Abstract
The adeno-associated virus (AAV) vector system is based on nonpathogenic and helper-virus-dependent parvoviruses. The vector system offers safe, efficient, and long-term in vivo gene transfer in numerous tissues. Clinical trials using AAV vectors have demonstrated vector safety as well as efficiency. The increasing interest in the use of AAV for clinical studies demands large quantities of vectors and hence a need for improvement in vector production. The commonly used transient-transfection method, although versatile and free of adenovirus (Ad), is not cost-effective for large-scale production. While the wild-type-Ad-dependent AAV producer cell lines seem to be cost-effective, this method faces the problem of wild-type Ad contamination. To overcome these shortcomings, we have explored the feasibility of creating inducible AAV packaging cell lines that require neither transfection nor helper virus infection. As a first step toward that goal, we have created a cell line containing highly inducible Ad E1A and E1B genes, which are essential for AAV production. Subsequently, the AAV Rep and Cap genes and an AAV vector containing a green fluorescent protein (GFP) reporter gene were stably introduced into the E1A-E1B cell line, generating inducible AAV-GFP packaging cell lines. Upon induction of E1A and E1B genes and infection with replication-defective Ad with E1A, E1B, and E3 deleted, the packaging cells yielded high-titer AAV-GFP vectors. Finally, the E2, E4, and VA genes of Ad, under the control of their endogenous promoters, were also introduced into these cells. A few producer cell lines were obtained, which could produce AAV-GFP vectors upon simple drug induction. Although future improvement is necessary to increase the stability and vector yield of the cells, our study has nonetheless demonstrated the feasibility of generating helper-virus-free inducible AAV producer cell lines.
The recombinant adeno-associated virus (AAV) vector system is derived from defective parvoviruses, which depend on essential helper functions provided by other viruses, such as adenovirus (Ad), for efficient viral replication and propagation (14). AAV has no etiologic association with any known diseases (2) and has been successfully used to establish efficient and long-term gene expression in vivo in a variety of tissues without significant cellular immune responses or toxicity (33, 36, 37). The success of preclinical studies has led to clinical trials using AAV vectors to treat genetic diseases such as cystic fibrosis (32) and hemophilia (5, 16). Although the use of AAV vectors for human gene therapy is promising, the present vector production methods may not meet the demand for both large-quantity and high-quality AAV vectors for clinical studies involving a wide range of genetic and acquired diseases (11, 25).
The traditional method for AAV vector production is a double plasmid transfection followed by a helper Ad infection. The vector plasmid contains the transgene expression cassette that is flanked by the AAV inverted terminal repeats, while the packaging plasmid provides AAV Rep and Cap genes for vector DNA replication and packaging (1, 17, 24, 28, 31, 38). After infection with wild-type (wt) helper Ad in the transfected cells, the AAV viral genes are expressed and the vector DNA is replicated and packaged into AAV viral particles. In the meantime, a large amount of wt helper Ad is also propagated. While generally useful, this method has two major drawbacks that restrict its utility. One drawback is the labor-intensive transient cotransfection of the vector and packaging plasmids, and the other is the requirement of wt Ad infection for essential helper functions. Even though the Ad can be removed during purification or can be heat inactivated in the purification process, it still poses the risk of contamination (21). Of the numerous approaches to improve AAV vector manufacture, two strategies, which differ in principle, are now widely used. One is based on transient cotransfection of all necessary elements required for AAV production (vector and packaging plasmids and essential helper genes isolated from Ad) into host cells such as 293 or HeLa cells (10, 20, 23, 38). The other strategy relies on wt Ad infection into cell lines that stably harbor AAV Rep and Cap genes along with the AAV vector DNA (or the vector DNA can be brought in by an Ad vector) (4, 14, 29). Although the transient-transfection method generates high titers of AAV vectors that are free of Ad, it is labor-intensive and expensive to scale up for clinical studies. On the other hand, the wt Ad-dependent AAV producer cell lines can be readily scaled up into suspension cultures and produce AAV vectors with titers comparable to those obtained by the transient-transfection method. However, this approach faces a problem of the traditional method, i.e., the propagation of wt helper Ad. Contamination with wt Ad is highly undesirable in view of vector safety. Therefore, novel strategies for AAV vector production, such as the creation of helper-virus-free producer cell lines, are sought to meet the demand for high-quality and high-quantity vectors in both preclinical and clinical studies (11).
The ultimate goal of our studies is to test the feasibility of creating ideal helper-virus-free AAV producer cell lines. The final AAV producer cell lines not only should harbor integrated and rescuable vector plasmid DNA and AAV Rep and Cap genes but also should contain essential helper genes from other viruses such as Ad. For example, five Ad genes, i.e., those for E1A, E1B, E2A, E4, and VA RNA, are required for efficient AAV gene expression, DNA replication, and packaging (22). The key player of these five helper genes is the gene for E1A (26), which the earliest gene product made during Ad infection. E1A not only positively controls the expression of numerous Ad genes such as those for E1B, E2A, and E4, but also trans activates AAV Rep and Cap genes. Therefore, leaky expression of E1A will turn on Ad genes as well as the AAV Rep gene. The latter is well known to be cytostatic and cytotoxic, making it extremely difficult to obtain a stable cell line from cells that constitutively express E1A, for example, 293 cells (40). While regulation of the p5 Rep gene products (Rep 78/68) is possible by replacing the p5 promoter with an inducible one (40), it is very difficult to regulate the two smaller Rep proteins by an inducible promoter, because the p19 promoter, which controls the two small Rep proteins (Rep 52/40), is embedded in the coding sequence of the two large Rep proteins. In addition, the inducible promoters most likely will not deliver temporal and quantitative control in the same manner as the endogenous p5 and p19 promoters. Furthermore, constitutive E1 gene expression will activate the Ad E2A and E4 genes, which are also toxic to the host cells. However, both the E2A and E4 genes are largely silent in the absence of E1A gene expression (26). All of these observations seem to point to a common theme that highly regulated E1A gene expression appears to be crucial to the success of a helper-virus-free AAV packaging cell line.
In the first step of this study, a highly inducible E1A-E1B cell line was established. Subsequently, the AAV Rep and Cap genes and AAV-green fluorescent protein (GFP) vector DNA were introduced into the inducible E1A-E1B cell line, which could produce high-titer AAV-GFP vector upon induction of the E1A gene and infection with a replication-defective Ad (with E1A, E1B, and E3 deleted). This improvement eliminated the tedious transient-transfection step as well as the requirement for wt Ad helper infection. To further test the feasibility of generating helper-virus-free AAV producer cells, the three additional helper genes (E2, E4, and VA RNA) from Ad were delivered into these cells. Our results indicate that creating a helper-virus-free AAV producer cell line is feasible, although the strategy needs further improvement.
MATERIALS AND METHODS
Plasmid construction.
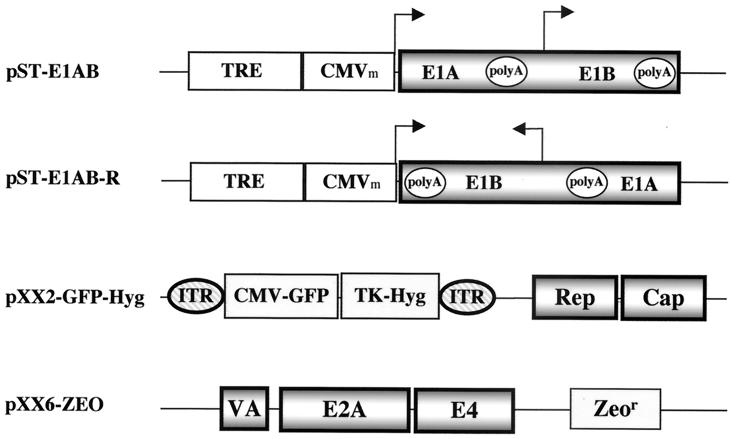
To construct the tetracycline (TET)-responsive plasmid for Ad E1A and E1B expression, the E1A-E1B gene fragment was cloned into the PBI vector (Clontech) downstream of the minimal cytomegalovirus (CMV) promoter. In detail, the E1A and -B coding sequence (nucleotides 498 to 3635 of Ad type 2) was obtained by PCR and inserted at the unique EcoRV site of the PBI vector. Plasmids containing the E1A and -B genes in both orientations were identified. Plasmid pST-E1AB (Fig. 1) had the E1A and -B genes in the sense orientation, where the E1A gene was driven by the minimal CMV promoter linked to seven tandem copies of the TetR-binding site (27). Plasmid pST-E1AB-R (Fig. 1) had the E1A and -B genes in the reverse orientation, where the E1A gene did not have a promoter. In both plasmids, the E1B gene was driven by its endogenous promoter.
FIG. 1.
Construction of inducible Ad gene expression plasmids and AAV plasmids. The Ad type 2 E1A gene (without a promoter) and the E1B gene (with the endogenous promoter) were PCR amplified and cloned into the EcoRV site of plasmid PBI (Clontech), which has a TET-inducible promoter consisting of a minimal CMV promoter and a TRE enhancer. Plasmid pST-E1AB contains the E1A and -B genes in the sense orientation with respect to the TET-inducible promoter, whereas plasmid pST-E1A/B-R has the E1A and -B genes in the reverse orientation. Plasmid pXX2-GFP-Hyg harbors an AAV vector with a GFP reporter gene and a hygromycin resistance gene flanked by two inverted terminal repeats (ITR) of AAV. This plasmid also harbors the AAV viral coding genes Rep and Cap at a different locus. Plasmid pXX6-ZEO contains a zeomycin resistance gene and three Ad genes (VA RNA, E2A, and E4) that can provide helper functions to AAV.
Plasmid pXX2-GFP-Hyg (Fig. 1) was constructed from pXX2 (38), pTK-Hyg (Clontech) and pXX-GFP (38). In detail, pTK-hyg was digested with HindIII and AflIII. The small fragment containing the hygromycin cassette was purified. This fragment was ligated to the AvrII fragment isolated from pXX-GFP containing the AAV-GFP vector cassette and plasmid backbone. The new construct, pXX-GFP-Hyg, was digested with BglII and XmnI. The larger fragment containing the hygromycin cassette and the AAV-GFP cassette was purified. This fragment was then ligated to the BglII fragment of pXX2 containing the AAV coding sequences (Rep and Cap genes).
Plasmid pXX6-Zeo was constructed from pXX6 (38) and pSV40/Zeo (Invitrogen). Briefly, the Xho-SalI fragment containing the zeocin cassette was inserted into the SalI site of plasmid pXX6. This plasmid contains the Ad E2A, E4, and VA RNA genes with a zeocin resistance marker.
Virus and cells.
Ad-GFP and Ad-LacZ were the commonly used first-generation Ad vectors that lacked the E1A, E1B, and E3 genes. Human 293 cells and the HeLa-tet-off cell line (Clontech) were propagated in Dulbecco's modified Eagle's medium (Gibco) supplemented with heat-inactivated 10% fetal bovine serum (Gibco). Stable transfection was done in HeLa-tet-off cells by the liposome (Gene Therapy Systems Inc.) method. Concentrations of antibiotics for selection of clones and for maintenance of resistant cell lines, respectively, were as follows: G418, 800 and 200 μg/ml; puromycin, 2 and 1 μg/ml; and hygromycin, 200 and 100 μg/ml. Doxycycline (DOX) (Clontech) was added to the medium at 2 μg/ml to repress tetracycline-controlled transactivator-dependent transcription.
To screen for E1A- and E1B-expressing clones, cells were infected with Ad-GFP (multiplicity of infection [MOI] = 5) in the presence or absence of DOX. After incubation for 48 to 72 h, the cells along with the medium were collected. Following four freeze-thaw cycles and removal of cell debris by centrifugation, the lysate was subjected to tests for the yield of Ad-GFP from each cell clone. Clones producing Ad-GFP with a yield of more than 108 transducing units (t.u.) per 10-cm-diameter plate of cells were selected.
To screen for clones expressing AAV Rep and Cap upon induction of the E1A and -B genes, cells were infected with Ad-LacZ (MOI = 5) following removal of DOX and addition of trichostatin A (TSA) (Sigma). After incubation for 12 h, the cells were fed with fresh medium to remove TSA, and they were harvested 48 h later. After freeze-thawing and removal of cell debris by centrifugation, the lysate was heated at 56°C for 1 h to inactivate the Ad-LacZ. The AAV-GFP titers were determined after infecting HeLa cells with Ad type 5 (MOI = 1) and counting the green cells under a florescence microscope. Clones with a yield of AAV-GFP of >5 × 106 t.u./10-cm-diameter plate were selected for further characterization.
Western analysis of Ad E1A protein and AAV Cap protein.
Western blotting was carried out by previously published methods with modifications (13). Briefly, the cell pellet from one well of a six-well plate was lysed in 200 μl of radioimmunoprecipitation assay buffer (10 mM Tris-Cl [pH 8.2], 1% Triton X-100, 1% sodium dodecyl sulfate, 150 mM NaCl). The samples were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (10% polyacrylamide) and transferred to a nitrocellulose membrane. After being subjected to blocking in 10% nonfat dry milk in Tris-buffered saline (TBS) (50 mM Tris-Cl [pH 7.5], 200 mM NaCl) for 1 h, the membranes were incubated at room temperature for 1 h with primary antibodies in TBS containing 0.5% Tween 20. The primary antibody for E1A was a monoclonal antibody (M58; PharMingen) which recognizes Ad type 5 E1A proteins. It was used at a 1:300 dilution. The primary antibody for AAV capsid proteins was a guinea pig polyclonal antibody against AAV type 2 (Braton Biotech, Inc.); it was used at a dilution of 1:400. Following primary antibody incubation and three rinses, the membranes were incubated with secondary antibodies at room temperature for 1 h. The secondary antibody for E1A was a goat anti-mouse immunoglobulin conjugated to horseradish peroxidase (Sigma) at a 1:5,000 dilution. The secondary antibody for AAV capsid proteins was a rabbit anti-guinea pig immunoglobulin conjugated to horseradish peroxidase at a 1:3,000 dilution. All of the antibodies were diluted with 2% dry milk in TBS. After three washes with TBS, the specific protein bands were visualized with chemiluminescence reagent and exposed to X-ray film.
RESULTS
Construction of inducible Ad E1 plasmid and AAV and Ad plasmids.
A hallmark of AAV is its requirement for coinfection with an unrelated virus, such as Ad, to provide essential helper functions for the productive life cycle. Five Ad genes (those for E1A, E1B, E2A, E4, and VA RNA) are sufficient to provide full helper functions, while the E1A gene plays a central role in up-regulating transcriptional activities of numerous Ad genes as well as the AAV Rep and Cap genes. However, E1A-mediated activation of the AAV Rep and Ad E2A and E4 genes will subsequently exert toxic effects on host cells and prevent the generation of producer cell lines. Therefore, it is essential to tightly control the E1A gene expression in an inducible manner to avoid leaky expression. For this purpose, we have constructed an inducible E1A gene expression plasmid, pST-E1AB, in which the E1A gene is under the control of a TET-regulated promoter (TET-responsive element [TRE]) (Fig. 1) (27). In another plasmid (pST-E1AB-R), the E1A and E1B genes are in the reverse orientation to the TRE promoter (Fig. 1). In both constructs, the E1B gene is driven by its endogenous promoter, which is positively regulated by E1A gene products.
We have also constructed an AAV plasmid (pXX2-GFP-Hyg) which not only contains the AAV Rep and Cap genes (38) to provide the viral gene products in trans but also harbors an AAV vector in cis that has a GFP reporter gene and a hygromycin resistance gene flanked by the inverted terminal repeats (Fig. 1). The Rep genes are under the control of their native promoters, p5 and p19, which are highly inducible by the Ad E1A gene products. Finally, to test the feasibility of creating helper-virus-free AAV producer cells, we have constructed an Ad plasmid (pXX6-ZEO) that contains the three additional Ad helper genes E2A, E4, and VA RNA under the control of their native promoters. The zeomycin resistance gene is included as a selection marker (Fig. 1).
Generation of TET-inducible E1-expressing cell lines.
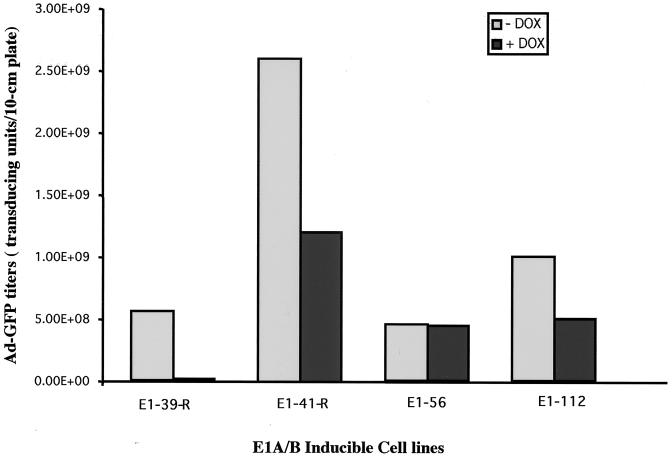
To generate inducible E1A-E1B cell lines, the tet-off system was used to regulate E1A gene expression. In the presence of TET or its analog DOX, the E1A gene should be repressed, while the removal of TET or DOX should turn on the E1A gene, subsequently activating the E1B gene. The inducible pST-E1AB plasmid was cotransfected with a puromycin resistance plasmid into a HeLa-tet-off cell line (Clontech). The cell line expresses a TET repressor-VP16 fusion protein that activates the TRE promoter, whereas the presence of DOX abolishes the activation and represses the TRE promoter. After selection with puromycin, more than 100 clones were obtained. To examine whether those cells could express the E1A and E1B genes upon removal of DOX, we used an Ad vector (Ad-GFP) with E1 deleted as an indicator because its efficient replication requires the E1A and E1B gene products. Upon infection, two of the cell clones (E1-56 and E1-112) were able to efficiently complement the propagation of the Ad vector with E1 deleted, demonstrating the functionality of the E1A and E1B genes in those clones (Fig. 2). However, the E1 gene expression was leaky in those two cell lines, because the yields of Ad-GFP in the presence of DOX were not dramatically decreased. Interestingly, after stable transfection of the pST-E1AB-R plasmid (with the E1 genes in a reverse orientation to the TRE promoter) into the HeLa-tet-off cell line, two cell clones (E1-41-R and E1-39-R) capable of complementing the Ad-GFP vector with E1 deleted were also found (Fig. 2). Unexpectedly, the E1 gene expression in the E1-39-R cell line was highly inducible upon removal of DOX from the tissue culture medium. The yield of the Ad-GFP vector in this cell line was 5 × 108 t.u. per 10-cm-diameter plate, while the vector yield decreased by nearly 2 log units to 7 × 106 t.u. per 10-cm-diameter plate in the presence of DOX (Fig. 2). A possible explanation for this phenomenon may be that the E1A gene in the E1A/B-39-R cell line had integrated in front of a weak cellular promoter, while the TRE became a strong, TET-inducible 3" enhancer. Southern analysis of genomic DNA isolated from the above-described cells revealed that the E1-39-R and E1-56 cell lines had a single copy of the E1A and -B genes, while E1-112 had 2 copies and E1-41-R had more than 10 copies (data not shown).
FIG. 2.
Complementation of the E1A-E1B cell lines for Ad-GFP vector production. Plasmid pST-E1AB or pST-E1AB-R was transfected into HeLa-tet-off cells together with a puromycin selection marker plasmid. Puromycin-resistant clones were screened for their ability to complement Ad-GFP production. The representative clones shown here were infected with Ad-GFP (MOI = 5) in the presence or absence of DOX and incubated for 72 h. The yields of Ad-GFP vector were determined by titer determination on 293 cells.
Reactivation of TET-inducible E1A and E1B expression after promoter shutoff.
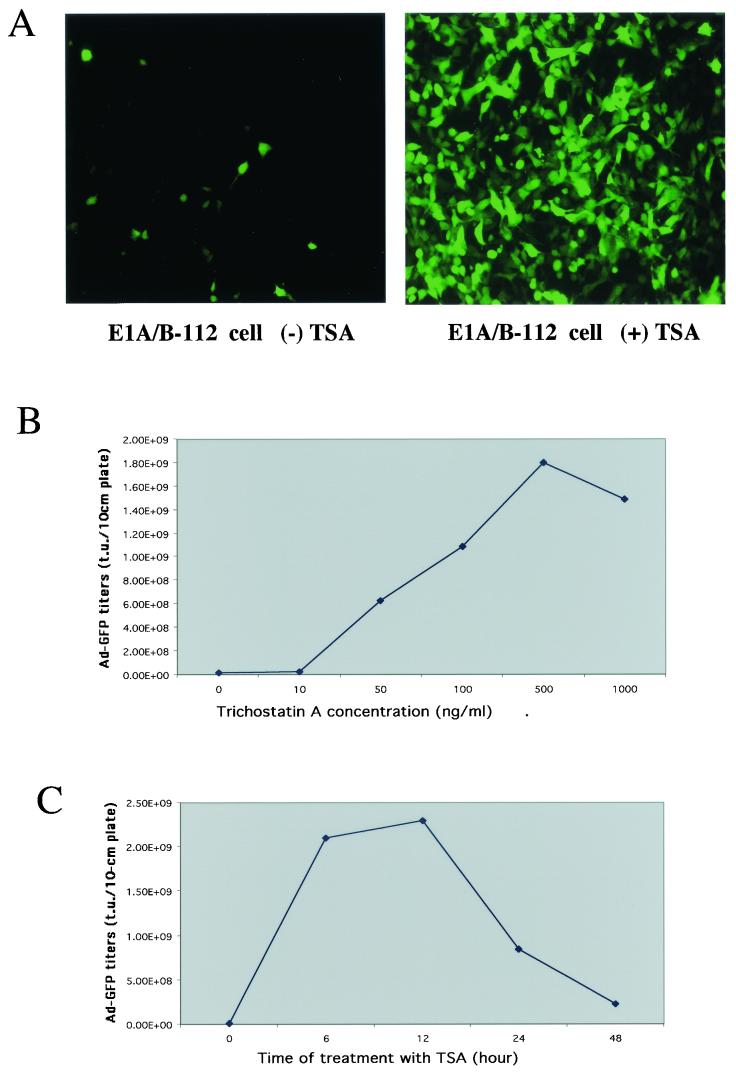
After continuous passages of the above-described E1 cell lines for over a month in the presence of DOX to repress gene expression, the inducibility of the E1A and -B genes decreased significantly, except in the E1-56 cells that had not been TET responsive. Upon induction of E1 genes by removal of DOX, cell lines E1-39-R, E1-41-R, and E1-112 failed to generate high titers of Ad-GFP vector after continuous passages (Table 1). For example, the initial yield of Ad-GFP from the E1-112 cell line was 109 t.u. per 10-cm-diameter plate. After eight passages in 1.5 months, the Ad-GFP yield diminished by 2 log units to 107 t.u. per 10-cm-diameter plate. PCR analysis revealed the presence of the E1 genes, indicating that the loss of E1 gene function might be due to the gene shutoff phenomenon. To test whether E1A and -B gene expression could be reactivated, TSA, a specific inhibitor of deacetylase (3), was added to the cell culture medium. Previous studies have shown that TSA can reactivate genes that were shut off after chromatin inactivation (19). As expected, Ad-GFP vector yields were effectively recovered in those cell lines after treatment with TSA and removal of DOX. For instance, the Ad-GFP yield in cell line E1-112 recovered from 1.0 × 107 t.u./10-cm-diameter plate before TSA treatment to 1.8 × 109 t.u./10-cm-diameter plate after TSA treatment (Fig. 3A).
TABLE 1.
Ad-GFP vector yields in E1A-E1B HeLa-tet-off cells after serial passages
| Cell line | Passage | Ad-GFP (t.u./10-cm-diameter plate)a |
|---|---|---|
| E1-41-R | 1 | 2.6 × 109 |
| 3 | 5.0 × 108 | |
| 6 | 1.6 × 107 | |
| E1-39-R | 1 | 5.6 × 108 |
| 3 | 2.0 × 108 | |
| 6 | 3.7 × 107 | |
| E1-112 | 1 | 1.0 × 109 |
| 3 | 4.2 × 108 | |
| 8 | 1.9 × 107 |
The Ad-GFP titers are mean values from three experiments performed with 10-cm-diameter plates of the indicated cell lines. The tranducing units were determined by infecting HeLa cells with various dilutions of Ad-GFP virus stock. Under the fluorescence microscope, each green cell was translated into 1 t.u.
FIG.3.
Reactivation of the TET-inducible TRE promoter by TSA after promoter shutoff. (A) Ad-GFP production before and after reactivation of the TRE promoter in E1-112 cells by TSA. The E1-112 cells were infected with Ad-GFP (MOI = 5) in the absence of DOX and without or with TSA treatment (see Materials and Methods). Shown here are the Ad-GFP vectors produced by the E1-112 cells and with titers determined on 293 cells. The Ad-GFP yields were 2.9 × 106 t.u./10-cm-diameter plate without induction and 1.8 × 109 t.u./10-cm-diameter plate with induction. (B) Effects of TSA concentrations on Ad-GFP production in the E1-112 cell line. (C) Effects of TSA incubation time on Ad-GFP production in the E1-112 cell line.
Since the E1-112 cell line could complement the Ad-GFP vector propagation most efficiently, we further characterized this cell line by optimizing the conditions of TSA treatment (Fig. 3B and C). The optimal conditions were 12 h of incubation with 500 ng of TSA per ml in the absence of DOX, yielding 1.8 × 109 t.u. of Ad-GFP per 10-cm-diameter plate of cells. This cell line could also be reactivated by another deacetylase inhibitor, sodium butyrate (3) (data not shown), confirming that gene shutoff was the reason for the gradual loss of the complementing capacity of the E1 gene during continuous cell passage.
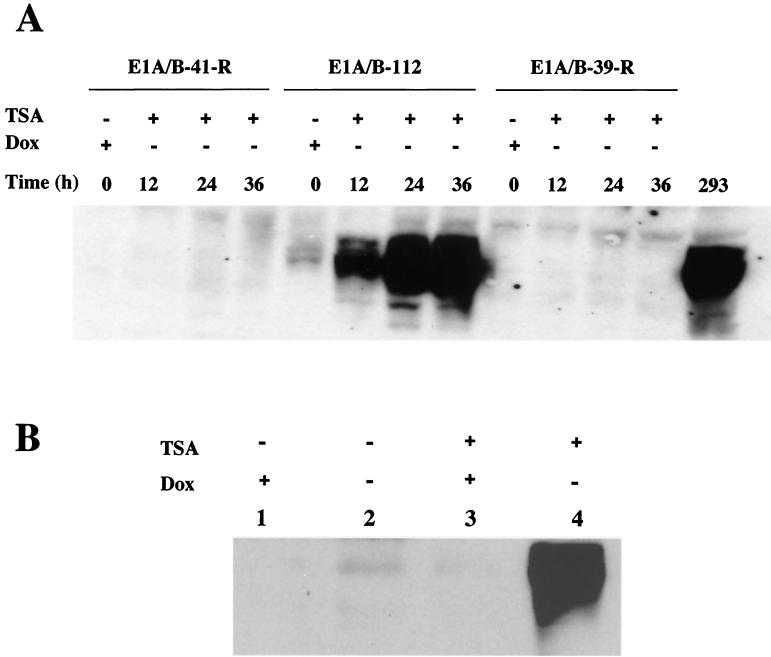
To verify the inducibility of E1A gene expression, Western blotting was performed on the E1 cell lines at different time points after TSA treatment. As shown in Fig. 4A, the E1A gene in the E1-112 cell line began to be expressed at 12 h after induction (removal of DOX and addition of TSA). At 24 h after induction, E1A gene expression reached levels comparable to those in 293 cells. In addition, the E1A gene expression was dependent not only on the activation by TSA but also on the removal of DOX repression, since either TSA treatment without removal of DOX (Fig. 4B, lane 3) or removal of DOX without TSA treatment (Fig. 4B, lane 2) failed to induce significant E1A gene expression. It is interesting that the E1A gene was initially very leaky in the E1-112 cell line. However, after gene shutoff, the transcription machinery driving the leaky expression of the E1A gene in this cell line seemed no longer to be accessible to TSA-mediated reactivation, whereas the TET-inducible TRE remained viable. As expected, E1B gene expression also increased after induction in E1-112 cells (data not shown). However, other cell lines gradually lost their inducibility of E1 gene expression (Fig. 4A).
FIG. 4.
Western analysis of E1A gene expression after TSA and DOX induction. (A) E1A gene expression in three E1 cell lines was examined to see if the gene shutoff can be reversed by TSA. The cells were treated with TSA for 12 h, and E1A gene expression was measured throughout the time course from the beginning of TSA treatment in the absence of DOX repression. 293 cells were used as the positive control for E1A expression without any treatment. (B) E1A gene reactivation and induction in the E1-112 cell line is dependent on both TSA treatment and removal of DOX repression.
Inducible AAV packaging cell lines that require Ad with E1A, E1B, and E3 deleted.
On the basis of our results with the highly inducible E1-expressing cell line E1-112, we set out to test whether the AAV Rep genes as well as the Cap genes can be stably incorporated into cells that contain the tightly controlled E1 genes. If so, infection of such E1 cell lines with a replication-defective Ad, which has E1A, E1B, and E3 deleted but can provide the other three helper functions (E2A, E4, and VA RNA), should lead to AAV vector replication and packaging. To create such E1-inducible Rep-Cap cell lines, the E1-112 cell clone was stably transfected with the AAV plasmid XX2-GFP-Hyg (Fig. 1), which had the AAV Rep and Cap genes, along with an AAV vector containing the GFP and hygromycin resistance genes. In the presence of DOX to prevent E1 gene expression, hygromycin-resistant clones were selected and expanded into 24-well tissue culture plates. To screen for the AAV-GFP packaging cells, an Ad-LacZ vector with E1A, E1B, and E3 deleted was used to provide the E2A, E4, and VA RNA helper functions. After induction of the E1 genes by addition of TSA and removal of DOX and infection with the Ad-LacZ helper virus, more than 40 of approximately 300 cell clones could produce AAV-GFP viruses. Three AAV-GFP producer cell clones (GFP-108, GFP-174, and GFP-214) that at the initial screening produced AAV-GPF vectors at around 107 t.u. per 10-cm-diameter plate were subjected to further characterization. Following optimization with different amounts of Ad-LacZ helper virus infection, the yields of AAV-GFP could reach more than 2 × 108 t.u./10-cm-diameter plate (Table 2 and Fig. 5), which was approximately 2 × 1011 to 3 × 1011 vector genome particles/10-cm-diameter plate, as determined by the DNA dot blot method (data not shown). In addition, the growth rates of cell lines GFP-174 and GFP-214 were essentially the same as that of the parental HeLa-tet-off cells after consecutive passages for more than 6 months. Equally important is the fact that these cells maintained their capacity to produce the AAV-GFP vector after gene induction and Ad-LacZ infection. These results therefore confirm the hypothesis that AAV Rep genes can coexist with Ad E1 genes without causing toxicity to the host cell as long as the latter are under tight transcriptional control.
TABLE 2.
Yields of AAV-GFP vector from different AAV-GFP packaging cell lines after infection with helper Ad with E1A, E1B, and E3 deleted
| Cell line | AAV vector yield (t.u./10-cm-diameter plate)a
|
|
|---|---|---|
| Initial | Optimized | |
| GFP-60 | 1.2 × 106 | NDb |
| GFP-113 | 3.3 × 106 | ND |
| GFP-108 | 7.0 × 106 | ND |
| GFP-174 | 1.2 × 107 | 6.3 × 107 |
| GFP-214 | 1.18 × 107 | 2.0 × 108 |
| GFP-265 | 6.6 × 105 | ND |
The AAV-GFP yields are mean values from two experiments. The tranducing units were determined by infecting HeLa cells with various dilutions of AAV-GFP virus stock. Each GFP-positive cell was translated into 1 t.u.
ND, not determined.
FIG. 5.
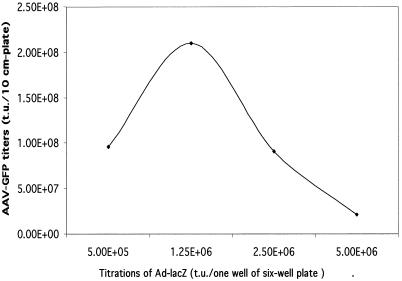
Optimization of AAV-GFP yields with different doses of Ad-LacZ as a helper. The AAV packaging cell line GFP-214 was infected with different doses of helper Ad-LacZ upon induction of the E1 gene (removal of DOX and addition of TSA). The AAV-GFP yields were measured by infecting HeLa cells with various dilutions of AAV. Each green cell was translated into one transducing unit. The results are averages from two separate experiments.
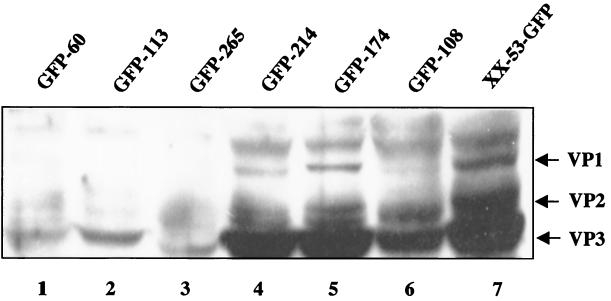
AAV capsid proteins are the essential structural components for AAV viral particle formation. Our previous studies (17) demonstrated a direct correlation between AAV vector yield and the quantity of capsid protein synthesis. However, the correlation between the quantity of Rep proteins and the AAV vector yield is more complex. Therefore, we examined the accumulation of capsid proteins in different packaging cell lines by Western analysis. As expected, the cell lines producing low levels of capsid proteins also produced low titers of AAV (Fig. 6, lanes 1, 2, and 3). Packaging cell lines GFP-214 and GFP-174 (Fig. 6, lanes 4 and 5) produced abundant capsid proteins and high AAV titers (Table 2). The lower levels of capsid protein synthesis in cell line GFP-108 (Fig. 6, lane 6) were also in accordance with its lower AAV vector yield (Table 2). Consistently, the control cell line XX-53-GFP produced the highest levels of capsid proteins (Fig. 6, lane 7), correlating with the highest titer of AAV-GFP (109 t.u. per 10-cm-diameter plate) generated by this cell line. Similar to the E1-inducible AAV packaging cell lines, the XX-53-GFP cells also stably harbored the AAV Rep and Cap genes along with an AAV vector containing the GFP and the Neor genes. However, because this cell line is based on the plain HeLa cell line that does not have Ad E1 genes, it requires wt Ad infection to provide helper functions, similarly to the cell lines generated by Clark et al. (4) (X. Xiao, unpublished observations). We need to caution that capsid gene expression is not the sole factor affecting AAV vector yields. The difference in AAV vector yields between XX-53-GFP cells and the E1-inducible cells may also be attributable to many other factors. Nonetheless, by using the first-generation Ad vector with E1A, E1B, and E3 deleted rather than the wt Ad as a helper for AAV production in the packaging cell lines, we have achieved an incremental and useful improvement toward the goal of generating transfection-free and helper-virus-free AAV producer cell lines. The use of a replication-defective Ad as the helper virus for AAV production should by itself offer an added safety advantage.
FIG. 6.
Western analysis of capsid gene expression from different cell lines. Various AAV-GFP cell lines were infected with Ad-LacZ in the absence of DOX. The E1A gene was induced by treating the cells with TSA for 12 h. The cell line XX-53-GFP is an AAV packaging cell line lacking the E1 genes. Only wt Ad infection can lead to AAV-GFP production in this cell line. Western analysis was performed with an anticapsid polyclonal antibody which recognizes all three capsid proteins.
Inducible AAV producer cell lines free of helper Ad infection.
To create helper-virus-free AAV producer cells, the pXX6-Zeo plasmid (Fig. 1) was further introduced into the GFP-214 cells, which already contained the inducible E1A and E1B genes along with the AAV Rep and Cap genes and an AAV GFP vector. The introduction of the plasmid pXX6-Zeo will add to the packaging cell line the last three essential genes (E2A, E4, and VA RNA) and fulfill the requirement for all components in a productive life cycle of AAV. Ideally, the E2A, E4, and VA RNA genes should also be regulated by the E1A gene products and therefore be indirectly inducible by TSA and the Tet system. After pXX6-Zeo transfection and zeocin selection, more than 100 zeocin-resistant cell clones were obtained in the presence of DOX to repress E1A gene expression. To screen for the helper-virus-free AAV-GFP producer cells, a simple induction experiment was done by removing DOX and adding TSA into the cell culture medium to activate the E1 genes. Two cell lines were identified for their ability to produce significant amounts of AAV-GFP vectors upon drug induction. The yields of AAV-GFP from the cell lines GFP-152-XX6 and GFP-155-XX6 were 3.8 × 106 and 3.0 × 107 t.u. per 10-cm-diameter plate, respectively, in the initial passages. Unfortunately, cell line GFP-152-XX6 gradually lost its capacity to produce AAV vector, while cell line GFP-155-XX6 grew too slowly and eventually stopped growing in the later passages, possibly due to leaky expression of the E2A and E4 genes, which are known to be toxic to the host cells. Apparently, a tighter control of the E2A and E4 genes using exogenous inducible promoters is highly desirable for the success of future inducible AAV producer cell lines. Despite the setback, our studies have demonstrated proof of principle and thus the feasibility of creating a completely inducible AAV producer cell line.
DISCUSSION
In vivo gene therapy, especially for diseases afflicting major organs such as muscle and liver, requires large quantities of high-titer and high-quality vector preparations (15, 34, 35, 39). One of the major hurdles limiting the application of AAV vectors is the efficiency of vector production and potential contamination with helper Ad. To further improve the AAV production technology, we explored the feasibility of creating ideal AAV producer cell lines that require neither transient transfection nor helper virus infection for vector production. We have devised a strategy that sequentially introduces the essential components, including the AAV Rep and Cap genes, the AAV vector DNA sequences, and the five essential Ad helper genes (those for E1A, E1B, E2A, E4, and VA RNA). Using the E1A gene as a general switch to control other viral gene expression, we have obtained stable AAV packaging cell lines that contain highly inducible E1A and E1B genes along with the AAV genes and AAV vectors. Upon infection by a first-generation Ad vector with E1A, E1B, and E3 deleted, the AAV packaging cell lines produced high-titer AAV-GFP vectors, avoiding the tedious transfection step as well as wt helper Ad contamination. Such a strategy and its resultant cell lines should provide immediately applicable tools for transfection-free and wt helper-Ad-free AAV vector production. Further introduction of the E2A, E4, and VA RNA genes generated two cell lines that were inducible by simple chemicals to produce AAV vectors. Much improvement is needed to increase both the stability and productivity of the helper-Ad-free producer cell lines before they can be used for preclinical and clinical purposes.
Previous attempts to establish AAV packaging cell lines have relied mostly on wt Ad to provide helper functions (4, 7, 18). Such strategies lead to the coproduction of AAV vectors along with the wt Ad helpers, thus creating the problem of wt Ad contamination in the recombinant AAV vector stocks. Even though the vast majority of the Ad particles can be removed during vector purification, the use of wt Ad is still highly undesirable (21). By contrast, the cell lines generated in this study have the E1A and E1B genes stably integrated into the chromosomes. Therefore, a replication-defective Ad with E1A and E1B deleted is sufficient to provide helper functions. Compared with the wt Ad, the use of replication-defective Ad vectors as the helper virus for AAV is safer for the production of clinical-grade AAV vectors. Given the fact that Ad vectors with E1A and E1B deleted have been directly delivered into human patients with high doses in numerous gene therapy protocols, a trace amount of contamination with defective Ad in AAV stocks, though undesirable, poses smaller risks than that with wt Ad does.
The E1A-E1B cell line described here may offer potential uses for production of first-generation Ad vectors free of replication-competent Ad, because the E1 gene sequence used in this cell line has less homology than the one used in an Ad packaging cell line free of replication-competent Ad previously published by another group (6). The inducible E1A-E1B cell line may also be helpful for the generation of Ad vector packaging cell lines containing both the E2 and E4 genes. Ad vectors are currently widely used for gene transfer in basic research as well as in gene therapy trials. mainly due to the ease of vector production. In addition, an Ad vector's ability to transduce a wide range of dividing and nondividing cells and its high-level expression of foreign proteins without chromosomal integration are also attractive features. Nonetheless, several factors have significantly limited the utility of earlier generations of Ad vectors (8). For example, toxicity and immunogenicity associated with viral genomic backbone gene expression are a major obstacle to successful gene therapy. Recently, efforts to improve Ad vectors for in vivo applications have been focused on the sequential deletion of essential early genes. Ad vectors in this category grow in packaging cell lines that complement the deleted viral genes (9). The Ad vector with E1 deleted (first generation) and Ad vectors with E1 and E2A (8) or E1 and E4 (9, 12) mutated (second generation) are propagated in packaging cell lines complementing the mutated Ad genes. The gutless Ad vectors that have all the Ad genes deleted also require the helper functions from the first- or second-generation Ad vectors. Therefore, cross contamination remains a technical concern. Dexamethasome-induced Ad E1-E2A cells (8) and an E1-E4-expressing cell line (12) have been described. However, complementing cells that express the E1 gene and both the E2 and E4 genes have not been established. Part of the reason has been the constitutive expression of E1A gene products, which serve as transactivators and up-regulate numerous Ad early and late genes. Expression of E2 and E4 genes is toxic to the cells (38). Therefore, the inducible E1A-E1B cells may be useful in establishing Ad packaging cell lines containing both E2 and E4 and possibly other genes.
Although we have demonstrated proof of principle that a complete inducible AAV producer cell line is feasible, the cell lines we obtained were not stable and the vector yields were not high. An ideal helper-virus-free AAV vector producer cell line not only should eliminate the costly transfection and undesirable helper virus infection but also should possess stability for large-scale cell expansion and high-efficiency vector production. In our study, the sequential introduction of the inducible E1 gene, AAV Rep and Cap genes, and AAV vector DNA did not affect the growth rate of the HeLa-based packaging cell line. The results suggest that tight control of the E1A gene is sufficient to prevent the expression of AAV Rep genes and the concurrent toxic effects. However, it is also apparent from our study that tight control of E1A gene is not sufficient to prevent the expression of E2A and E4 genes driven by their own viral promoters (38). It seems that basal levels of E2A and E4 expression were still toxic to the cells even without the E1A-mediated activation. We believe that this is the primary reason why we failed to obtain stable AAV producer cell lines harboring all of the essential components, including the E2 and E4 genes. To create an ideal helper-virus-free AAV producer cell line, it is necessary to explore other inducible systems that confer tight regulation of the transgenes, such as E2A and E4 gene expression (30, 41). Alternatively, the E2A and E4 genes may be introduced into the E1A inducible cell line prior to the stable transfection of the AAV Rep and Cap genes and AAV vector DNA. Such a sequence of events should be more useful for establishing a more versatile packaging cell line suitable for different AAV vectors as well as for Ad vectors.
In summary, we have established inducible AAV packaging cell lines that produced high-titer AAV vectors after induction of the E1A gene and infection with a replication-defective Ad. In addition, inducible AAV vector producer cell lines, which harbored integrated AAV-GFP vector DNA, AAV coding genes, and the Ad helper genes in a highly inducible manner, were also created. These prototype producer cell lines were able to generate the AAV-GFP vector without either transient plasmid transfection or helper virus infection. Additional efforts are required to further explore and improve the helper-virus-free AAV producer cell lines.
Acknowledgments
We thank Michael Y. Xiao for his critical reading of the manuscript.
This work was supported by NIH grant AR 45967.
REFERENCES
- 1.Bartlett, J. S., X. Xiao, and R. J. Samulski. 1996. Adeno-associated virus vectors for gene transfer, p. 115-127. In P. R. Lowenstein and L. W. Enquist (ed.), Protocols for gene transfer in neuroscience: towards gene therapy of neurological disorders. John Wiley & Sons Ltd., Chichester, United Kingdom.
- 2.Berns, K. I., and R. A. Bohenzky. 1987. Adeno-associated viruses: an update. Adv. Virus Res. 32:243-306. [DOI] [PubMed] [Google Scholar]
- 3.Chen, W. Y., E. C. Bailey, S. L. McCune, J. Y. Dong, and T. M. Townes. 1997. Reactivation of silenced, virally transduced genes by inhibitors of histone deacetylase. Proc. Natl. Acad. Sci. USA 94:5798-5803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clark, K. R., F. Voulgaropoulou, D. M. Fraley, and P. R. Johnson. 1995. Cell lines for the production of recombinant adeno-associated virus. Hum. Gene Ther. 6:1329-1341. [DOI] [PubMed] [Google Scholar]
- 5.Fabb, S. A., and J. G. Dickson. 2000. Technology evaluation: AAV factor IX gene therapy, Avigen Inc. Curr. Opin. Mol. Ther. 2:601-606. [PubMed] [Google Scholar]
- 6.Gao, G. P., R. K. Engdahl, and J. M. Wilson. 2000. A cell line for high-yield production of E1-deleted adenovirus vectors without the emergence of replication-competent virus. Hum. Gene Ther. 11:213-219. [DOI] [PubMed] [Google Scholar]
- 7.Gao, G. P., G. Qu, L. Z. Faust, R. K. Engdahl, W. Xiao, J. V. Hughes, P. W. Zoltick, and J. M. Wilson. 1998. High-titer adeno-associated viral vectors from a Rep/Cap cell line and hybrid shuttle virus. Hum. Gene Ther. 9:2353-2362. [DOI] [PubMed] [Google Scholar]
- 8.Gorziglia, M. I., M. J. Kadan, S. Yei, J. Lim, G. M. Lee, R. Luthra, and B. C. Trapnell. 1996. Elimination of both E1 and E2 from adenovirus vectors further improves prospects for in vivo human gene therapy. J. Virol. 70:4173-4178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gorziglia, M. I., C. Lapcevich, S. Roy, Q. Kang, M. Kadan, V. Wu, P. Pechan, and M. Kaleko. 1999. Generation of an adenovirus vector lacking E1, E2a, E3, and all of E4 except open reading frame 3. J. Virol. 73:6048-6055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grimm, D., A. Kern, K. Rittner, and J. A. Kleinschmidt. 1998. Novel tools for production and purification of recombinant adeno-associated virus vectors. Hum. Gene Ther. 9:2745-2760. [DOI] [PubMed] [Google Scholar]
- 11.Grimm, D., and J. A. Kleinschmidt. 1999. Progress in adeno-associated virus type 2 vector production: promises and prospects for clinical use. Hum. Gene Ther. 10:2445-2450. [DOI] [PubMed] [Google Scholar]
- 12.He, T. C., S. Zhou, L. T. da Costa, J. Yu, K. W. Kinzler, and B. Vogelstein. 1998. A simplified system for generating recombinant adenoviruses. Proc. Natl. Acad. Sci. USA 95:2509-2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hunter, L. A., and R. J. Samulski. 1992. Colocalization of adeno-associated virus Rep and capsid proteins in the nuclei of infected cells. J. Virol. 66:317-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inoue, N., and D. W. Russell. 1998. Packaging cells based on inducible gene amplification for the production of adeno-associated virus vectors. J. Virol. 72:7024-7031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jung, S. C., I. P. Han, A. Limaye, R. Xu, M. P. Gelderman, P. Zerfas, K. Tirumalai, G. J. Murray, M. J. During, R. O. Brady, and P. Qasba. 2001. Adeno-associated viral vector-mediated gene transfer results in long-term enzymatic and functional correction in multiple organs of Fabry mice. Proc. Natl. Acad. Sci. USA 98:2676-2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kay, M. A., C. S. Manno, M. V. Ragni, P. J. Larson, L. B. Couto, A. McClelland, B. Glader, A. J. Chew, S. J. Tai, R. W. Herzog, V. Arruda, F. Johnson, C. Scallan, E. Skarsgard, A. W. Flake, and K. A. High. 2000. Evidence for gene transfer and expression of factor IX in haemophilia B patients treated with an AAV vector. Nat. Genet. 24:257-261. [DOI] [PubMed] [Google Scholar]
- 17.Li, J., R. J. Samulski, and X. Xiao. 1997. Role for highly regulated rep gene expression in adeno-associated virus vector production. J. Virol. 71:5236-5243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu, X. L., K. R. Clark, and P. R. Johnson. 1999. Production of recombinant adeno-associated virus vectors using a packaging cell line and a hybrid recombinant adenovirus. Gene Ther. 6:293-299. [DOI] [PubMed] [Google Scholar]
- 19.Liu, Z., J. Wong, S. Y. Tsai, M. J. Tsai, and B. W. O'Malley. 1999. Steroid receptor coactivator-1 (SRC-1) enhances ligand-dependent and receptor-dependent cell-free transcription of chromatin. Proc. Natl. Acad. Sci. USA 96:9485-9490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsushita, T., S. Elliger, C. Elliger, G. Podsakoff, L. Villarreal, G. J. Kurtzman, Y. Iwaki, and P. Colosi. 1998. Adeno-associated virus vectors can be efficiently produced without helper virus. Gene Ther. 5:938-945. [DOI] [PubMed] [Google Scholar]
- 21.Monahan, P. E., R. J. Samulski, J. Tazelaar, X. Xiao, T. C. Nichols, D. A. Bellinger, M. S. Read, and C. E. Walsh. 1998. Direct intramuscular injection with recombinant AAV vectors results in sustained expression in a dog model of hemophilia. Gene Ther. 5:40-49. [DOI] [PubMed] [Google Scholar]
- 22.Muzyczka, N. 1992. Use of adeno-associated virus as a general transduction vector for mammalian cells. Curr. Top. Microbiol. Immunol. 158:97-129. [DOI] [PubMed] [Google Scholar]
- 23.Salvetti, A., S. Oreve, G. Chadeuf, D. Favre, Y. Cherel, P. Champion-Arnaud, J. David-Ameline, and P. Moullier. 1998. Factors influencing recombinant adeno-associated virus production. Hum. Gene Ther. 9:695-706. [DOI] [PubMed] [Google Scholar]
- 24.Samulski, R. J., L. S. Chang, and T. Shenk. 1989. Helper-free stocks of recombinant adeno-associated viruses: normal integration does not require viral gene expression. J. Virol. 63:3822-3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Samulski, R. J., M. Sally, and N. Muzyczka. 1999. Adeno-associated viral vectors, p. 131-172. In T. Friedmann (ed.), Development of human gene therapy. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 26.Shenk, T. (ed.). 1996. Adenoviridae: the viruses and their replication. Lippincott-Raven, Philadelphia, Pa.
- 27.Shockett, P. E., and D. G. Schatz. 1996. Diverse strategies for tetracycline-regulated inducible gene expression. Proc. Natl. Acad. Sci. USA 93:5173-5176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Snyder, R., X. Xiao, and R. J. Samulski. 1996. Production of recombinant adeno-associated viral vectors, p. 12.1.1-12.2.23. In N. Dracopoli, J. Haines, B. Krof, D. Moir, C. Seidman, and J. S. Seidman (ed.), Current protocols in human genetics. John Wiley & Sons Ltd., New York, N.Y. [DOI] [PubMed]
- 29.Tamayose, K., Y. Hirai, and T. Shimada. 1996. A new strategy for large-scale preparation of high-titer recombinant adeno-associated virus vectors by using packaging cell lines and sulfonated cellulose column chromatography. Hum. Gene Ther. 7:507-513. [DOI] [PubMed] [Google Scholar]
- 30.Urlinger, S., U. Baron, M. Thellmann, M. T. Hasan, H. Bujard, and W. Hillen. 2000. Exploring the sequence space for tetracycline-dependent transcriptional activators: novel mutations yield expanded range and sensitivity. Proc. Natl. Acad. Sci. USA 97:7963-7968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vincent, K. A., S. T. Piraino, and S. C. Wadsworth. 1997. Analysis of recombinant adeno-associated virus packaging and requirements for rep and cap gene products. J. Virol. 71:1897-1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wagner, J. A., A. H. Messner, M. L. Moran, R. Daifuku, K. Kouyama, J. K. Desch, S. Manley, A. M. Norbash, C. K. Conrad, S. Friborg, T. Reynolds, W. B. Guggino, R. B. Moss, B. J. Carter, J. J. Wine, T. R. Flotte, and P. Gardner. 1999. Safety and biological efficacy of an adeno-associated virus vector-cystic fibrosis transmembrane regulator (AAV-CFTR) in the cystic fibrosis maxillary sinus. Laryngoscope 109:266-274. [DOI] [PubMed] [Google Scholar]
- 33.Wang, B., J. Li, and X. Xiao. 2000. Adeno-associated virus vector carrying human minidystrophin genes effectively ameliorates muscular dystrophy in mdx mouse model. Proc. Natl. Acad. Sci. USA 97:13714-13719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang, L., T. C. Nichols, M. S. Read, D. A. Bellinger, and I. M. Verma. 2000. Sustained expression of therapeutic level of factor IX in hemophilia B dogs by AAV-mediated gene therapy in liver. Mol. Ther. 1:154-158. [DOI] [PubMed] [Google Scholar]
- 35.Xiao, W., S. C. Berta, M. M. Lu, A. D. Moscioni, J. Tazelaar, and J. M. Wilson. 1998. Adeno-associated virus as a vector for liver-directed gene therapy. J. Virol. 72:10222-10226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiao, X., W. DeVlaminick, and J. Monahan. 1993. Adeno-associated virus (AAV) vectors for gene transfer. Adv. Drug Delivery Rev. 12:201-215. [Google Scholar]
- 37.Xiao, X., J. Li, and R. J. Samulski. 1996. Efficient long-term gene transfer into muscle tissue of immunocompetent mice by adeno-associated virus vector. J. Virol. 70:8098-8108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xiao, X., J. Li, and R. J. Samulski. 1998. Production of high-titer recombinant adeno-associated virus vectors in the absence of helper adenovirus. J. Virol. 72:2224-2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xiao, X., J. Li, T.-Y. Tsao, D. Dressman, E. P. Hoffman, and J. F. Watchko. 2000. Full functional rescue of a complete muscle (TA) in dystrophic hamsters by adeno-associated virus vector-directed gene therapy. J. Virol. 74:1436-1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang, Q., F. Chen, and J. P. Trempe. 1994. Characterization of cell lines that inducibly express the adeno-associated virus Rep proteins. J. Virol. 68:4847-4856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ye, X., V. M. Rivera, P. Zoltick, F. Cerasoli, Jr., M. A. Schnell, G. Gao, J. V. Hughes, M. Gilman, and J. M. Wilson. 1999. Regulated delivery of therapeutic proteins after in vivo somatic cell gene transfer. Science 283:88-91. [DOI] [PubMed] [Google Scholar]