Abstract
Turnip crinkle virus (TCV) is a small, plus-sense, single-stranded RNA virus of plants. A virus-coded protein, p88, which is required for replication has been expressed and purified from Escherichia coli. In vitro assays revealed that the recombinant p88 has an RNA-dependent RNA polymerase (RdRp) activity and can also bind to RNA. Deletion of the N-terminal region in p88 resulted in a more active RdRp, while further deletions abolished RdRp activity. Comparison of the E. coli-expressed p88, the N-terminal deletion mutant of p88, and a TCV RdRp preparation obtained from infected plants revealed that these preparations show remarkable similarities in RNA template recognition and usage. Both the recombinant and the plant TCV RdRp preparations are capable of de novo initiation on both plus- and minus-strand satC and satD templates, which are small parasitic RNAs associated with TCV infections. In addition, these RdRp preparations can efficiently recognize the related Tomato bushy stunt virus promoter sequences, including the minus- and plus-strand initiation promoters. Heterologous viral and artificial promoters are recognized poorly by the recombinant and the plant TCV RdRps. Further comparison of the single-component recombinant TCV RdRp and the multicomponent plant TCV RdRp will help dissect the functions of various components of the TCV replicase.
Replication of RNA viruses is carried out by membrane-bound multisubunit replicase complexes, which consist of virus- and host-coded proteins (5, 6, 19). The catalytic subunit of the viral replicase complexes is a virus-coded RNA-dependent RNA polymerase (RdRp). Biochemical features of RdRps for several positive-strand RNA viruses, including poliovirus (3), flaviviruses (12, 18, 22, 23, 45), plant potyviruses (16), and potexviruses (21) have been examined in some detail by using purified preparations obtained from heterologous expression systems.
Another approach to obtain viral RdRps is to purify them from virus-infected cells. Indeed, this approach has been documented for several viruses, including bacteriophages (4), brome mosaic virus (17, 35), cucumber mosaic virus (15), tobacco mosaic virus (32), turnip yellow mosaic virus (10, 38), alfalfa mosaic virus (36), potexviruses (34) and tombusviruses (30). The RdRp preparations were found to initiate cRNA synthesis either with short primers or in the absence of primers (de novo synthesis).
Specificity in template selection and the stringency of promoter recognition vary among the purified RdRps (reviewed in references 5, 6, and 11). However, it has been shown that common sequence elements, such as CCA repeats, can be recognized by several RdRps (11, 44), and this suggests that there are significant similarities among some RdRps. This is further supported by the similar structures of three RdRps (1, 7, 14, 20).
Turnip crinkle virus (TCV), a carmovirus, is a well-characterized model plus-strand RNA virus (reviewed in references 5 and 37). TCV has a small genome (4,054 bases) with five genes, of which two are required for replication, p28 and p88 (43). p88 overlaps p28 and contains the signature RdRp motifs (reviewed in reference 31) in the unique C-terminal portion (Fig. 1). The role of the p28 is not known. In addition, TCV infections are associated with several satellite (sat) RNAs (37), including satD (194 nucleotides [nt]) and satC (356 nt). With the help of an in vitro replicase assay based on an RdRp preparation obtained from TCV-infected plants, these satellite RNAs were widely used to dissect cis-acting elements involved in RNA accumulation (13, 24, 29, 37, 39, 40).
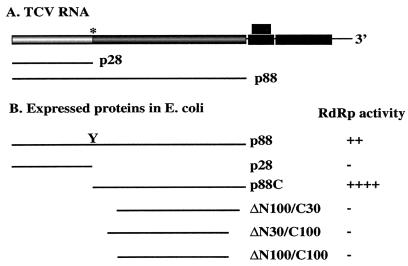
FIG. 1.
RdRp activity of the recombinant TCV p88 and its derivatives obtained from E. coli. (A) Schematic representation of the TCV genome. TCV RNA contains five open reading frames, of which two are expressed from the genomic RNAs (shown by shaded boxes) and three (shown by black boxes) are expressed from two subgenomic RNAs. p88 is expressed by leaky termination of the stop codon (marked with an asterisk) of the p28 gene. (B) Schematic representation and RdRp activities of p88 and its derivatives expressed and purified from E. coli. The leaky termination codon in p88 was altered to a tyrosine (Y) codon (see Materials and Methods), which was shown to be functional in vivo (43). The RdRp activity of each of the six separately expressed and purified proteins (fused to MBP) was tested in a standard in vitro assay using 32P-labeled UTP and PAGE analysis of the RdRp products (not shown). Only p88 and p88C showed detectable levels of RdRp activity.
In vitro analyses of sequences that affect minus- and plus-strand synthesis revealed the presence of promoter and enhancer elements that are required for or enhance accumulation of satC. The plus strand contains a 3"-terminal 29-base hairpin promoter that is required for complementary strand synthesis in vitro (40). The minus-strand satC contains two RNA elements, one called the 3"-proximal element, and the other the 5"-proximal element, both of which can function as independent promoters in vitro (13). Another cis-acting element present on minus strands of satC, which is important for plus-strand synthesis, is a 30-base hairpin (positions 180 to 209), designated the motif 1 hairpin. The motif 1 hairpin was found to function as an RNA replication enhancer for primer-independent cRNA synthesis (de novo initiation) (29). In addition, the motif 1 hairpin also facilitates primer-dependent RNA synthesis by the TCV RdRp in vitro (26-28), which can lead to the generation of recombinant RNA molecules by a template switching mechanism (8, 25).
To dissect the functions of the TCV replicase proteins in more detail, we expressed and purified them from Escherichia coli. We found that p88 alone possessed RdRp activity. Interestingly, the unique C-terminal fragment of p88, designated p88C, also showed RdRp activity in vitro. Both p88 and p88C were capable of both de novo and primer-dependent RNA synthesis, similar to the plant TCV RdRp. In addition, all three RdRp preparations showed similar template selectivity as well as promoter recognition in vitro.
MATERIALS AND METHODS
Construction of expression plasmids.
To express the p88 gene, which has a UAG termination codon behind the overlapping p28 gene (see Fig. 1), a mutation was introduced at position 817 to generate a tyrosine codon (43) using sequential PCR. First, the N- and C-terminal portions of p88 were generated separately using Pfu turbo polymerase (Stratagene) and either primer pair p88F (CCTCTTCTACACACACTC) and p28TYR (GTAGCGGACAAAAGAGATC) or primer pair p88TYR (GGGTGCTTGCGGGAGCTG) and p88-stop (CCGTAAGCTTGATTAGAGAGTTGTAGGGAATTCG). The N- and C-terminal PCR products were ligated to generate the modified p88 gene. The ligated product was treated with HindIII, followed by cloning into pMAL-c2X (NEB) at the XmnI and HindIII sites. Construct p88C was generated by ligating the PCR product (digested with HindIII and XbaI) obtained with primers 818F (GGAGTCTAGAGATACCATCAAGAGGATG) and p88-stop into pMAL-c2X between the HindIII and XbaI sites.
The other constructs, which were used to express the proteins shown in Fig. 1B, were generated using a method similar to that described for p88C, except using EcoRI and XbaI sites. The primers used were the following: for construct p28, 88F and 28-stop (GGAGTCTAGACTAGCGGACAAAAGAGATC); for ΔN30/C100, aa281F (GAG GAA TTC AAG GTT CGA CGC ATC TTC) and aa674F (GAG TCT AGA CTA TGA TTG TAA CAC TGG TAC); for ΔN100/C30, aa351F (GAG GAA TTC GGA AAT CAT ACC CCT GTG) and aa744F (GAG TCT AGA CTA GTC GTA GTA CTC CTC AAG); and for ΔN100/C100, aa351F and aa674F. The resulting constructs expressed proteins ΔN100/C30 (amino acid positions 352 to 745) (7), ΔN30/C100 (amino acid positions 282 to 675), and ΔN100/C100 (amino acid positions 352 to 675).
Purification of p88 and its derivatives from E. coli.
The generated expression constructs (see above), which were used to obtain the proteins shown in Fig. 1B, were introduced into Epicurian BL21-CodonPlus (DE3)-RIL (Stratagene). Protein expression was induced as recommended by the supplier using IPTG (isopropylthiogalactopyranoside). After 8 to 10 h of induction at 14°C, the cells were harvested, collected by centrifugation (5,000 rpm for 5 min), resuspended, and sonicated as recommended by the supplier, except the amount of NaCl was reduced to 25 mM. The samples were then centrifuged again (15,000 rpm for 5 min), followed by affinity-based chromatography (amylose column from NEB) following the supplied procedure. After thorough washing with the column buffer (containing 25 mM NaCl), the proteins were eluted with maltose-containing column buffer (NEB). All steps were carried out on ice or in the cold room. The quality of the proteins obtained was checked by sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis (SDS-PAGE) analysis (36). Most of the RdRp studies (see below) were done with the fusion proteins (except where it is noted otherwise).
Plant TCV RdRp preparation.
Turnip plants inoculated with TCV transcripts were used 10 days after inoculation to isolate partially purified TCV RdRp preparations as described previously (30, 39). All steps were carried out on ice or in the cold room.
Preparation of RNA templates.
RNA templates were prepared with T7 RNA polymerase (36), followed by removal of unincorporated nucleotides as described previously (24). Constructs containing full-length cDNAs of satC, satD, and MDV were obtained from Anne Simon, while DI-72 was from Andy White (42). The series of promoter-containing constructs shown in Fig. 7 were obtained from Tadas Panavas (Panavas et al., submitted for publication).
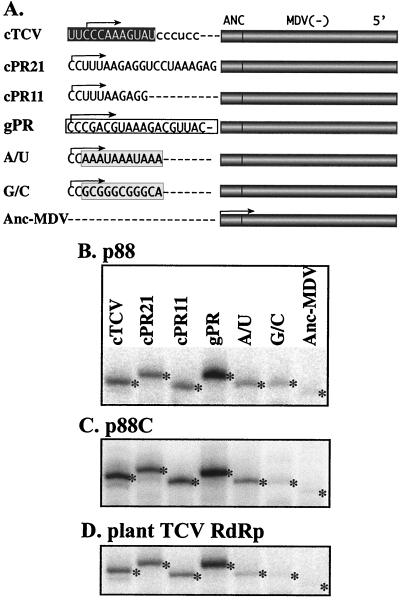
FIG. 7.
Comparison of promoter recognition by the recombinant p88 and p88C and the plant TCV RdRps. (A) Schematic representation of constructs tested in in vitro RdRp assays. The actual 3"-end sequence of each construct is shown in 3"→5" orientation. The core plus-strand initiation promoter for satC(−) (13) is shown in a black box (construct cTCV). Constructs cPR21 and cPR11 contain the extended and the core plus-strand initiation promoter for minus-strand DI-72 of TBSV, respectively (Panavas et al., submitted). The core minus-strand synthesis promoter for DI-72 of TBSV is boxed (construct gPR). The artificial AU-rich and GC-rich promoter sequences are shown in gray boxes (constructs A/U and G/C). The sites of expected initiation products are shown with arrows. In addition to the sequences shown, each construct contains the same 5" sequences derived from minus-strand MDV (221 nt) and a 17-nt anchor (Anc) sequence from TBSV (positions 4666 to 4682). (B to D) Representative denaturing gel analyses of radiolabeled RNA products synthesized by in vitro transcription with p88, p88C, and the TCV plant RdRp. Template-sized products are labeled with asterisks. The order of samples is the same in each panel.
RdRp assay.
RdRp reactions were carried out as previously described for the plant TCV RdRp (30) for 1 h at 25°C (unless indicated otherwise). Briefly, the RdRp reactions were performed in the presence of 50 mM Tris-HCl (pH 8.2), 10 mM MgCl2, 10 mM dithiothreitol, 100 mM potassium glutamate, 1.0 mM each ATP, CTP, and GTP, 0.01 mM UTP (final concentration), and 0.5 μl of [32P]UTP (ICN) in a 50-μl total volume. In addition, RdRp reaction mixtures contained 165 nM template RNA. In some experiments, the amount of template RNA was reduced to 80 nM for p88C due to its high RdRp activity (Fig. 4 and 5). The amounts of p88 and p88C (used as fusion proteins with MBP) were 3 μg/assay.
FIG. 4.
Effect of temperature on the RdRp activity of recombinant p88 and p88C and the plant TCV RdRp. (A) Representative denaturing gel analyses of radiolabeled RNA products synthesized by in vitro transcription with p88, p88C, and the plant TCV RdRp using satC(−) as the template. Products are marked as shown in the legend to Fig. 3. The exposure time was different for each RdRp assay due to the increased activity for p88C. (B) The relative amounts of M and D RdRp products were measured at various temperatures, as shown. The amount of product D obtained at 25°C was selected as 100% separately for each RdRp preparation. Note that the increased level of product D at 30 and 37°C is due to terminal transferase activity present only in the p88 preparation, as confirmed by S1 nuclease digestion (not shown). The terminal transferase activity was not significant at 25°C (not shown). Terminal transferase activity in the p88 preparation was confirmed by obtaining template-sized product (similar in size to product D) when the RdRp reaction contained [32P]UTP in the absence of the other three nucleotides. This terminally labeled product, unlike product D, was fully S1 nuclease sensitive (not shown). Product E was not included because its amount varied in separate experiments.
After phenol-chloroform extraction and ammonium acetate-isopropanol precipitation, half the amount of the RdRp products was treated with S1 nuclease as described previously (27, 28). Subsequently, the RdRp products were analyzed on 20-cm-long denaturing 5% polyacrylamide-8 M urea gels, followed by analysis with a phosphorimager as described (24, 39). During the competition experiments, the same amount (50 nM) of template RNA and increasing amounts (between 1- and 16-fold excess) of competitor RNAs were used in RdRp reactions similar to those described above.
RNA binding studies.
RNA probes labeled with [32P]UTP were prepared using T7 RNA polymerase as described above, except 100-fold less unlabeled UTP was used. Approximately 20 ng of labeled RNA was mixed with 2 μg of p88 or p88C preparation for 20 min at 25°C in the presence of the RdRp buffer (see above) (30, 39). The volume of the plant TCV RdRp preparation used for RNA binding was five times less than that used during the RdRp assays. The samples were analyzed by electrophoresis on native 1% agarose gels, performed at 100 V for 1 h at 4°C in Tris-acetate-EDTA (TAE) buffer (36). Dried gels were analyzed using a phosphorimager. During the competition experiments, severalfold excess amounts of unlabeled competitor RNAs were added simultaneously with the 32P-labeled probe to similar amounts of p88 (as shown in Fig. 9).
FIG. 9.
(A) Representative gel mobility shift analysis of radiolabeled RNA bound to p88, p88C, or the plant TCV RdRp. The bound and free RNAs are marked at the sides. Comparable amounts of p88 and p88C were used for RNA binding (see Materials and Methods for details). Note that the probe bound to p88C resulted in two types of products, a slow-migrating product at the top of the gel and a diffuse fast-migrating product above the free probe. The first lane on the left shows the 32P-labeled satD(−) template without added proteins (probe). (B) Template competition experiments for RdRp binding. A representative gel mobility shift analysis of radiolabeled satC(+) RNA bound to p88 in the absence or presence of unlabeled competitor RNAs (the competitors are shown above the gels). The bound and free RNAs are marked at the sides. The first lane on the left shows the 32P-labeled template bound to p88 without added competitors (no competitor). (C) Graphic presentation of the competition experiments. The relative amount of competitor (in comparison with the constant amount of 32P-labeled RNA probe) is shown below the graph.
RESULTS
Expression and purification of TCV p88 in E. coli.
The N-terminal region of the p88 gene of TCV overlaps the p28 gene (Fig. 1), while the unique C-terminal portion codes for the putative RdRp, based on sequence comparison (reviewed in references 5 and 31). A mutated p88 gene of TCV in which the readthrough stop codon was changed to a tyrosine codon (43) was cloned into an expression vector (pMAL c2X; NEB), and p88 was expressed as a C-terminal fusion protein with the maltose-binding protein (MBP) in E. coli as described in Materials and Methods.
After affinity-based purification of the p88 fusion protein (Fig. 2), we tested its RdRp activity first using minus-strand satC as the template in an RdRp buffer described for TCV RdRp preparations purified from TCV-infected plants (24, 39). The RdRp products were analyzed by denaturing PAGE, which revealed RdRp activity for the p88 preparation only when external template was added (not shown). The use of minus-strand satC templates in the p88-based RdRp assay resulted in three major bands in denaturing PAGE gels (Fig. 3B). The major bands obtained with p88 were identical to the bands obtained with a control TCV RdRp preparation purified from plants (Fig. 3B; note that we use the term plant TCV RdRp for the control TCV RdRp preparation purified from plants).
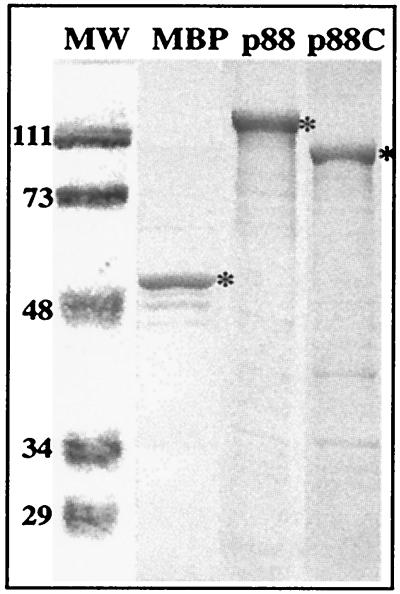
FIG. 2.
Representative SDS-PAGE analysis of the purified recombinant proteins from E. coli. The gel was stained with Coomassie blue. Asterisks mark the full-length proteins. Due to the expression strategy with pMAL c2X, the MBP contains a C-terminal extension, resulting in an ≈50-kDa protein. Note that p88 and p88C are fused with the MBP to aid affinity-based purification. The smaller products are likely generated by either premature termination of translation of p88 and p88C genes or protein degradation, since their amounts were not reduced by intensive washing of the columns during affinity-based purification (not shown). Lane MW, size markers (in thousands).
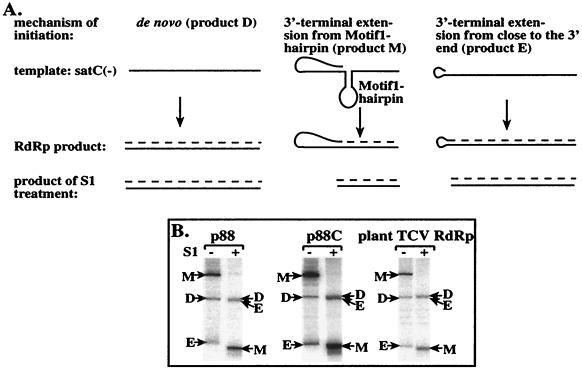
FIG. 3.
Comparison of the RdRp products obtained with the recombinant p88 and p88C and with the TCV RdRp purified from plants using satC(−) as the template. (A) Schematic representation of the three major products generated by the plant TCV RdRp in vitro using satC(−) templates. The mechanism of the formation of these products is explained schematically based on data from Song and Simon (39, 41). Solid lines represent the template, while the broken line depicts the cRNA product (labeled during the RdRp reaction). S1 nuclease treatment removes the single-stranded regions of the RdRp products under the conditions used (28, 41). The names of the products reflect their generation: product D, de novo initiation; product M, motif 1 hairpin-mediated primer extension; product E, 3"-end-mediated primer extension. (B) Representative denaturing gel analyses of radiolabeled RNA products synthesized by in vitro transcription with the recombinant p88 and p88C and the plant TCV RdRp. The − and + signs above the lanes indicate untreated and S1 nuclease-treated samples, respectively, prior to PAGE analysis. Products M, D, and E are marked with arrows. Note that product E runs aberrantly (i.e., much faster) under these conditions in the untreated samples due to the highly stable hairpin structure of this product (see panel A and also reference 41). Also, S1-treated product E migrates close to product D under the conditions used. The exposure time was different for each set of RdRps due to the different activities of the RdRp preparations. The amount of p88C applied in the RdRp assay was 40% of that of p88, based on Coomassie-stained SDS-PAGE gels (not shown).
S1 nuclease digestion of the RdRp products revealed the presence of three major types of RdRp products. The first type constitutes nuclease-resistant template-sized cRNA, and the second type includes partially nuclease-sensitive, shorter-than-template-sized RNA products (Fig. 3B). Earlier work with the plant TCV RdRp preparation demonstrated that the template-sized RdRp product is generated by de novo initiation from the very 3" end of the minus-strand satC RNA [product D (de novo) in Fig. 3A] (41). The shorter-than-template-sized product is generated by primer extension (self-priming from the 3" end of the template) initiating internally close to the motif 1 hairpin replication enhancer [product M (supported by the motif 1 hairpin replication enhancer) in Fig. 3A; also termed the large RNA in reference 41].
The third type, which migrates aberrantly on denaturing PAGE gels, is also partially S1 nuclease sensitive and is likely generated by primer extension starting close to the 3" end of the template [product E (priming from close to the 3" end) in Fig. 3A; also termed the small RNA in reference 41]. The S1 nuclease digestion profile of the in vitro products obtained with the plant TCV RdRp preparation was similar to that obtained with p88 (Fig. 3B, lane +). The above data, based on the similar-sized RdRp products and on the similar S1 nuclease digestion profile, suggest that RdRp products are likely generated by the same mechanism as described for the plant TCV RdRp products (39, 41).
Five different N- and C-terminally truncated versions of p88 were also generated, expressed, purified, and tested as MBP fusion proteins in vitro for RdRp activity (Fig. 1B). First, p28, which contains the N-terminal portion of p88 but lacks the putative polymerase domain of p88 and represents one of the two naturally expressed TCV replicase proteins (Fig. 1) (43), had no RdRp activity in vitro (Fig. 1B and data not shown). Second, p88C, which has the complete polymerase-like domain of p88 (Fig. 1B), showed unusually high RdRp activity (Fig. 3B). Removal of the MBP domain by cleavage with factor Xa from the p88C fusion protein had no effect on RdRp activity of p88C (not shown). Third, three different truncations within the p88C domain (constructs ΔN100/C30, ΔN30/C100, and ΔN100/C100, Fig. 1B) made the preparations inactive in standard RdRp assays (Fig. 1B and data not shown).
Overall, these data confirm that the C-terminal portion of p88 contains the functional RdRp domain and also suggest that the entire 88C domain of p88 is required for RdRp activity in vitro. In addition, the lack of TCV-specific RdRp activities of p28, the three truncated derivatives of p88C (Fig. 1B), and MBP alone (data not shown) excludes the possibility that we purified a contaminating putative E. coli RdRp, which would be responsible for labeling the externally added templates in vitro. This is because the putative E. coli-derived RdRp, if any, should be present in all of the above preparations, which were obtained from the same E. coli strain and purified by exactly the same procedure (Materials and Methods). Yet only the p88 and p88C preparations showed RdRp activities. In addition, the RdRp products obtained with p88 and p88C preparations were similar to those generated with the plant TCV RdRp (Fig. 3B). From the above observations, we conclude that p88 and p88C preparations contain functional TCV RdRp. The RdRp activities of p88 and p88C were characterized further, as shown below.
Characterization of RdRp activities of TCV p88 and p88C.
To test the stability and activity of p88 and p88C, we performed the RdRp assays at various temperatures, as shown in Fig. 4 using minus-strand satC templates. Preparations of p88 and p88C showed RdRp activity at temperatures from 4 to 37°C (Fig. 4A and B). The plant TCV RdRp was also active at these temperatures, although much longer exposure of the gels was needed to detect the small amount of products synthesized (Fig. 4A and B). All three preparations produced mainly primer extension products (product M in Fig. 4A) at low temperatures (4 and 12°C), while de novo initiation was hardly detectable (product D in Fig. 4A). Primer extension (product M in Fig. 4A) was the highest for all three preparations at 30°C.
De novo initiation occurred with the highest efficiency at 25 and 30°C for p88C and the plant TCV RdRp, while p88 generated the most template-sized products at 37°C (product D, Fig. 4A). S1 digestion of the RdRp products, however, revealed that a portion of the template-sized RdRp products (product D) for p88 obtained at 30 and 37°C but not at 25°C were S1 nuclease sensitive (data not shown), suggesting the presence of terminal transferase-like activity in the p88 preparation. Similar activity was not detected in the p88C or the plant TCV RdRp preparation. It is known that other viral RdRp preparations, such as the RdRp of hepatitis C virus, obtained from E. coli may also contain terminal transferase activity (22). Because the above terminal transferase-like activity in the p88 preparations was not detectable at 25°C, we used this temperature in all of the following studies.
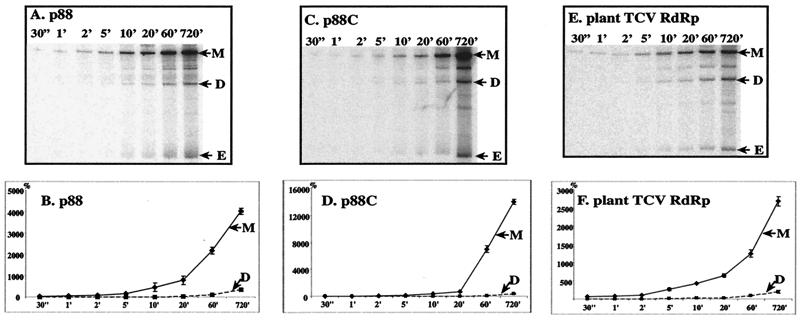
To compare the kinetics of RNA synthesis by p88, p88C, and the plant TCV RdRp, we conducted time course experiments at 25°C. All three preparations produced primer extension products (product M) as quickly as 30 s (Fig. 5). In contrast, detection of the template-sized de novo-initiated products (product D) required at least 5 min of incubation with all three preparations. The amounts of both de novo-initiated (product D) and primer extension products (product M) for each preparation increased over time, suggesting that each RdRp molecule can likely produce several nascent RNA products sequentially (RdRp is reused). The data in Fig. 5 also demonstrate that p88C is different from p88 and the plant TCV RdRp by favoring primer extension from the internal replication enhancer (the motif 1 hairpin, product M) over the production of template-sized de novo products (compare products M and D).
FIG. 5.
Comparison of the kinetics of RNA synthesis by the recombinant p88 and p88C with that of the plant TCV RdRp. Standard RdRp reactions were performed at 25°C for a given amount of time using satC(−) as the template. Products are marked as described in the legend to Fig. 3. Panels A, C, and E show representative denaturing gel analyses of radiolabeled RNA products synthesized by in vitro transcription with p88, p88C, and the plant TCV RdRp. Panels B, D, and F show the relative amounts of M and D RdRp products made during various incubation times as shown. The amount of product D obtained after 60 min of incubation was selected as 100% separately for each RdRp preparation.
For example, at the 60-min time point, the amount of product M was ≈70-fold more (normalized value) than that of product D in the p88C assays, while product M was only 13- and 21-fold more abundant for the plant TCV RdRp and the p88 RdRp, respectively, than product D (Fig. 5B, D, and F; also see Fig. 4B). More diluted p88C preparations also resulted in ≈70-fold differences between products M and D (not shown). The profiles of RdRp products obtained with p88 and the plant TCV RdRp were more similar to each other than to that obtained with p88C. These observations indicate that the N-terminal portion of p88, which is not present in p88C, may somehow inhibit primer extension by more than twofold. Further experiments will be needed to demonstrate a direct role for the N-terminal region of p88 in affecting selection between de novo synthesis and primer extension.
Comparison of template use by p88, p88C, and the plant TCV RdRp.
To compare the template use by p88, p88C, and plant TCV RdRp, we performed RdRp assays using four pairs of plus- and minus-strand RNA templates. Compared to the above-tested minus-strand satC, the plus-strand satC RNA was used inefficiently in all three assays (Fig. 6A to C). All three preparations synthesized predominantly the full-length complementary products for the satC(+) template, based on the size and S1 nuclease-resistant nature of the products [Fig. 6, lane satC(+)]. The second pair of templates were for satD RNA, which is 194 nt long and is associated with TCV infections (37). The third set of templates were for DI-72 RNA, which is a 621-nt defective interfering RNA associated with Tomato bushy stunt virus (TBSV; a tombusvirus related to TCV) infections. The fourth pair of templates were for the heterologous MDV RNA, a 221-nt satellite RNA associated with Qβ bacteriophage (2).
FIG. 6.
Comparison of template use by recombinant p88 and p88C and the plant TCV RdRps. Representative denaturing gel analyses of radiolabeled RNA products synthesized by in vitro transcription with (A) p88, (B) p88C, and (C) the plant TCV RdRp are shown. Full-length plus or minus strands of the TCV-associated satC and satD RNAs, the TBSV-associated DI-72 RNA, and the Qβ-associated MDV RNA were used in equal amounts (1 μg) as templates. Half the amount of RdRp products was treated with single-strand-specific S1 nuclease (lanes +). Template-sized RdRp products that were resistant to S1 nuclease treatment are marked by arrows. The primer extension products that were partially S1 nuclease sensitive are depicted by asterisks. These products changed their migration pattern after S1 nuclease treatment. Lanes MW, single-stranded RNA size markers (in bases) obtained by T7 transcription using the satC (356 nt) and MDV (221 nt) clones as templates.
The plus-strand satD, DI-72, and MDV were found to be poor templates in all three RdRp assays [Fig. 6, lanes satD(+), DI-72(+), and MDV(+)]. In contrast, the minus-strand satD and DI-72 were used efficiently in all three RdRp systems [Fig. 6, lanes satD(−) and DI-72(−)]. In addition to the full-length complementary product (arrows in Fig. 6), primer extension (i.e., extension from the 3" end of the template, marked with asterisks in Fig. 6) products were also obtained in all three RdRp assays with satD(−) and DI-72(−). Easily detectable levels of internal initiation (bracketed) at three positions were detected for DI-72(−) in all three RdRp assays. The nature of these products was characterized in more detail in a previous paper (30).
Plus-strand MDV RNA was not used as a template for de novo initiation from the authentic 3" end in any of the three assays [there are no S1 nuclease-resistant bands in Fig. 6, lane MDV(+)]. Low levels of partially S1 nuclease-sensitive products were observed in all three systems, although p88C gave the largest amount of these products. The above products obtained with MDV(+) are likely the result of inefficient primer extension, based on their partially S1 nuclease-resistant nature.
The lack of detectable amounts of de novo-initiated products for MDV(+) suggests that all three RdRp preparations have comparable levels of selectivity in template usage. Surprisingly, however, the minus-strand MDV RNA was a rather efficient template in all three assays [Fig. 6, lanes MDV(−)]. We also tested an additional heterologous template, yeast tRNA, which was not used as a template in any of the three RdRp assays (data not shown).
Overall, these experiments demonstrated that, in general, plus-strand RNAs were poorer templates than minus-strand RNAs. In addition, the three RdRp preparations were shown to have comparable levels of template specificity. This is somewhat surprising since p88C includes only the polymerase domain, while the plant TCV RdRp preparation contains a small amount of p88 and an excess amount of free p28 and some host components (39; P. D. Nagy, data not shown).
Comparison of promoter recognition by E. coli-expressed and plant TCV RdRps.
The TCV RdRp has been shown to recognize short (11-nt) linear sequences and a hairpin sequence with a short single-stranded tail for plus- and minus-strand RNA synthesis, respectively (13, 40). To test whether p88 and p88C are also capable of recognizing a minimal TCV promoter and other viral and artificial sequences, we tested RdRp activity with seven different RNA sequences, as shown in Fig. 7. The selected sequences were separately fused to the 3" end of a chimeric heterologous sequence (see construct Anc-MDV, Fig. 7). The rationale for use of heterologous sequences is that they can facilitate correct RNA folding, which in turn reduces the extent of primer extension in the in vitro RdRp system (13; T. Panavas and P. D. Nagy, unpublished).
The heterologous sequence used in this work consists of negative-strand MDV sequence and a 3" anchor sequence derived from TBSV(+) (Fig. 7). This anchor sequence was found previously to reduce the level of RNA synthesis from MDV sequences with the tombusvirus RdRps (Panavas et al., unpublished). Accordingly, the heterologous sequence alone supported RNA synthesis only at a very low level in all three in vitro TCV systems tested here (Fig. 7, lane Anc-MDV). By adding the 11-nt minimal promoter for positive-strand synthesis (i.e., the 3"-proximal sequence [13]) derived from the minus-strand satC to the 3" end of Anc-MDV, the extent of de novo initiation was increased 12-, 143-, and 25-fold for p88, p88C, and the plant TCV RdRp, respectively (see construct cTCV, Fig. 7).
The proper and efficient recognition of the 3"-proximal sequence of satC(−) suggests that all three in vitro systems can recognize the TCV promoter sequence in a similar manner. The higher level of increase for the p88C preparation, which has 10-fold higher activity in RNA synthesis for satC(−) templates (Fig. 4) than p88 or the plant TCV RdRp (not shown), may be the result of the presence of a larger fraction of active RdRp molecules in the p88C preparation (see Fig. 4).
To test whether the in vitro systems can recognize heterologous promoter sequences, we included cPR21, cPR11, and gPR sequences, which are derived from the related TBSV. cPR21 and cPR11 represent the minimal promoters for plus-strand (constructs cPR21 and cPR11, Fig. 7), while gPR represents the “core” promoter sequence for minus-strand synthesis for TBSV (Panavas et al., submitted). These experiments demonstrated efficient de novo initiation for the three constructs in all three in vitro systems (Fig. 7, lanes cPR21, cPR11, and gPR). Addition of artificial AU-rich and GC-rich sequences (constructs A/U and G/C, Fig. 7) to the 3" end of the Anc-MDV sequence resulted in reduction in RNA synthesis (Fig. 7, lanes A/U and G/C) compared to the level obtained with cTCV. The above experiments demonstrated that the promoter selectivity of all three preparations is comparable, based on the tested promoter sequences.
Template competition experiments.
To further study the role of sequences in template selection by p88 and p88C, we used template competition experiments, which may be more similar to in vivo conditions, where the viral replicase can encounter different templates and promoters and must choose among them. The template RNA was cPR11 (shown in Fig. 7), which consists of Anc-MDV at the 5" end and the TBSV-derived 11-nt core plus-strand synthesis promoter at the 3" end.
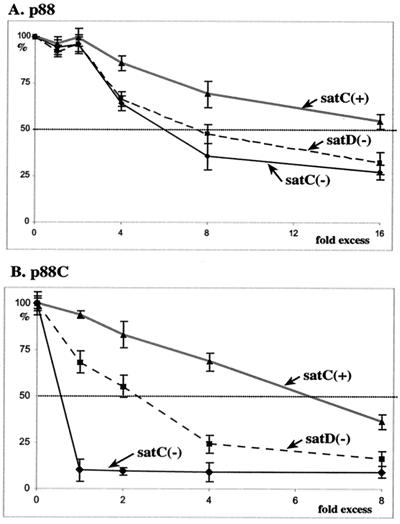
cPR11 was recognized efficiently by both p88 and p88C when present as a single template in the in vitro assay (Fig. 7). Three different competitor RNAs were used in these experiments, including satC(−), satD(−), and satC(+), which are excellent, good, and poor templates for the TCV RdRp, respectively (Fig. 6). Compared to the template RNA, the above competitor RNAs give different-sized RdRp products to allow separation of template and competitor-derived products.
By applying the same amount of template RNA while increasing the amount of the competitor RNA in the RdRp assays, we calculated the 50% inhibitory concentration (IC50) for the above templates. The IC50 determines the amount of competitor RNA needed to reduce de novo initiation from the given template RNA to 50% of the level obtained in the absence of the competitor (modified from references 9 and 33). The data obtained in the competition experiments revealed that satC(−) was the most competitive, and satD(−) showed a moderate level of competitiveness, while satC(+) was the least competitive in both the p88 and p88C in vitro assays (Fig. 8). The IC50s for the three competitors, however, were markedly different in the p88 and the p88C assay systems, with lower levels of competitors competing more efficiently in the p88C assays (Fig. 8).
FIG. 8.
Determination of IC50s for satC(+), satC(−), and satD(−) in RdRp reactions containing p88 (A) or p88C (B). The amount of radiolabeled, template-sized RdRp products synthesized on cPR11 templates (Fig. 7) was measured in the absence (100%) or in the presence of a competitor RNA. The three competitor RNAs, satC(+), satC(−), and satD(−), were used separately. The relative amount of competitor (in comparison with the constant amount of template RNA) is shown below the graph. Panels A and B show the results obtained with recombinant p88 and p88C, respectively.
The reason for the observed differences in template competition between p88 and p88C is not known. We speculate that p88 and p88C may differ in the kinetics of cRNA synthesis (i.e., how quickly the RdRp is released from the template after termination) or need different amounts of time for RNA binding, both of which can affect the chance for the repeated use of the RdRps.
RNA binding by p88 and p88C.
Gel mobility shift assays were used to characterize the ability of p88, p88C, and the plant TCV RdRp to bind RNA. p88 and the plant TCV RdRp were found to bind efficiently to the minus-strand satD (Fig. 9A). Binding of p88C to the minus-strand satD was also significant, although the pattern of the shift was different from that observed with p88 and the plant TCV RdRp (Fig. 9A). For example, in addition to the fully shifted band (which is the major band obtained with p88 and the plant TCV RdRp), a fraction of RNAs were only partially shifted by binding to p88C. This suggests that not only p88 but also p88C contains an RNA binding domain. Future experiments will map the RNA binding domain(s) in these proteins.
RNA binding by p88 was also tested in competition experiments in which increasing amounts of unlabeled competitor RNAs, either satC(+) or tRNA, were used in addition to a constant amount of labeled satC(+) template (Fig. 9B). This experiment demonstrated that while satC(+) was a good competitor, tRNA was a poor competitor (Fig. 9B and C).
DISCUSSION
Viral RdRps purified from infected plant cells are multisubunit enzymes (5, 6). Comparison of activities associated with the plant-derived viral RdRps and the single-subunit RdRps obtained from heterologous systems should help define functions for the subunits. We undertook these studies using TCV, for which the plant-derived RdRp is available (39). This preparation contains a small amount of p88 and excess amount of p28 and some host components (39; Nagy, not shown). We have shown in this paper that the heterologously expressed TCV p88 protein, in the absence of free p28 and eukaryotic host factors, has RdRp activity. In addition, the N-terminally truncated p88, designated p88C, which lacks the p28 overlapping domain (Fig. 1), not only is an active RdRp but also has 10-fold-higher activity than the full-length p88 when applied in the same amount (not shown). The approximately 60-kDa p88C is one of the smallest RdRps, similar in size to polymerase 3D of poliovirus (3). In contrast to polymerase 3D, p88C can initiate cRNA synthesis de novo (see below).
Comparison of template recognition and use by the E. coli-expressed p88 and p88C with that of the well-characterized plant TCV RdRp (39) revealed surprising similarities among the single-unit p88 and p88C and the multisubunit plant TCV RdRp preparations. The similarities include (i) the ability to initiate de novo synthesis on both plus- and minus-strand templates (this was confirmed indirectly by showing the nuclease-resistant nature of the template-sized products in Fig. 3), (ii) the ability to extend on primers (self-priming), (iii) the ability to use the minus-strand templates more efficiently than the plus-strand templates, and (iv) the ability to use the TCV-associated satC and satD as well as the related TBSV-derived DI-72 templates more efficiently than the heterologous MDV RNA (Fig. 6) or tRNA (not shown).
It is possible that free p88, which dissociated from other subunits that are normally part of the replicase, is present in our plant TCV RdRp preparation. The presence of free and active p88 in our preparation would explain the similar results obtained with p88 obtained from E. coli and the plant TCV RdRp preparation. This explanation, however, is unlikely, since the plant TCV RdRp was purified as a large complex (>500,000 Da). Further experiments will be conducted to address this possibility.
Comparison of promoter recognition by p88 and p88C with the plant TCV RdRp revealed that all three RdRps recognized efficiently and correctly the 3"-proximal minimal positive-strand initiation promoter of satC (Fig. 7) defined previously by Guan et al. (13). In addition, the minimal positive-strand initiation promoter for the related TBSV was also recognized by all three RdRps as efficiently as the corresponding TCV promoter. In contrast, the unrelated Anc-MDV template and a template carrying artificial GC-rich sequences at the 3" end were recognized poorly by all three RdRps. These data suggest that these RdRps have the ability to discriminate against some templates at comparable levels. The discrimination against some host RNAs may be manifested by their lack of binding to the RdRp, since tRNA was found to be a poor competitor in RdRp binding (Fig. 9) and is not used as a template for cRNA synthesis by p88 and p88C (not shown). Overall, it is surprising that no significant difference in template recognition was found among these preparations (Fig. 6). This suggests that the minimal p88C RdRp has many of the features characteristic of the multisubunit plant TCV RdRp preparation in vitro. This will make the small, single-component p88C an attractive system for future in vitro studies.
The most significant difference between p88C and either p88 or the plant TCV RdRp was the enhanced ability of p88C to generate a primer extension (self-primed) product that is facilitated by the presence of the motif 1 hairpin replication enhancer (29). It was found that ≈70-fold more primer extension product than the de novo-initiated product was obtained with p88C after 60 min of incubation. The corresponding values for p88 and the plant TCV RdRp were only 21- and 13-fold, respectively. Since p88C lacks the p28 region, while it is present in both p88 and the plant TCV RdRp, it is possible that the role of the overlapping region (or, in the case of the plant TCV RdRp, free p28 may also play a role) is to inhibit the ability of the RdRp to use (self-)primers. Since self-priming is probably an in vitro artifact, it is possible that free p28 or the overlapping region of p88 may be involved in reduction of incorrectly initiated products or in stimulating correctly initiated products. Further experiments will be needed to dissect the role of the free p28 and the overlapping region of p88 in TCV replication.
Kinetic studies with the three RdRp preparations demonstrated that RNA synthesis is relatively rapid in these systems. It took 30 s to obtain detectable amounts of the self-primed product (product M in Fig. 5) in all three systems. In contrast, 5 min were needed in all three systems to detect the template-sized de novo-initiated products (product D in Fig. 5). This observation suggests that initiation of RNA synthesis by self-priming at the motif 1 hairpin replication enhancer is a faster process than de novo initiation at the 3"-terminal promoter. Also, the increasing amounts of RdRp products during prolonged incubations suggest, although they do not prove, that the RdRp molecules are reused sequentially several times during the reaction. This also suggests that these RdRp preparations are stable during prolonged incubations.
Acknowledgments
The first two authors contributed equally to this work.
We thank J. Shaw and T. Panavas for critical reading of the manuscript and very helpful suggestions. cDNA clones of satC, satD, and MDV were generous gifts from Anne Simon, while DI-72 was from Andy White.
This work was supported by the University of Kentucky and by the NSF (MCB0078152).
Footnotes
Publication no. 01-12-115 of the Kentucky Agricultural Experiment Station
REFERENCES
- 1.Ago, H., T. Adachi, A. Yoshida, M. Yamamoto, N. Habuka, K. Yatsunami, and M. Miyano. 1999. Crystal structure of the RNA-dependent RNA polymerase of hepatitis C virus. Structure Folding Design 7:1417-1426. [DOI] [PubMed] [Google Scholar]
- 2.Axelrod, V. D., E. Brown, C. Priano, and D. R. Mills. 1991. Coliphage Q beta RNA replication: RNA catalytic for single-strand release. Virology 184:595-608. [DOI] [PubMed] [Google Scholar]
- 3.Barton, D. J., B. J. Morasco, and J. B. Flanegan. 1996. Assays for poliovirus polymerase, 3DPol, and authentic RNA replication in HeLa S10 extracts. Methods Enzymol. 275:35-57. [DOI] [PubMed] [Google Scholar]
- 4.Blumenthal, T., and G. G. Carmichael. 1979. RNA replication: function and structure of Qβ-replicase. Annu. Rev. Biochem. 48:525-548. [DOI] [PubMed] [Google Scholar]
- 5.Buck, K. W. 1996. Comparison of the replication of positive-stranded RNA viruses of plants and animals. Adv. Virus Res. 47:159-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buck, K. W. 1999. Replication of tobacco mosaic virus RNA. Philos. Trans. R. Soc. Lond. B Biol. Sci. 354:613-627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butcher, S. J., J. M. Grimes, E. V. Makeyev, D. H. Bamford, and D. I. Stuart. 2001. A mechanism for initiating RNA-dependent RNA polymerization. Nature 410:235-240. [DOI] [PubMed] [Google Scholar]
- 7a.Carrington, J. C., L. A. Heaton, D. Zuidema, B. I. Hillman, and T. J. Morris. 1989. The genome structure of turnip crinkle virus. Virology 170:219-226. [DOI] [PubMed] [Google Scholar]
- 8.Cascone, P. J., T. F. Haydar, and A. E. Simon. 1993. Sequences and structures required for recombination between virus-associated RNAs. Science 260:801-805. [DOI] [PubMed] [Google Scholar]
- 9.Chapman, M. R., and C. C. Kao. 1999. A minimal RNA promoter for minus-strand RNA synthesis by the brome mosaic virus polymerase complex. J. Mol. Biol. 286:709-720. [DOI] [PubMed] [Google Scholar]
- 10.Deiman, B. A., K. Seron, E. M. Jaspars, and C. W. Pleij. 1997. Efficient transcription of the tRNA-like structure of turnip yellow mosaic virus by a template-dependent and specific viral RNA polymerase obtained by a new procedure. J. Virol. Methods 64:181-195. [DOI] [PubMed] [Google Scholar]
- 11.Deiman, B. A., P. W. Verlaan, and C. W. Pleij. 2000. In vitro transcription by the turnip yellow mosaic virus RNA polymerase: a comparison with the alfalfa mosaic virus and brome mosaic virus replicases. J. Virol. 74:264-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11a.Dreher, T. W. 1999. Functions of the 3"-untranslated regions of positive strand RNA viral genomes. Annu. Rev. Phytopathol. 37:151-174. [DOI] [PubMed] [Google Scholar]
- 12.Ferrari, E., J. Wright-Minogue, J. W. Fang, B. M. Baroudy, J. Y. Lau, and Z. Hong. 1999. Characterization of soluble hepatitis C virus RNA-dependent RNA polymerase expressed in Escherichia coli. J. Virol. 73:1649-1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guan, H., C. Song, and A. E. Simon. 1997. RNA promoters located on (−)-strands of a subviral RNA associated with turnip crinkle virus. RNA 3:1401-1412. [PMC free article] [PubMed] [Google Scholar]
- 14.Hansen, J. L., A. M. Long, and S. C. Schultz. 1997. Structure of the RNA-dependent RNA polymerase of poliovirus. Structure 5:1109-1122. [DOI] [PubMed] [Google Scholar]
- 15.Hayes, R. J., and K. W. Buck. 1990. Complete replication of a eukaryotic virus RNA in vitro by a purified RNA-dependent RNA polymerase. Cell 63:363-368. [DOI] [PubMed] [Google Scholar]
- 16.Hong, Y., and A. G. Hunt. 1996. RNA polymerase activity catalyzed by a potyvirus-encoded RNA-dependent RNA polymerase. Virology 226:146-151. [DOI] [PubMed] [Google Scholar]
- 17.Kao, C. C., and J. H. Sun. 1996. Initiation of minus-strand RNA synthesis by the brome mosaic virus RNA-dependent RNA polymerase: use of oligoribonucleotide primers. J. Virol. 70:6826-6830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kao, C. C., X. Yang, A. Kline, Q. M. Wang, D. Barket, and B. A. Heinz. 2000. Template requirements for RNA synthesis by a recombinant hepatitis C virus RNA-dependent RNA polymerase. J. Virol. 74:11121-11128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lai, M. M. 1998. Cellular factors in the transcription and replication of viral RNA genomes: a parallel to DNA-dependent RNA transcription. Virology 244:1-12. [DOI] [PubMed] [Google Scholar]
- 20.Lesburg, C. A., M. B. Cable, E. Ferrari, Z. Hong, A. F. Mannarino, and P. C. Weber. 1999. Crystal structure of the RNA-dependent RNA polymerase from hepatitis C virus reveals a fully encircled active site. Nat. Struct. Biol. 6:937-943. [DOI] [PubMed] [Google Scholar]
- 21.Li, Y. I., Y. M. Cheng, Y. L. Huang, C. H. Tsai, Y. H. Hsu, and M. Meng. 1998. Identification and characterization of the Escherichia coli-expressed RNA-dependent RNA polymerase of bamboo mosaic virus. J. Virol. 72:10093-10099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lohmann, V., F. Korner, U. Herian, and R. Bartenschlager. 1997. Biochemical properties of hepatitis C virus NS5B RNA-dependent RNA polymerase and identification of amino acid sequence motifs essential for enzymatic activity. J. Virol. 71:8416-8428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luo, G., R. K. Hamatake, D. M. Mathis, J. Racela, K. L. Rigat, J. Lemm, and R. J. Colonno. 2000. De novo initiation of RNA synthesis by the RNA-dependent RNA polymerase (NS5B) of hepatitis C virus. J. Virol. 74:851-863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nagy, P. D., C. D. Carpenter, and A. E. Simon. 1997. A novel 3"-end repair mechanism in an RNA virus. Proc. Natl. Acad. Sci. USA 94:1113-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagy, P. D., and A. E. Simon. 1997. New insights into the mechanisms of RNA recombination. Virology 235:1-9. [DOI] [PubMed] [Google Scholar]
- 26.Nagy, P. D., and A. E. Simon. 1998. In vitro characterization of late steps of RNA recombination in turnip crinkle virus. II. The role of the priming stem and flanking sequences. Virology 249:393-405. [DOI] [PubMed] [Google Scholar]
- 27.Nagy, P. D., and A. E. Simon. 1998. In vitro characterization of late steps of RNA recombination in turnip crinkle virus. I. Role of motif 1 hairpin structure. Virology 249:379-392. [DOI] [PubMed] [Google Scholar]
- 28.Nagy, P. D., C. Zhang, and A. E. Simon. 1998. Dissecting RNA recombination in vitro: role of RNA sequences and the viral replicase. EMBO J. 17:2392-2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagy, P. D., J. Pogany, and A. E. Simon. 1999. RNA elements required for RNA recombination function as replication enhancers in vitro and in vivo in a plus-strand RNA virus. EMBO J. 18:5653-5665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagy, P. D., and J. Pogany. 2000. Partial purification and characterization of Cucumber necrosis virus and tomato bushy stunt virus RNA-dependent RNA polymerases: similarities and differences in template usage between tombusvirus and carmovirus RNA-dependent RNA polymerases. Virology 276:279-288. [DOI] [PubMed] [Google Scholar]
- 31.O'Reilly, E. K., and C. C. Kao. 1998. Analysis of RNA-dependent RNA polymerase structure and function as guided by known polymerase structures and computer predictions of secondary structure. Virology 252:287-303. [DOI] [PubMed] [Google Scholar]
- 32.Osman, T. A., and K. W. Buck. 1996. Complete replication in vitro of tobacco mosaic virus RNA by a template-dependent, membrane-bound RNA polymerase. J. Virol. 70:6227-6234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Osman, T. A., C. L. Hemenway, and K. W. Buck. 2000. Role of the 3" tRNA-like structure in tobacco mosaic virus minus-strand RNA synthesis by the viral RNA-dependent RNA polymerase in vitro. J. Virol. 74:11671-11680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Plante, C. A., K. H. Kim, N. Pillai-Nair, T. A. Osman, K. W. Buck, and C. L. Hemenway. 2000. Soluble, template-dependent extracts from Nicotiana benthamiana plants infected with potato virus X transcribe both plus- and minus-strand RNA templates. Virology 275:444-451. [DOI] [PubMed] [Google Scholar]
- 35.Quadt, R., and E. M. Jaspars. 1990. Purification and characterization of brome mosaic virus RNA-dependent RNA polymerase. Virology 178:189-194. [DOI] [PubMed] [Google Scholar]
- 36.Quadt, R., H. J. Rosdorff, T. W. Hunt, and E. M. Jaspars. 1991. Analysis of the protein composition of alfalfa mosaic virus RNA-dependent RNA polymerase. Virology 182:309-315. [DOI] [PubMed] [Google Scholar]
- 36a.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 37.Simon, A. E. 1999. Replication, recombination, and symptom-modulation properties of the satellite RNAs of turnip crinkle virus. Curr. Top. Microbiol. Immunol. 239:19-36. [DOI] [PubMed] [Google Scholar]
- 38.Singh, R. N., and T. W. Dreher. 1997. Turnip yellow mosaic virus RNA-dependent RNA polymerase: initiation of minus strand synthesis in vitro. Virology 233:430-439. [DOI] [PubMed] [Google Scholar]
- 39.Song, C., and A. E. Simon. 1994. RNA-dependent RNA polymerase from plants infected with turnip crinkle virus can transcribe (+)- and (−)-strands of virus-associated RNAs. Proc. Natl. Acad. Sci. USA 91:8792-8796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Song, C., and A. E. Simon. 1995. Requirement of a 3"-terminal stem-loop in in vitro transcription by an RNA-dependent RNA polymerase. J. Mol. Biol. 254:6-14. [DOI] [PubMed] [Google Scholar]
- 41.Song, C., and A. E. Simon. 1995. Synthesis of novel products in vitro by an RNA-dependent RNA polymerase. J. Virol. 69:4020-4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.White, K. A., and T. J. Morris. 1994. Recombination between defective tombusvirus RNAs generates functional hybrid genomes. Proc. Natl. Acad. Sci. USA 91:3642-3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.White, K. A., J. M. Skuzeski, W. Li, N. Wei, and T. J. Morris. 1995. Immunodetection, expression strategy and complementation of turnip crinkle virus p28 and p88 replication components. Virology 211:525-534. [DOI] [PubMed] [Google Scholar]
- 44.Yoshinari, S., P. D. Nagy, A. E. Simon, and T. W. Dreher. 2000. CCA initiation boxes without unique promoter elements support in vitro transcription by three viral RNA-dependent RNA polymerases. RNA 6:698-707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhong, W., A. S. Uss, E. Ferrari, J. Y. Lau, and Z. Hong. 2000. De novo initiation of RNA synthesis by hepatitis C virus nonstructural protein 5B polymerase. J. Virol. 74:2017-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]