to synthetic peptides, are mediated by T cells in other species. In self-limited WHV infection of adult woodchucks, the kinetics of the PBMC proliferative responses to viral antigens (33) are comparable to the well-defined T-cell proliferative responses in HBV-infected humans and chimpanzees (for examples, see references 2, 3, 11, 12, 24, and 29). rWHcAg-stimulated PBMC that do not adhere to nylon wool were CD3+ when stained with a universal anti-CD3 antibody reagent (34). In a more detailed study, PBMC from 4 acutely infected woodchucks were expanded first with rWHcAg or WHc peptide C97-110, and IL-2 (i.e., a T-cell growth factor), and were then passed over nylon wool. This provided nonadherent cells (i.e., T cells) that were directly and reciprocally restimulated in vitro with rWHcAg or C97-110 (33, 34). Moreover, immunization of adult woodchucks with the defined WHcAg epitope (i.e., WHc peptide C97-110) was able to protect against experimental WHV infection in a manner consistent with the priming of virus-specific T cells (34).
In the present study, in vitro proliferation of PBMC was used to measure vCMI throughout the acute phase of neonatal WHV infections. The results in resolving woodchucks were compared to those of carriers with or without significant acute-phase vCMI. There were no resolved W7 woodchucks in this particular study for inclusion in the comparisons. Given the identical nature of most W8 and W7 antigens, including the core and the small and middle surface proteins (4, 18), it was reasonable to assume that the vCMI in successful recovery responses in W8 and W7 infections of neonates will be similar with respect to these antigens. Thus, resolution of experimental neonatal W8 infection was associated with the development of a robust acute-phase vCMI to WHV antigens (i.e., rWHcAg, WHsAg, and rWHxAg) and to at least 4 nonoverlapping epitopes of WHcAg (C1-20, C28-47, C97-110, and C112-128). These were located within 3 distinct regions of the core protein (sites I, II, and IV) (Fig. 3) and included the protective epitope within C97-110 (33, 34) in site IV. In contrast, chronicity as an outcome of neonatal WHV infection (W8 and W7) involved complete or partial deficiencies in the primary vCMI response to WHV antigens. The results suggest that the onset of chronic HBV infection in humans may be associated with deficiencies in the primary T-cell response during the acute phase of infection.
The highest peak acute-phase responses (SI values) to rWHcAg were observed in 3 of 11 W8 woodchucks with resolved infection, and these 3 had generally higher initial viral loads in serum before vCMI was detectable than did the other 8 (Fig. 1). When woodchucks mount an immune response leading to recovery, the more rapid initial development of viral load may stimulate a more robust vCMI to WHcAg. In contrast, the high initial viral loads observed in vCMI− carriers did not produce an enhanced vCMI to WHcAg. The early and incomplete vCMI to WHcAg in vCMI+ carriers was associated with reduced initial viremia. In either case, the level of acute-phase vCMI to WHcAg in carriers did not appear to be driven by higher viral loads, as observed in W8 resolved woodchucks. Our present experiments cannot differentiate whether the rapid initial increase in WHV and WHsAg inhibits the vCMI or whether a deficient immune response enables development of a high viral load. Previous studies (reviewed in references 6 and 7), however, have shown that transient immunosuppression with cyclosporine A at the time of experimental WHV infection of adult woodchucks leads to increased viral load and to high rates of chronicity compared to those of untreated woodchucks. This suggests that a primary deficiency in the neonatal immune response could be an important mechanism responsible for the development of chronic infection.
In resolving WHV infections, the onset and peak of vCMI were associated temporally with clearance of WHV from the serum. In the evolving chronic infection, acute-phase vCMI appeared aberrant in one or more respects. Of the carriers (W8 and W7), 44% had significant vCMI to one or more viral antigens and 39% had detectable, but significantly diminished, acute-phase vCMI to the intact core protein. The responses to surface and x proteins, and to certain WHc peptides, were observed mainly among the carriers that responded to the core protein. The peak magnitudes of vCMI to rWHcAg and WHc peptide C97-110 were diminished compared to those of woodchucks that resolved infection, although the magnitudes of the vCMI to surface and x proteins were similar in the two outcomes. The reduction in frequency of vCMI to WHsAg and rWHxAg was characteristic and most remarkable among carriers. The epitope specificity of vCMI to WHcAg also differed (Fig. 3). Thus, chronicity can develop in the face of an early acute-phase cell-mediated immune response to WHV but one that is diminished and incomplete compared with that observed in recovery. Further studies are needed to define a possible role in the induction of chronicity for vCMI to certain sites of WHcAg (e.g., sites IV and V) (Fig. 3).
Of the carriers, 56% appeared to be unresponsive altogether to WHV antigens during the early stages of acute WHV infection, indicating unequivocally that acute-phase vCMI is required for resolution. The failure to respond was not due to some general unresponsiveness of the PBMC, since PBMC samples from such carriers responded to several polyclonal activators in a manner similar to that of woodchucks in which infection was resolved and uninfected control woodchucks. The lack of significant vCMI in this group of carriers could be the result of early induction of tolerance. Possible mechanisms for early tolerance may include complete deletion of virus-specific Th cells, partial depletion of such cells to below the levels needed for detection in the in vitro proliferation assay, clonal anergy, clonal exhaustion due to rapid increases in viral load, or abnormal mechanisms of T-cell apoptosis (1, 21, 36, 37, 46).
Chronicity develops not only in association with the lack of acute-phase vCMI but also in the face of suboptimal responses, which could suggest that multiple mechanisms lead to chronicity. Alternatively, such results could be explained by a continuum of responses in which chronicity develops in woodchucks with no responses and in woodchucks with responses below a threshold required for resolution of WHV infection. Another explanation involves the potential differential response to certain epitopes of WHcAg. The woodchucks used in this study were outbred, and genetic factors could have played a role in determining the variability in the vCMI observed.
One goal of these studies is the eventual identification of early events in the liver or periphery that predict outcome. This could greatly facilitate identification of early mechanisms that determine chronicity or resolution (6). Most (95%) of the neonatal WHV infections that resolve serologically do so by 20 to 24 weeks postinfection, whereas most (95%) of the woodchucks that are still antigenemic at this time are in fact already chronic carriers (5; reviewed in reference 6). A useful predictive marker of outcome should be detectable before 20 to 24 weeks. In the present study, we expected that robust vCMI would be detected very early within the first 8 weeks postinfection in resolving infections but not detected in chronic infections. This was not always the case. The early vCMI detected in almost half of carriers could complicate predictions of outcome. The first detection and peak of vCMI in resolving woodchucks were observed cotemporally with the appearance and then disappearance of WHV DNA in serum. The onset and peak of intrahepatic effector responses probably occur at the same time as or slightly after the peak of peripheral vCMI (6, 38).
T-cell responses to viral antigens are undetectable in most patients with established chronic HBV infection, and asymptomatic carriers of HBV appear to be immunologically tolerant to HBV (11, 14, 21, 22, 24-26, 44). T-cell responses to HBV antigens have been detected in some chronically infected HBV patients but usually in association with transient exacerbation of chronic hepatitis resulting from modulation in viremia or seroconversion from HBV e antigen to antibody against HBV e antigen (13, 14, 21, 22, 24-26, 29, 30, 44, 45). Studies of such T-cell responses provide information on the relationship between the immune response and maintenance of chronic infection, on host mechanisms of clearance of chronic infection, and on the role of the immune response in the progression of chronic liver disease. However, such responses may not be representative of the initial response pattern that determines chronic outcome. Nearly all carrier woodchucks with long-standing chronic WHV infection examined thus far also appear immunologically tolerant (34, 35). The onset and development of chronic neonatal WHV infection without evidence of vCMI in approximately half of the carriers indicates that such tolerance is evident from the earliest stages of infection but also may develop shortly after a transient and weak, acute vCMI in the remaining half of the carriers. How such a response pattern may relate to the subsequent progression of chronic liver disease or to the response to therapy in these categories of chronic carriers warrants further studies.
The partial response in some carriers indicated that the onset of chronic infection is not linked entirely with an inability to generate early WHV-specific CMI. In previous studies, at least half of carrier woodchucks infected neonatally had histologic and immunohistologic evidence of mild acute hepatitis with portal infiltrates containing CD3+ T cells (6, 38). This suggested that not all chronic carriers are completely tolerant to WHV during the onset of chronic infection. However, the overall inflammatory response was diminished compared with that of age-matched woodchucks that resolved WHV infection. Moreover, there was a uniform deficiency in the intrahepatic expression of gamma interferon and tumor necrosis factor alpha, which correlated with a markedly reduced sinusoidal infiltration by CD3+ T cells. The deficiencies in acute-phase peripheral vCMI in the carriers of the present study would be consistent with less-severe acute hepatitis, decreased acute CD3+ T-cell inflammation, and reduced intrahepatic type 1 effector responses. A hypothesis suggested by the results of this study is that the acute-phase response defect in the carriers (6, 38) stems from suboptimal induction of Th1 cells early following infection.
In conclusion, a robust and consistently detectable acute-phase vCMI was associated with resolving (or self-limited) neonatal WHV infection. In contrast, absent or incomplete early vCMI was associated with increasing viral load and with the onset of chronic infection. Further studies in woodchucks now can be focused on identifying the T-cell subsets and molecular mechanisms responsible for these differences in response and on how to induce or enhance the vCMI of chronic WHV carriers to achieve therapeutic benefit. Such studies should be aimed at differentiating between mechanisms of T-cell clonal deletion and T-cell clonal anergy or exhaustion, since a difference in therapeutic approach would be indicated, e.g., adoptive cell transfer versus therapeutic vaccination, respectively. Interestingly, WHc peptides C97-110 and C100-119 were recognized by 100% of the woodchucks in which WHV infection resolved, strengthening the conclusion reached previously that this sequence contains an immunodominant epitope for woodchucks (34) important in controlling acute WHV infection (33). This is remarkable when considering that the laboratory woodchuck is outbred with likely variation in major histocompatibility complex class I and class II antigen presentation alleles. Of the carriers in this study, 17% recognized C97-110, suggesting that extensive clonal deletion of responding WHV-specific cells is not an essential mechanism responsible for all chronic infections and, therefore, would not be an impediment to therapeutic vaccination. Identification and testing of such viral peptide sequences in the woodchuck model under controlled laboratory conditions should facilitate development of immunotherapeutic vaccines that target existing WHV-specific cells present in chronic hepadnavirus infection.
Acknowledgments
This work was supported by contracts NO1-AI-35164 and NO1-AI-05399 to the College of Veterinary Medicine, Cornell University, with the National Institute of Allergy and Infectious Diseases (NIAID) and contract NO1-AI-45179 to the Division of Molecular Virology and Immunology, Georgetown University, School of Medicine, with the NIAID.
We are grateful to Betty H. Baldwin, Chris Bellezza, Lou A. Graham, and Rich Moore (Cornell University) for excellent technical assistance.
REFERENCES
- 1.Bertoletti, A., A. Sette, F. V. Chisari, A. Penna, M. Levrero, M. De Carli, F. Fiaccadori, and C. Ferrari. 1994. Natural variants of cytotoxic epitopes are T-cell receptor antagonists for antiviral cytotoxic T-cells. Nature (London) 369:407-410. [DOI] [PubMed] [Google Scholar]
- 2.Chang, K. M., and F. V. Chisari. 1999. Immunopathogenesis of hepatitis B virus infection. Clin. Liver Dis. 3:221-239. [DOI] [PubMed] [Google Scholar]
- 3.Chisari, F. V., and C. Ferrari. 1997. Viral hepatitis, p. 745-778. In N. Nathanson (ed.), Viral pathogenesis, Lippincott-Raven Publisher, Philadelphia, Pa.
- 4.Cohen, J. I., R. H. Miller, B. Rosenblum, K. Denniston, J. L. Gerin, and R. H. Purcell. 1988. Sequence comparison of woodchuck hepatitis virus replicative forms shows conservation of the genome. Virology 182:12-20. [DOI] [PubMed] [Google Scholar]
- 5.Cote, P. J., B. E. Korba, R. H. Miller, J. R. Jacob, B. H. Baldwin, W. E. Hornbuckle, R. H. Purcell, B. C. Tennant, and J. L. Gerin. 2000. Effects of age and viral determinants on chronicity as an outcome of experimental woodchuck hepatitis virus infection. Hepatology 31:190-200. [DOI] [PubMed] [Google Scholar]
- 6.Cote, P. J., I. Toshkov, C. Bellezza, M. Ascenzi, C. Roneker, L. A. Graham, B. H. Baldwin, K. Gaye, I. Nakamura, B. E. Korba, B. C. Tennant, and J. L. Gerin. 2000. Temporal pathogenesis of experimental neonatal woodchuck hepatitis virus infection: increased initial viral load and decreased severity of acute hepatitis during the development of chronic viral infection. Hepatology 32:807-817. [DOI] [PubMed] [Google Scholar]
- 7.Cote, P. J., and J. L. Gerin. 1996. The woodchuck as a model of hepadnavirus infection, pathogenesis and therapy. Forum Trends Exp. Clin. Med. 6:131-159. [Google Scholar]
- 8.Cote, P. J., and J. L. Gerin. 1995. In vitro activation of woodchuck lymphocytes measured by radiopurine incorporation and interleukin-2 production: implications for modeling immunity and therapy in hepatitis B virus infection. Hepatology 22:687-699. [PubMed] [Google Scholar]
- 9.Cote, P. J., C. Roneker, K. Cass, F. Schoedel, D. Peterson, B. C. Tennant, F. De Noronha, and J. L. Gerin. 1993. New enzyme immunoassays for the serologic detection of woodchuck hepatitis virus infection. Viral Immunol. 6:161-169. [DOI] [PubMed] [Google Scholar]
- 10.Cote, P. J., M. Shapiro, R. E. Engle, H. Popper, R. H. Purcell, and J. L. Gerin. 1986. Protection of chimpanzees from type B hepatitis by immunization with woodchuck hepatitis virus surface antigen. J. Virol. 60:895-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diepolder, H. M., M.-C. Jung, E. Wierenga, R. M. Hoffmann, R. Zachoval, T. J. Gerlach, S. Scholz, G. Heavner, G. Riethmueller, and G. R. Pape. 1996. Anergic TH1 clones specific for hepatitis B virus (HBV) core peptides are inhibitory to other HBV core-specific CD4+ T cells in vitro. J. Virol. 70:7540-7548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferrari, C., A. Bertoletti, A. Penna, A. Cavalli, A. Valli, G. Missale, M. Pilli, P. Fowler, T. Giuberti, F. V. Chisari, and F. Fiaccadori. 1991. Identification of immunodominant T-cell epitopes of the hepatitis B virus nucleocapsid antigen. J. Clin. Investig. 88:214-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferrari, C., A. Penna, A. Bertoletti, A. Valli, A. D. Antoni, T. Giuberti, A. Cavalli, M. A. Petit, and F. Fiaccadori. 1990. Cellular immune response to hepatitis B virus-encoded antigens in acute and chronic hepatitis B virus infection. J. Immunol. 145:3442-3449. [PubMed] [Google Scholar]
- 14.Ferrari, C., A. Penna, P. Sansoni, T. Giuberti, T. M. Neri, F. V. Chisari, and F. Fiaccadori. 1986. Selective sensitization of peripheral blood T lymphocytes to hepatitis B core antigen in patients with chronic active hepatitis type B. Clin. Exp. Immunol. 66:497-506. [PMC free article] [PubMed] [Google Scholar]
- 15.Franco, A., C. Ferrari, A. Sette, and F. V. Chisari. 1995. Viral mutations, TCR antagonism and escape from the immune response. Curr. Opin. Immunol. 7:524-531. [DOI] [PubMed] [Google Scholar]
- 16.Galibert, F., T. N. Chen, and E. Mandart. 1982. Nucleotide sequence of a cloned woodchuck hepatitis virus sequence. J. Virol. 41:51-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gerin, J. L., R. M. Faust, and P. V. Holland. 1975. Biophysical characterization of the adr subtype of hepatitis B antigen and preparation of anti-r sera in rabbits. J. Immunol. 115:100-105. [PubMed] [Google Scholar]
- 18.Girones, R., P. J. Cote, W. E. Hornbuckle, B. C. Tennant, J. L. Gerin, R. H. Purcell, and R. H. Miller. 1989. Complete nucleotide sequence of a molecular clone of woodchuck hepatitis virus that is infectious in the natural host. Proc. Natl. Acad. Sci. USA 86:1846-1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guidotti, L. G., R. Rochford, J. Chung, M. Shapiro, R. Purcell, and F. V. Chisari. 1999. Viral clearance without destruction of infected cells during acute HBV infection. Science 284:825-829. [DOI] [PubMed] [Google Scholar]
- 20.Guo, J. T., H. Zhou, C. Liu, C. Aldrich, J. Saputelli, T. Whitaker, M. I. Barrasa, W. S. Mason, and C. Seeger. 2000. Apoptosis and regeneration of hepatocytes during recovery from transient hepadnavirus infections. J. Virol. 74:1495-1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsu, H. Y., M. H. Chang, K. H. Hsieh, C. Y. Lee, H. H. Lin, L. H. Hwang, P. J. Chen, and D. S. Chen. 1992. Cellular immune response to HBcAg in mother-to-infant transmission of hepatitis B virus. Hepatology 15:770-776. [DOI] [PubMed] [Google Scholar]
- 22.Inoue, M., S. Kakumu, K. Yoshioka, Y. Tsutsumi, T. Wakita, and M. Arao. 1989. Hepatitis B core antigen-specific IFN-gamma production of peripheral blood mononuclear cells in patients with chronic hepatitis B virus infection. J. Immunol. 142:4006-4011. [PubMed] [Google Scholar]
- 23.Jacob, J. R., M. A. Ascenzi, C. A. Roneker, I. A. Toshkov, P. J. Cote, J. L. Gerin, and B. C. Tennant. 1997. Hepatic expression of the woodchuck hepatitis virus x-antigen during acute and chronic infection and detection of a woodchuck hepatitis virus x-antigen antibody response. Hepatology 26:1607-1615. [DOI] [PubMed] [Google Scholar]
- 24.Jung, M.-C., H. M. Diepolder, U. Spengler, E. A. Wierenga, R. Zachoval, R. M. Hoffmann, D. Eichenlaub, G. Froesner, H. Will, and G. R. Pape. 1995. Activation of a heterogeneous hepatitis B (HB) core and e antigen-specific CD4+ T-cell population during seroconversion to anti-HBe and anti-HBs in hepatitis B virus infection. J. Virol. 69:3358-3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jung, M.-C., U. Spengler, W. Schraut, R. Hoffmann, R. Zachoval, J. Eisenburg, D. Eichenlaub, G. Riethmueller, G. Paumgartner, H. W. L. Ziegler-Heitbrock, H. Will, and G. R. Pape. 1991. Hepatitis B virus antigen specific T-cell activation in patients with acute and chronic hepatitis B. J. Hepatol. 13:310-317. [DOI] [PubMed] [Google Scholar]
- 26.Jung, M.-C., M. Stemler, T. Weimer, U. Spengler, J. Dohrmann, R. Hoffmann, D. Eichenlaub, J. Eisenburg, G. Riethmueller, H. Will, and G. R. Pape. 1991. Immune response of peripheral blood mononuclear cells to HBx-antigen of hepatitis B virus. Hepatology 13:637-643. [PubMed] [Google Scholar]
- 27.Kodama, K., N. Ogasawara, H. Yoshikawa, and S. Murikami. 1985. Nucleotide sequence of a cloned woodchuck hepatitis virus genome: evolutional relationship between hepadnaviruses. J. Virol. 56:978-986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Korba, B. E., P. J. Cote, and J. L. Gerin. 1988. Mitogen-induced replication of woodchuck hepatitis virus in cultured peripheral blood lymphocytes. Science 241:1213-1216. [DOI] [PubMed] [Google Scholar]
- 29.Loehr, H. F., W. Weber, J. Schlaak, B. Goergen, K.-H. Meyer zum Bueschenfelde, and G. Gerken. 1995. Proliferative response of CD4+ T-cells and hepatitis B virus clearance in chronic hepatitis with or without hepatitis B e-minus hepatitis B virus mutants. Hepatology 22:61-68. [DOI] [PubMed] [Google Scholar]
- 30.Marinos, G., F. Torre, S. Chokshi, M. Hussain, B. E. Clarke, D. J. Rowlands, A. L. Eddleston, N. V. Naoumov, and R. Williams. 1995. Induction of T-helper cell response to hepatitis B core antigen in chronic hepatitis B: a major factor in activation of the host immune response to the hepatitis B virus. Hepatology 22:1040-1049. [DOI] [PubMed] [Google Scholar]
- 31.Maschke, J., S. Menne, J. R. Jacob, E. Kreuzfelder, B. C. Tennant, M. Roggendorf, and H. Grosse-Wilde. 2001. Thymidine kinases and thymidine utilization in proliferating peripheral blood lymphocytes and hepatic cells of the woodchuck (Marmota monax). Vet. Immunol. Immunopathol. 78:279-296. [DOI] [PubMed] [Google Scholar]
- 32.Menne, S., and B. C. Tennant. 1999. Unraveling hepatitis B virus infection of mice and men (and woodchucks and ducks). Nat. Med. 10:1125-1126. [DOI] [PubMed] [Google Scholar]
- 33.Menne, S., J. Maschke, M. Lu, H. Grosse-Wilde, and M. Roggendorf. 1998. T-cell response to woodchuck hepatitis virus (WHV) antigens during acute self-limited WHV infection and convalescence and after viral challenge. J. Virol. 72:6083-6091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Menne, S., J. Maschke, T. K. Tolle, M. Lu, and M. Roggendorf. 1997. Characterization of T-cell response to woodchuck hepatitis virus core protein and protection of woodchucks from infection by immunization with peptides containing a T-cell epitope. J. Virol. 71:65-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Menne, S., J. Maschke, R. Klaes, H. Grosse-Wilde, and M. Roggendorf. 1997. Cellular immune response of woodchucks to woodchuck hepatitis virus surface protein during acute WHV infection, p. 453-457. In M. Rizzetto, R. H. Purcell, J. L. Gerin, and G. Verme (ed.), Viral hepatitis and liver disease, Edizioni Minerva Medica, Turin, Italy.
- 36.Milich, D. R., M. K. Chen, J. L. Hughes, and J. E. Jones. 1998. The secreted hepatitis B precore antigen can modulate the immune response to the nucleocapsid: a mechanism for persistence. J. Immunol. 160:2013-2021. [PubMed] [Google Scholar]
- 37.Milich, D. R., J. E. Jones, J. L. Hughes, J. Price, A. K. Raney, and A. McLachlan. 1990. Is a function of the secreted hepatitis B e antigen to induce immunologic tolerance in utero? Proc. Natl. Acad. Sci. USA 487:6599-6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakamura, I., J. T. Nupp, M. Cowlen, W. C. Hall, B. C. Tennant, J. L. Casey, J. L. Gerin, and P. J. Cote. 2001. Pathogenesis of neonatal woodchuck hepatitis virus infection: chronicity as an outcome of infection is associated with a diminished acute hepatitis that is temporally deficient for the expression of interferon-gamma and tumor necrosis factor-alpha messenger RNAs. Hepatology 33:439-447. [DOI] [PubMed] [Google Scholar]
- 39.Penna, A., F. V. Chisari, A. Bertoletti, G. Missale, P. Fowler, T. Giuberti, F. Fiaccadori, and C. Ferrari. 1991. Cytotoxic T lymphocytes recognize an HLA-A2-restricted epitope within the hepatitis B virus nucleocapsid antigen. J. Exp. Med. 174:1565-1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rawson, R. E., P. W. Concannon, P. J. Roberts, and B. C. Tennant. 1998. Seasonal differences in resting oxygen consumption, respiratory quotient, and free thyroxine in woodchucks. Am. J. Physiol. 274:R963-R969. [DOI] [PubMed]
- 41.Rehermann, B., C. Ferrari, C. Pasquinelli, and F. V. Chisari. 1996. The hepatitis B virus persists for decades after patients' recovery from acute viral hepatitis despite active maintenance of a cytotoxic T-lymphocyte response. Nat. Med. 2:1104-1108. [DOI] [PubMed] [Google Scholar]
- 42.Roggendorf, M., and T. K. Tolle. 1995. The woodchuck: an animal model for hepatitis B virus infection in man. Intervirology 38:100-112. [DOI] [PubMed] [Google Scholar]
- 43.Tennant, B. C. 1999. Animal models of hepatitis B virus infection. Clin. Liver Dis. 3:241-266. [Google Scholar]
- 44.Tiku, M. L., K. R. Beutner, K. Tiku, and P. L. Ogra. 1987. Cell-mediated immune response to liver tissue antigen and hepatitis B surface antigen after infection with hepatitis B virus in humans. J. Infect. Dis. 138:587-596. [DOI] [PubMed] [Google Scholar]
- 45.Tsai, S. L., P. J. Chen, M. Y. Lai, P. M. Yang, J. L. Sung, J. H. Huang, L. H. Hwang, T. H. Chang, and D. S. Chen. 1992. Acute exacerbations of chronic type B hepatitis are accompanied by increased T cell responses to hepatitis B core and e antigens. Implications for hepatitis B e antigen seroconversion. J. Clin. Investig. 89:87-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vingerhoets, J., P. Michielsen, G. Vanham, E. Bosmans, W. Paulij, A. Ramon, P. Pelckmans, L. Kestens, and G. Leroux-Roels. 1998. HBV-specific lymphoproliferative and cytokine responses in patients with chronic hepatitis B. J. Hepatol. 28:8-16. [DOI] [PubMed] [Google Scholar]
- 47.Webster, G. J., S. Reignat, M. K. Maini, S. A. Whalley, G. S. Ogg, A. King, D. Brown, P. L. Amlot, R. Williams, D. Vergani, G. M. Dusheiko, and A. Bertoletti. 2000. Incubation phase of acute hepatitis B in man: dynamic of cellular immune mechanisms. Hepatology 32:1117-1124. [DOI] [PubMed] [Google Scholar]