Abstract
The ability of recombinant rhesus interleukin-12 (rMamu-IL-12) administration during acute simian immunodeficiency virus SIVmac251 infection to influence the quality of the antiviral immune responses was assessed in rhesus macaques. Group I (n = 4) was the virus-only control group. Group II and III received a conditioning regimen of rMamu-IL-12 (10 and 20 μg/kg, respectively, subcutaneously [s.c.]) on days −2 and 0. Thereafter, group II received 2 μg of IL-12 per kg and group III received 10 μg/kg s.c. twice a week for 8 weeks. On day 0 all animals were infected with SIVmac251 intravenously. While all four group I animals and three of four group II animals died by 8 and 10 months post infection (p.i.), all four group III animals remained alive for >20 months p.i. The higher IL-12 dose led to lower plasma viral loads and markedly lower peripheral blood mononuclear cell and lymph node proviral DNA loads. During the acute viremia phase, the high-IL-12-dose monkeys showed an increase in CD3− CD8α/α+ and CD3+ CD8 α/α+ cells and, unlike the control and low-IL-12-dose animals, did not demonstrate an increase in CD4+ CD45RA+ CD62L+ naive cells. The high-IL-12-dose animals also demonstrated that both CD8α/α+ and CD8α/β+ cells produced antiviral factors early p.i., whereas only CD8α/β+ cells retained this function late p.i. Long-term survival correlated with sustained high levels of SIV gag/pol and SIV env cytotoxic T lymphocytes and retention of high memory responses against nominal antigens. This is the first study to demonstrate the capacity of IL-12 to significantly protect macaques from SIV-induced disease, and it provides a useful model to more precisely identify correlates of virus-specific disease-protective responses.
Immunosuppression is one of the hallmarks of both human immunodeficiency virus type 1 (HIV-1) infection and experimental simian immunodeficiency virus (SIV) infection of macaque monkeys (17, 30, 40, 47). Prominent among the constellation of immune abnormalities that is induced by these lentiviral infections is the profound suppressive effect on cell-mediated immunity (CMI) (6, 37). One of the major factors essential to the generation and orchestration of CMI is the heterodimeric cytokine interleukin-12 (IL-12), synthesized primarily by monocytes and macrophages (57, 59). A number of studies have documented not only marked decreases in the levels of IL-12 synthesized following cell activation but also a marked decrease in the response to IL-12 in vitro by peripheral blood mononuclear cells (PBMCs) from HIV-1-infected patients and SIV-infected rhesus macaques (9, 11, 46, 48, 62, 65). While the precise mechanisms involved in the down regulation of IL-12 synthesis by HIV-1 remain to be established, it is known that HIV-1 infection interferes with the regulatory pathways involved in the gene transcription of this cytokine (44). IL-12 not only influences the generation of CMI but also has been shown to influence innate immune effector mechanisms (20, 34, 58, 71) whose down regulation contributes to the development of AIDS and opportunistic infections (28, 41). These findings have prompted a number of studies aimed at the exogenous replenishment of this important cytokine either by using recombinant IL-12 alone or as an adjuvant coadministered with and/or incorporated into a DNA-based vaccine (7, 23, 25, 29, 35, 55, 61, 65; K. Okuda, T. Tsuji, K.-Q. Xin, Y. Asakura, T. Kaneko, S. Kawamoto, J. Fukushima, H. Bukawa, and K. Hamajima, Conference on Advances in AIDS Vaccine Development, abstr. 190, 1996).
Initial studies of the use of this cytokine in patients with malignancies and in HIV-1-infected patients showed significant toxicity and led to skepticism with regards to its use in the clinical setting (2, 29, 43). However, not much was known at that time about the in vivo biological effects of this cytokine in humans and, in particular, its effect on immunocompromised individuals, such as HIV-1-infected patients and patients with malignancies. In addition, there was limited knowledge regarding the requirements for dosing and route of administration (21, 22, 39) that were later shown to be critical. Clearly, the nonhuman primate model of AIDS provides an important tool to define in more detail the optimal dose and route of administration of this cytokine and to assess it objectively as an adjuvant. The knowledge that virus-specific cytotoxic T lymphocytes (CTLs) play an important role in containing HIV and SIV replication in vivo combined with the fact that IL-12 has been shown to markedly augment CTL function in PBMCs from HIV-1-infected patients in vitro (13, 16, 36, 48, 70, 73) and SIV infection in vivo (65, 69) provides a compelling rationale for additional studies aimed at defining a more optimal in vivo use of this cytokine. The fact that IL-12 has been shown to activate and augment both innate and acquired immune responses and severely limit infection of rodents and monkeys challenged with a number of intracellular pathogens (1, 7, 15, 26, 27, 38, 72) provides additional support for this view. In addition, while it is the general consensus that a preferential Th1 cytokine milieu is important for mediating and maintaining an effective antiviral response against HIV and SIV, formal in vivo proof of this concept has been lacking. It was thus reasoned that administration of IL-12 prior to and during early acute SIV infection should provide the appropriate Th1 prototype cytokine environment that would promote bridging of both the innate and acquired immune responses and would thus provide a means to test this hypothesis. Results of these studies suggest that IL-12 is indeed a potent cytokine and if administered properly leads to lower viral loads, lower proviral DNA levels, and, importantly, a marked increase in the period of disease-free survival.
MATERIALS AND METHODS
Animals.
A group of 12 healthy adult rhesus macaques weighing 5 to 9 kg at the initiation of the study were utilized and housed at the Yerkes Regional Primate Research Center, Emory University. All animals were maintained in accordance with the instructions of the Committee on the Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources, National Research Council (51).
Immunizations.
After baseline bleeding, all 12 animals were immunized and given boosters twice with influenza virus (Flu), keyhole limpet hemocyanin (KLH), and tetanus toxoid (TT); routes and doses are described elsewhere (3). The monkeys were then each bled at 2 and 4 weeks after the final booster dose, and their humoral and cellular responses against Flu, KLH, and TT were determined and monitored at intervals specified in Results.
SIV infection.
All animals were given 200 50% tissue culture infective doses of pathogenic uncloned SIVmac251 intravenously 4 weeks following the last immunization. Prior to infection, the monkeys were divided into three groups (four animals/group). Group I animals served as Flu/KLH/TT-immunized and SIV-infected controls, group II animals were the low-IL-12-dose test group, and group III animals were the high-IL-12-dose test group.
IL-12 administration.
Initial studies carried out by several laboratories, including ours, have clearly demonstrated that administration of one or several doses of recombinant human IL-12 induces the rapid development of readily detectable but variable levels of human IL-12 neutralizing-antibody responses (27, 50, 69). These data precluded the repeated use of this xenogeneic protein in monkeys and required that we clone and prepare rhesus macaque-derived IL-12. The cloning, sequencing, and preparation of recombinant rhesus IL-12 (rMamu-IL-12) has been described by our laboratory elsewhere (63). In addition, preliminary studies showed that the gamma interferon (IFN-γ) response to repeated administration of IL-12 at 2-day intervals was indistinguishable from a single-dose response in terms of serum IFN-γ protein and PBMC IFN-γ message (27). In marked contrast, biweekly administration of 5 μg of rMamu-IL-12 per kg to asymptomatic rhesus macaques chronically infected with SIVmac239 was found to induce sustained high levels of plasma IFN-γ (65). The magnitudes of these IFN-γ levels were equivalent to those of IFN-γ levels induced by a 10- or 20-μg/kg dose, although individual differences in responses were noted and the minimum amount of IL-12 required to induce detectable levels of IFN-γ in plasma was 1 μg/kg (153 ± 46 pg of IFN-γ/ml) (27). In addition, preliminary studies utilizing staggered dosing schedules (i.e., daily, every 2, 3, 4, or 5 days, or weekly; 10 μg of rMamu-IL-12/kg administered subcutaneously [s.c.]) showed that there appears to be a “refractory period” following administration during which PBMCs from monkeys would not respond to IL-12 in vitro. This refractory period was determined to be between 3 to 4 days at this dose. Based on these initial findings, a dosing schedule was devised in which group II received a loading dose of 10 μg of IL-12 per kg on days −2 and 0 and 2 μg/kg s.c. twice a week thereafter for a total of 8 weeks (low-dose group) and group III animals received a loading dose of 20 μg/kg on days −2 and 0 and 10 μg/kg s.c. twice a week thereafter for 8 weeks (high-dose group). Day 0 was the day of infection with SIVmac251. The purpose of using low- and high-dose schedules was to determine whether the lower doses of IL-12, which would be sufficient to induce readily detectable but low levels of IFN-γ with potential reduced toxicity, would be as effective in influencing the quality of virus-specific immune responses as the higher doses of IL-12.
Plasma viral load measurements.
Viral RNA was purified from 0.25 ml of plasma by utilizing the viral RNAid isolation kit (Bio-101, Carlsbad, Calif.). Viral RNA was quantitated with our laboratory-established quantitative competitive reverse transcription-PCR, which included standard gag primers as previously described (49). The lower limit of sensitivity for this plasma viral RNA quantitation was determined to be 500 copies per ml.
Proviral DNA load.
PBMCs were isolated from peripheral blood samples of each of the SIV-infected monkeys by standard Ficoll-Hypaque gradient centrifugation techniques. Lymph node cells (LNC) were isolated from lymph node biopsy samples obtained from each monkey at 3 weeks postinfection (p.i.) (sample 1), 24 weeks p.i. (sample 2), and 58 weeks p.i. (sample 3) if the monkeys were alive or at autopsy (sample 2 or 3). Cellular DNA was isolated from 106 PBMCs or LNC with a commercially available DNA purification kit (Qiagen, Valencia, Calif.), and proviral DNA quantitation was performed as previously described (49). The sensitivity of the assay was determined to be one copy per 105 cells, and results are expressed as the number of DNA copies per 105 cells.
Determination of SIV Env-, SIV Gag/Pol-, and Flu-specific pCTLs.
Herpesvirus papio-transformed lymphocytoblast cell lines were established from each of the 12 monkeys prior to SIV infection. Sequential PBMC aliquots obtained from each monkey were assayed for the presence and frequencies of SIV Gag/Pol-, SIV Env-, and Flu matrix protein (Flu-MP)-specific CTL activity by utilizing autologous vaccinia virus expressing SIV Gag/Pol (Vacc-SIV Gag/Pol), Vacc-SIV Env, Vacc-Flu-MP, and, as a control, wild-type vaccinia virus-infected target lymphocyte/blasts and a classical limiting dilution assay described previously (3, 66, 67). The frequencies of CTL precursors (pCTLs) and confidence intervals (CI) were derived using the Jackknife program and expressed as numbers of pCTLs per 106 effector cells (14, 56). Data are net pCTL values, where the values for SIV Gag/Pol, SIV Env, and Flu-MP have been adjusted for the values obtained against targets pulsed with wild-type vaccinia virus by the same effector cell population. An assay was considered valid only if the mean spontaneous release for a given target cell was less than 20% of the maximal release for the same target cell population. The values obtained with the wild-type vaccinia virus varied but were in all cases <120 pCTLs/106 effector cells.
Antigen-specific proliferation assays.
PBMCs were washed and adjusted to 4 × 106 cells/ml in complete medium, and 0.1 ml was dispensed into each well of a 96-well microtiter plate. To triplicate wells was added either medium (control) or 0.1, 1, or 5 μg of KLH or the immunodominant promiscuous P2 peptide of TT (residues 830 to 843; QYIKANSKFIGITE). Cultures were incubated at 37°C in a 7% CO2 humidified atmosphere for 72 h, pulsed with 1 μCi of [methyl-3H]thymidine (specific activity of 2 Ci/mmol), and harvested after 16 h. The mean uptake of [3H]thymidine was calculated. The mean radioactivity (counts per minute) of the antigen-containing cultures was divided by the mean radioactivity of the medium-only control cultures to derive a proliferation index.
MHC typing.
DNA isolated from the PBMCs from each of the 12 monkeys in the present study were subjected to rhesus macaque major histocompatibility complex (MHC) Mamu-A01 typing as previously described (33).
Evaluation of CD8+-T-cell-synthesized soluble antiviral mediators.
Secretion of soluble antiviral activity was evaluated on sequential PBMC cultures from the animals by using a dual Transwell chamber assay (54). Briefly, PBMCs were stimulated with 2 μg of phytohemagglutinin (PHA; Difco Laboratories, Detroit, Mich.) per ml for 3 days before being added to the top chamber of a Transwell culture system (Costar, Corning, N.Y.) containing CD4+ PBMCs from a single SIV-naive donor freshly infected with SIVmac239 (MOI of 0.1) in vitro in the bottom chamber. All cultures were set up in duplicate as a single experiment to avoid interassay variability. Culture supernatant fluids collected on days 5, 10, 15, 20, and 25 were analyzed for p27 content. Controls consisted of cultures containing PHA-stimulated PBMCs from SIV-naive monkeys or Transwells with no effector cells in the top chamber. PBMCs to be evaluated for antiviral factors were unfractionated or were depleted of either CD8α+ cells (to deplete both NK and traditional cytotoxic CD8+ T cells) or CD8β+ cells (to deplete only the traditional CD8+ T cells) by using immunomagnetic beads prior to PHA stimulation. The monoclonal antibodies used to deplete CD8α- and CD8β-expressing cells included clones SK1/SK2 and 2ST8.5H7, respectively. Aliquots of the depleted cells were examined by flow cytometry with the same antibodies in addition to anti-mouse immunoglobulin to define the efficiency of depletion. Results from these studies showed that the depletion efficiency ranged from 92 to 98%.
Flow microfluorometric analyses.
The monoclonal antibodies utilized for flow microfluorometric analyses included biotin-conjugated anti-CD3 (clone FN18; Biosource Int., Camarillo, Calif.); phycoerythrin (PE)-conjugated anti-CD4, fluorescein isothiocyanate (FITC)-conjugated or peridinin chlorophyll protein (PerCP)-conjugated anti-CD8α, FITC-anti-CD45RA, FITC-anti-CD56, PE-anti-CD49d, PE-anti-CD62L, and PerCP-conjugated streptavidin (all from Becton Dickinson, San Jose, Calif.); FITC-anti-CD3 and CyChrome-anti-CD56 (Pharmingen, San Diego, Calif.); and PE-anti-CD8β (Immunotech, Hialeah, Fla.). A minimum of 10,000 cells were analyzed and coupled with the white blood cell count of the blood sample, and Bioquest software was utilized to derive frequency and absolute values of each subset.
Tetramer analyses.
The Mamu-A01+ animals (one each in group I and group II and all four in group III) were monitored for the frequencies of CD8β+ T cells that were positive for the SIV Gag p11CM tetramer. The frequency of cells staining positive for tetramer was determined by evaluating a minimum of 100,000 and in most cases 200,000 PBMCs and using CellQuest software.
IFN-γ EIA.
In efforts to monitor the effect of rMamu-IL-12 in vivo, levels of IFN-γ in plasma were quantitated with an IFN-γ enzyme immunoassay (EIA) as previously described (27, 65).
Statistical analyses.
Statistically significant differences in the viral loads, time of survival, antiviral effects mediated by CD8α- versus CD8β-expressing cells, and pCTL values were each determined by one-way analysis of variance (ANOVA) with contrast by using the Analyze-It statistical package for Microsoft Excel. Student's one-tailed t test was utilized in the analysis of selected sets of data as outlined below.
RESULTS
Effect of rMamu-IL-12 administration on survival.
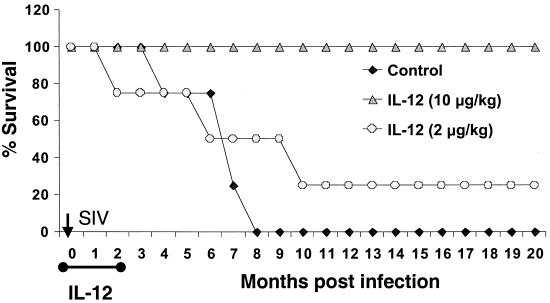
As depicted in Fig. 1, IL-12 administration during the acute phase of SIV infection markedly enhanced survival. While all four group I (virus-only control) monkeys died by week 35 (8 months) p.i. and three of four group II (low-IL-12-dose) monkeys died by week 42 p.i. (10 months), one of four group II and all four of the group III monkeys remained alive and healthy up to the present (90 weeks p.i.). The survival time of the group III monkeys was found to be significantly longer (P < 0.004 at Tukey's 95% CI) than that of group I and II monkeys. Deaths in the group I monkeys were due to Pneumocystis carinii pneumonia (n = 2), cholecystitis and liver fibrosis (n = 1), and non-P. carinii pneumonia (n = 1). Deaths in group II animals were due to cholecystitis and wasting (n = 3).
FIG. 1.
Survival in months of the three groups of monkeys. Group I (n = 4) was SIVmac251-infected control monkeys, group II (n = 4) was SIV infected and given a loading dose of 10 μg of rMamu-IL-12 per kg s.c. on days −2 and 0 followed by 2 μg/kg s.c. twice a week for 8 weeks (low dose), and group III was SIV infected and given a loading dose of 20 μg of rMamu-IL-12 per kg s.c. on days −2 and 0 and 10 μg/kg s.c. twice a week for 8 weeks (high dose). Survival of group III monkeys was statistically significant (P < 0.001 by ANOVA at Tukey's 95% CI).
Effect of rMamu-IL-12 on plasma viral loads.
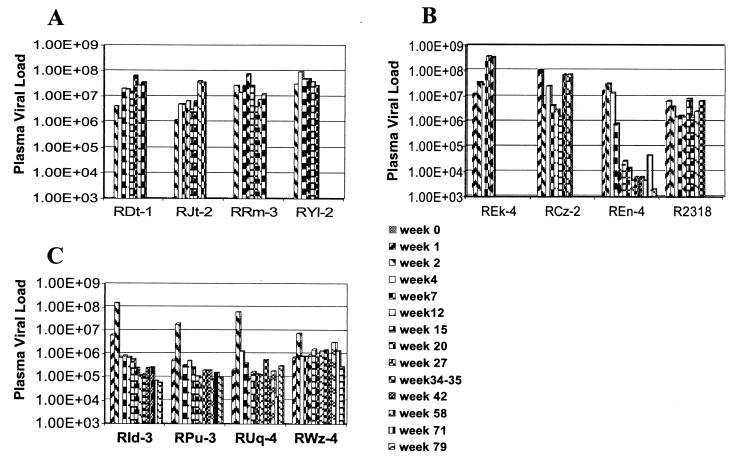
Plasma viral loads were monitored in samples from all 12 monkeys over the entire period of the study or until the death of the respective animals. As seen in Fig. 2, while all 12 monkeys showed variable plasma viral loads but comparable peaks of acute plasma viremia, the plasma viral load set points in the group III monkeys appeared to be generally lower than those in the group I and II monkeys. Thus, while all four group I monkeys and three of four group II monkeys showed high (>106 copies/ml) or even increasing plasma viral loads until death, one of the four group II monkey (REn-4) continues to show decreased plasma viral loads. Each of the four monkeys in group III showed a markedly low viral load set point compared to the acute peak of viremia. These set points appeared to be relatively stable over time and were between 105 and 106 copies/ml, which were significantly lower than those in the plasma of group I monkeys (P = 0.004 at Tukey 95% CI) as determined by one-way ANOVA with contrasts to normally distributed (by the Shapiro-Wilk W normality test) log-transformed measurements. It is noteworthy that the peak of acute viral replication may have been delayed in monkey REn-4 and the group III monkeys, although the frequency of sampling during the acute infection period in each of the three groups in this study does not provide conclusive evidence for this delay. Nevertheless, the plasma viral load findings show that the IL-12 dosing regimen in the group III monkeys clearly influenced the rate of viral replication.
FIG. 2.
Sequential analysis of plasma viral loads in group I (A), group II (B), and group III (C) monkeys. Statistical analysis of the viral loads showed significantly lower (P < 0.004 by ANOVA at Tukey's 95% CI) plasma viral loads in group III monkeys than in group I monkeys.
Effect of rMamu-IL-12 on PBMC and LNC proviral DNA loads.
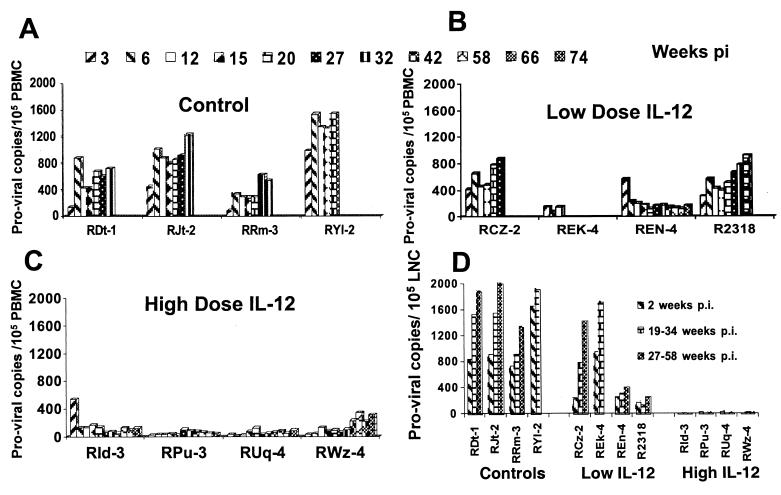
PBMC samples from all 12 monkeys were monitored for levels of SIV proviral DNA at various times p.i. As seen in Fig. 3A to C, proviral DNA loads in PBMCs from the group III monkeys were markedly lower than those in PBMCs from the control animals throughout the studies. The differences in mean proviral DNA loads between group III and group I animals were statistically significant at all time points (range, P < 0.00003 for week 27 to P < 0.04 for week 15; for the other weeks, P values were in this range). In addition, lymph node biopsy samples were also obtained from the monkeys at 3 weeks p.i. (acute viremia stage, sample 1), at 24 weeks or at autopsy (sample 2), and at 58 weeks or at autopsy (sample 3). As seen in Fig. 3D, there was an even more marked difference in proviral DNA levels in the lymph nodes of the group III monkeys as early as week 3 (6 to 36 copies/105 LNC) compared to group I and II monkeys (738 to 1,668 copies/105 LNC in group I and 178 to 968 copies/105 LNC in group II). The differences between the proviral DNA levels in the LNC from group III and those in the LNC from group I were highly significant (P < 0.008, 0.01, and 0.0006 for samples 1, 2, and 3, respectively). These data indicate that the higher dose of IL-12 had a more marked effect on levels of proviral DNA in PBMCs and an even more significant effect on LNC proviral loads than on the plasma viremia levels. To confirm that the analysis was indeed being performed on mononuclear cells isolated from the lymph nodes, an aliquot of the cell preparation was subjected to fluorescence-activated cell sorting analysis utilizing standard T-cell (CD3)-, B-cell (CD20)-, and NK cell (CD8α/α)-specific monoclonal antibody reagents. This analysis confirmed the fact that the cell suspension was >99.0% lymphoid (data not shown).
FIG. 3.
Proviral DNA loads in PBMC samples and LNC from biopsies or at autopsy from the three groups of monkeys. The sensitivity of the assay was 1 copy per 105 cells. (A) PBMC samples from group I at weeks 3 to 32, except monkey RYl-2, which died at week 20. (B) PBMC samples from group II monkeys obtained up to weeks 27, 12, 74, and 42. Monkey REn-4 is still alive. (C) PBMC samples from group III monkeys up to week 74 (still alive). (D) LNC from each of the three groups of monkeys obtained from biopsy samples at 3 weeks p.i. (sample 1); at 24 weeks p.i. or at autopsy (REk-4 at 12 weeks, RYl-2 at 20 weeks) (sample 2); and at 58 weeks p.i. or at autopsy for monkeys RDt-1, RJt-2, RRm-3, RCz-2, and R2318 (weeks 27 to 35) (sample 3). Statistical analysis of PBMC proviral DNA levels was performed by comparison of viral loads on individual days p.i. from each group of monkeys. Data for group III monkeys were statistically highly significantly different from those for group I monkeys for all data points (range, P < 0.00003 at week 27 to P < 0.04 at week 15). Similarly, the proviral DNA levels in LNC samples of the group III monkeys were also statistically significantly different from those for group I (P < 0.008, 0.01, and 0.0006 for samples 1, 2, and 3, respectively).
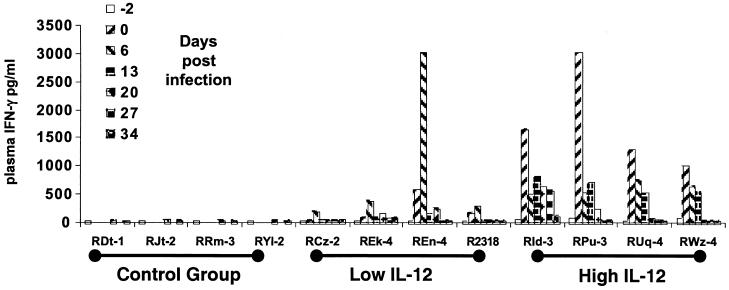
Effect of IL-12 on the induction of plasma IFN-γ levels.
As illustrated in Fig. 4, each of the group III animals responded to the initial high-dose regimen of IL-12 with vigorous IFN-γ responses. While three of four group II animals also exhibited detectable serum IFN-γ levels, monkeys in this group showed markedly lower response and delayed kinetics with the exception of monkey REn-4, which exhibited IFN-γ level profiles similar to those of the group III monkeys. The reason for the increased sensitivity of this one monkey is not clear at present. However, the rapid and vigorous IFN-γ response to the IL-12 administration correlated with time of survival p.i. and decreased plasma viral loads. In contrast, untreated group I animals showed essentially little or no detectable serum IFN-γ levels during the acute infection phase. It is interesting that the high initial levels of IFN-γ were followed by low levels despite continued biweekly administration of IL-12. This is probably a reflection of the refractory period (although due to the dosing schedule, this refractoriness was not as marked) observed during treatment with this immune modulator. It remains to be established whether this refractory period is restricted to IFN-γ synthesis or whether it includes other pleiotropic functions mediated by IL-12.
FIG. 4.
Plasma IFN-γ levels in samples from the three groups of monkeys collected at day −2 (prior to IL-12 administration) and at various times thereafter.
Effect of IL-12 administration on SIV Gag/Pol- and SIV Env-specific pCTL values.
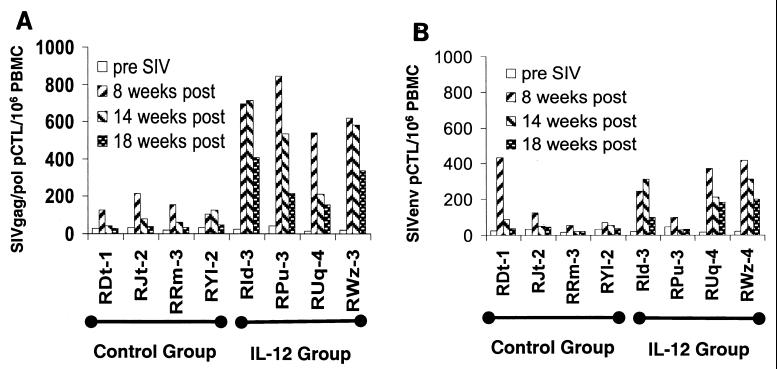
PBMCs from all 12 monkeys were assessed for SIV Gag/Pol- and Env-specific pCTL frequencies at 8, 14, and 18 weeks p.i. For brevity, only the data from groups I and III are presented (Fig. 5A and B). While low but readily detectable levels of SIV Gag/Pol- and SIV Env-specific pCTLs were seen in the group I animals at 8 weeks, these values were markedly reduced in samples obtained at 14 and 18 weeks p.i. In contrast, PBMC samples from each of the group III monkeys showed a marked augmentation of SIV Gag/Pol-specific and, to a lower level, SIV Env-specific pCTL levels at 8 weeks p.i.; levels gradually decreased but remained significantly higher than the maximum levels in samples from the group I animals (P < 0.0001). These data, in general, support the view that viral load appears to be inversely correlated with the ability of the SIV-infected monkeys to generate and maintain a SIV-specific CTL response. The CTL response measured was determined to be MHC class I restricted, since the addition of anti-MHC class I monomorphic antibody, unlike addition of anti-MHC class II antibody, inhibited the CTL function monitored in the experiments performed on the 8-week sample by >78% (data not shown). This was deemed important, since IL-12 has also been noted to augment NK cell function.
FIG. 5.
PBMC samples from groups I and III obtained prior to SIV infection and at 8, 14, and 18 weeks p.i. were assayed for levels of SIV Gag/Pol-specific (A) and SIV Env-specific (B) pCTLs as described in Materials and Methods. The pCTL values from group III versus group I at 8, 14, and 18 weeks are statistically significant (P < 0.0001) by the t test.
Effect of IL-12 administration on Flu-, TT-, and KLH-specific humoral and cellular memory responses.
Our laboratory has previously shown that SIV infection rapidly leads to marked qualitative and quantitative diminution in the Flu-, TT-, and KLH-specific memory T-cell responses (27, 65, 68) in rhesus macaques. As described above, all 12 monkeys were immunized and given boosters with live attenuated Flu, TT, and KLH prior to IL-12 administration and SIV infection. For brevity, only the responses in group I and group III monkeys are described herein.
Flu memory response.
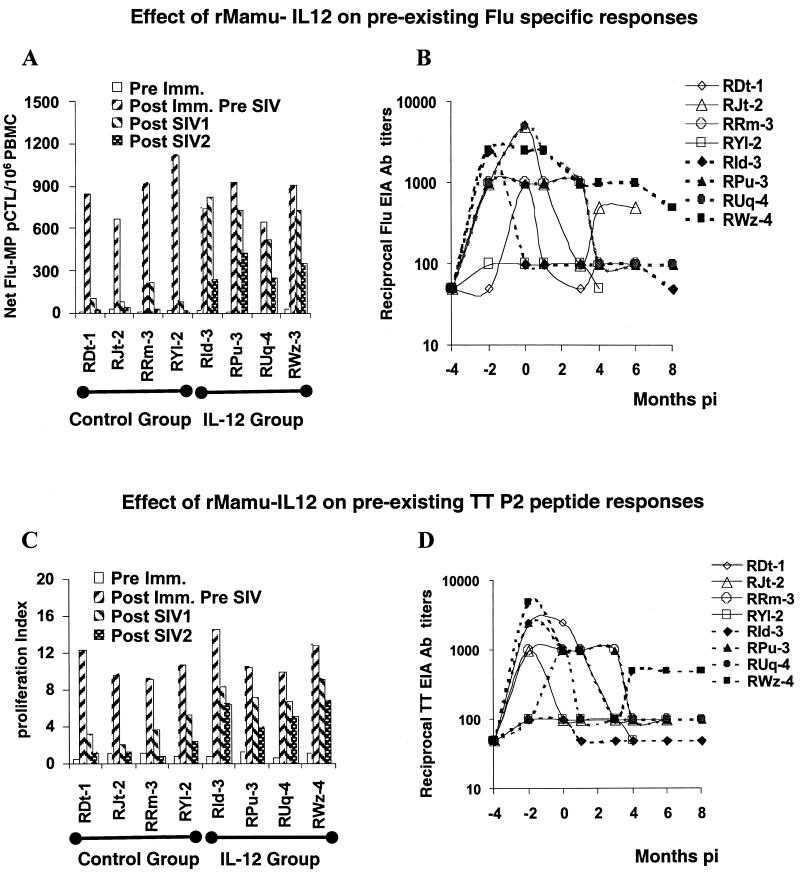
Following the last booster Flu immunization and prior to IL-12 administration and SIV infection, each of the group I and group III monkeys mounted significant (>650 net pCTLs/106 PBMCs) Flu-MP-specific CTL responses using autologous Vacc-Flu-MP-infected target cells (Fig. 6A). However, soon after SIV infection (by week 12 p.i.), there was a marked reduction and disappearance of Flu-MP pCTLs in each of the group I control monkeys. In contrast, all four group III monkeys initially retained equivalent Flu-MP-specific pCTL frequencies and still exhibited significant frequencies of Flu-MP-specific pCTLs at 20 weeks p.i. (Fig. 6A). A similar analysis of Flu-MP-specific pCTL function in the PBMC samples obtained at week 20 showed basically the same trend (Fig. 6A). These decreases were actually similar to decreases our lab has observed in control non-SIV-infected monkeys and thus a reflection of a normal decay in the antigen-specific memory CD8+-CTL response in rhesus macaques (data not shown). Thus, while no major differences were noted in the Flu-MP-specific CTL values in samples from the group I and III monkeys prior to SIV infection, the IL-12 administration appears to have preserved this immune mechanism following SIV infection. These differences were not correlated with titers of Flu-specific antibodies (Fig. 6B), which appeared to be similar among group I and III monkeys.
FIG. 6.
Cellular and humoral responses to Flu (A and B) and TT (C and D). PBMC and serum samples obtained from group I and group III prior to immunization with Flu, TT, or KLH and SIV infection (Pre Imm. or −4), after Flu, TT, and KLH immunization but prior to SIV infection (Post Imm. Pre SIV or −2 and 0) and at 12 and 20 weeks after SIV infection (Post SIV1 and Post SIV2, respectively) (A and C) or at the indicated months p.i. (B and D) were assayed for Flu-MP-specific net pCTL values (A) and their proliferative response to the P2 TT peptide (C) as described in Materials and Methods. The values of pCTLs for group III and the proliferative response to P2 TT peptide at week 12 p.i. compared to the values for group I are statistically significant, with a P value of 0.0001 (t test). Differences in humoral responses to Flu or TT between IL-12-conditioned (filled symbols) and control monkeys (open symbols) were not significant.
TT- and KLH-specific proliferative responses.
Similar to the analysis of Flu-specific responses, the pre-SIV post-TT-immunization proliferative responses of group I and group III monkeys were comparable in magnitude (Fig. 6C). Shortly after SIV infection (week 12) and at 22 weeks p.i., once again, while there was a marked diminution in the proliferative response of PBMC samples from group I animals against the P2 TT peptide (Fig. 6C), PBMC samples from the group III animals retained significantly higher TT-specific memory T-cell responses. The proliferative responses against KLH showed the same profile as the response to TT (data not shown). The proliferative responses were blocked (>80%) with the addition of anti-MHC class II but not anti-MHC class I antisera, indicating that the response was likely a reflection of CD4+ T cells (data not shown). Similar to the humoral responses to Flu, titers of antibody to TT (and KLH [data not shown]) did not show any significant difference between group I and III animals (Fig. 6D), with the decrease in titers corresponding to the natural decay of such responses in non-SIV-infected monkeys (data not shown). Thus, SIV does not appear to significantly impair humoral responses to antigens encountered prior to SIV infection. In addition, the data presented herein indicate that IL-12 administration did not seem to significantly influence overall titers of antibody to antigens administered 4 weeks before the cytokine therapy was initiated.
Effect of IL-12 administration on CD8+ antiviral factor synthesis.
Besides antigen-specific humoral and cell-mediated SIV-specific immune responses, it has been known for some time that noncytolytic antiviral factors are also synthesized by CD8+ T cells, which regulate viral replication (42, 54). These include the chemokines MIP-1α, MIP-1β, and RANTES, potentially the α/β interferons, IL-16, and likely a number of other, as-yet-undefined molecules (4, 5, 19, 24). Production and/or release of such effector molecules are induced soon after SIV infection and have been shown to be predominantly synthesized by conventional CD8α/β+ T cells but not CD8α/α+ NK cells and, as such, are reasoned to be part of the acquired immune effector mechanisms (60, 64). Since IL-12 has been shown to promote Th1 immune responses and CD8+ effector T cells, PBMCs from these animals were assayed for the production of such effector molecules. Unfractionated PBMCs and aliquots of CD8α- and CD8β-depleted PBMCs from each of the 12 SIV-infected monkeys were assayed early p.i. (week 4 to 6) and at week 30 p.i. (from the surviving monkeys). Unfractionated PBMCs from non-SIV-infected control animals demonstrate <5% inhibition of viral replication as shown by our laboratory previously (62). As seen in Table 1, unfractionated PBMC samples from all 12 SIV-infected monkeys at week 6 clearly inhibited viral replication. However, the inhibition induced by PBMC samples from group III monkeys (mean ± standard deviation, 83.88% ± 4.45%) was significantly (P < 0.001) higher than the inhibition induced by PBMC samples from group I (63.99% ± 5.05%) and group II (65.69% ± 9.84%) monkeys. Of further interest was the finding that whereas the entire viral replication-inhibitory activity could be removed by the depletion of either the CD8α+ or CD8β+ effectors from group I and group II monkeys (REn-4 excepted), depletion of CD8α+ PBMCs was far more effective than depletion of CD8β+ PBMCs from the group III monkeys (for values for CD8α versus CD8β, P < 0.0001) to enhance viral replication. It should be noted that monkey REn-4 in group II was the lone long-term survivor of the group II animals and appeared to show a differential pattern of CD8α- and CD8β-mediated inhibition similar to that of the group III monkeys. Similar analyses of CD8+-cell function in samples from the surviving monkeys at week 30 showed somewhat different results. Thus, while unfractionated PBMCs from all three surviving monkeys in group I and one of the two monkeys from group II showed weak CD8+-T-cell-mediated viral replication inhibitory activity (correlating with their deteriorating clinical status) compared to samples from the same monkeys obtained at 6 weeks p.i., the long-term survivor monkey REn-4 of group II and all four group III monkeys appeared to retain CD8+ antiviral effector function at levels essentially similar to that at 6 weeks p.i. There was, however, a difference in the relative contribution of the CD8 lineage producing the soluble inhibitors. Thus, while removal of CD8α+ PBMCs of these four group III monkeys and monkey REn-4 was more effective in depletion of CD8 antiviral activity in the week 6 samples, the week 30 samples did not show any marked difference with the removal of either CD8α+ or CD8β+ PBMCs, suggesting that CD8α/α+ PBMCs had lost the ability to secrete soluble antiviral replication inhibitors over time. These data suggest that IL-12 administration appears to enhance CD8+ antiviral activity and that such cytokine administration also induces antiviral activity in both CD8α/α+ and CD8α/β+ cells, at least early p.i.
TABLE 1.
Noncytolytic CD8+-cell-mediated inhibition of viral replication
| Group and monkey | p27 level (ng/ml) in cell type ata:
|
||||||
|---|---|---|---|---|---|---|---|
| 6 wk p.i.
|
30 wk p.i.
|
||||||
| Unfractionated | CD8β depleted | CD8α depleted | Unfractionated | CD8β depleted | CD8α depleted | ||
| Group I | |||||||
| RDt-1 | 43.4 | 138.9 | 136.2 | 126.3 | 151.9 | 157.4 | |
| RJt-2 | 56.3 | 142.2 | 140.9 | 99.8 | 134.4 | 138.6 | |
| RRm-3 | 62.9 | 145.2 | 139.6 | 129.3 | 157.3 | 148.9 | |
| RYl-2 | 59.2 | 150.2 | 148.3 | ND | ND | ND | |
| Group II | |||||||
| RCz-2 | 54.3 | 128.9 | 125.4 | ND | ND | ND | |
| REk-4 | 61.2 | 142.7 | 138.4 | ND | ND | ND | |
| REn-4 | 31.4 | 112.1 | 143.8b | 35.4 | 144.9 | 141.2 | |
| R2318 | 65.7 | 150.6 | 145.2 | 88.9 | 140.4 | 132.9 | |
| Group III | |||||||
| RId-3 | 21.1 | 118.6 | 144.5b | 30.6 | 135.9 | 132.3 | |
| RPu-3 | 18.8 | 122.3 | 147.9b | 27.8 | 138.3 | 126.4 | |
| RUq-4 | 34.4 | 109.5 | 150.2b | 41.9 | 154.2 | 150.9 | |
| RWz-4 | 25.6 | 115.3 | 147.8b | 26.3 | 144.4 | 132.9 | |
ND, not determined.
Statistically significantly different (P < 0.0001) from value for CD8β-depleted cells at the same time point.
Effect of IL-12 on the frequency of lymphoid-cell subsets.
In brief, while all 12 animals in the three groups showed an initial decline during the acute viremia period in the frequency and absolute numbers of CD3+ CD4+ T cells (week 2 p.i.) followed by an increase in the frequency and absolute numbers of CD3+ CD8β+ T cells (week 4 to 6), this decline (an approximately 20 to 38% decrease in frequency and absolute number of CD4+ T cells) was followed by a significant period of stabilized CD4+-cell counts in all animals. This was followed eventually by a decline first in the CD4+ T cells and then CD3+ CD8β+ T cells and an increase in the frequency of CD20+ cells (B cells) just prior to development of clinical AIDS in the virus control and three of four group II animals. While a considerable amount of data was collected, for brevity, only the highlights are presented herein. Several interesting trends were observed. First, while there was a marked sustained increase in the frequencies of CD4+ CD45RA+ CD62L+ cells (CD4+ naive cells) in the virus control monkeys after SIV infection, this increase was not noted in samples from the group III monkeys, particularly during the acute viremia phase (Table 2). There was some increase in this subset in the group II monkeys following SIV infection and in the group III monkeys at 1 year p.i. Second, while all 12 monkeys showed an increase in the frequencies and absolute numbers of CD3− CD8α+ CD8β− cells (NK cells) during the acute viremia period (Table 3), this increase was much more marked in the group III monkeys (P < 0.01). A similar set of data was also noted with the use of a HLA-E tetramer reagent that appears to bind to killer inhibitory receptors expressed by NK cells (8) (data not shown). This increase in the frequency of NK cells following IL-12 administration has been noted in other species (20, 34, 65). Third, there was also an increase in the frequency and absolute numbers of CD3+ CD8α+ CD8β− during the acute viremia period in the group III monkeys (Table 4) but not the other two groups. This initial increase was followed by values similar to those for samples obtained prior to SIV infection and IL-12 administration.
TABLE 2.
Regulation of the frequencies of CD4+ CD45RA+ CD62L+ naive CD4+ T cells by rMamu-IL-12 administration during acute SIV infection
| Group (status) | Monkey | Frequency (%) of naive CD4+ T cellsa
|
|||
|---|---|---|---|---|---|
| Pre-infection | At 2-3 mo p.i. | At 5-6 mo p.i. | At 8-12 mo p.i. | ||
| I (control) | RDt-1 | 8.4 | 22.2b | 17.1b | ND |
| RJt-2 | 3.3 | 16.6b | 38.8b | ND | |
| RRm-3 | 5.8 | 53.1b | 47.3b | 49.5 | |
| RYl-2 | 1.7 | 31.7b | 37.8b | ND | |
| II (low IL-12) | RCz-2 | 7.6 | 18.7 | 19.8 | ND |
| REk-4 | 3.6 | 14.6 | ND | ND | |
| REn-4 | 0.8 | 1.5 | 3.5 | 4.9 | |
| R2318 | 2.5 | 12.5 | 17.1 | ND | |
| III (high IL-12) | RId-3 | 4.2 | 5.4 | 8.8 | 10.3 |
| RPu-3 | 4.0 | 6.8 | 8.1 | 12.3 | |
| RUq-4 | 2.7 | 3.0 | 2.1 | 9.4 | |
| RWz-4 | 2.3 | 3.3 | 8.1 | 16.4 | |
ND, not determined.
Highly statistically significantly different from the values for the high-dose group at the same time point (P < 0.0001).
TABLE 3.
Changes in the frequency of CD3− CD8α+ CD8β− cells in the three groups of monkeys after SIV infection
| Group (status) | Monkey | Frequencya
|
|||
|---|---|---|---|---|---|
| Pre-infection | At 2-3 mo p.i. | At 5-6 mo p.i. | At 12 mo p.i. | ||
| I (control) | RDt-1 | 7.0 | 10.8 | 6.5 | ND |
| RJt-2 | 10.7 | 10.1 | 5.2 | ND | |
| RRm-3 | 4.7 | 5.4 | 9.0 | ND | |
| RYl-2 | 5.2 | 3.2 | 1.6 | ND | |
| II (low IL-12) | RCz-2 | 1.8 | 6.6 | 5.1 | ND |
| REk-4 | 4.8 | 8.4 | ND | ND | |
| REn-4 | 3.7 | 9.5 | 7.2 | 5.9 | |
| R2318 | 3.2 | 9.2 | 7.1 | ND | |
| III (high IL-12) | RId-3 | 3.8 | 17.8b | 16.4 | 12.1 |
| RPu-3 | 6.6 | 19.0b | 4.7 | 7.9 | |
| RUq-4 | 4.8 | 11.5b | 6.0 | 5.0 | |
| RWz-4 | 5.8 | 19.5b | 11.1 | 9.6 | |
Absolute numbers (ranges) for the control, low-IL-12, and high-IL-12 groups were 146 to 712, 126 to 384, and 112 to 138 prior to SIV infection, 136 to 523, 382 to 674, and 746 to 848 at 2 to 3 months p.i., and 112 to 338, 337 to 566, and 321 to 731 at 5 to 6 months p.i. ND, not determined.
Values for the high-IL-12-dose monkeys (mean, 16.95 ± 3.7) are statistically significantly different (P < 0.01) from the values for the control monkeys (mean, 7.375 ± 3.67).
TABLE 4.
Changes in the frequency of CD3+ CD8α+ CD8β− cells in the PBMC of the three groups of monkeys after SIV infection
| Group (status) | Monkey | Frequency
|
|||
|---|---|---|---|---|---|
| Pre-infection | At 2-3 mo p.i. | At 5-6 mo p.i. | At 8-12 mo p.i. | ||
| I (control) | RDt-1 | 5.4 | 6.6 | 3.1 | NDb |
| RJt-2 | 3.9 | 3.1 | 4.3 | ND | |
| RRm-3 | 3.7 | 3.7 | 5.6 | 4.9 | |
| RYl-2 | 1.9 | 2.2 | 0.6 | ND | |
| II (low IL-12) | RCz-2 | 4.4 | 5.6 | 5.1 | ND |
| REk-4 | 6.4 | 7.3 | ND | ND | |
| REn-4 | 2.4 | 2.3 | 3.0 | 1.8 | |
| R2318 | 5.5 | 5.3 | 4.7 | ND | |
| III (high IL-12) | RId-3 | 1.8 | 7.5a | 2.5 | 2.1 |
| RPu-3 | 3.2 | 6.4a | 3.4 | 6.5 | |
| RUq-4 | 1.9 | 19.3a | 3.7 | 4.3 | |
| RWz-4 | 2.9 | 11.6a | 2.0 | 1.8 | |
Mean values were statistically higher than values for the two other groups of monkeys (P < 0.001).
ND, not determined.
Antigen-specific tetramer analysis.
The rMamu-IL-12 studies were initiated prior to our ability to MHC type rhesus macaques, and thus, all 12 monkeys were typed for Mamu-A01 after SIV infection. Interestingly, six of the 12 monkeys included in this study were Mamu-A01+. These six included all four group III monkeys, one group II monkey (REn-4), and one virus control monkey (RJt-2). This allowed the evaluation of frequencies of Mamu-A01 SIV Gag p11CM tetramer-positive cells in these six animals before and after infection (Table 5). While all four group III monkeys showed a marked sustained increase in the frequency of Gag p11CM tetramer-positive cells, it is unclear at present whether the IL-12 administration significantly enhanced such responses, since monkey RJt-2 showed levels of p11CM tetramer-positive cells similar to those of two of the IL-12-treated monkeys. In addition, REn-4 showed the most prominent Gag p11CM response despite receiving only low doses of IL-12 (Table 5), suggesting that the levels of these responses may be specific for each individual monkey.
TABLE 5.
Frequency of pIICM peptide Mamu-A01 tetramer binding CD8+ T cells in the PBMC of the three groups of monkeys after SIVmac251 infection
| Group (status) | Monkey | Frequencya
|
|||
|---|---|---|---|---|---|
| Pre-infection | At 2-3 mo p.i. | At 5-6 mo p.i. | At 12 mo p.i. | ||
| I (control) | RJt-2 | 0.08 | 1.18 | 0.75 | ND |
| II (low IL-12) | REn-4 | 0.09 | 6.15 | 9.98 | 12.2 |
| III (high IL-12) | RId-3 | 0.01 | 1.3 | 1.2 | 3.2 |
| RPu-3 | 0.01 | 3.0 | 5.1 | 4.8 | |
| RUq-4 | 0.04 | 1.2 | 1.5 | 3.9 | |
| RWz-4 | 0.02 | 2.4 | 2.8 | 3.7 | |
ND, not determined.
DISCUSSION
The results of the present study demonstrate that rMamu-IL-12 at the doses and regimen used here is tolerated well and leads to the induction of a spectrum of innate and acquired SIV-specific immune responses that contribute to prolonged disease-free survival, lower plasma viremia, and lower proviral DNA levels in both the PBMCs and lymph nodes. Since a number of previous studies have reported a variety of abnormalities that are induced by IL-12 administration in vivo, in the present study, the monkeys were carefully monitored for physical signs of toxicity and by hematologic and urine analysis. No detectable anemia, leukopenia, lymphopenia, thrombocytopenia, hyperglycemia, hypoalbuminemia, or abnormal transaminases or bilirubin were noted in either group II or group III monkeys during and up to 4 weeks following IL-12 administration (data not shown). The exquisite sensitivity of humans to IL-12 toxicity could be due to the differences that have been described for the levels of IL-12 expressed by gut lymphoid tissues such as Peyer's patches in humans and rodents (45) and presumably nonhuman primates. Thus, while rodents fed antigens orally appear to synthesize predominantly immunosuppressive cytokines such as transforming growth factor β, human Peyer's patch and gut T-cell responses appear to be biased towards a Th1 response associated with higher levels of IL-12 (45). Thus, the beneficial effects of IL-12 demonstrated by the data presented herein should be interpreted and extrapolated to humans with caution, especially with regard to oral tolerance and the role of gut-associated lymphoid tissues (GALT). Other factors could also have contributed to the lack of observed toxicity. Thus, in the present study, rMamu-IL-12 was administered s.c., which may be less toxic than intravenous administration, since the bioavailability of s.c. administered IL-12 appears to be approximately 20% that of IL-12 administered intravenously (43). In addition, a “conditioning” dose of 10 μg/kg was given to the low-dose group and 20 μg/kg was given to the high-dose group at days −2 and 0 prior to SIV infection. Such conditioning doses may also have prevented the occurrence of some of the toxic effects, as has been previously noted in humans and rodents (22, 39, 43). The dosage of rMamu-IL-12 utilized was carefully selected following a series of preliminary studies aimed at defining the optimal dose. Thus, dosing of non-SIV-infected normal monkeys with 10 μg of IL-12 per kg daily, every other day, and to a certain extent every third day led to various periods of refractoriness (little or no plasma IFN-γ), which could explain the failure of previous studies which employed such dosing schemes (data not shown). In the present study, we chose to utilize two different doses, a dose that would induce and maintain maximal IFN-γ levels with a risk of toxicity (10 μg/kg twice a week) and a minimal dose required to induce IFN-γ response which may not have maximal biological effect (2 μg/kg twice a week). We submit that the doses chosen in fact did provide minimal compared with optimal benefit as far as animal survival and viral loads, although individual differences in the capacity to respond to IL-12 were evidenced in the group II animals.
The mechanisms by which chronic IL-12 administration leads to biological refractoriness has been a subject of study by a number of laboratories, including ours. IL-12 mediates its biological effect by signaling through its cognate heterodimeric receptor (IL-12Rβ1/IL-12Rβ2), leading to the activation of the Janus kinase (JAK)-signal transduction and activation of transcription (STAT) pathways. These include the Janus kinase family Jak2 and Tyk2 and several members of the STAT family, including STAT1, STAT4, and STAT5 (52). STAT4 has been shown to play the most critical role, as STAT4 knockout mice demonstrate complete abolition of all major functions attributed to IL-12 (32), including the loss of IFN-γ responses. This finding prompted us to examine the levels of STAT4 mRNA. Samples of PBMC corresponding to time points when high and low plasma IFN-γ were noted (days 2 and 34) (Fig. 4) were subjected to STAT4 mRNA quantitation with STAT4-specific primer pairs and real-time PCR. However, such studies failed to demonstrate any detectable difference in the levels of STAT4 mRNA in these samples. This was difficult to reconcile. Of interest is the recent finding by Wang et al. (67) that such down regulation of IL-12 signaling is not due to a decrease in STAT4 mRNA levels but appears to be secondary to phospho-STAT4 protein degradation, possibly via the proteasome degradation pathway. Studies are currently under way in our lab to confirm the levels of STAT4 protein in the IL-12-permissive and refractory samples from our nonhuman primates.
One of the primary sets of issues arising from the studies outlined above is the mechanisms by which IL-12 administration during the acute viremia period led to the prolongation of survival and relative decreases in plasma viral loads and to a larger extent proviral DNA loads. In other words, what can be learned from the findings with regard to correlates of immunity? We submit that early p.i., the relative increase in the frequencies of CD8α/α+ and CD8α/β+ cells (Tables 3 and 4), the preservation of the CD4+-T-cell profile (i.e., lack of an increase in the CD4+ CD45RA+ CD62L+ subset) (Table 2), the generation of both CD8α/α+- and CD8α/β+-T-cell-secreted soluble antiviral factors (Table 1), and the generation of significant levels of Gag/Pol-specific CTL responses (Fig. 5A) all correlate with increased survival and lower viral loads. It is not possible at present to uncouple any of these findings, and in our view it is likely that several such responses may be required in concert and that each contributes to the increased survival and lower viral loads. In addition, it is likely that the presence of high IL-12 levels during acute viremia may have prevented the development of eventual tolerance mechanisms similar to the reported effect of IL-12 in the gastrointestinal tracts of mice (10).
There are a number of issues that need to be addressed. What is the contribution of the Mamu-A01 allele to the levels of viremia, since all four monkeys in group III and one of four monkeys in group II had lower viral loads and showed disease protection? While the numbers in each group are in fact quite small and the data need to be interpreted with caution, it is noteworthy that the one animal in the control group that had a rapid disease course was Mamu-A01+. In addition, a number of studies with other SIVs administered intravenously to Mamu-A01+ monkeys (such as SIVmac239 [n = 4] or SHIV 89.6p [n = 11] and to a limited extent SIVmac251[n = 4]) failed to show any difference in the viral load profile in these animals. Thus, the Mamu-A01 allele cannot by itself contribute to such a viral load profile, as not all Mamu-A01+ monkeys show low viral loads. Clearly, additional studies utilizing Mamu-A01+ and Mamu-A01− animals are warranted to resolve this issue.
Another issue that needs addressing is the discrepancy in the plasma virus loads and the levels of proviral DNA in the IL-12-treated monkeys. A number of other laboratories have in fact shown a good correlation between proviral loads and plasma viremia levels (12, 18). It is our working hypothesis that IL-12 administration must contribute to the early selective homing of the infected cells in tissues other than regional lymph nodes, such as GALT. Thus, infected GALT cells may have high continuous levels of viral replication, which maintain the plasma viral loads while preventing recirculation of these cells. Since each of these five monkeys (four in group III and one in group II) are still alive, we shall wait and examine the GALT from these animals for levels of viral RNA and DNA at autopsy.
The third issue is the significance of the fact that the CD4+ naive population (CD4+ CD45RA+ CD62L+) increased in group I and three of the four group II monkeys but not in the group III and one of four group II monkeys. Due to a decrease in the absolute number of CD4+ T cells in all 12 animals, this change in the naive population takes on added significance, since an increase in the frequency of naive CD4+ T cells appears to be associated with poor disease prognosis. It has been known for some time that a depletion of the memory CD4+-T-cell subset occurs early after HIV-1 infection, which is associated with loss of helper T-cell function and decreased ability to synthesize IL-2 (13, 36, 48). Although IL-2 is synthesized by both CD45RA+ and CD45RA− populations, it is known that the CD45RA− (in humans, CD45RO+) population synthesizes IL-2 much more rapidly, a reflection of the rapid response required from memory T cells (53). Our data suggest that there is a rapid decrease of memory CD4+ T cells in the group I and three of four group II monkeys. However, there is also a decrease in CD4+ T cells in the group III and the remaining one of four group II monkeys without an accompanying increase in the CD4+ naïve subset. It is difficult at present to address these distinct outcomes, since they could be a reflection of mobilization of lymphoid cell stores, differential homing, enhanced maturation, and differentiation of new CD4+ T cells versus proliferation of memory T cells and selective increased susceptibility of certain CD4+-T-cell subsets to undergo apoptosis. Additional studies such as the examination of T-cell receptor excision circles, the expression of α4β7 (gut homing marker for which no commercially available antibody exists), and Vβ T-cell receptor repertoire distribution may provide some clues to this issue. It is interesting that since the initial depletion of peripheral recirculating CD4+ T cells occurs in both the virus control and the high-IL-12-dose group, these data suggest that this loss is not critical for long-term survival or for inducing and maintaining lower viral loads. However, as suggested by the naive versus memory phenotype, there may be a qualitative difference in the subsets depleted in control monkeys versus IL-12 treated animals.
Fourth, there also appeared to be a population of both CD8α/α+ and CD8α/β+ cells that had the capacity to regulate virus replication selectively present in the PBMCs of the group III and of the one group II monkey but not the group I and three of four group II monkeys (Table 1). This is the first documented finding of a role for nonconventional CD3− CD8+ cells (presumably NK, γ/δ+, or an as-yet-undefined lineage of cells that express CD8α/α) to demonstrate such virus replication control function acquired following IL-12 administration. This is an interesting finding, since such a function was previously ascribed solely to CD8α/β+ T cells (31, 60). This functional capacity to inhibit virus replication by CD3− CD8α/α cells, however, disappeared at later stages of infection. Whether the mechanisms by which this subset mediates its antiviral effect are similar to and/or different from those of conventional CD3 CD8α/β+ T cells is not known at present, and its relevance in the overall antiviral mechanisms is a subject of further study. It would also be of great interest to determine whether the effector mechanisms that regulate virus replication are directed at all the infected cell lineages or specific for either virus-infected CD4+ T cells or macrophage and/or dendritic-cell lineages. Fifth, it is of interest that one of the four animals in group II appears to behave similarly to the four animals in group III. The simplest explanation is that this particular animal is exquisitely sensitive to the effects of IL-12, similar to humans.
It is important to note that all five monkeys that have so far survived demonstrate an inverse correlation between levels of p11CM Gag tetramer binding cells and plasma viral loads. It is currently not known whether the p11CM tetramer response represents a reflection of overall CTL responses or whether the CTL response is by and large restricted to this dominant epitope in Mamu-A01 macaques. The pCTL data in Fig. 5A suggest that the relative levels of p11CM tetramer-positive cells did not directly correlate with the relative Gag/Pol pCTL frequencies observed in these Mamu-A01+ monkeys (Fig. 5A and Table 5). This important issue is currently being examined by using enzyme-linked immunospot assays against SIV-specific peptides spanning SIV Env, Gag, Rev, Tat, Nef, and Vif. Data from such studies may provide a better understanding of the eventual contribution of the breadth of the cellular response to the protection of these animals from disease development. It is noteworthy that monkey REn-4 demonstrates up to 10% or more of the entire CD8+-T-cell pool with specificity for p11CM Gag peptide. However, whereas a high proportion (>90%) of these CD8+ T cells synthesized IFN-γ upon p11CM peptide incubation 12 months p.i., at present (>18 months p.i.), the frequency of PBMCs responding to p11CM stimulation with IFN-γ synthesis appears to be markedly decreased (data not shown). Thus, these cells appear to have gradually lost their function, as recently reported (67). An additional issue that is currently being studied is the role of virus-specific neutralizing antibodies and the relative affinity of such antibodies in controlling the levels of viremia. Crude EIA titers of antibody against SIVmac251 in sera collected at various times p.i. were higher in the group III animals than the group II and group I animals (data not shown).
Finally, do the findings reported herein have clinical significance and can the outcome be improved? It should be kept in mind that this study was essentially initiated to establish proof of principle that indeed IL-12 has the capacity to significantly improve and enhance immune function and hence a protocol was chosen to maximize our ability to document such a function. The fact that such an outcome has been noted prompts the study of its application in monkeys already infected with SIV. We have previously attempted such short-term IL-12 administration in SIV-infected monkeys during the asymptomatic and late AIDS stages (65). While the asymptomatic monkeys did appear to demonstrate almost intact functional response to IL-12 administration, the late-AIDS-stage monkeys did not. The important issue is whether IL-12 would have the beneficial effect reported in the present communication when administered shortly after SIV infection or in the setting of therapy involving structured treatment interruption. In addition, it is important to investigate the use of other cytokines that may increase the pool of virus-specific effector cells in concert with IL-12 and to look for means to keep such a pool alive for prolonged periods of time, such as the intermittent use of IL-2 and IL-15. Clearly, such attempts have to be made in light of the limitations that are currently being observed with highly active antiretroviral therapies in human HIV-1 infection.
Acknowledgments
This work was supported by a grant (NIH1RO1 27057) from the National Institutes of Health.
We are highly grateful to the veterinary and support staff of the Yerkes Regional Primate Research Center and for the help of Nathaniel Chikkala in providing assistance to carry out the studies reported herein. We acknowledge the help and guidance provided by Joe Miller and Mike Hulsey for the tetramer analyses, and we acknowledge the NIH AIDS Research and Reference Reagent Repository and the NIH-supported Tetramer Core Facility for providing reagents critical for the performance of these studies.
REFERENCES
- 1.Ahuja, S. S., R. L. Reddick, N. Sato, E. Montalbo, V. Kostecki, W. Zhao, M. J. Dolan, P. C. Melby, and S. K. Ahuja. 1999. Dendritic cell (DC)-based anti-infective strategies: DCs engineered to secrete IL-12 are a potent vaccine in a murine model of an intracellular infection. J. Immunol. 163:3890-3897. [PubMed] [Google Scholar]
- 2.Anonymous. 1995. Genetics Institute suspends phase II study of rhIL-12. J. Int. Assoc. Physicians AIDS Care 1:34.. [PubMed] [Google Scholar]
- 3.Ansari, A. A., P. Bostik, A. E. Mayne, and F. Villinger. 2001. Failure to expand influenza and tetanus toxoid memory T cells in vitro correlates with disease course in SIV infected rhesus macaques. Cell. Immunol. 210:125-142. [DOI] [PubMed] [Google Scholar]
- 4.Baier, M., A. Werner, N. Bannert, K. Metzner, and R. Kurth. 1995. HIV suppression by interleukin-16. Nature 378:563.. [DOI] [PubMed] [Google Scholar]
- 5.Barker, E., K. N. Bossart, and J. A. Levy. 1998. Primary CD8+ cells from HIV-infected individuals can suppress productive infection of macrophages independent of beta-chemokines. Proc. Natl. Acad. Sci. USA 95:1725-1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bowen, D. L., H. C. Lane, and A. S. Fauci. 1985. Immunopathogenesis of the acquired immunodeficiency syndrome. Ann. Intern. Med. 103:704-709. [DOI] [PubMed] [Google Scholar]
- 7.Boyer, J. D., A. D. Cohen, K. E. Ugen, R. L. Edgeworth, M. Bennett, A. Shah, K. Schumann, B. Nath, A. Javadian, M. L. Bagarazzi, J. Kim, and D. B. Weiner. 2000. Therapeutic immunization of HIV-infected chimpanzees using HIV-1 plasmid antigens and interleukin-12 expressing plasmids. AIDS 14:1515-1522. [DOI] [PubMed] [Google Scholar]
- 8.Braud, V. M., D. S. Allan, C. A. O'Callaghan, K. Soderstrom, A. D'Andrea, G. S. Ogg, S. Lazetic, N. T. Young, J. I. Bell, J. H. Phillips, L. L. Lanier, and A. J. McMichael. 1998. HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C. Nature 391:795-799. [DOI] [PubMed] [Google Scholar]
- 9.Chehimi, J., S. E. Starr, I. Frank, A. D'Andrea, X. Ma, R. R. MacGregor, J. Sennelier, and G. Trinchieri. 1994. Impaired interleukin 12 production in human immunodeficiency virus-infected patients. J. Exp. Med. 179:1361-1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen, Y., K. Song, and S. L. Eck. 2000. An intra-Peyer's patch gene transfer model for studying mucosal tolerance: distinct roles of B7 and IL-12 in mucosal T cell tolerance. J. Immunol. 165:3145-3153. [DOI] [PubMed] [Google Scholar]
- 11.Chougnet, C., T. A. Wynn, M. Clerici, A. L. Landay, H. A. Kessler, J. Rusnak, G. P. Melcher, A. Sher, and G. M. Shearer. 1996. Molecular analysis of decreased interleukin-12 production in persons infected with human immunodeficiency virus. J. Infect. Dis. 174:46-53. [DOI] [PubMed] [Google Scholar]
- 12.Chun, T. W., L. Carruth, D. Finzi, X. Shen, J. A. DiGiuseppe, H. Taylor, M. Hermankova, K. Chadwick, J. Margolick, T. C. Quinn, Y. H. Kuo, R. Brookmeyer, M. A. Zeiger, P. Barditch-Crovo, and R. F. Siliciano. 1997. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature 387:183-188. [DOI] [PubMed] [Google Scholar]
- 13.Clerici, M., D. R. Lucey, J. A. Berzofsky, L. A. Pinto, T. A. Wynn, S. P. Blatt, M. J. Dolan, C. W. Hendrix, S. F. Wolf, and G. M. Shearer. 1993. Restoration of HIV-specific cell-mediated immune responses by interleukin-12 in vitro. Science 262:1721-1724. [DOI] [PubMed] [Google Scholar]
- 14.Does, R. J., L. W. Strijbosch, and W. Albers. 1988. Using Jackknife methods for estimating the parameter in dilution series. Biometrics 44:1093-1102. [PubMed] [Google Scholar]
- 15.Doolan, D. L., and S. L. Hoffman. 1999. IL-12 and NK cells are required for antigen-specific adaptive immunity against malaria initiated by CD8+ T cells in the Plasmodium yoelii model. J. Immunol. 163:884-892. [PubMed] [Google Scholar]
- 16.Dybul, M., G. Mercier, M. Belson, C. W. Hallahan, S. Liu, C. Perry, B. Herpin, L. Ehler, R. T. Davey, J. A. Metcalf, J. M. Mican, R. A. Seder, and A. S. Fauci. 2000. CD40 ligand trimer and IL-12 enhance peripheral blood mononuclear cells and CD4+ T cell proliferation and production of IFN-gamma in response to p24 antigen in HIV-infected individuals: potential contribution of anergy to HIV-specific unresponsiveness. J. Immunol. 165:1685-1691. [DOI] [PubMed] [Google Scholar]
- 17.Fauci, A. S. 1993. Multifactorial nature of human immunodeficiency virus disease: implications for therapy. Science 262:1011-1018. [DOI] [PubMed] [Google Scholar]
- 18.Fujii, T., Y. Kato, N. Takata, and A. Kimura. 2001. Change in plasma viral load, and viral DNA and mRNA burdens, in peripheral blood mononuclear cells from patients infected with HIV-1. J. Infect. 42:20-26. [DOI] [PubMed] [Google Scholar]
- 19.Garzino-Demo, A., A. L. DeVico, K. E. Conant, and R. C. Gallo. 2000. The role of chemokines in human immunodeficiency virus infection. Immunol. Rev. 177:79-87. [DOI] [PubMed] [Google Scholar]
- 20.Gately, M. K., A. G. Wolitzky, P. M. Quinn, and R. Chizzonite. 1992. Regulation of human cytolytic lymphocyte responses by interleukin-12. Cell. Immunol. 143:127-142. [DOI] [PubMed] [Google Scholar]
- 21.Gherardi, M. M., J. C. Ramirez, and M. Esteban. 2000. Interleukin-12 (IL-12) enhancement of the cellular immune response against human immunodeficiency virus type 1 Env antigen in a DNA prime/vaccinia virus boost vaccine regimen is time and dose dependent: suppressive effects of IL-12 boost are mediated by nitric oxide. J. Virol. 74:6278-6286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gherardi, M. M., J. C. Ramirez, and M. Esteban. 2001. Towards a new generation of vaccines: the cytokine IL-12 as an adjuvant to enhance cellular immune responses to pathogens during prime-booster vaccination regimens. Histol. Histopathol. 16:655-667. [DOI] [PubMed] [Google Scholar]
- 23.Gherardi, M. M., J. C. Ramirez, D. Rodriguez, J. R. Rodriguez, G. Sano, F. Zavala, and M. Esteban. 1999. IL-12 delivery from recombinant vaccinia virus attenuates the vector and enhances the cellular immune response against HIV-1 Env in a dose-dependent manner. J. Immunol. 162:6724-6733. [PubMed] [Google Scholar]
- 24.Haas, D. W., J. Lavelle, J. P. Nadler, S. B. Greenberg, P. Frame, N. Mustafa, M. St Clair, R. McKinnis, L. Dix, M. Elkins, and J. Rooney. 2000. A randomized trial of interferon alpha therapy for HIV type 1 infection. AIDS Res. Hum. Retrovir. 16:183-190. [DOI] [PubMed] [Google Scholar]
- 25.Haicheur, N., B. Escudier, T. Dorval, S. Negrier, P. H. De Mulder, J. M. Dupuy, D. Novick, T. Guillot, S. Wolf, P. Pouillart, W. H. Fridman, and E. Tartour. 2000. Cytokines and soluble cytokine receptor induction after IL-12 administration in cancer patients. Clin. Exp. Immunol. 119:28-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamajima, K., J. Fukushima, H. Bukawa, T. Kaneko, T. Tsuji, Y. Asakura, S. Sasaki, K. Q. Xin, and K. Okuda. 1997. Strong augment effect of IL-12 expression plasmid on the induction of HIV-specific cytotoxic T lymphocyte activity by a peptide vaccine candidate. Clin. Immunol. Immunopathol. 83:179-184. [DOI] [PubMed] [Google Scholar]
- 27.Hoffman, S. L., J. M. Crutcher, S. K. Puri, A. A. Ansari, F. Villinger, E. D. Franke, P. P. Singh, F. Finkelman, M. K. Gately, G. P. Dutta, and M. Sedegah. 1997. Sterile protection of monkeys against malaria after administration of interleukin-12. Nat. Med. 3:80-83. [DOI] [PubMed] [Google Scholar]
- 28.Howie, S., R. Ramage, and T. Hewson. 2000. Innate immune system damage in human immunodeficiency virus type 1 infection. Implications for acquired immunity and vaccine design. Am. J. Respir. Crit. Care Med. 162:S141-S145. [DOI] [PubMed] [Google Scholar]
- 29.Jacobson, M. A., D. Hardy, E. Connick, J. Watson, and M. DeBruin. 2000. Phase 1 trial of a single dose of recombinant human interleukin-12 in human immunodeficiency virus-infected patients with 100-500 CD4 cells/μL. J. Infect. Dis. 182:1070-1076. [DOI] [PubMed] [Google Scholar]
- 30.Johnson, P. R., and V. M. Hirsch. 1992. SIV infection of macaques as a model for AIDS pathogenesis. Int. Rev. Immunol. 8:55-63. [DOI] [PubMed] [Google Scholar]
- 31.Kannagi, M., L. V. Chalifoux, C. I. Lord, and N. L. Letvin. 1988. Suppression of simian immunodeficiency virus replication in vitro by CD8+ lymphocytes. J. Immunol. 140:2237-2242. [PubMed] [Google Scholar]
- 32.Kaplan, M. H., Y. L. Sun, T. Hoey, and M. J. Grusby. 1996. Impaired IL-12 responses and enhanced development of Th2 cells in Stat4-deficient mice. Nature 382:174-177. [DOI] [PubMed] [Google Scholar]
- 33.Knapp, L. A., E. Lehmann, M. S. Piekarczyk, J. A. Urvater, and D. I. Watkins. 1997. A high frequency of Mamu-A*01 in the rhesus macaque detected by polymerase chain reaction with sequence-specific primers and direct sequencing. Tissue Antigens 50:657-661. [DOI] [PubMed] [Google Scholar]
- 34.Kobayashi, M., L. Fitz, M. Ryan, R. M. Hewick, S. C. Clark, S. Chan, R. Loudon, F. Sherman, B. Perussia, and G. Trinchieri. 1989. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J. Exp. Med. 170:827-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuwata, T., T. Miura, T. Haga, I. Kozyrev, and M. Hayami. 2000. Construction of chimeric simian and human immunodeficiency viruses that produce interleukin 12. AIDS Res. Hum. Retrovir. 16:465-470. [DOI] [PubMed] [Google Scholar]
- 36.Landay, A. L., M. Clerici, F. Hashemi, H. Kessler, J. A. Berzofsky, and G. M. Shearer. 1996. In vitro restoration of T cell immune function in human immunodeficiency virus-positive persons: effects of interleukin (IL)-12 and anti-IL-10. J. Infect. Dis. 173:1085-1091. [DOI] [PubMed] [Google Scholar]
- 36a.Lane, H. C. 1995. The generation of CD4 T lymphocytes in patients with HIV infection. J. Biol. Regul. Homeost. Agents 9:107-109. [PubMed] [Google Scholar]
- 37.Lane, H. C., and A. S. Fauci. 1985. Immunologic abnormalities in the acquired immunodeficiency syndrome. Annu. Rev. Immunol. 3:477-500. [DOI] [PubMed] [Google Scholar]
- 38.Lee, J. H., G. Teuber, M. von Wagner, W. K. Roth, and S. Zeuzem. 2000. Antiviral effect of human recombinant interleukin-12 in patients infected with hepatitis C virus. J. Med. Virol. 60:264-268. [PubMed] [Google Scholar]
- 39.Leonard, J. P., M. L. Sherman, G. L. Fisher, L. J. Buchanan, G. Larsen, M. B. Atkins, J. A. Sosman, J. P. Dutcher, N. J. Vogelzang, and J. L. Ryan. 1997. Effects of single-dose interleukin-12 exposure on interleukin-12-associated toxicity and interferon-gamma production. Blood 90:2541-2548. [PubMed] [Google Scholar]
- 40.Letvin, N. L., and N. W. King. 1990. Immunologic and pathologic manifestations of the infection of rhesus monkeys with simian immunodeficiency virus of macaques. J. Acquir. Immune Defic. Syndr. 3:1023-1040. [PubMed] [Google Scholar]
- 41.Levy, J. A. 2001. The importance of the innate immune system in controlling HIV infection and disease. Trends Immunol. 22:312-316. [DOI] [PubMed] [Google Scholar]
- 42.Levy, J. A., C. E. Mackewicz, and E. Barker. 1996. Controlling HIV pathogenesis: the role of the noncytotoxic anti-HIV response of CD8+ T cells. Immunol. Today 17:217-224. [DOI] [PubMed] [Google Scholar]
- 43.Lotze, M. T., L. Zitvogel, R. Campbell, P. D. Robbins, E. Elder, C. Haluszczak, D. Martin, T. L. Whiteside, W. J. Storkus, and H. Tahara. 1996. Cytokine gene therapy of cancer using interleukin-12: murine and clinical trials. Ann. N. Y. Acad. Sci. 795:440-454. [DOI] [PubMed] [Google Scholar]
- 44.Ma, X., and L. J. Montaner. 2000. Proinflammatory response and IL-12 expression in HIV-1 infection. J. Leukoc. Biol. 68:383-390. [PubMed] [Google Scholar]
- 45.MacDonald, T. T., and G. Monteleone. 2001. IL-12 and Th1 immune responses in human Peyer's patches. Trends Immunol. 22:244-247. [DOI] [PubMed] [Google Scholar]
- 46.Marshall, J. D., J. Chehimi, G. Gri, J. R. Kostman, L. J. Montaner, and G. Trinchieri. 1999. The interleukin-12-mediated pathway of immune events is dysfunctional in human immunodeficiency virus-infected individuals. Blood 94:1003-1011. [PubMed] [Google Scholar]
- 47.McClure, H. M., D. C. Anderson, P. N. Fultz, A. A. Ansari, E. Lockwood, and A. Brodie. 1989. Spectrum of disease in macaque monkeys chronically infected with SIV/SMM. Vet. Immunol. Immunopathol. 21:13-24. [DOI] [PubMed] [Google Scholar]
- 48.McFarland, E. J., P. A. Harding, S. MaWhinney, R. T. Schooley, and D. R. Kuritzkes. 1998. In vitro effects of IL-12 on HIV-1-specific CTL lines from HIV-1-infected children. J. Immunol. 161:513-519. [PubMed] [Google Scholar]
- 48a.Meyaard, L., S. A. Otto, I. P. Keet, R. A. van Lier, and F. Miedema. 1994. Changes in cytokine secretion patterns of CD4+ T-cell clones in human imunodeficiency virus infection. Blood 84:4262-4268. [PubMed] [Google Scholar]
- 49.Mori, K., Y. Yasutomi, S. Sawada, F. Villinger, K. Sugama, B. Rosenwirth, J. L. Heeney, K. Uberla, S. Yamazaki, A. A. Ansari, and H. Rubsamen-Waigmann. 2000. Suppression of acute viremia by short-term postexposure prophylaxis of simian/human immunodeficiency virus SHIV-RT-infected monkeys with a novel reverse transcriptase inhibitor (GW420867) allows for development of potent antiviral immune responses resulting in efficient containment of infection. J. Virol. 74:5747-5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nadeau, R. R., C. Ostrowski, G. Ni-Wu, and D. J. Liberato. 1995. Pharmacokinetics and pharmacodynamics of recombinant human interleukin-12 in male rhesus monkeys. J. Pharmacol. Exp. Ther. 274:78-83. [PubMed] [Google Scholar]
- 51.National Research Council. 1996. Guide for the care and use of laboratory animals. National Academy of Sciences, Washington, D.C.
- 52.O'Shea, J. J. 1997. Jaks, STATs, cytokine signal transduction, and immunoregulation: are we there yet? Immunity 7:1-11. [DOI] [PubMed] [Google Scholar]
- 53.Picker, L. J., M. K. Singh, Z. Zdraveski, J. R. Treer, S. L. Waldrop, P. R. Bergstresser, and V. C. Maino. 1995. Direct demonstration of cytokine synthesis heterogeneity among human memory/effector T cells by flow cytometry. Blood 86:1408-1419. [PubMed] [Google Scholar]
- 54.Powell, J. D., D. P. Bednarik, T. M. Folks, T. Jehuda-Cohen, F. Villinger, K. W. Sell, and A. A. Ansari. 1993. Inhibition of cellular activation of retroviral replication by CD8+ T cells derived from non-human primates. Clin. Exp. Immunol. 91:473-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stobie, L., S. Gurunathan, C. Prussin, D. L. Sacks, N. Glaichenhaus, C. Y. Wu, and R. A. Seder. 2000. The role of antigen and IL-12 in sustaining Th1 memory cells in vivo: IL-12 is required to maintain memory/effector Th1 cells sufficient to mediate protection to an infectious parasite challenge. Proc. Natl. Acad. Sci. USA 97:8427-8432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Strijbosch, L. W., W. A. Buurman, R. J. Does, P. H. Zinken, and G. Groenewegen. 1987. Limiting dilution assays. Experimental design and statistical analysis. J. Immunol. Methods 97:133-140. [DOI] [PubMed] [Google Scholar]
- 57.Trinchieri, G. 1998. Immunobiology of interleukin-12. Immunol. Res. 17:269-278. [DOI] [PubMed] [Google Scholar]
- 58.Trinchieri, G. 1994. Interleukin-12: a cytokine produced by antigen-presenting cells with immunoregulatory functions in the generation of T-helper cells type 1 and cytotoxic lymphocytes. Blood 84:4008-4027. [PubMed] [Google Scholar]
- 59.Trinchieri, G. 1995. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu. Rev. Immunol. 13:251-276. [DOI] [PubMed] [Google Scholar]
- 60.Tsubota, H., C. I. Lord, D. I. Watkins, C. Morimoto, and N. L. Letvin. 1989. A cytotoxic T lymphocyte inhibits acquired immunodeficiency syndrome virus replication in peripheral blood lymphocytes. J. Exp. Med. 169:1421-1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tsuji, T., K. Hamajima, J. Fukushima, K. Q. Xin, N. Ishii, I. Aoki, Y. Ishigatsubo, K. Tani, S. Kawamoto, Y. Nitta, J. Miyazaki, W. C. Koff, T. Okubo, and K. Okuda. 1997. Enhancement of cell-mediated immunity against HIV-1 induced by coinoculation of plasmid-encoded HIV-1 antigen with plasmid expressing IL-12. J. Immunol. 158:4008-4013. [PubMed] [Google Scholar]
- 62.Vanham, G., L. Penne, J. Devalck, L. Kestens, R. Colebunders, E. Bosmans, K. Thielemans, and J. L. Ceuppens. 1999. Decreased CD40 ligand induction in CD4 T cells and dysregulated IL-12 production during HIV infection. Clin. Exp. Immunol. 117:335-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Villinger, F., S. S. Brar, A. Mayne, N. Chikkala, and A. A. Ansari. 1995. Comparative sequence analysis of cytokine genes from human and nonhuman primates. J. Immunol. 155:3946-3954. [PubMed] [Google Scholar]
- 64.Villinger, F., G. T. Brice, A. Mayne, P. Bostik, and A. A. Ansari. 1999. Control mechanisms of virus replication in naturally SIVsmm infected mangabeys and experimentally infected macaques. Immunol. Lett. 66:37-46. [DOI] [PubMed] [Google Scholar]
- 65.Villinger, F., S. Bucur, N. Chikkala, S. S. Brar, P. Bostik, A. E. Mayne, J. Adams, M. E. Lee, F. J. Novembre, M. K. Gately, A. A. Ansari, and C. D. Hillyer. 2000. In vitro and in vivo responses to interleukin-12 are maintained until the late SIV infection stage but lost during AIDS. AIDS Res. Hum. Retrovir. 16:751-763. [DOI] [PubMed] [Google Scholar]
- 66.Vogel, T. U., T. M. Allen, J. D. Altman, and D. I. Watkins. 2001. Functional impairment of simian immunodeficiency virus-specific CD8+ T cells during the chronic phase of infection. J. Virol. 75:2458-2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang, K. S., E. Zorn, and J. Ritz. 2001. Specific down-regulation of interleukin-12 signaling through induction of phospho-STAT4 protein degradation. Blood 97:3860-3866. [DOI] [PubMed] [Google Scholar]
- 68.Watanabe, N., J. P. Sypek, S. Mittler, K. A. Reimann, P. Flores-Villanueva, G. Voss, C. I. Lord, and N. L. Letvin. 1998. Administration of recombinant human interleukin 12 to chronically SIVmac-infected rhesus monkeys. AIDS Res. Hum. Retrovir. 14:393-399. [DOI] [PubMed] [Google Scholar]
- 69.Wilson, C. C., W. C. Olson, T. Tuting, C. R. Rinaldo, M. T. Lotze, and W. J. Storkus. 1999. HIV-1-specific CTL responses primed in vitro by blood-derived dendritic cells and Th1-biasing cytokines. J. Immunol. 162:3070-3078. [PubMed] [Google Scholar]
- 70.Wolf, S. F., P. A. Temple, M. Kobayashi, D. Young, M. Dicig, L. Lowe, R. Dzialo, L. Fitz, C. Ferenz, and R. M. Hewick. 1991. Cloning of cDNA for natural killer cell stimulatory factor, a heterodimeric cytokine with multiple biologic effects on T and natural killer cells. J. Immunol. 146:3074-3081. [PubMed] [Google Scholar]
- 71.Yap, G., M. Pesin, and A. Sher. 2000. Cutting edge: IL-12 is required for the maintenance of IFN-gamma production in T cells mediating chronic resistance to the intracellular pathogen, Toxoplasma gondii. J. Immunol. 165:628-631. [DOI] [PubMed] [Google Scholar]
- 72.Young, J. M., R. A. Ffrench, J. D. Clarkson, G. J. Stewart, T. Liang, R. L. Tideman, D. Packham, D. A. Fulcher, and E. M. Benson. 2001. In vitro HIV-specific CTL activity from HIV-seropositive individuals is augmented by interleukin-12 (IL-12). AIDS Res. Hum. Retrovir. 17:233-242. [DOI] [PubMed] [Google Scholar]
- 73.Zou, W., A. A. Lackner, M. Simon, I. Durand-Gasselin, P. Galanaud, R. C. Desrosiers, and D. Emilie. 1997. Early cytokine and chemokine gene expression in lymph nodes of macaques infected with simian immunodeficiency virus is predictive of disease outcome and vaccine efficacy. J. Virol. 71:1227-1236. [DOI] [PMC free article] [PubMed] [Google Scholar]